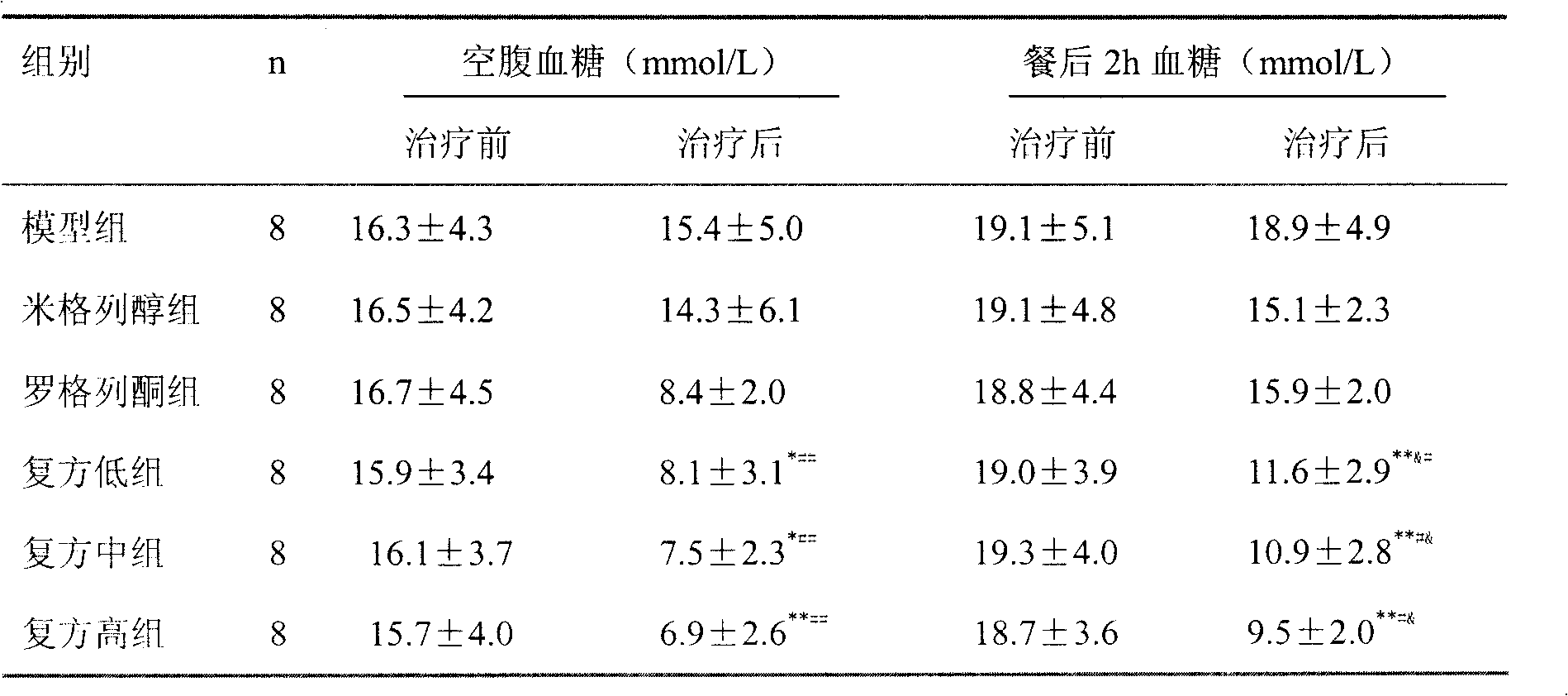
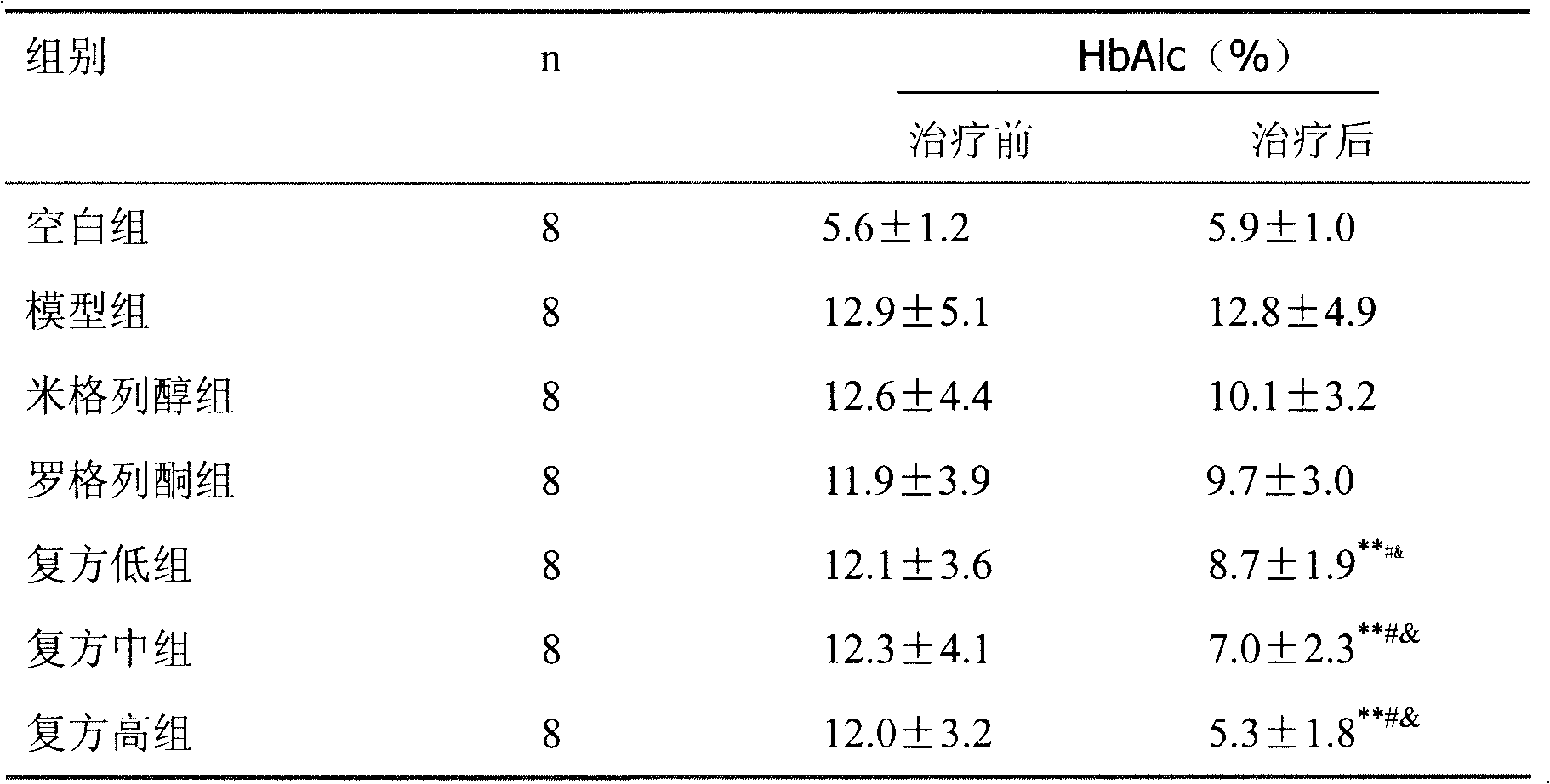
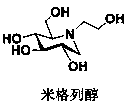
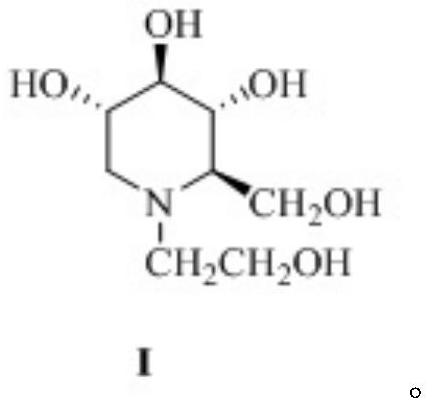
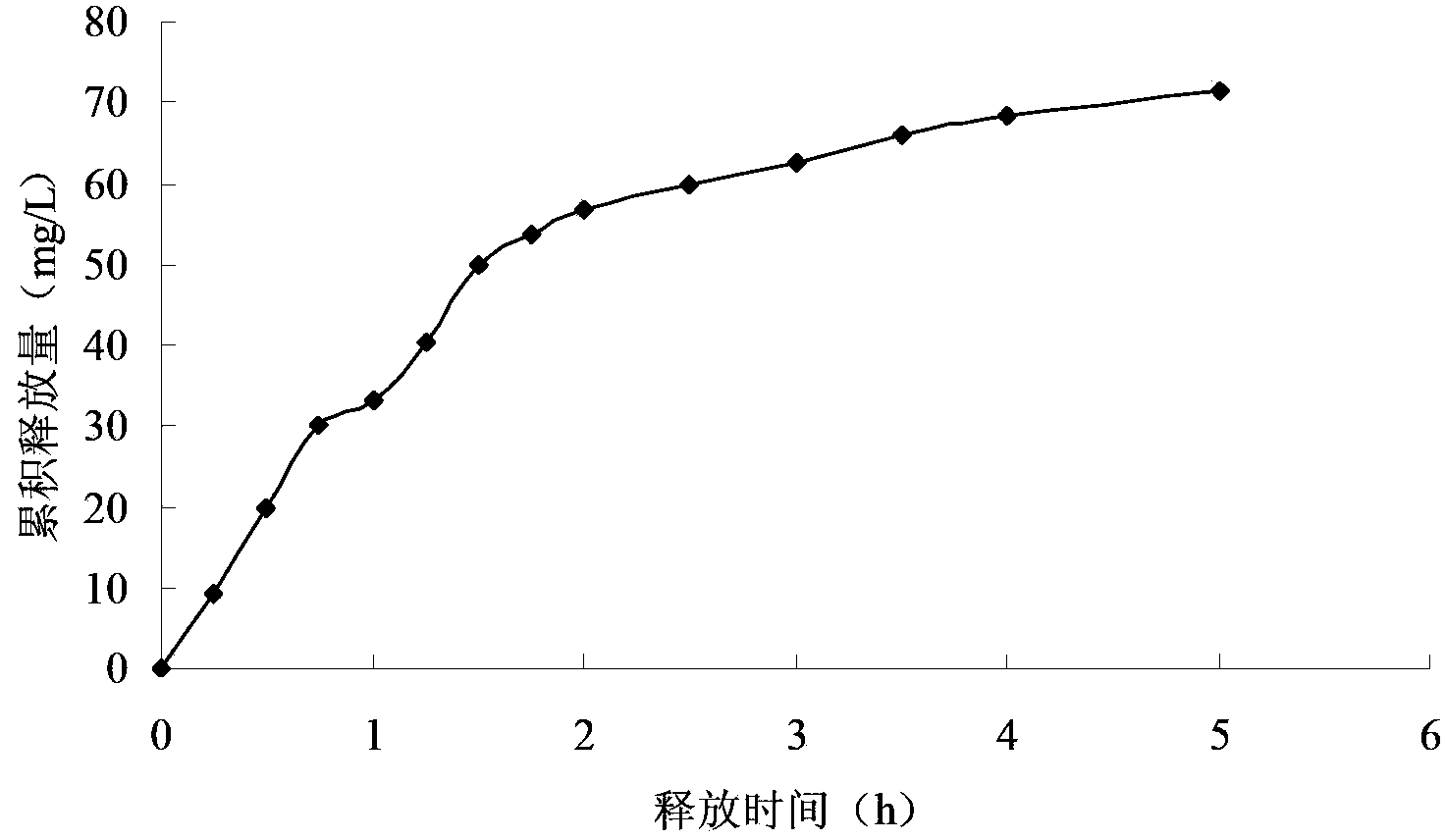
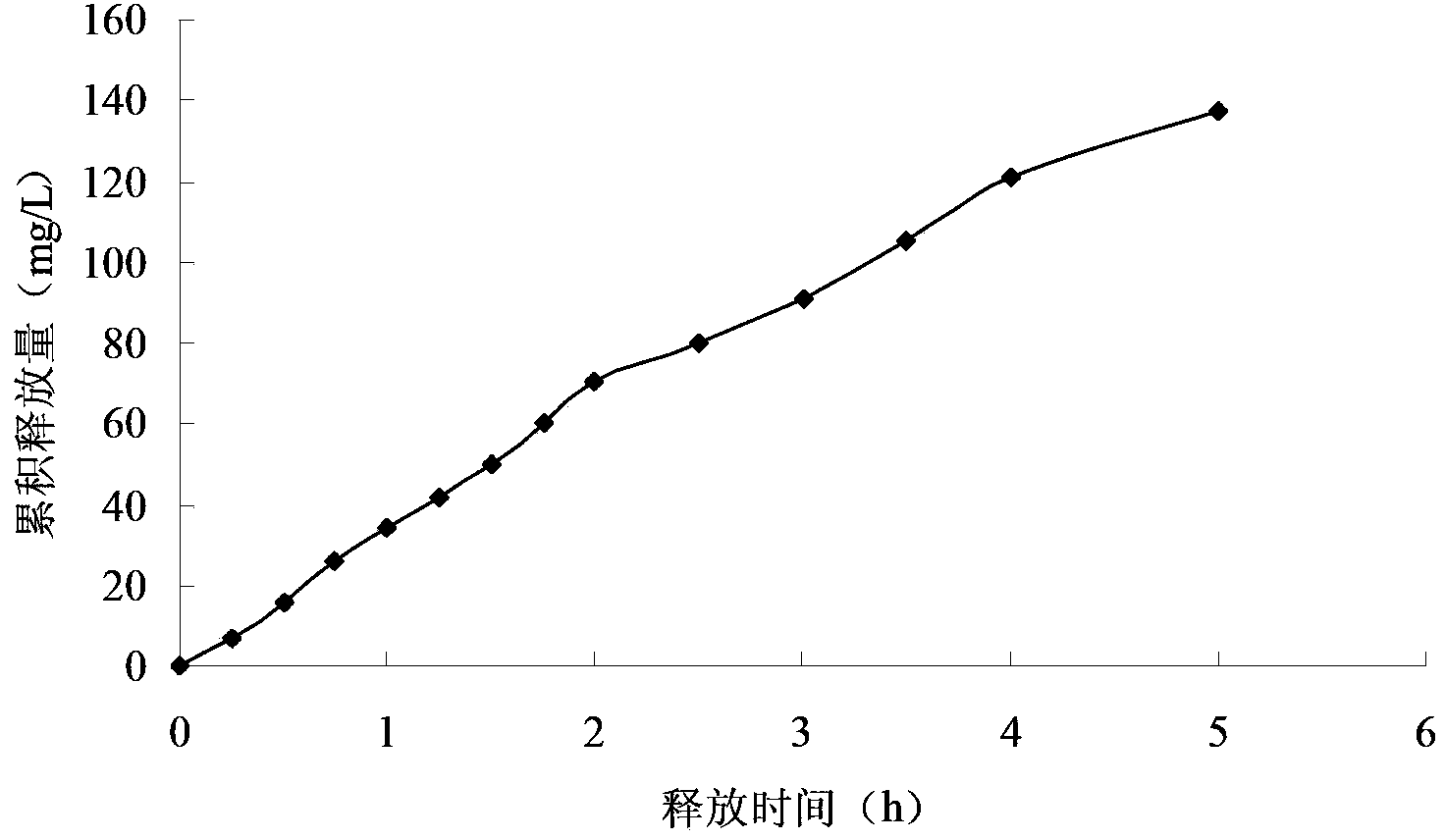
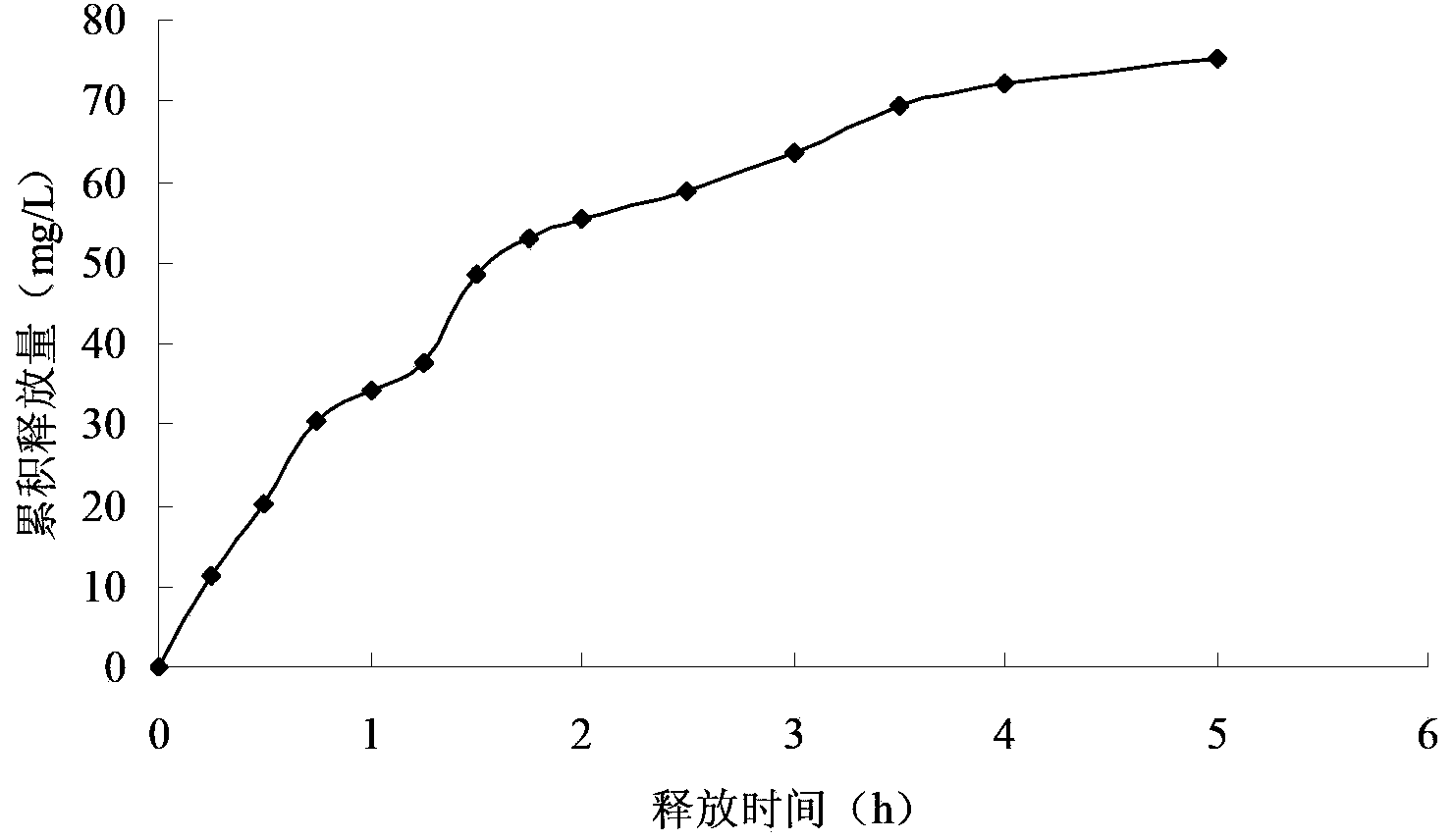
