Ciclosporin A microemulsion formulation for injection and preparation method thereof
A technology for injection and cyclosporine, which is applied in the field of medicine, can solve the problems of decreased renal blood flow and glomerular filtration rate, poisoning cells, and cholestasis, and achieves good absorption and bioavailability and low emulsifier dosage. Minimal effects with few side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 microemulsion preparation
[0033] Heat 9g of miglitol, 12g of HS 15 and 6g of lecithin in a water bath at 40°C to make them evenly mixed. After cooling to room temperature, add 6g of PEG-400 and 12g of ethanol, and then dissolve 2.5g of cyclosporine A In the above mixture of oil and surfactant, add water for injection to 100g, adjust the pH value to 5.0-7.0, stir well to obtain the microemulsion concentrate, and filter the obtained product with a 0.45μm microporous membrane, potting , Sterilize.
[0034] Particle size investigation: The particle size of the microemulsion was measured by a Malvern laser scattering particle size analyzer (Zetasizer 3000, Malvern, UK), the light scattering degree was set at 90°, and the measurement temperature was 25±1°C. The particle diameter of prescription 1 drug-containing microemulsion is measured within 100nm (55.7nm), which is suitable for injection.
Embodiment 2
[0036] Heat 10g medium carbon chain triglycerides (GTCC), 15g HS15 and 5g lecithin in a water bath at 40°C to mix them evenly, add 5g PEG-400 and 10g ethanol after cooling to room temperature, and then add 2.5g Dissolve cyclosporin A in the mixture of the above-mentioned oil and surfactant, add water for injection to 100g, and adjust the pH value to 5.0-7.0, stir well to obtain the microemulsion concentrate, and the obtained product is filtered through a 0.45 μm microporous filter Membrane filtration, potting and sterilization.
Embodiment 3
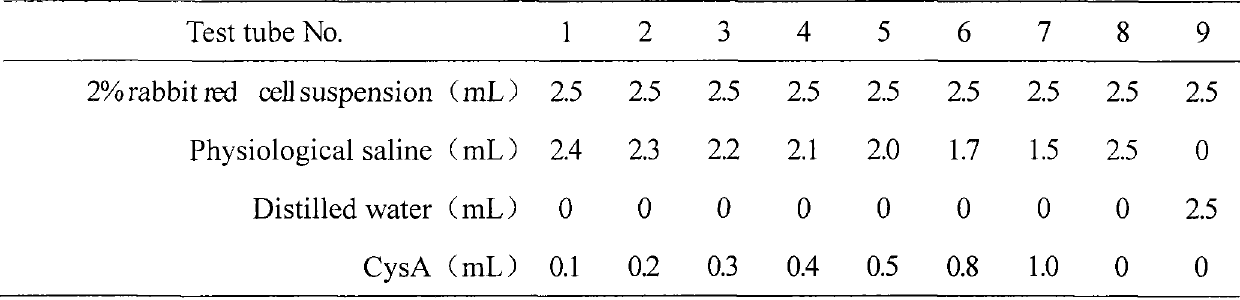
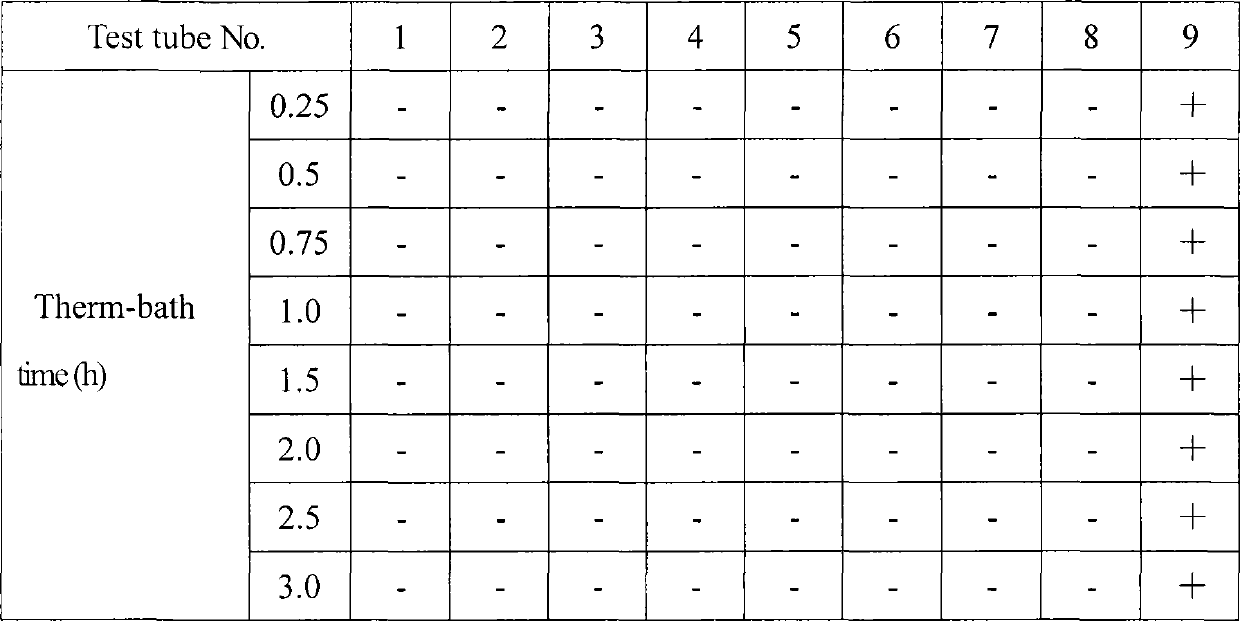
[0037] Embodiment 3: hemolytic test of cyclosporin A injection microemulsion preparation of the present invention
[0038] (1) Test materials and conditions:
[0039] Test material: prepare cyclosporin A microemulsion preparation for injection according to the method of above-mentioned embodiment 1, the concentration of this microemulsion prescription is 2.5% (w / w), dilutes 25 times with 0.9% sodium chloride injection, serves as Test solution; the control group is normal saline;
[0040] Experimental animal: 1 clean grade New Zealand white rabbit, weighing 2-3 kg (purchased from the Animal Experiment Center of Shenyang Pharmaceutical University);
[0041] Animal feeding environment: Room temperature: 20°C
[0042] Humidity: 30%-70%
[0043] Lighting: artificial light, 12 hours daylight, 12 hours dark
[0044] Laboratory animal facility certificate number: SYXK (Liao) 2006-0012
[0045] (2) Test method
[0046]Take 1 female New Ze...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com