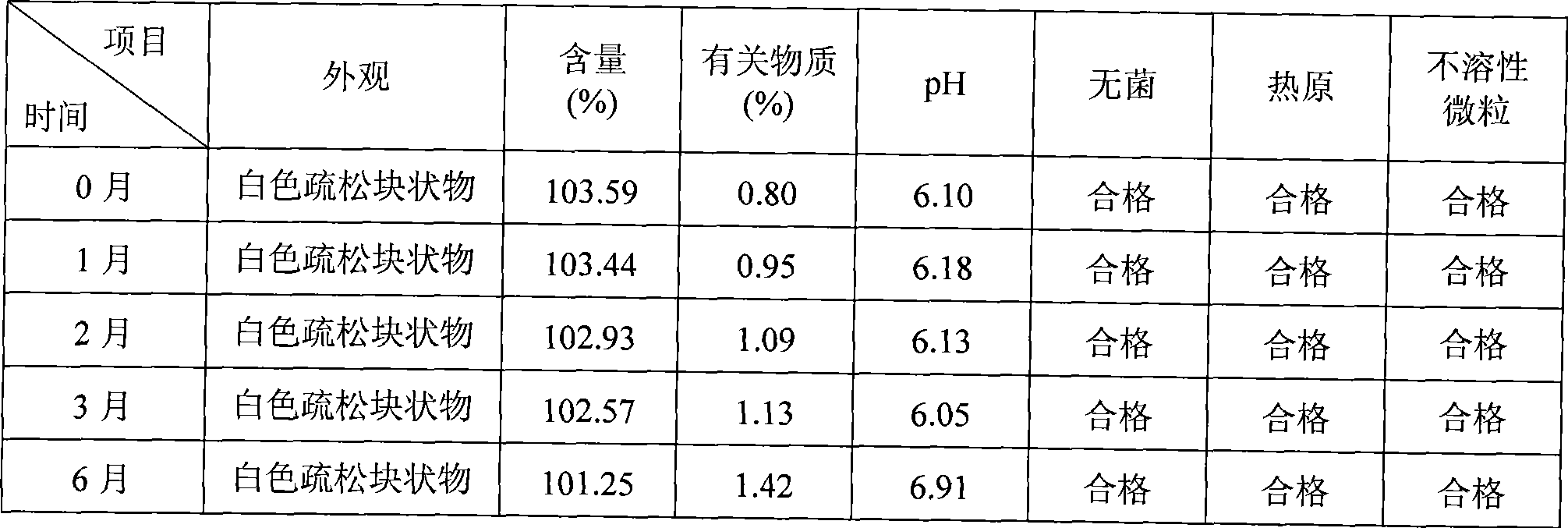
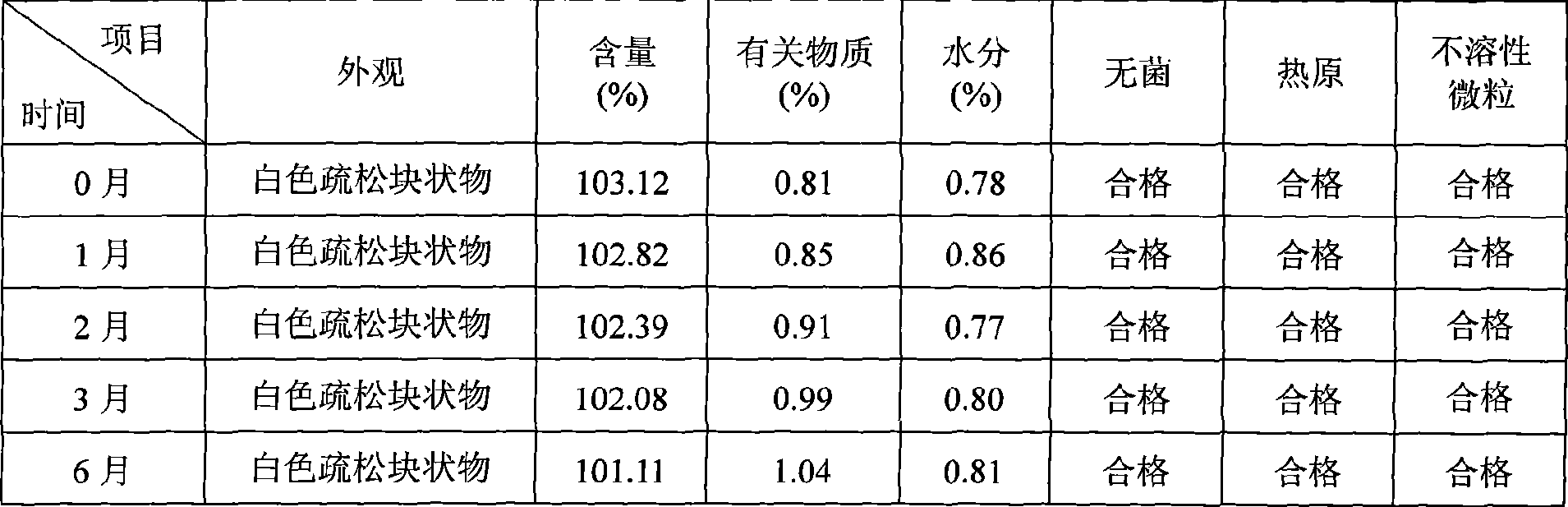
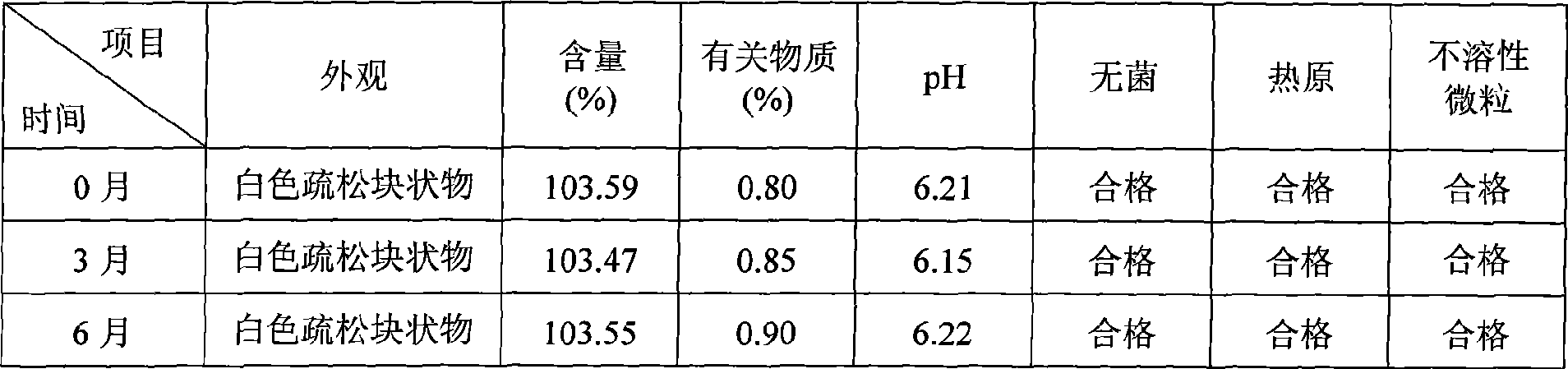
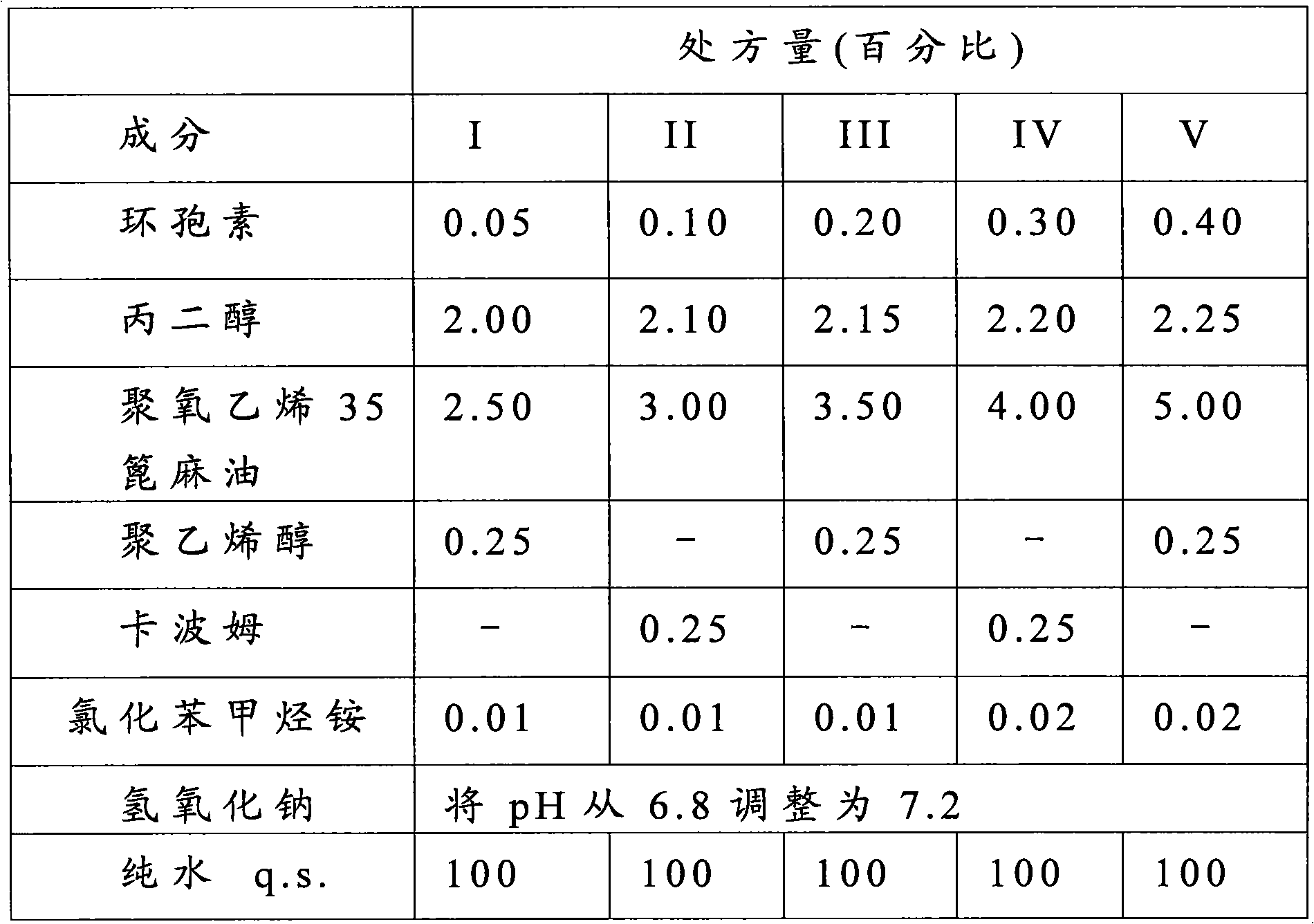
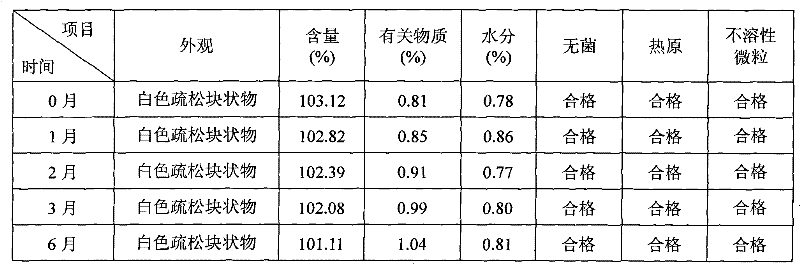
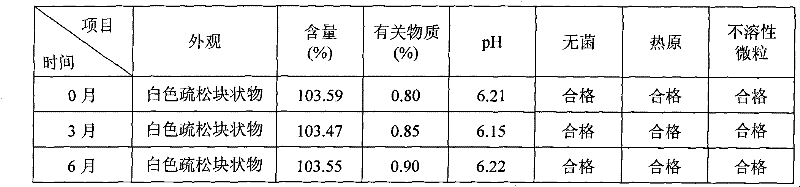
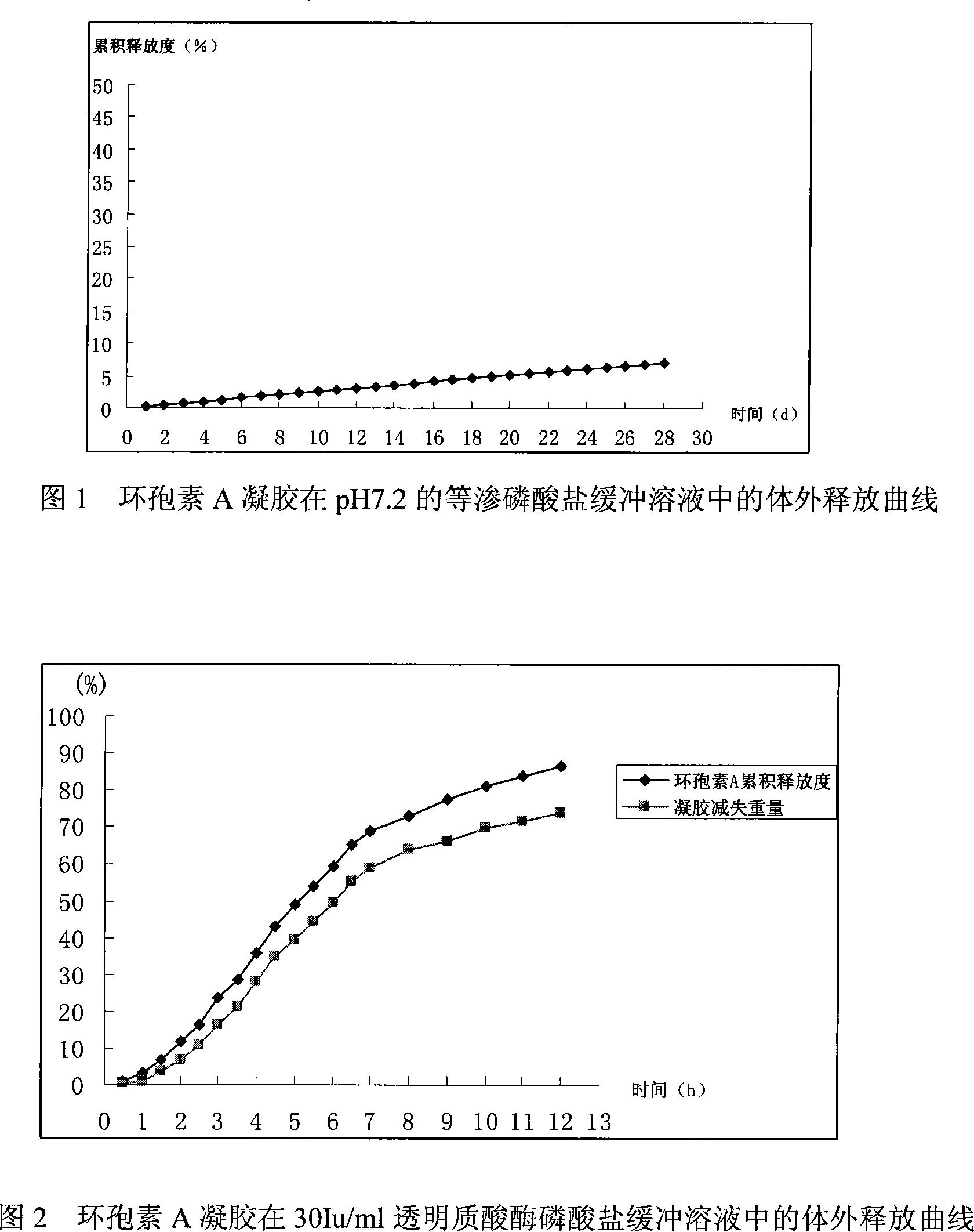
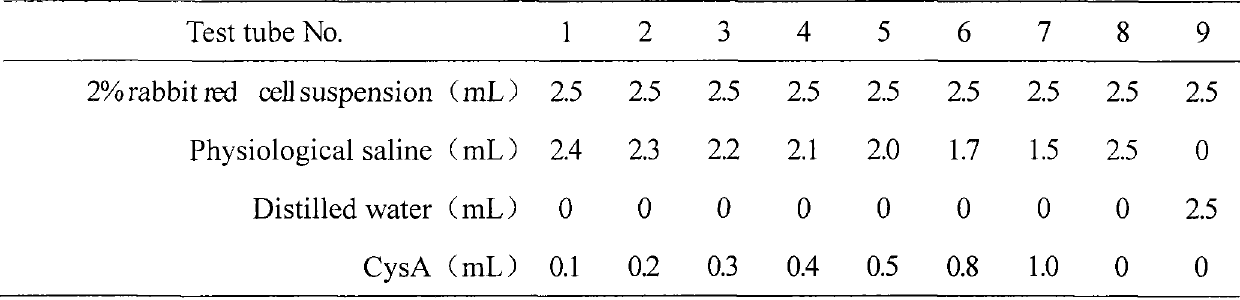
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "Cyclosporin C" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
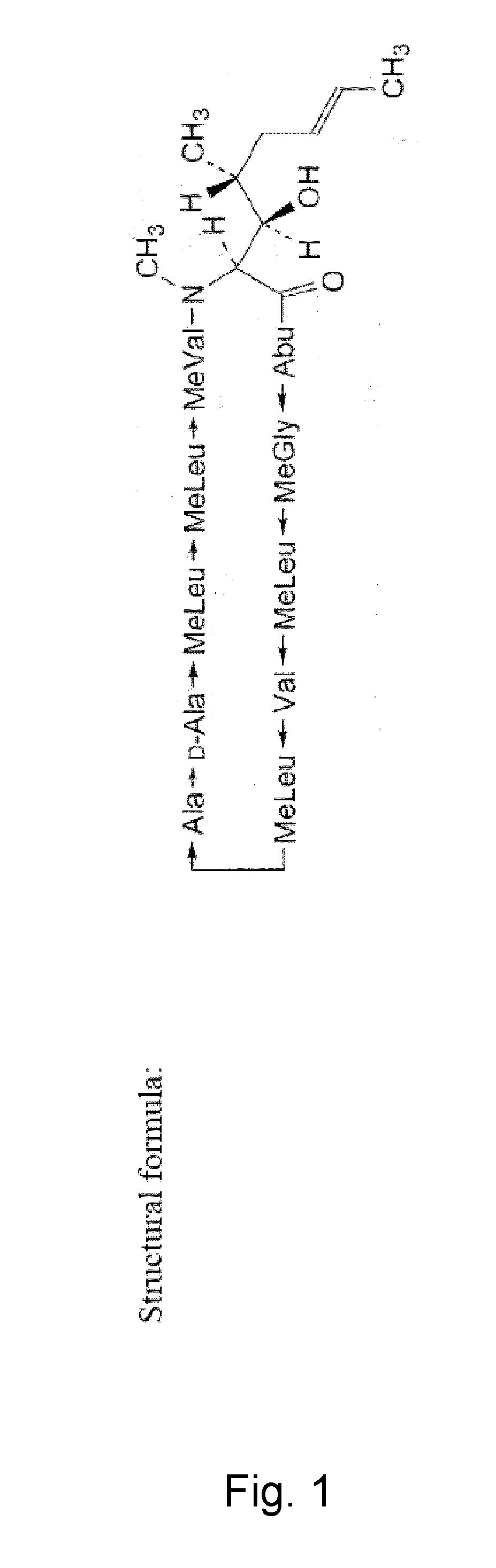
Ciclosporin, also spelled cyclosporine and cyclosporin, is an immunosuppressant medication and natural product. It is taken by mouth or by injection into a vein for rheumatoid arthritis, psoriasis, Crohn's disease, nephrotic syndrome, and in organ transplants to prevent rejection.
