Novel HBB overexpression vector and design method and application thereof
An overexpression vector and design method technology, applied to a novel HBB overexpression vector and its design method and application field, can solve the problems of low lentiviral packaging titer and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] The optimization of embodiment 1HS2, HS3 and HS4
[0089] In this example, the reported ChIP-seq data is systematically mined, the binding motifs (Consensus motif) of trans-acting factors are analyzed, and the regulatory motifs of HS2, HS3 and HS4 are precisely located and streamlined and optimized. The specific optimization features are as follows: :
[0090] (1) As shown in Figure 3(A), the length of HS2 remains unchanged at 0.6kb, the nucleotide sequence is shown in SEQ ID NO: 1, the box is the conserved binding motif of the transcription factor, and the bold is highly conserved Base, the corresponding transcription factor name is below the box, and the condensed HS2 contains binding motifs of TAL1, GATA1, KLF1, NFE2, GR and SOX6;
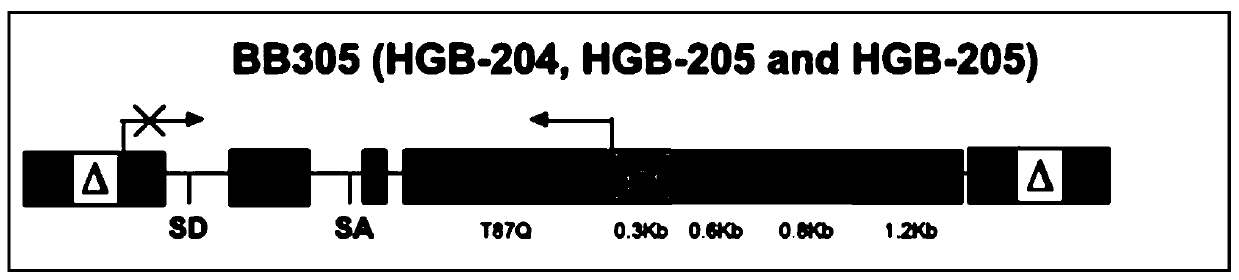
[0091] (2) As shown in Figure 3(B), the length of HS3 is reduced from 0.8kb of the BB305 vector to 0.6kb, the nucleotide sequence is shown in SEQ ID NO: 2, and the box is the conserved binding motif of the transcription factor, The base...
Embodiment 2
[0093] Embodiment 2 promoter optimization
[0094] In this example, the HBB promoter was re-analyzed, and the first 0.29kb was found to be the core transcription initiation element. According to the enrichment degree of trans-acting factors in the promoter region, the first 0.29bp sequence was extracted, as shown in Figure 3 (D) The HBB promoter shown in (D), the nucleotide sequence is shown in SEQ ID NO: 4, the box is the conserved binding motif of the transcription factor, the bold is the highly conserved base, and the corresponding transcription factor is below the box Name, the condensed HBB promoter contains binding motifs of TAL1, GATA1, KLF1 and TBP;
[0095] In this embodiment, further referring to the characteristics of HPFH, the combined mutation of the HBG promoter is carried out. The mutation site is shown in Table 1. The innovative 0.3kb mutant HBG promoter obtained is shown in Figure 3 (E), and the nucleotide sequence is as follows As shown in SEQ ID NO:5, the b...
Embodiment 3
[0098] Example 3 Optimization of HBB expression cassette
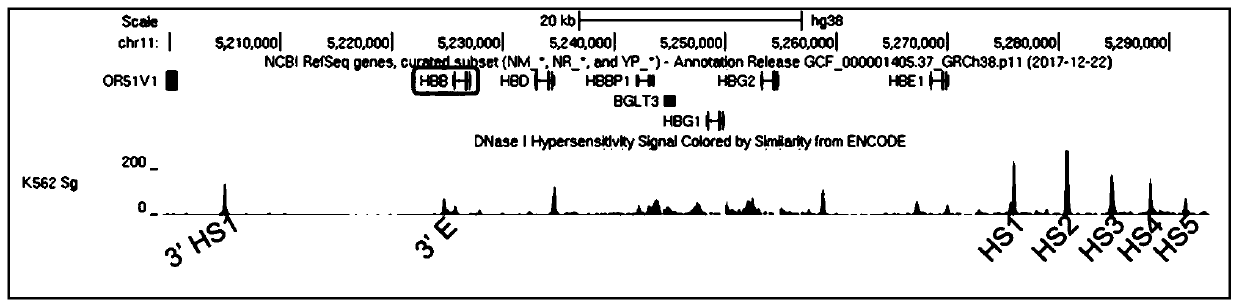
[0099] In this example, the HBB locus was located in the UCSC database, and the HBB expression frame was re-analyzed: the binding motifs of a large number of cis-acting elements were enriched in the No. 1 intron and the length was relatively short, so no adjustment was made; The middle part of the intron has almost no binding motifs of important trans-acting factors, but there is a binding motif of MIER1 that represses transcription, so the 387bp MIER1 binding region was deleted in intron 2 ( Figure 4 The shaded area in ), shortening the length of the HBB expression box. As shown in Figure 3(F), the length of the simplified HBB expression frame is 1.2kb, the nucleotide sequence is shown in SEQ ID NO: 6, the uppercase italics are 5'-UTR and 3'UTR, and the uppercase is ORF box, The lowercase is an intron, the bold lowercase is the conserved sequence at both ends of the intron, the uppercase in the No. 2 intron is the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com