Cyclosporin A eye drops and preparation method thereof
A technology of eye drops and cyclosporine, which is applied in the fields of polymer chemistry, biomedical materials and pharmacy to achieve the effects of reducing irritation, increasing tolerance and improving apparent solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
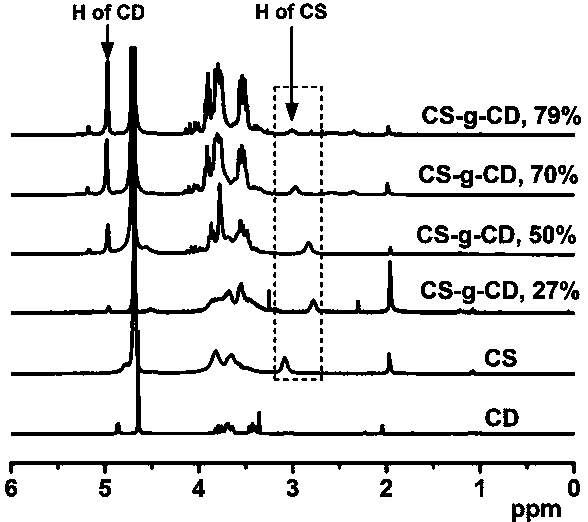
[0025] Embodiment 1--the synthesis of graft copolymer
[0026] High molecular graft copolymer, its molecular formula is: CS-g-CD.
[0027] The synthetic route is:
[0028]
[0029] Synthetic steps:
[0030] S1. Synthesis of CD-COOH: First, put 9.73 g α-CD and 7.2 g sodium hydroxide into a flask and dissolve with 30 mL distilled water; add 1.165 g sodium chloroacetate and react at 50°C for 5 h; then dissolve the system with hydrochloric acid Adjust the pH to 7, add excess methanol for precipitation, filter the precipitate, and dry it overnight at 40°C in a vacuum dryer to obtain a white powder CD-COOH, with a yield of > 96%;
[0031] S2. Synthesis of CS-g-CD: Dissolve 10 mmol CD-COOH and 11 mmol NHS in 50 mL of distilled water, place in a flask equipped with a magnetic stirring bar, place the flask in a water bath at 4°C, add 11 mmol EDC, React at 4°C for 2 h; slowly add the calculated amount of chitosan aqueous solution (CS containing -NH 2 The amount is 12~33 mmol), re...
Embodiment 2--0
[0035] Embodiment 2--Preparation of 0.06 wt% cyclosporin A eye drops
[0036] First, take 2 grams of the graft copolymer CS-g-CD with a CD grafting rate of 50% in Example 1, add 500 mL of water for injection therein, stir and dissolve to a transparent solution; then, anhydrous cyclosporin A Add 50 mL of ethanol solution (4 mg / mL) to the above solution (1:10, v / v), stir at low temperature (4°C) until the system becomes completely clear, and filter to remove insoluble matter (for CS-g-CD The amount is small, cyclosporine A is not completely complexed with it, and the filtration here mainly removes the cyclosporin A that has not been complexed); again, remove absolute ethanol under reduced pressure at low temperature (4°C), ultrafiltration tube (molecular weight cut-off 3.5k) and washed 3 times; finally, add water for injection to a volume of 250 mL, and sterilize to obtain eye drops. The concentration of the eye drops measured by high performance liquid chromatography is 0.06 wt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com