Composition
The invention provides microparticles comprising an immunosuppressant, such as tacrolimus, sirolimus, pimecrolimus, ciclosporin, everolimus or a derivative thereof, and optionally a pharmaceutically acceptable excipient or carrier, such as a saccharide, amino acid, a sugar alcohol or a mixture thereof, and having a median geometric diameter of less than, or equal to, about 10 μm and which have a tap density of less than or equal to about 0.3 g / cm3.
Owner:INNOVATA LTD
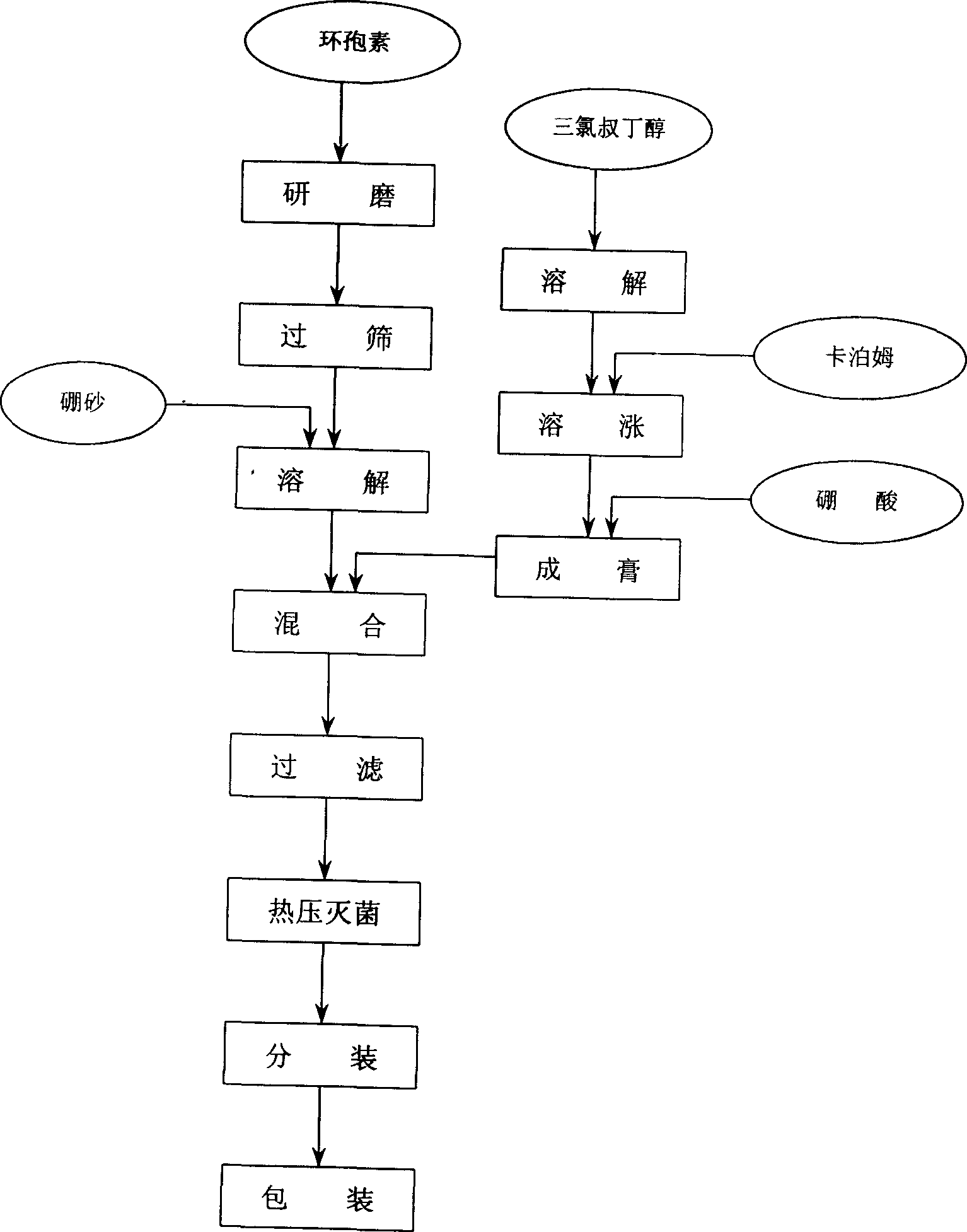
Eye cyclosporin gel
InactiveCN1456350ANot easy to diluteGood water solubilitySenses disorderPharmaceutical delivery mechanismCyclosporinsWhole body
A cyclosporin eye gel for treating the rejection reaction of corneal transplantation, keratoconjunctival xerosis, catarrhal conjunctivitis, and chemical burn of eye is prepared from cyclosporin, boric acid, borax, trichloro-tert-butanol, etc. Its advantages are long stay time in eye and high curative effect.
Owner:刘继东
Novel sacculus dilating catheter
The present invention provides a new type balloon dilation catheter which includes ballon and medication material coated on stent. Said medication material comes from one or two and more than two mixtures of heparin sodium, fiber degrading enzyme, serine proteinase, batroxobin, aspirin, genistein, hirudin and its recombined product, colchicine, sirolimus, biolimus, zotarolimus, tracrolimus, pimecrolimus, simvastatin, atorvastatin, pravastatin, ciclosporin, Anti-CD34, dexamethasone, bleomycin, plicamycin, daunomycin, mitomycin C, actinomycin D, taxol, celastrol, methopterin, 5-fluorouracil, cytarabine and 6-purinethol. The balloon is made of macromolecule nylon material, and the stimulation to blood vessel is far lower than the stent with metal structure.
Owner:上海赢生医疗科技有限公司
Preparation method of ciclosporin ophthalmic solution
InactiveCN103656617ASolve solubilitySmall local irritationSenses disorderPharmaceutical delivery mechanismSolubilityEmulsion
The invention relates to an ophthalmic solution which comprises ciclosporin. Due to adoption of a micro emulsion technique, the problem of solubility of ciclosporin in water is solved. The ophthalmic solution is applicable to ophthalmic preparations, and has the characteristics of small irritation and good stability. The ophthalmic solution provided by the invention is reasonable in formula and stable in process, and is applicable to industrial production and clinical application.
Owner:SHENYANG XINGQI PHARM CO LTD
Ophthalmic ciclosporin emulsion
InactiveCN105726479AHigh partition coefficientHigh content of active ingredientsSenses disorderCyclic peptide ingredientsEmulsionCiclosporin
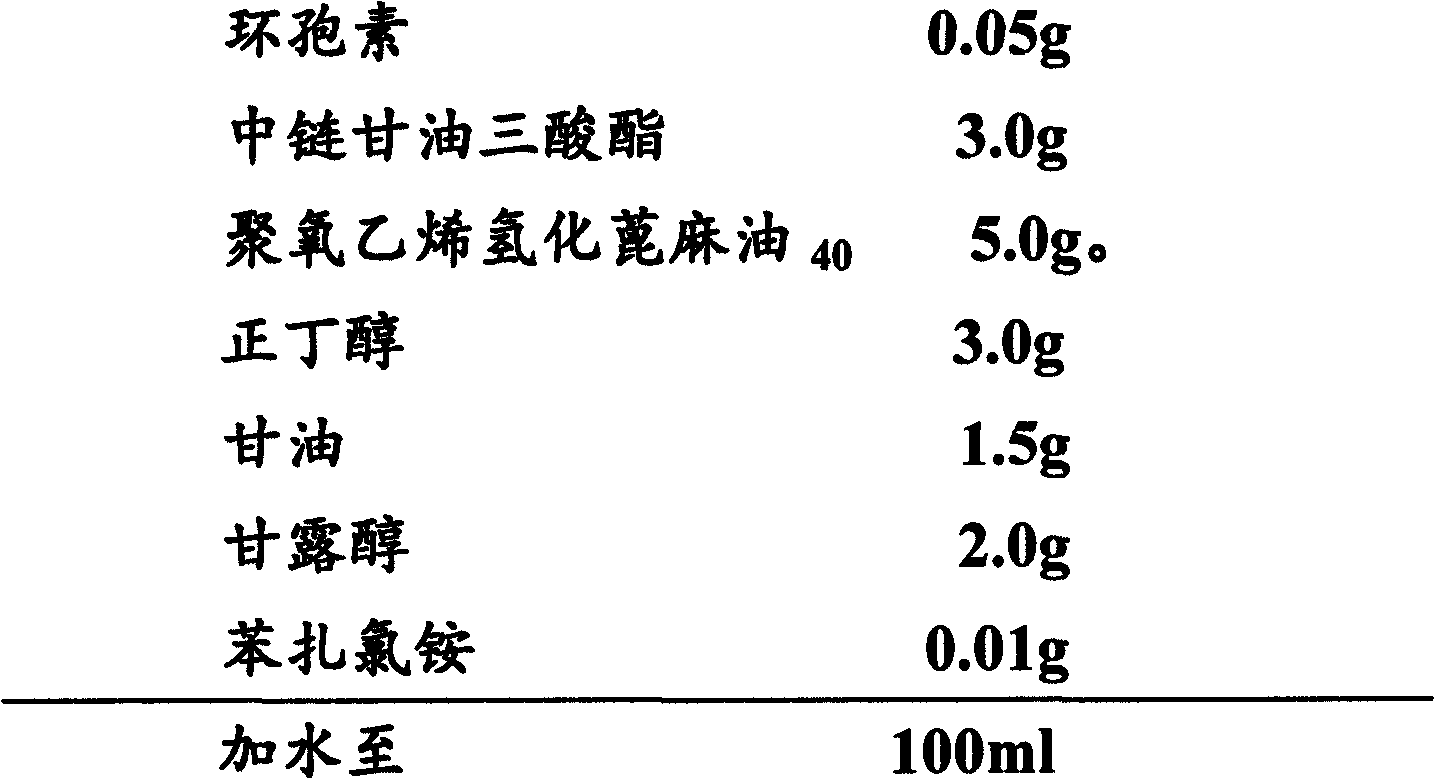
The invention provides ophthalmic ciclosporin emulsion. The ophthalmic emulsion is characterized by comprising the following components by weight percent: 0.02-0.1% of ciclosporin, 2.5-4% of polyoxyethylated castor oil, 1-3% of middle-chain fatty glyceride, 2-5% of glycerin, 0.2-0.5% of sodium alginate and the balance of water.