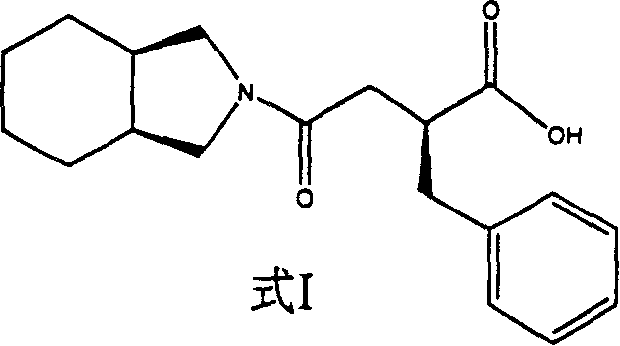
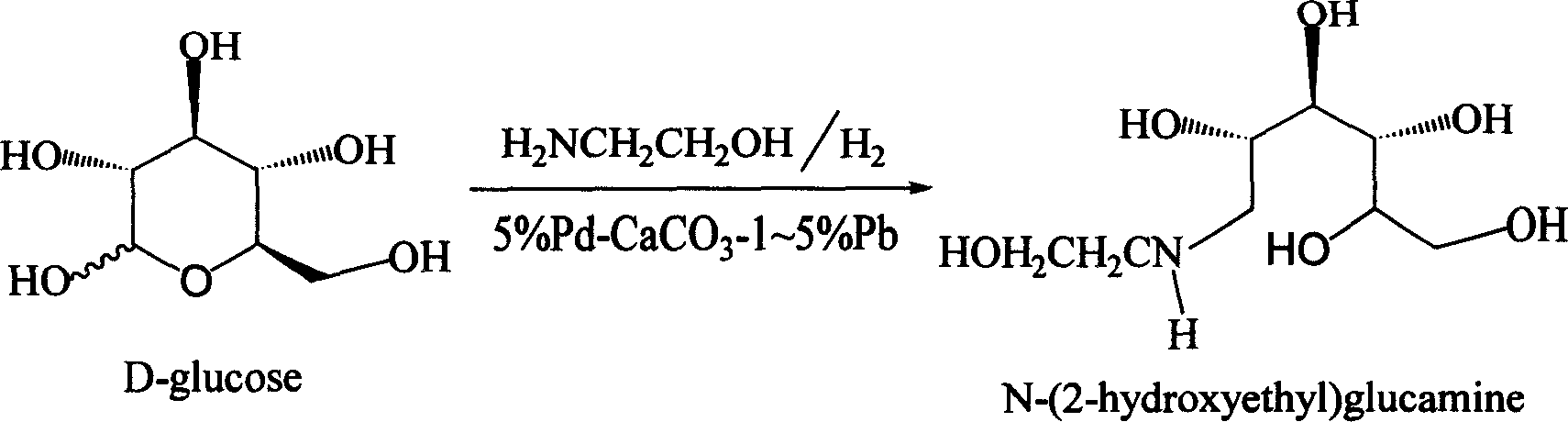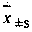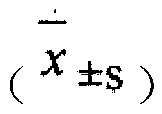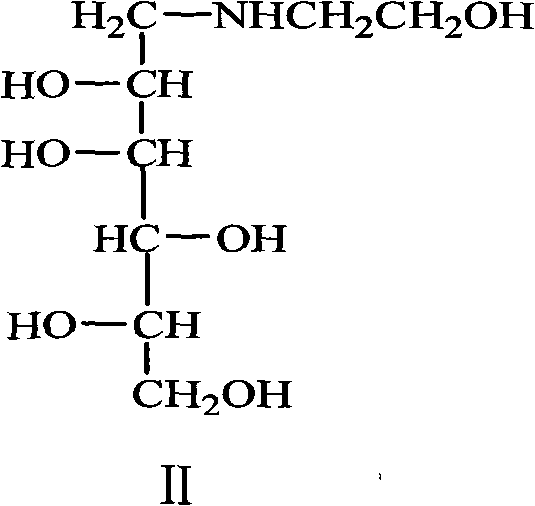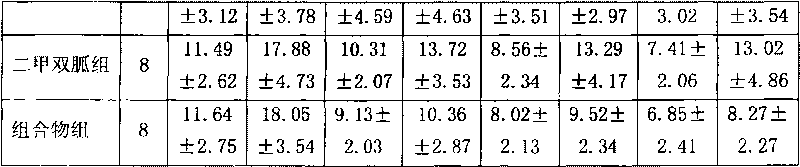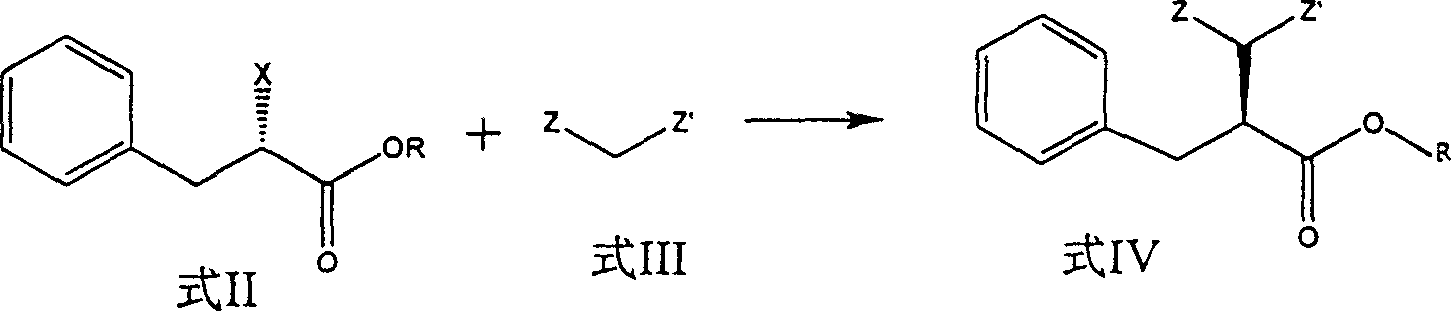Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Miglitol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
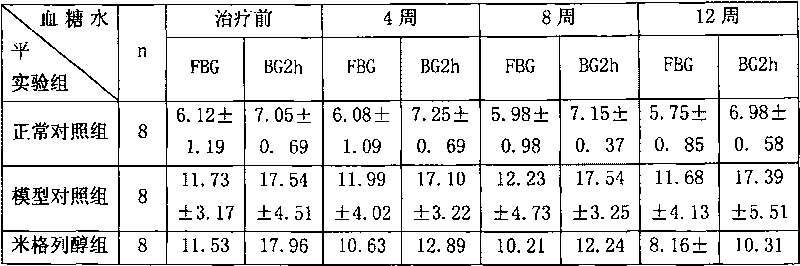
Miglitol is used with a proper diet and exercise program to control high blood sugar in people with type 2 diabetes.