Owner:北京茗泽中和药物研究有限公司
Application of HMGCoA reductase inhibitor in preparation of medicine used for treating xerophthalmia
InactiveCN103007284AImprove permeabilityPromote secretionSenses disorderEster active ingredientsSide effectOphthalmic drug
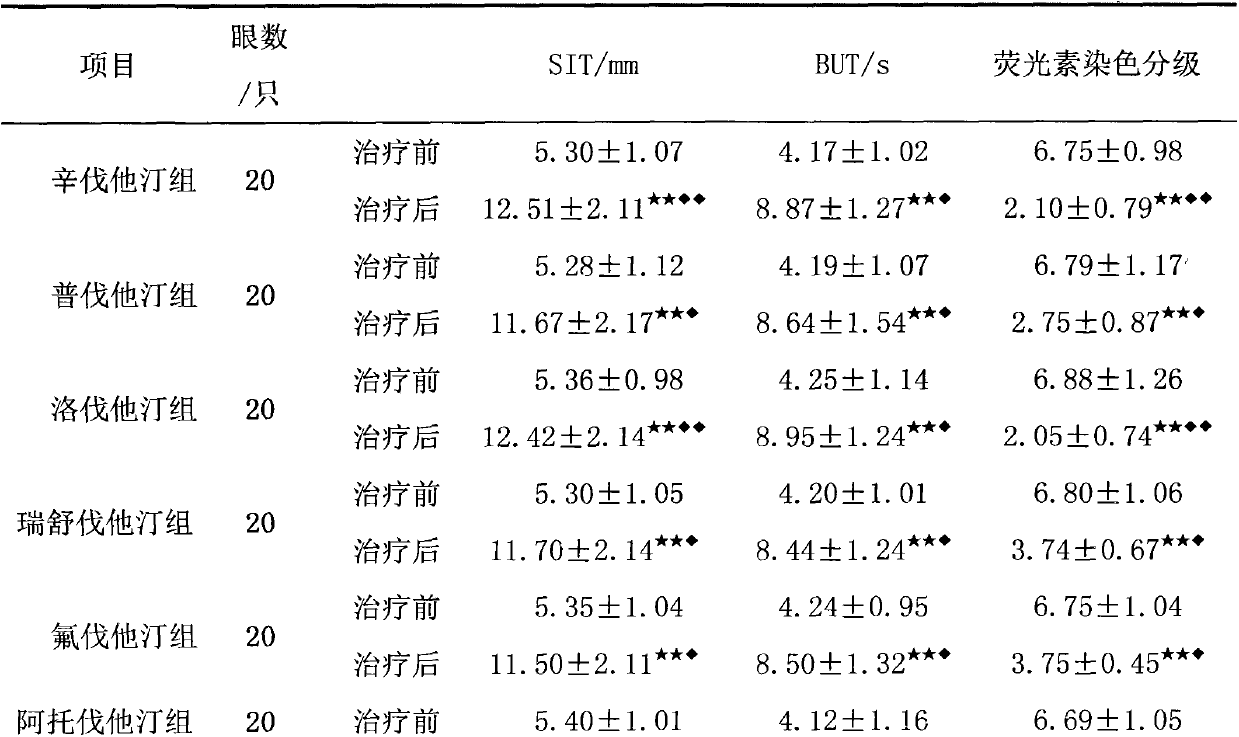
The invention relates to an application of an HMGCoA reductase inhibitor in preparation of a medicine used for treating xerophthalmia and belongs to the field of medicines. The inventor finds that a frequently-used lipid regulation medicine, the HMGCoA reductase inhibitor, can obviously prolong SIT time and BUT time and obviously reduce an FLS score when being used for treating the xerophthalmia, and the HMGCoA reductase inhibitor is better than ciclosporin or artificial tears in the aspect of improving the indexes, wherein simvastatin and lovastatin have the best treatment effect. The HMGCoA reductase inhibitor also can be prepared into oral preparation used for treatment or adjuvant therapy of the xerophthalmia. The HMGCoA reductase inhibitor has a definite curative effect and less toxic and side effects, compliance of a patient is high, and clinical ophthalmic drugs are further enriched.
Owner:闫莹
Cyclosporine emulsion and the preparing method
The present invention provides one kind of self-emulsified ciclosporin preparation and its preparation process. The self-emulsified ciclosporin preparation contains ciclosporin 5-25 wt%, vitamin E-TPGS 25-70 wt% and Pharmasolve 5-50 wt%. It has high stability, high medicine concentration, great capacity of self emulsifying, high bioactivity and easy taking, and may be used as orally taken liposoluble medicine.
Owner:SHANGHAI KAIZHAO PHARMA TECH
Composition of ciclosporin A and amphipathic chitosan derivatives and preparation thereof
InactiveCN102120027AHigh drug loadingHigh encapsulation efficiencyPharmaceutical delivery mechanismCyclic peptide ingredientsCholic acidSide effect
The invention relates to the field of pharmaceutic preparations, and discloses a composition of ciclosporin A and amphipathic chitosan derivatives (N,O-carboxymethyl N-cholic acid chitosan) and a preparation method thereof. The composition has the characteristics of high medicament loading rate, high stability and high possibility of being absorbed, and can be used for overcoming the defects such as serious toxic or side effect of the existing ciclosporin A. The preparation method of the composition is simple, and the process is mature, thus the preparation method id applicable to large-scale industrial production.
Owner:CHINA PHARM UNIV
Composition
The invention provides microparticles comprising an immunosuppressant, such as tacrolimus, sirolimus, pimecrolimus, ciclosporin, everolimus or a derivative thereof, and optionally a pharmaceutically acceptable excipient or carrier, such as a saccharide, amino acid, a sugar alcohol or a mixture thereof, and having a median geometric diameter of less than, or equal to, about 10 μm and which have a tap density of less than or equal to about 0.3 g / cm3.
Owner:INNOVATA LTD
Method for extracting umbilical cord blood hematopoietic stem cells under low-oxygen environment
ActiveCN107475197AOvercoming non-physiological oxygen stressPrecise temperature controlCulture processBlood/immune system cellsHydroxyethyl starchBlood collection
The invention discloses a method for extracting umbilical cord blood hematopoietic stem cells under a low-oxygen environment. The method specifically comprises the following steps: adding 80ng / ml to 120ng / ml of ciclosporin A into an anticoagulant added into a sterile blood collection bag; adding 30ng / ml to 70ng / ml of the ciclosporin A into a hydroxyethyl starch solution sedimentation agent; carrying out a whole-process extraction process of the hematopoietic stem cells in a sealed low-oxygen working station. The low-oxygen working station is provided with independent operation platforms at two sides and a transferring gate; the oxygen concentration of the operation platforms at two sides is set to 3 percent to 8 percent; the temperature of the operation platform at one side is set to 3 DEG C to 10 DEG C and the operation platform is used for carrying out preparation and sub-packaging of a reagent, transferring umbilical cord blood and cryopreserving the umbilical cord blood hematopoietic stem cells; the temperature of the operation platform at the other side is set to 18 DEG C to 25 DEG C and the operation platform is used for carrying out sedimentation of the umbilical cord blood, carrying out blood serum separation on the umbilical cord blood and centrifuging after the blood serum separation. According to the method disclosed by the invention, the hematopoietic stem cells with more quantity, more original state and strong proliferation and differentiation capabilities can be extracted.
Owner:希瑞干细胞科技有限公司
Method for detecting plasma concentration of ciclosporin A by adopting competition method
The invention relates to a method for detecting plasma concentration of ciclosporin A by adopting a competition method, which comprises the steps of: coupling the ciclosporin A on the surface of a magnetic bead, adding a plasma sample after anticoagulation and dilution in a sample hole, adding a ciclosporin A monoclonal antibody marked by fluorescein, eluting the mixture, detecting the fluorescence intensity under excitation of exciting light, and obtaining the plasma concentration of ciclosporin A. The method for detecting plasma concentration of ciclosporin A is simple to operate, has high detection speed, uses less samples and has low cost, higher sensitivity and wider linear range compared with an ELISA (Enzyme-Linked Immuno Sorbent Assay) method, and good application prospect.
Owner:SHANGHAI GENEXT MEDICAL TECH
Injection for treating hyperthyroidism
InactiveCN101474397AReduce volumeHigh remission rateOrganic active ingredientsCyclic peptide ingredientsLarynxSevere hypothyroidism
The invention relates to an injection medicament for treating hyperthyroid, which comprises the following components by weight part: 2 to 5 milligrams of dexamethasone injection, 0.5 to 1 milligram of octreotide injection, and 10 to 20 milligrams of ciclosporin A. Immune preparations such as the dexamethasone injection, the octreotide injection, the ciclosporin A and the like are adopted for treating the hyperthyroid by locally injecting the thyroid, the volume of the tumid thyroid is remarkably shrunk, and the function of the thyroid basically recovers within two months, so the injection medicament has remarkable curative effect, and can remarkably improve the remission rate of hyperthyroid and reduce the rate of relapse. Serious abnormal change, hypothyroidism and parathyroid hypofunction are not caused, and laryngeal recurrent nerve injury is also not caused. Through more than 580 cases of clinical therapy in related hospitals, the volume of the tumid thyroid is remarkably shrunk after therapy, clinical symptoms disappear, allergic response and complications are not caused, the effective percentage is nearly 100 percent, and the cure rate is as high as 98 percent.