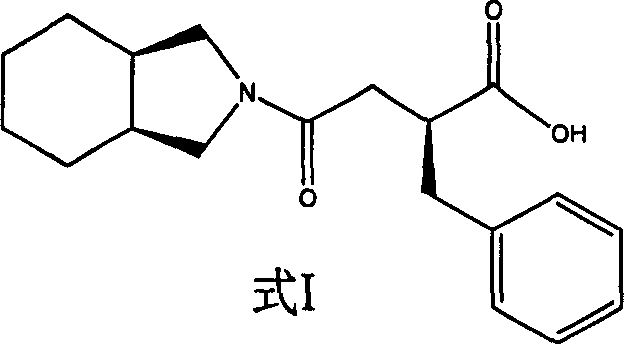
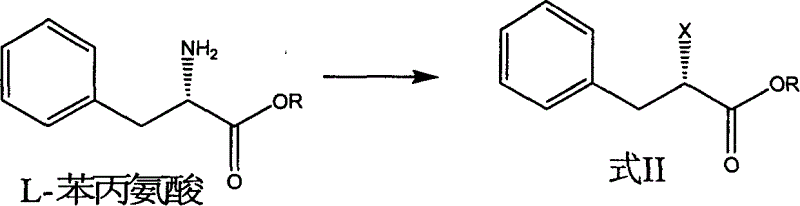
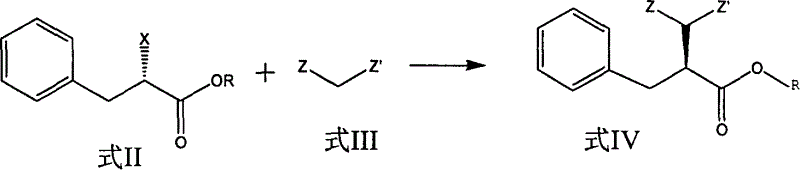
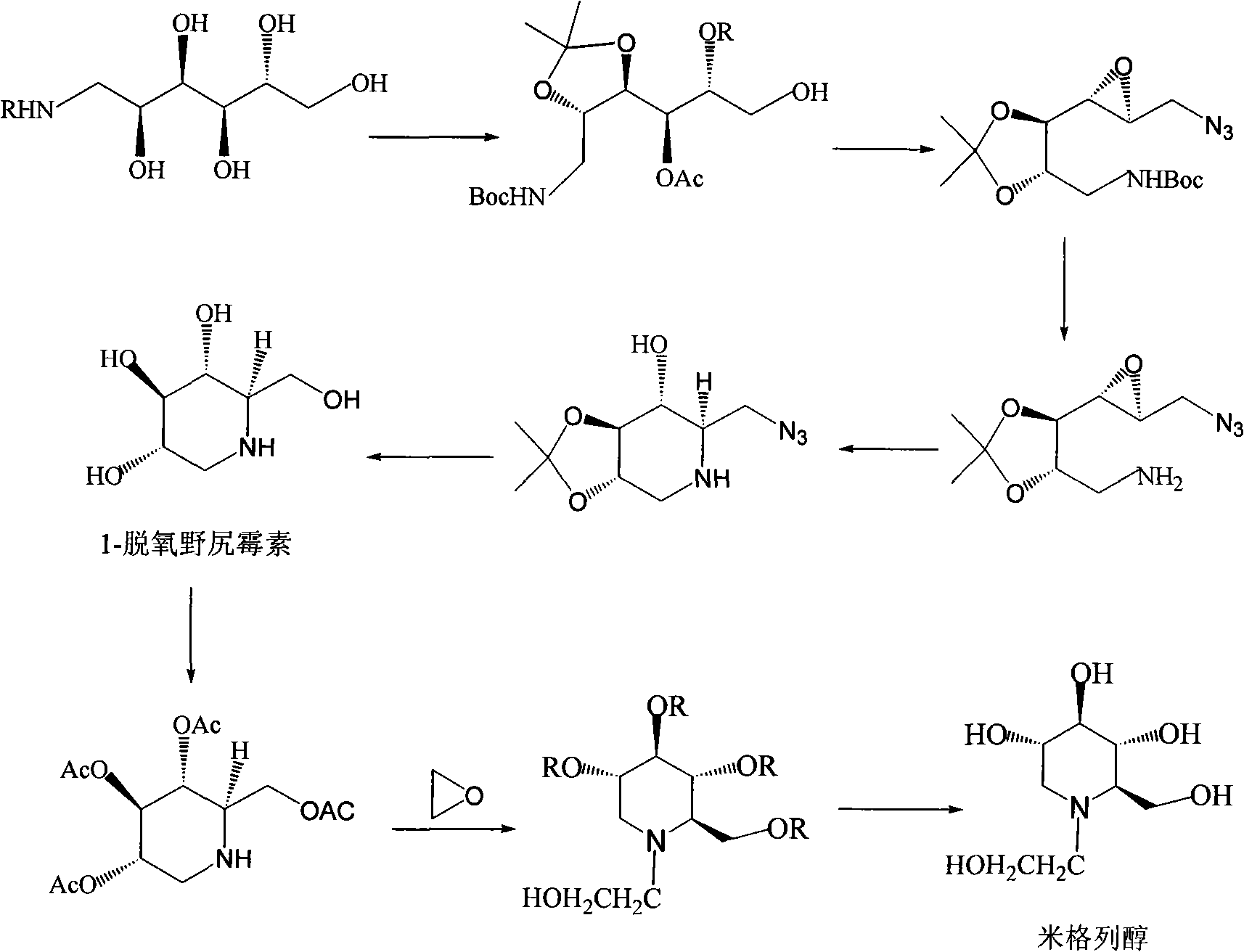
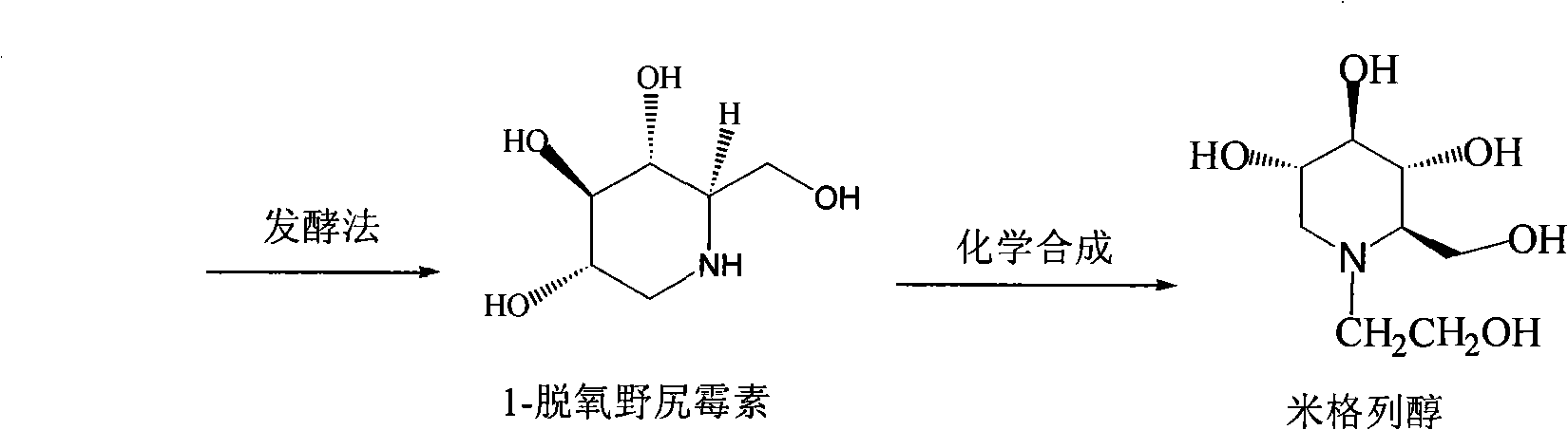
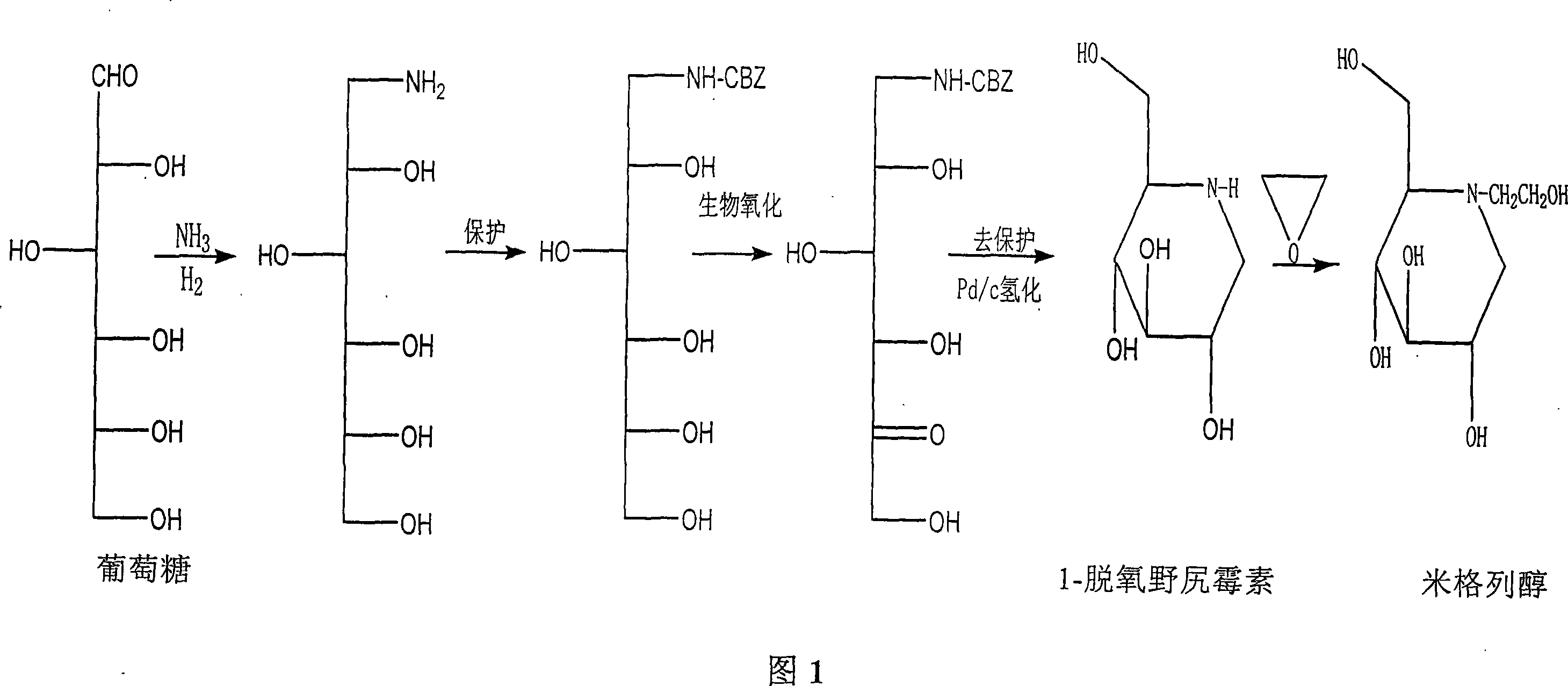
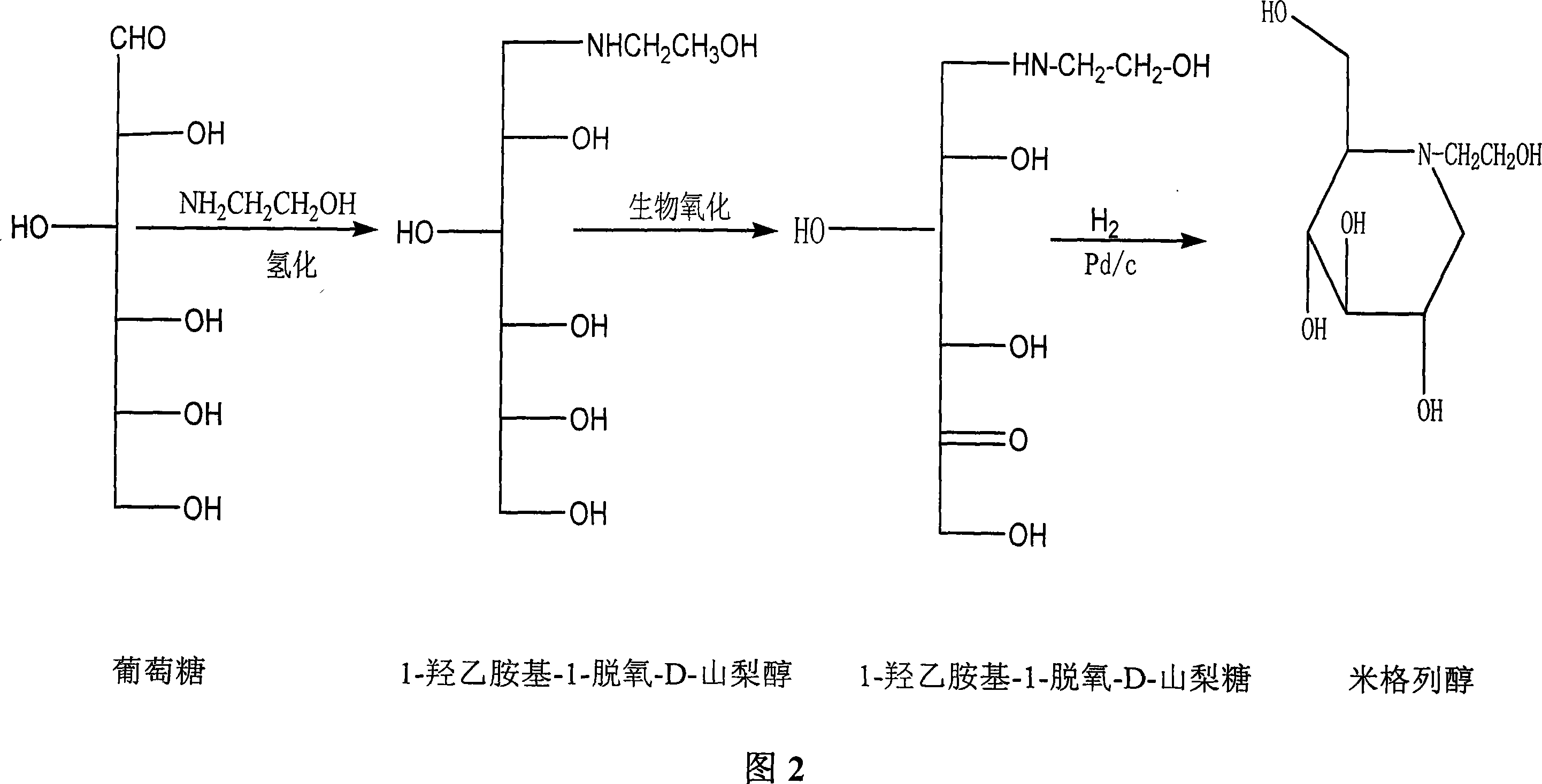
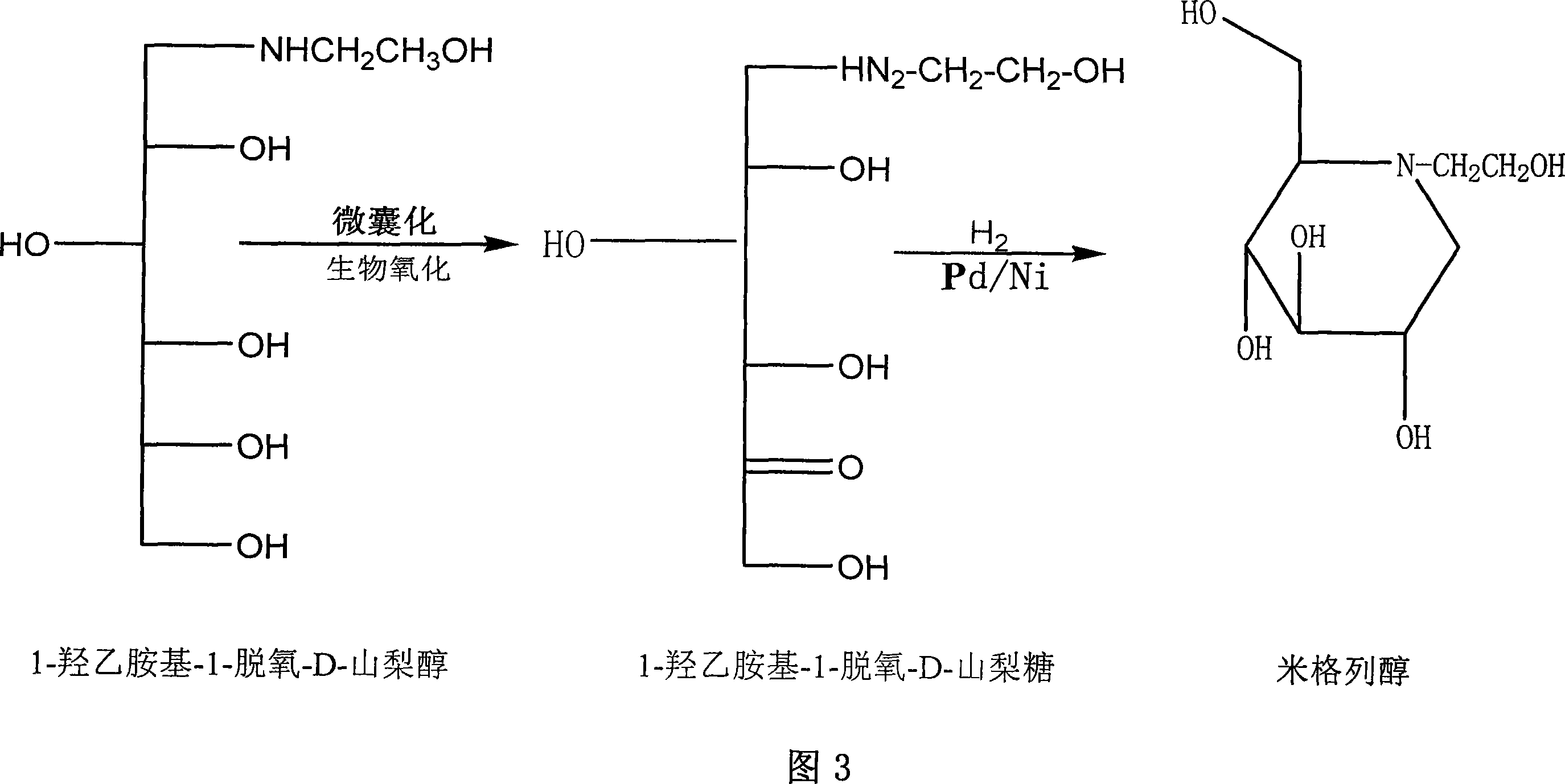
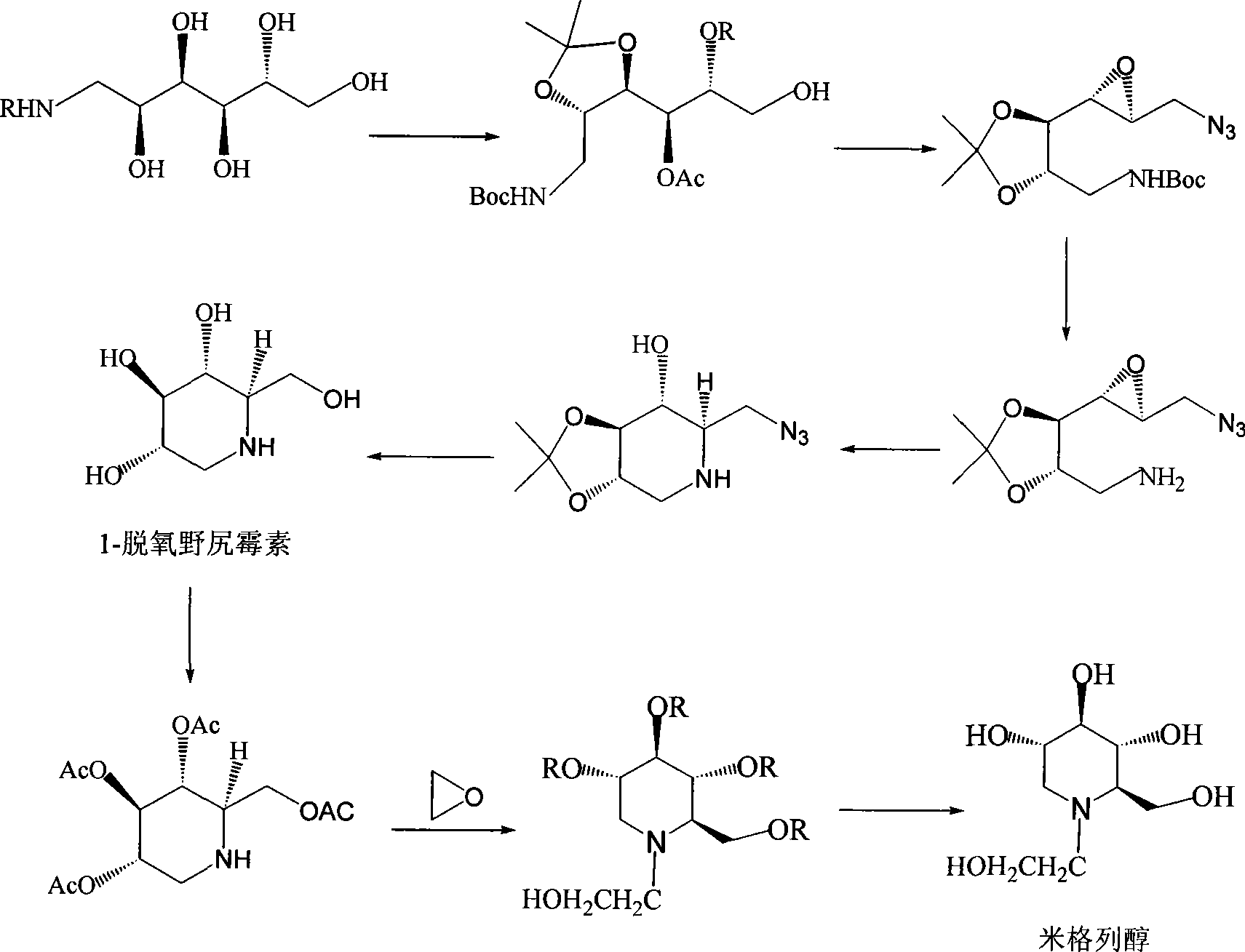
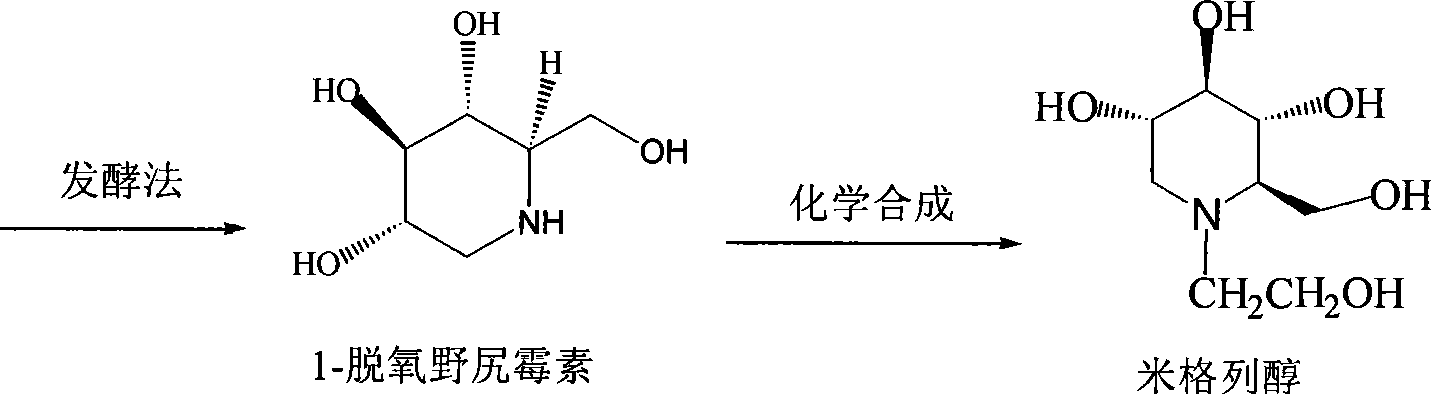
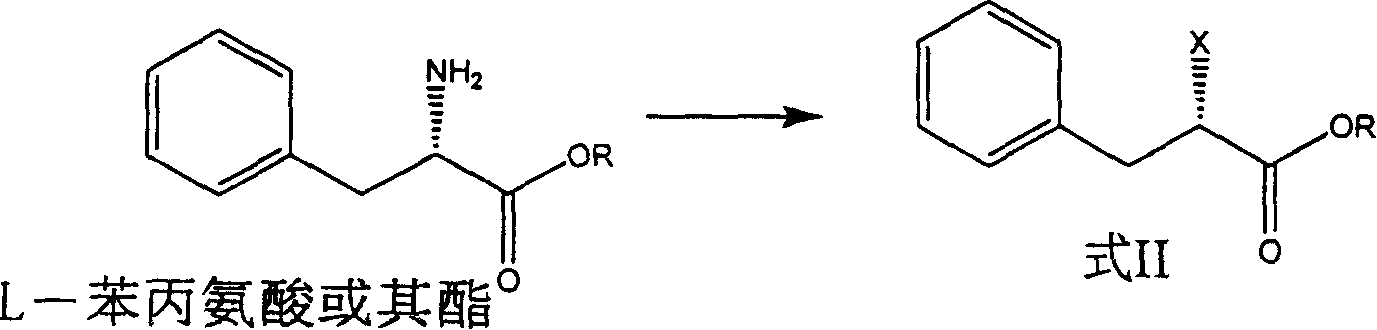
Preparation of miglitol
InactiveCN1680321AUnavailable solutionMild process conditionsOrganic chemistryMiglitolAsymmetric hydrogenation
Production of miglina is carried out by providing chiral center by L-phenylalanine. It achieves high efficiency and good optical purity, and to avoid chemical resolution and asymmetric hydrogenation.