Owner:陈小国
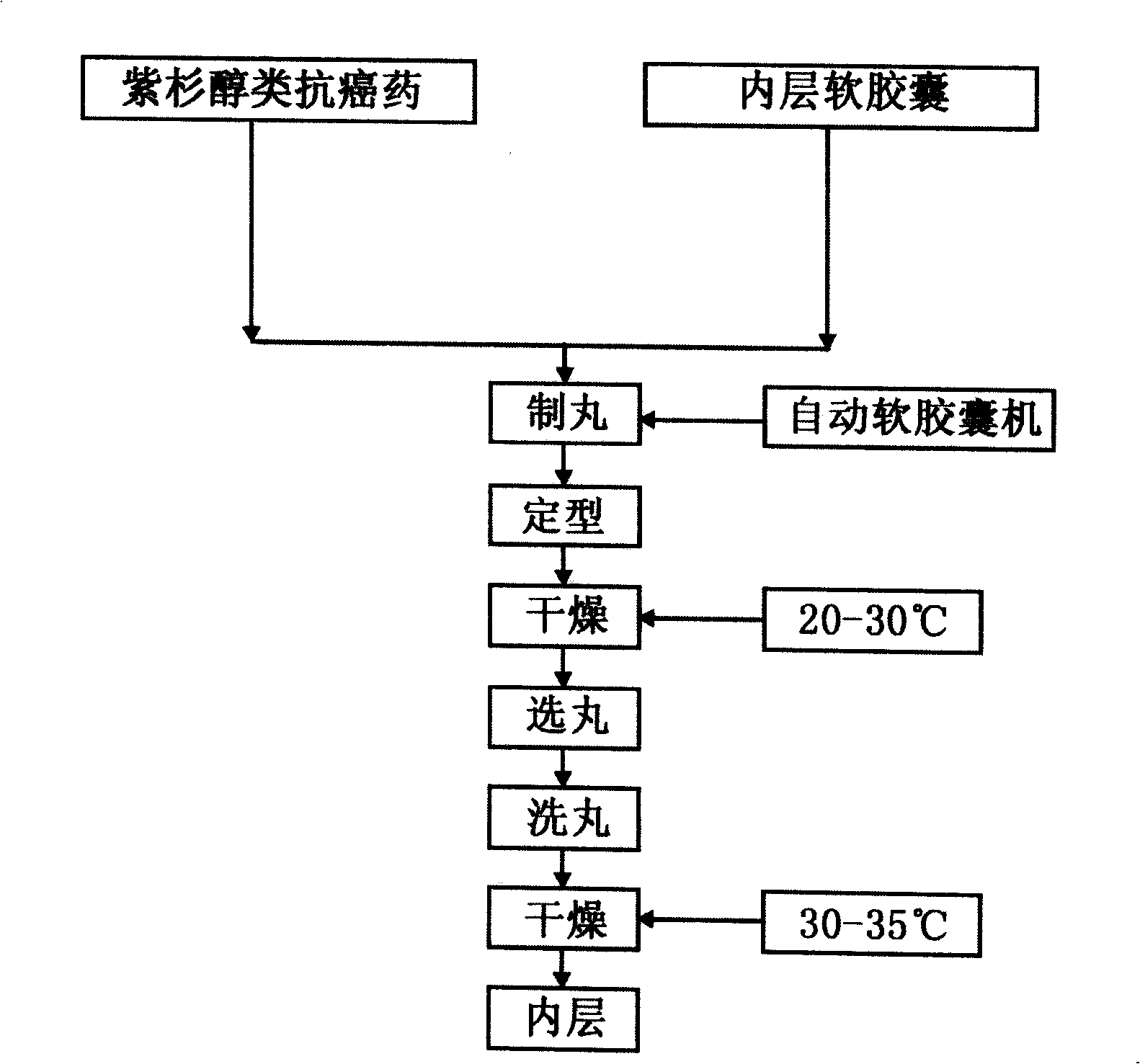
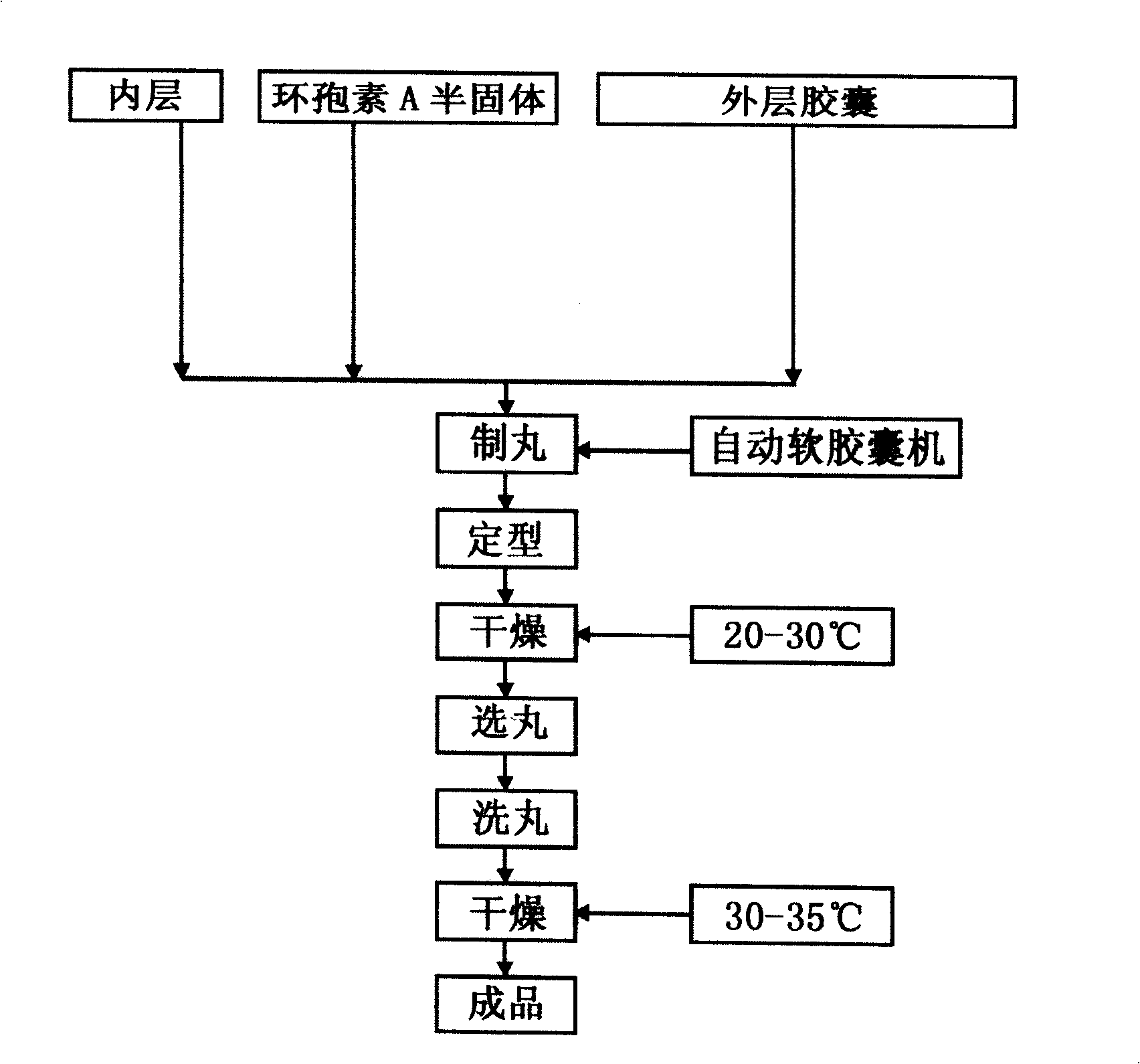
Taxol double-layer soft capsule oral preparation medicament
ActiveCN101513395APrevent volatilizationSimple preparation processOrganic active ingredientsPharmaceutical delivery mechanismCyclosporin CDrug carrier
The invention relates to a taxol double-layer soft capsule oral preparation medicament. The taxol double-layer soft capsule oral preparation medicament adopts the technical proposal that an inner layer is a taxol anticancerogen soft capsule, and an outer layer is a ciclosporin A solid or semisolid capsule; and the taxol double-layer soft capsule oral preparation medicine comprises taxol anticancerogen, the inner layer soft capsule, the ciclosporin A solid or semisolid and an outer layer capsule from the inside to the outside, wherein the taxol anticancerogen comprises taxol active substances and medicinal carriers; the ciclosporin A solid or semisolid comprises ciclosporin A and matched carriers; and the weight ratio of the taxol active substances to the ciclosporin A is 1:2-1:20 in unit dose. The medicament preparation method of the invention comprises the following steps: preparing the taxol anticancerogen; wrapping the taxol anticancerogen in the inner layer soft capsule; preparing the ciclosporin A solid or semisolid; coating the ciclosporin A solid or semisolid outside the inner layer soft capsule; and then wrapping the outer layer capsule outside the ciclosporin A solid or semisolid. The taxol double-layer soft capsule oral preparation medicament has the advantages of small toxicity, convenient taking and good anticancer treatment effect.
Owner:辽宁奥佳生物制药有限公司
Ciclosporin A proliposome, pharmaceutical composition and preparation method thereof
ActiveCN102008713AImprove stabilityHigh dissolution ratePowder deliverySolution deliveryLipid formationDispersity
The invention discloses a ciclosporin A proliposome for oral administration, a pharmaceutical composition and a preparation method thereof. The ciclosporin A proliposome comprises the following pharmaceutical active ingredients and non-active ingredients by weight percent: 1-25% of ciclosporin A, 2-40% of lecithin, 0.5-12.5% of cholesterol, 21-96.45% of polyol and 0.05-1.50% of vitamin E. The proliposome is prepared from the ciclosporin A, a lipid membrane material, a hydrophilic matrix and the like through the ultrasonic dispersion method and the reverse phase evaporation method, and a liposome is formed spontaneously after the dispersion in water. The proliposome can improve the dispersity and the oral bioavailability of ciclosporin A which is an insoluble drug, lead the stability to be good and be more applicable to industrial production in comparison with the ordinary liposome.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
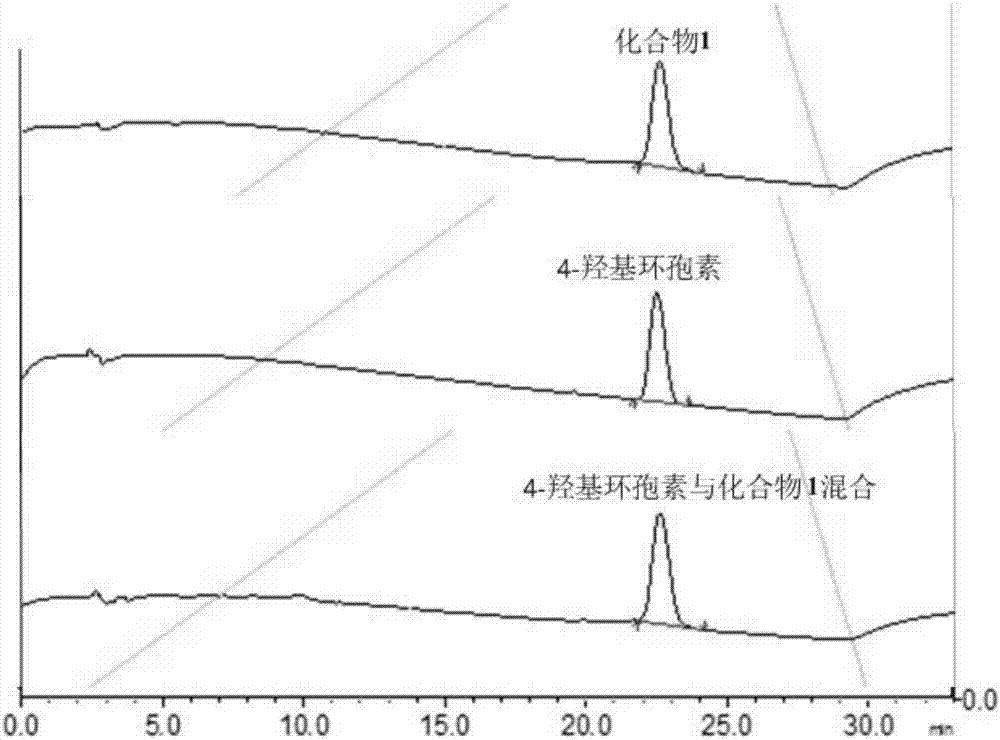
Preparation method of 4-hydroxy ciclosporin
ActiveCN107226843AHigh purityFast chemical synthesisPeptide preparation methodsCyclosporinsChemical synthesisCyclosporin C
The invention relates to a preparation method of 4-hydroxy ciclosporin. 4-hydroxy ciclosporin is prepared from cyclosporine A as a main raw material through chemical synthesis. In preparation of 4-hydroxy ciclosporin, the preparation method has a fast chemical synthesis rate, realizes a low cost and is easy to operate. The 4-hydroxy ciclosporin has high purity. The preparation method realizes large-scale preparation of 4-hydroxy ciclosporin.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL +1
Cyclosporine microball preparation for treating endophthalmitis
InactiveCN1853717ANon-irritatingNo toxic side effectsSenses disorderCyclic peptide ingredientsUveitisMicrosphere
An injection containing suspended microspheres carrying ciclosporin for treating endophthalmitis (such as uveitis) by injecting it into the vitreous body of eyeball is disclosed. Its advantages are slow release, long acting time and high curative effect.
Owner:PEKING UNIV
Bitter gourd and Chinese caterpillar fungus and ganoderma lucidum powder
The present invention provides bitter gourd and Chinese caterpillar fungus and ganoderma lucidum powder. Dried bitter gourd sheets, Chinese caterpillar fungus powder, ganoderma lucidum powder and propolis are crushed and uniformly mixed to prepare the bitter gourd and Chinese caterpillar fungus and ganoderma lucidum powder, which is an edible health product for high blood glucose patients. The bitter gourd has effects of heat clearing, detoxification and significant blood glucose level decreasing. The Chinese caterpillar fungus provides a significant blood glucose level decreasing effect for blood glucose level increasing induced by ciclosporin A, and provides a certain inhibition effect for insulin secretion decreasing induced by the ciclosporin A. With the ganoderma lucidum, utilization of the glucose of the organization can be improved. After taking the ganoderma lucidum, the ganoderma lucidum can replace the insulin and inhibit the fatty acid releasing, such that the high blood glucose, urine glucose and other symptoms can be changed. With the propolis, the blood glucose level is regulated, the therapeutic effect of the hypoglycemic agent is improved, the symptoms of diabetes mellitus are improved, and complications of diabetes mellitus patients are prevented and controlled. The powder provided by the present invention has the following advantages: the formula is reasonable; the amount of the raw materials is proper; after the diabetes mellitus patient takes the powder, the effects of blood glucose level decreasing and blood glucose level stabilizing are provided; compared to the tablet and the capsule, the powder is the powder food, and is easy to be absorbed by the human body, such that the better and faster positive effects can be provided for the human body; no side effect is generated; a plurality of the complications induced by taking the western medicine can be avoided.
Owner:NANNING DADAN BIOLOGICAL TECH
Construction method and application of synovial sarcoma xenograft mouse model with sound immunity
ActiveCN110547250AImproved issues with no immune systemCompounds screening/testingAnimal husbandrySynovial sarcomaCyclosporin C
The invention provides a method for constructing a synovial sarcoma xenograft mouse model with sound immunity. The method comprises the steps of culture of a P0 generation, wherein firstly, fresh tumor tissue masses of a patient with a synovial sarcoma are collected and then transplanted into the body of a mouse with immune deficiency under a sterile condition; a mouse with normal immunity is subjected to ciclosporin pretreatment; culture of Px generations, wherein tumor tissue masses growing in the body of a mouse of any generation from P0-P<x-1> are taken to be inoculated into the body of amouse subjected to the ciclosporin pretreatment to form a Px-generation mouse; culture of Py generations, wherein synovial sarcoma tumor tissue masses growing in the body of a mouse of any generationfrom Px-P<y-1> are taken to be inoculated into the body of the mouse with the normal immunity until tumor tissue grows in the body of the mouse with the normal immunity, and the culture of the Py generations is completed. The method solves the problem that a traditional PDX model has no immune system, provides an in-vivo model for a tumor immunotherapy, and broadens the application range of the PDX model.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
Self-microemulsifying soft capsule content combination of ciclosporin A and preparation method thereof
ActiveCN104055749AImprove bioavailabilityImprove clinical efficacyCyclic peptide ingredientsCapsule deliverySide effectClinical efficacy
The invention belongs to the technical field of medicines and particularly relates to a self-microemulsifying soft capsule content combination of ciclosporin A. The self-microemulsifying soft capsule content combination of ciclosporin A comprises 1-12 parts of ciclosporin A, 1-12 parts of phosphatidylcholine, 5-20 parts of anhydrous ethanol, 5-20 parts of propylene glycol, 30-80 parts of polyoxyethylene hydrogenated castor oil, 5-30 parts of span 80 and 10-30 parts of medium-chain triglycerides. The invention further provides a preparation method of the self-microemulsifying soft capsule content combination of ciclosporin A. The raw materials selected by the invention are easily available and low in cost, and the preparation process is simple, so that the self-microemulsifying soft capsule content combination of ciclosporin A is suitable for large-scaled industrialized production. The bad character that ciclosporin A is low in bioavailability can be overcome and the disparity of bioavailability of individuals can be reduced as far as possible. Dissolubility of water insoluble medicines is greatly improved, and the bioavailability of the medicines is improved. Particularly, combination with phosphatidylcholine increases the clinical curative effect of ciclosporin and reduces severe side effects of renal toxicity and hepatotoxicity in clinical application of ciclosporin.