Owner:江苏省药物研究所有限公司
Miglitol oral disintegration tablet for treating diabetes II and its preparing method
InactiveCN1615862AReduce degradationReduce absorptionMetabolism disorderPill deliveryMiglitolAdhesive
The orally disintegrated miglitol tablet for type-II diabetes consists of main medicine and supplementary material, the main medicine miglitol accounts for 5-50 % and is 10-200 mg in each tablet, and supplementary material consists of stuffing 5-30 wt%, disintegrating agent 5-45 wt%, disintegrating assistant 5-30 wt% and fine silica gel powder 0.1-3 wt% or consists of stuffing 5-30 wt%, disintegrating agent 5-45 wt%, disintegrating assistant 5-30 wt%, adhesive 5-15 wt% and lubricant 0.1-3 wt%. The disintegrated miglitol tablet is prepared with the material powder and through direct or wet tabletting. It is easy to take and can make full use of miglitol in treating diabetes effectively to lower the concentration of blood sugar after meal.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Pharmaceutical composition having alpha-glucosidase inhibition activity, and applications thereof
ActiveCN104984346AGood hypoglycemic effectHypoglycemic effect achievedOrganic active ingredientsMetabolism disorderSide effectHypoglycemia
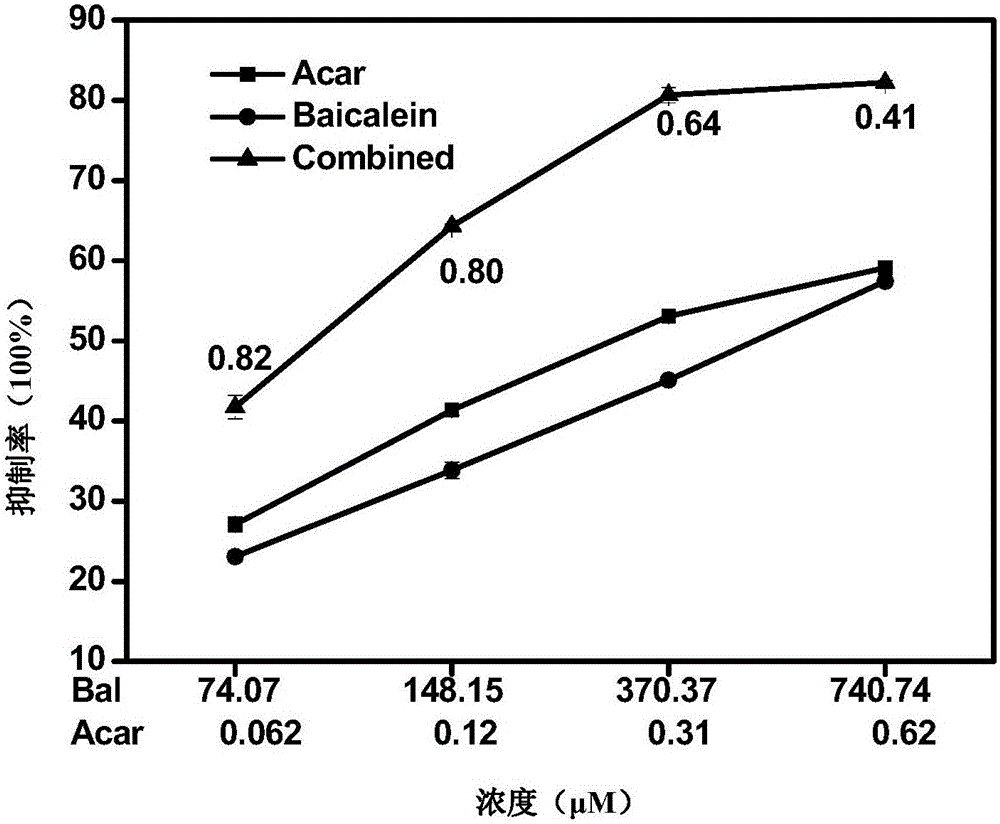
The invention relates to a pharmaceutical composition having alpha-glucosidase inhibition activity, wherein the pharmaceutical composition comprises a flavone compound and an alpha-glucosidase inhibitor, the flavone compound is at least one selected from a monomer such as baicalein, quercetin, luteolin, baicalein-7-O-glucoside and catechin, an organic salt of the monomer, and an inorganic salt of the monomer, and the alpha-glucosidase inhibitor is at least one selected from a monomer such as acarbose, voglibose and miglitol, an organic salt of the monomer, and an inorganic salt of the monomer. According to the present invention, the pharmaceutical composition can effectively reduce postprandial blood glucose, can inhibit the activity of alpha-glucosidase adopting starch, maltose and sucrose as substrates, and less uses the alpha-glucosidase inhibitor, such that the efficacy can be improved, the side effect of the alpha-glucosidase inhibitor can be effectively reduced, and hypoglycemia and other problems easily caused by drug combination are effectively solved.
Owner:上海皋鱼医药科技有限公司
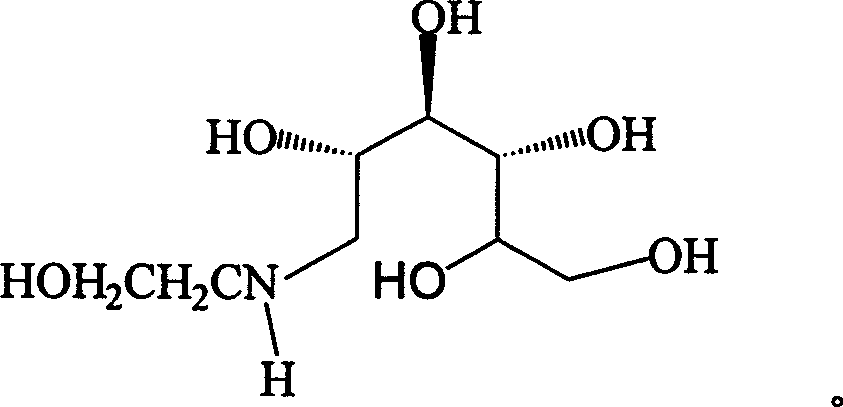
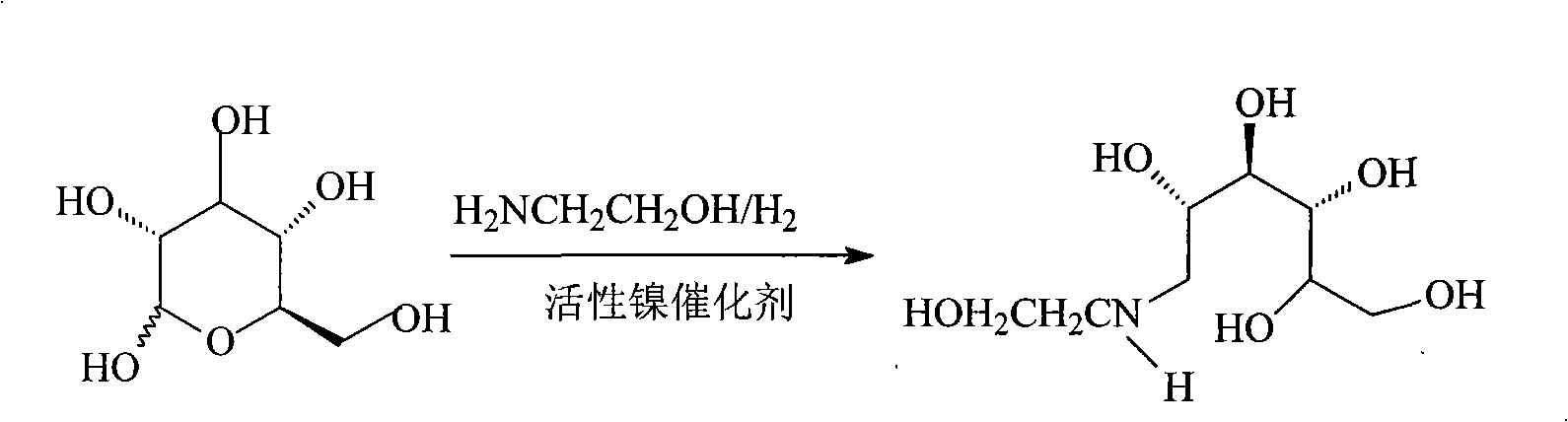