Owner:无锡曙辉药业有限公司
Treatment process of cyclosporine eye gel
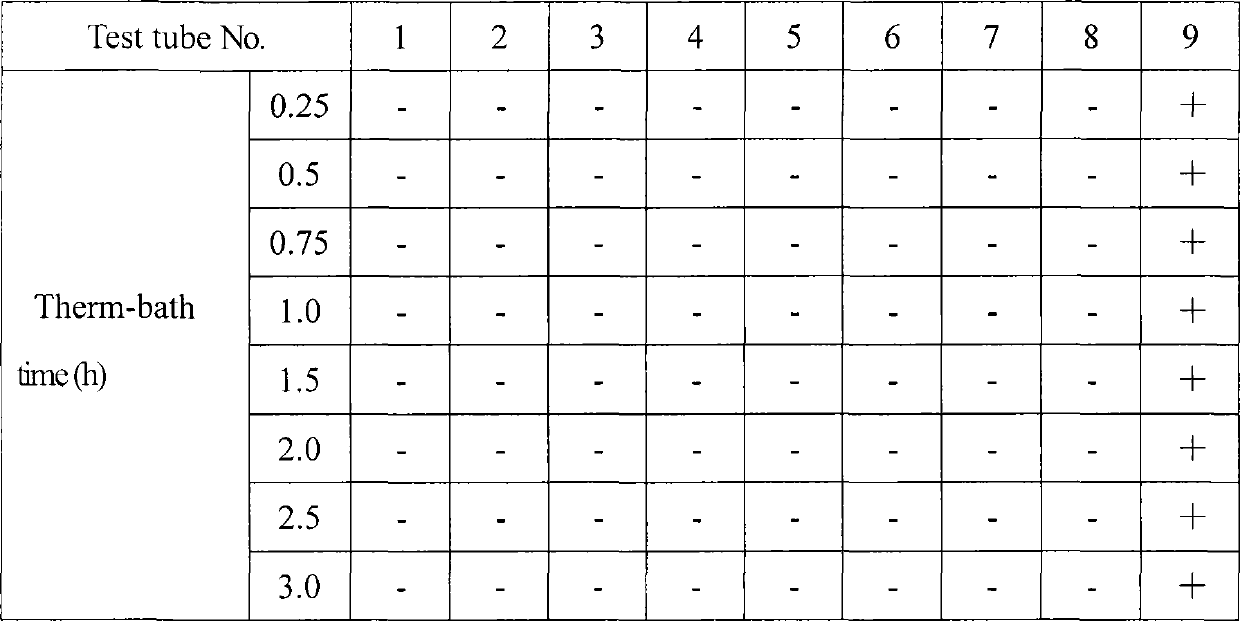
InactiveCN108066745ALow viscosityEase of industrial productionSenses disorderAerosol deliveryWater bathsFiltration
The invention relates to the field of medicines, and discloses a treatment process of cyclosporine eye gel. The treatment process comprises the following steps: adding water into carbomer for full stirring and homogenization, swelling a prepared carbomer matrix, and performing stirring and homogenization; performing moist heat sterilization on the matrix, then cooling the matrix, and performing vacuumizing debubbling treatment; adjusting the pH of the matrix to 5.0 to 9.0 through a filtered sodium hydroxide solution; fully mixing castor oil polyoxyhydrocarbon ester (35), ciclosporin A raw material, 1,2-propylene glycol and water to prepare a clarified solution under a water bath condition at 35 to 45 DEG C; filtering the clarified solution, uniformly mixing filtrate with the matrix, and performing vacuumizing debubbling; performing filtration again and sterile filling. According to the process disclosed by the invention, before the carbomer is added, sterilization and filtration are performed, so that industrial production is facilitated; only partial germfree treatment is required; purchasing is facilitated, and the cost can be reduced; furthermore, thermal degradation of ciclosporin is avoided, and product impurities are reduced; all indexes are qualified through detection; the treatment process has a good industrial application prospect.
Owner:ZHAOKE GUANGZHOU OPTHALMIC DRUG
Ciclosporin medicament composition for injection administration
InactiveCN101502641AFix stability issuesSolve the technical problems of resolubilityCyclic peptide ingredientsImmunological disordersSolubilityIrritation
The invention relates to ciclosporin medicine composition of injection drug administration, comprising ciclosporin which serves as an active ingredient, Carmowax15-hydroxystearate (solutol HS 15) which serves as a solubilizer, alcohols or esters which serve as latent solvent and water for injection which serves as solvent. The medicine composition can be prepared into injecta or lyophilized preparation. The medicine composition of the invention not only solves the problem that severe anaphylactic reaction exists as the existing ciclosporin injecta contains much Cremophor EL, but also prevents severe anaphylactic reaction from occurring and causes little acrimony; the medicine composition also solves the problem that the previous technology can not solve the stability and solubility of the ciclosporin injecta and avoids the insufficiencies of complex preparation technique and low cost caused by the adoption of technologies such as nanometer technology and lipidosome technology; the medicine composition further solves the technical problem of the stability and solubility of lyophilized powder injecta, which can not be solved by the previous technology, as a result, the medicine composition features good stability and solubility; the preparation technique of the injecta is simple, the quality control is simple and convenient, the production cost is relatively low, thus greatly reducing economic burden of the patients in terms of medicine use.
Owner:姚定全
Ophthalmic medicament composition for forming low-irritation transparent emulsion formulation for surface immune adjustment and inflammation reduction of relevant tissue of eyes or eye periphery
ActiveCN101897949AImprove solubilityLess irritatingSenses disorderCyclic peptide ingredientsPolyoxyethylene castor oilKERATOCONJUNCTIVITIS SICCA
The invention provides an ophthalmic medicament composition for forming a low-irritation transparent emulsion formulation for the surface immune adjustment and inflammation reduction of the relevant tissue of eyes or eye periphery. The ophthalmic medicament composition is used for treating the inflammatory reaction of a serious keratoconjunctivitis sicca and cornea epithelium pathological change patient, forms an emulsion formulation, contains at least one ciclosporin, propylene glycol and polyoxyethylene castor oil derivative, such as polyoxyethylene 35 castor oil derivative, and the like, is transparent, has the characteristics of low irritation, stability and no crystallization phenomenon and is suitable for more sensitive areas, such as eye tissue, and the like.
Owner:RXVISION PHARMA CO LTD
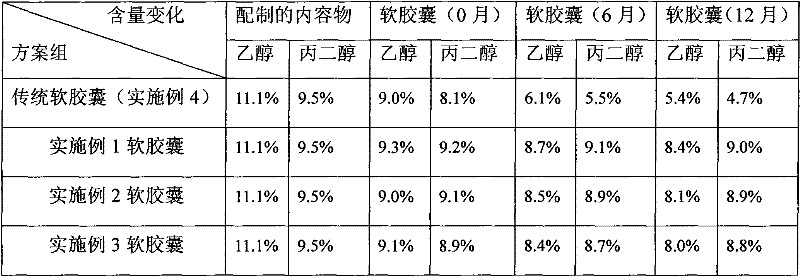
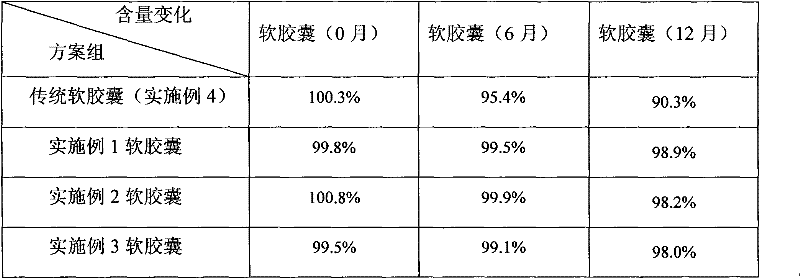
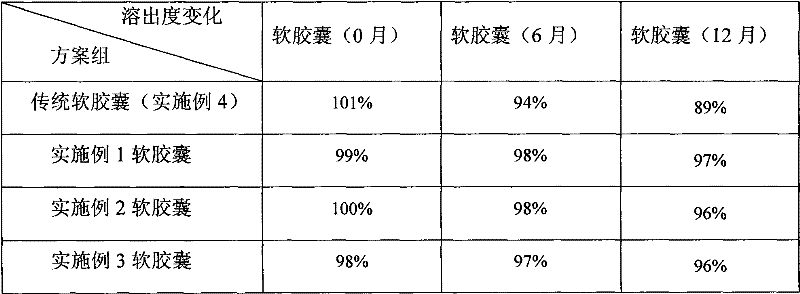
Cyclosporine-containing soft capsule capsule shell composition and preparation method, and cyclosporine-containing soft capsule prepared therefrom
InactiveCN102284062ALower ethanolReduced Propylene Glycol ContentCyclic peptide ingredientsCapsule deliveryCyclosporinsCyclosporin C
The invention relates to a technology of a soft capsule containing ciclosporin, in particular to a capsule shell composition suitably prepared into a soft capsule containing the ciclosporin as well as a preparation method thereof and the soft capsule containing the ciclosporin prepared thereby. Gelatin, glycerin, sorbierite, propanediol, water, hydroxyphenyl ethylester and talcum powder are addedin a glue digester and heated to be completely dissolved to obtain a capsule shell; and the capsule shell and a content solution are wrapped by a soft capsule machine to obtain the soft capsule. According to the capsule shell composition for the soft capsule containing the ciclosporin, which is disclosed by the invention, the contents of ethanol and the propanediol in the content of the soft capsule are ensured not to be greatly reduced in the storage process, the medicament dissolving of the ciclosporin can be ensured and the bioavailability of the ciclosporin is improved. Various raw materials provided for the capsule composition are common varieties and have low price, thus the capsule composition is suitable for large-scale industrial production.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Ciclosporin medicament composition for injection administration
The invention relates to ciclosporin medicine composition of injection drug administration, comprising ciclosporin which serves as an active ingredient, Carmowax15-hydroxystearate (solutol HS 15) which serves as a solubilizer, alcohols or esters which serve as latent solvent and water for injection which serves as solvent. The medicine composition can be prepared into injecta or lyophilized preparation. The medicine composition of the invention not only solves the problem that severe anaphylactic reaction exists as the existing ciclosporin injecta contains much Cremophor EL, but also preventssevere anaphylactic reaction from occurring and causes little acrimony; the medicine composition also solves the problem that the previous technology can not solve the stability and solubility of theciclosporin injecta and avoids the insufficiencies of complex preparation technique and low cost caused by the adoption of technologies such as nanometer technology and lipidosome technology; the medicine composition further solves the technical problem of the stability and solubility of lyophilized powder injecta, which can not be solved by the previous technology, as a result, the medicine composition features good stability and solubility; the preparation technique of the injecta is simple, the quality control is simple and convenient, the production cost is relatively low, thus greatly reducing economic burden of the patients in terms of medicine use.
Owner:姚定全
Eye implanted gel containing cyclosporin
InactiveCN101085341APromote formationAvoid scaringPharmaceutical delivery mechanismCyclic peptide ingredientsCross-linkDisease
The invention relates to ocular implantation gel containing ciclosporin and its preparation method and application. The gel is characterized in that ciclosporin disperses in cross linked hyaluronic acid gel, and the weight ratio of cross linked hyaluronic acid and ciclosporin is 1:5-5:1.The preparation method of the gel comprises suspending ciclosporin in sodium hyaluronateaqueous solution, mixing with cross-linking agent for reaction to form gel, purifying, cutting, packaging, and sterilizing to obtain final product. The gel can be be used for preparation of ocular drug implants for preventing adhesion of humor aquosus through filtering channel in non penetrable joist operation of glaucoma, improving filterability and keeping filtering channel, controlling intraocular pressure after operation, preventing rejection reaction after corneal transplantation and some ocular autoimmune disease.
Owner:凌沛学
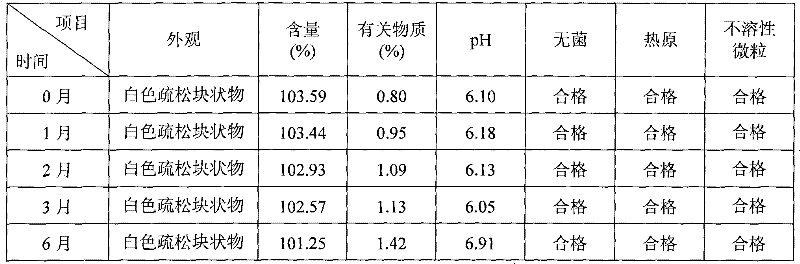
Ciclosporin A microemulsion formulation for injection and preparation method thereof
InactiveCN101406452BReduce allergic reactionsReduce allergic reactions and other side effectsCyclic peptide ingredientsEmulsion deliveryWater bathsPolyoxyethylene castor oil
The invention belongs to the technical field of medicine, and discloses an injection microemulsion preparation containing ciclosporin A and a preparation method thereof. The preparation consists of the following components: 1 to 5 percent of basic medicine, 1 to 30 percent of oil phase, 2 to 40 percent of a surfactant, 2 to 40 percent of a cosurfactant, and the balance being water for injection. The preparation method comprises the following steps: dissolving the oil phase and the surfactant of the recipe dosage into liquid through a water bath at 40 DEG C, adding the cosurfactant at a room temperature into the liquid and mixing the mixture evenly, adding the medicine into the mixture, stirring the mixture slightly to dissolve the medicine, dropping water so as to regulate the pH value from 5.0 to 7.0, and obtaining the microemulsion preparation. The invention takes the safe and nonirritant non-ion surfactant to replace polyoxyethylene castor oil capable of easily causing an allergic reaction in the prior injection, thereby improving the safety of the medicine, and reducing toxicity and adverse reactions.
Owner:SHENYANG PHARMA UNIVERSITY
Cyclosporin A eye drops and preparation method thereof
ActiveCN105997863BPayloadImprove apparent solubilitySenses disorderPharmaceutical delivery mechanismIrritationCyclodextrin
The invention belongs to the technical field of high polymer chemistry, biomedical materials and pharmacy, and particularly discloses ciclosporin A eye drops and a preparation method thereof. Every 1,000 mL of the eye drops are prepared from the following substances: ciclosporin A 30-630 mg, a polymer-grafted copolymer 1-10 g, and the balance of injection water, wherein the polymer-grafted copolymer is chitosan-g-cyclodextrin. The preparation method comprises the following steps: firstly, weighing the chitosan-g-cyclodextrin as the polymer-grafted copolymer, adding and dissolving equivalent injection water in the chitosan-g-cyclodextrin, and stirring and dissolving until a solution is transparent; secondly, adding an ethanol solution of the ciclosporin A in the solution, stirring at the temperature of 0-4 DEG C until a system becomes completely clear; thirdly, removing ethanol under reduced pressure at the temperature of 0-4 DEG C and washing; and finally, adding the injection water and determining the volume, and sterilizing to obtain the final product. The eye drops have the advantages that irritation of the medicine is reduced, the residence time of the medicine on an ocular surface is prolonged, the corneal permeability of the medicine is improved, and the treatment effect of the medicine is also improved.
Owner:河南省眼科研究所
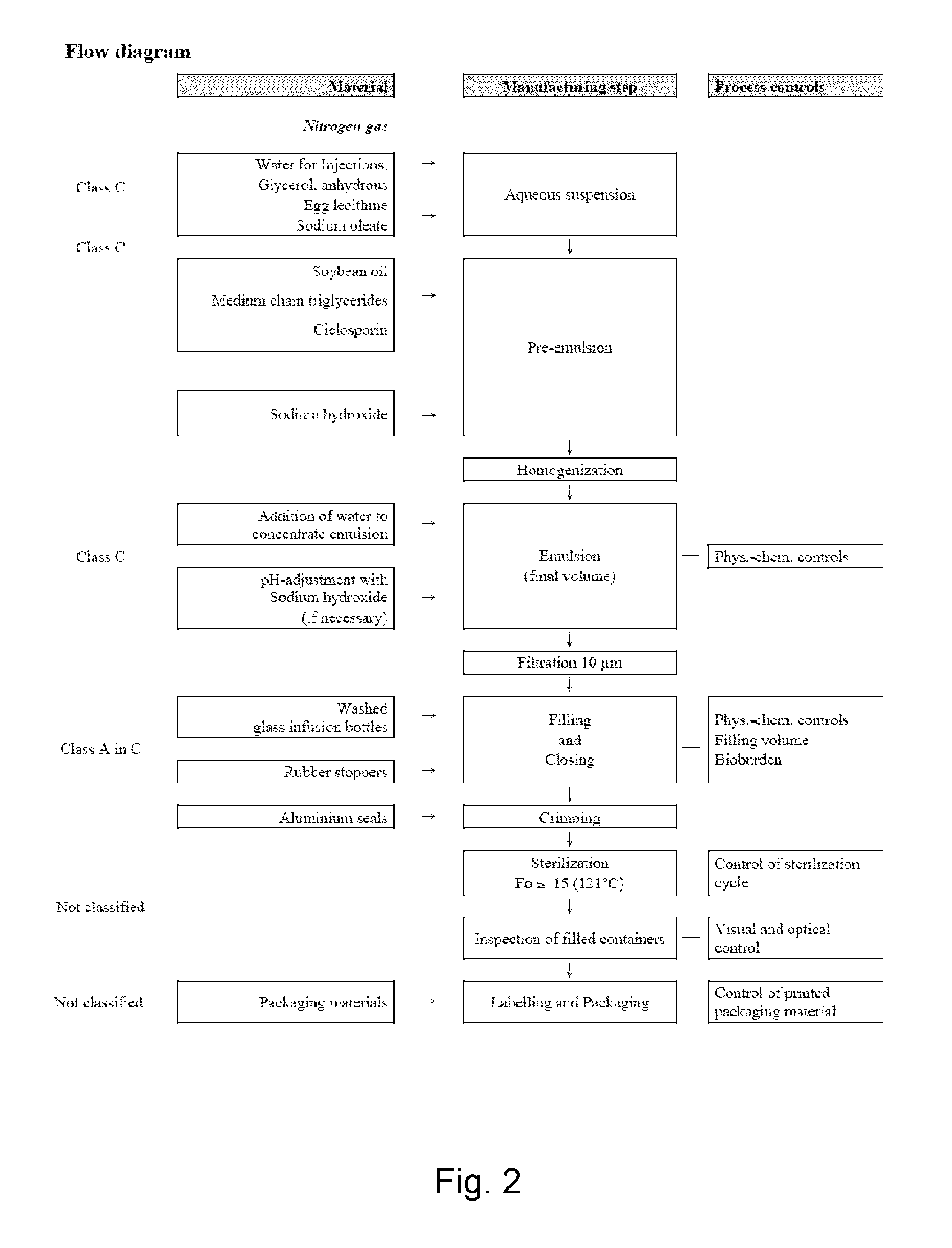
Cyclosporine emulsion
ActiveUS20130323270A1Increase stickinessEliminate needNervous disorderMetabolism disorderGlycerolCyclosporin C
The present invention relates to a cyclosporine emulsion containing: i) a cyclosporine ii) a natural oil (long chain triglyceride) iii) a phosphatidylcholine, iv) glycerol, v) a pharmaceutically tolerable alkali salt of a free fatty acid, vi) a medium chain triglyceride-oil vii) optionally, hydrochloric acid or sodium hydroxide for pH adjustment viii) water.
Owner:ABLIVA AB
Composition of ciclosporin micro-emulsion soft capsules and preparation method thereof
InactiveCN107737116AReduce nephrotoxicityReduce liver toxicityCyclic peptide ingredientsCapsule deliverySide effectClinical efficacy
The invention relates to the technical field of ciclosporin micro-emulsion soft capsules and in particular relates to a composition of ciclosporin micro-emulsion soft capsules and a preparation methodthereof. The ciclosporin micro-emulsion soft capsules comprise the following compositions in parts by weight: 5-18 parts of ciclosporin A, 3-20 parts of phosphatidylcholine, 7-26 parts of a surfactant, 36-74 parts of polyoxyethylene hydrogenated castor oil, 11-26 parts of Span 80, 10-30 parts of sorbitan and 11-28 parts of medium-chain triglycerides. According to the composition of ciclosporin micro-emulsion soft capsules disclosed by the invention, due to the addition of the surfactant, the polyoxyethylene hydrogenated castor oil and the sorbitan, dissolution of water-insoluble drugs is promoted, the bioavailability of the drugs is improved, and disparity of the bioavailability among individuals and other poor properties are reduced, so that clinical curative effects of the ciclosporin micro-emulsion soft capsules are increased, and renal toxicity, hepatotoxicity and other severe side effects in clinical application of ciclosporin are decreased.
Owner:温州中壹技术研究院有限公司
Method to detect blood creatinine and an immunosuppressive drug
InactiveUS20180259514A1Easy to monitorReduce the amount requiredDisease diagnosisBiological testingImmunosuppressive drugCreatinine rise
The invention provides a method for detecting or quantifying creatinine and an immunosuppressive drug selected from tacrolimus and ciclosporin in a blood sample, devices, including lateral flow assay devices, and kits, as well as methods that enables the reduction of nephrotoxicity and / or enables the maintenance of good renal function in an organ or tissue transplant patient who is undergoing treatment with an immunosuppressive drug selected from tacrolimus and ciclosporin, and methods for monitoring nephrotoxicity and / or kidney function in transplant patients.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com