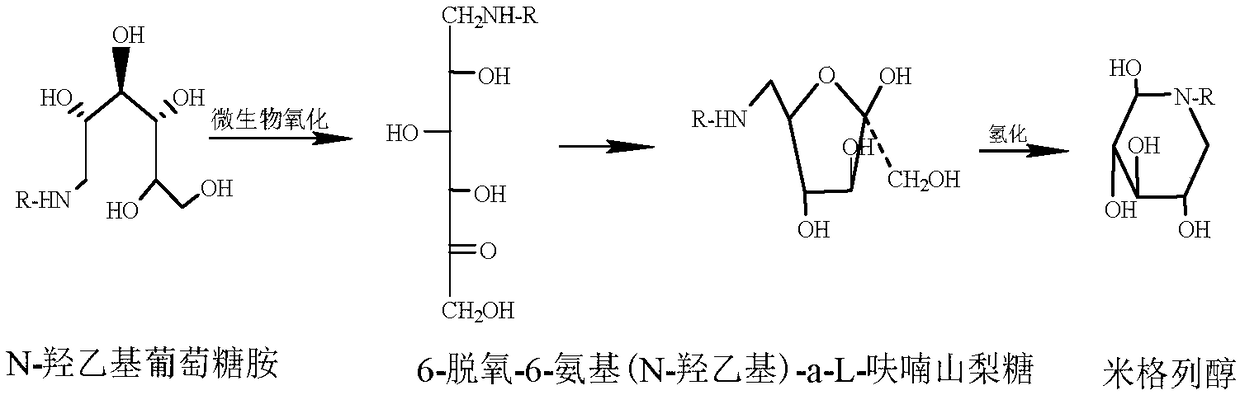
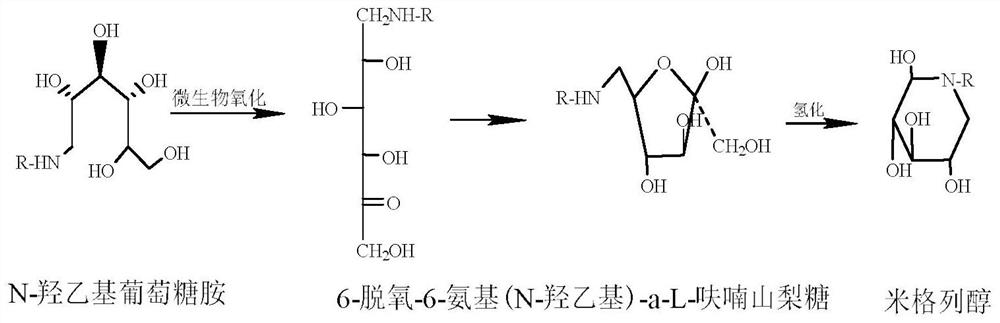
Method for synthesizing N- (2-hydroxyethyl)-glucosamine
InactiveCN1611485AMild reaction conditionsImprove responseOrganic compound preparationAmino-hyroxy compound preparationDiabetes TherapyMiglitol
The invention relates to a kind of method for synthesizing midbody N-(2-ethoxyl)-glucosamine of diabetes therapy medicine miglitol alcohol. The existing literature has no synthesizing method recordation of midbody N-(2-ethoxyl)-glucosamine. The reaction steps of the invention are as follows: A) Stuff casting: in autoclave, add D-glucose and ethanolamine according to molar ratio of D-glucose / ethanolamine with 1 / 1.05-1.10; then add 70-95% alcohols solvent with 5-10 times weight of glucose; and after nitrogen displace, add 5%Pd-CaCO3-1-5%Pb catalyst with 2-10% weight of glucose; inject high purity hydrogen, keep hydrogen pressure to 2-3Mpa, and then intensify the temperature slowly up to reaction temperature; B) Reaction: control reaction temperature in 35-45deg.C, hydrogen pressure in 2-4Mpa, and reaction by 20-24 hours; C) Get white pulverous solid by after-treatment. The invention selects the appropriate catalyst and reasonable hydrogenization technology, so that it can make the reaction condition of glucose and ethanolamine with relatively gentleness, make the reaction easy to proceed, make by-product and other impurity generated badly, and also it has high conversion rate, and more than or equal to 98% purity.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
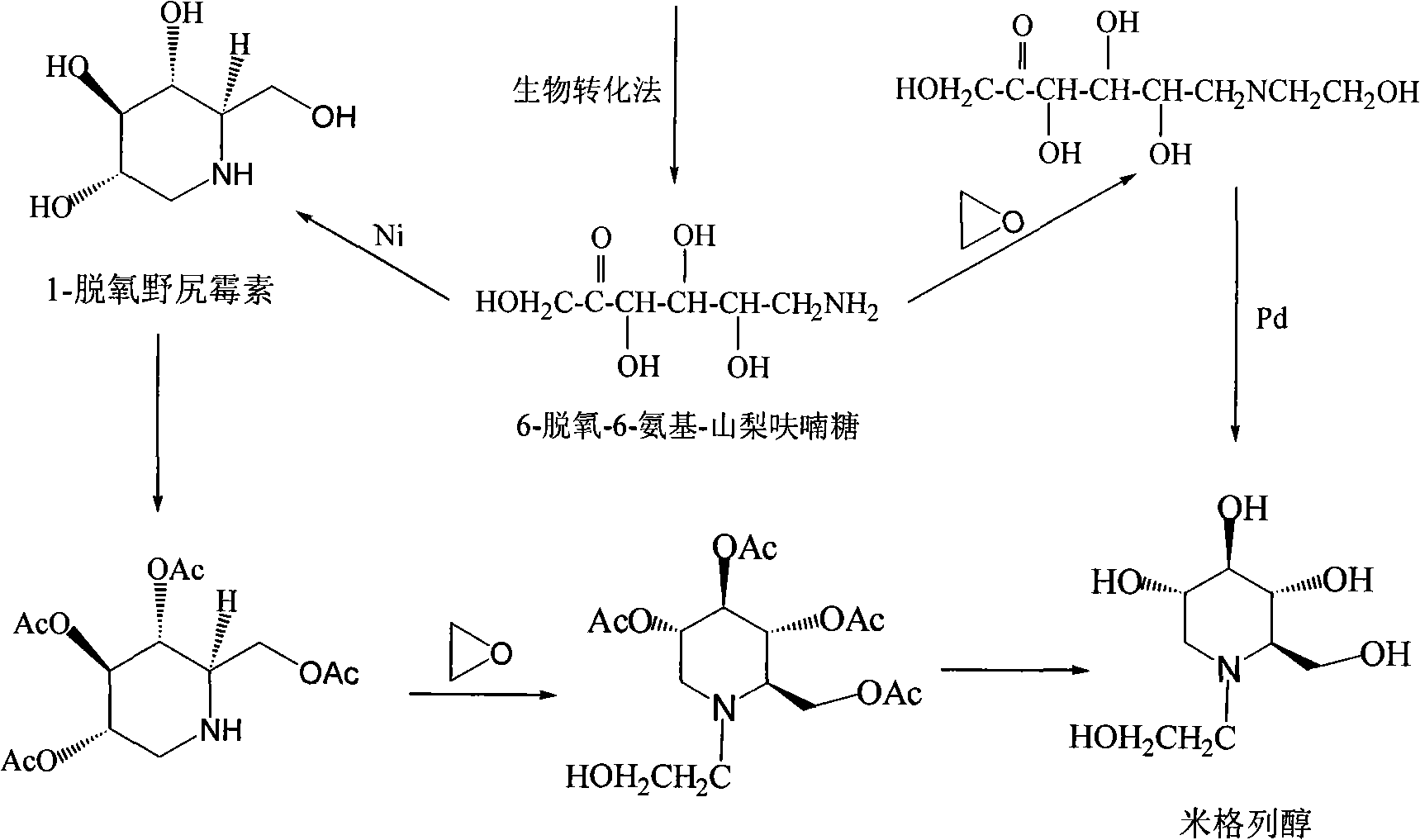
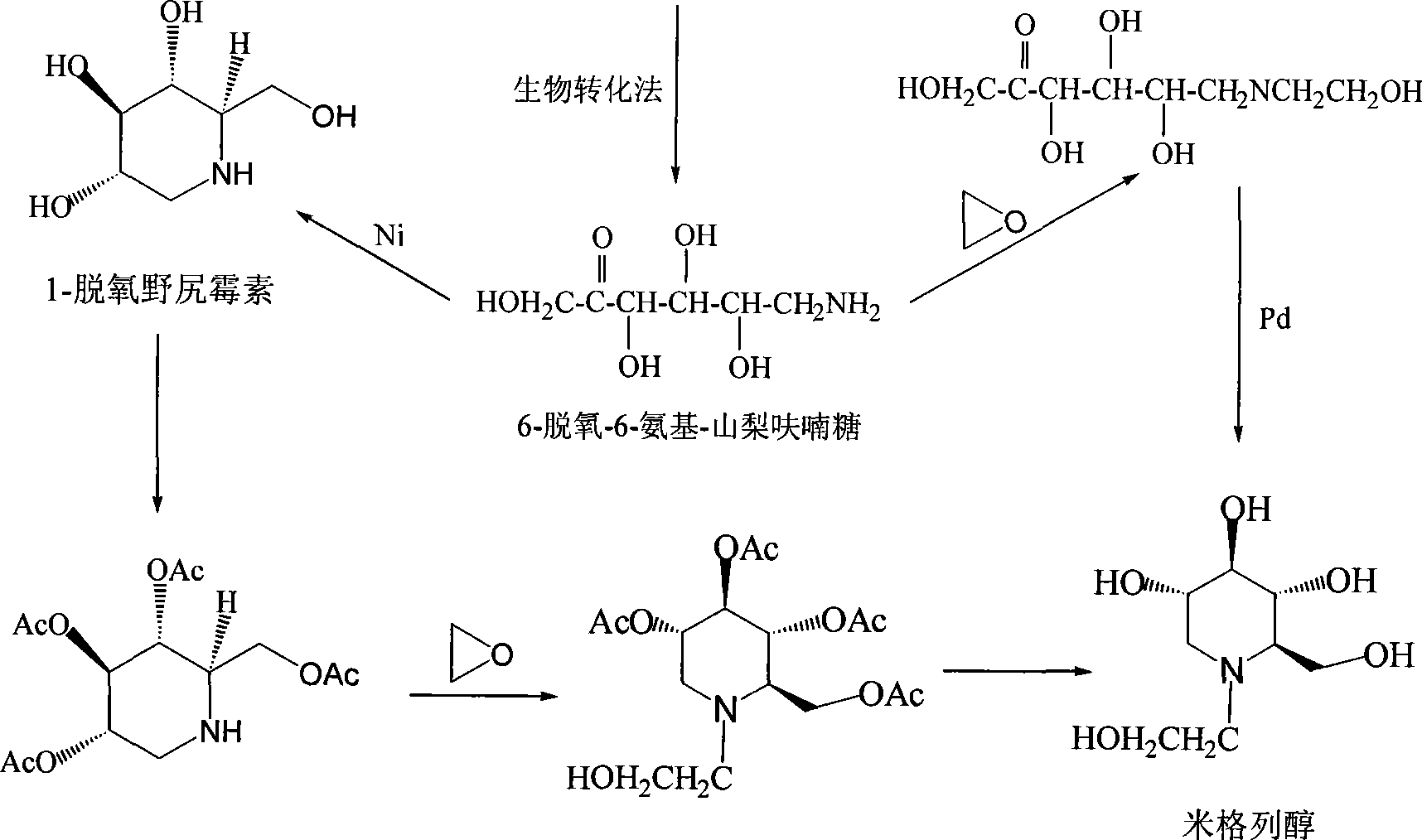
High-purity miglitol production process
ActiveCN101302549AEfficient purificationRealize industrializationOrganic chemistryBacteriaMiglitolDesorption
The invention relates to a method for producing high-purity miglitol. The method comprises the following stages that: Stage a. a miglitol producing strain is obtained through the cultivation of a culture medium consisting of D-sorbierite, a yeast extract and KH2PO4 and microfiltration separation; Stage b. the miglitol producing strain performs biotransformation, microfiltration, hyperfiltration, nanofiltration, and activated carbon decolorization to a substrate to obtain an intermediate of the miglitol; Stage c. hydrogenation reaction, ion fractionating, activated carbon decolorization, desorption, condensation and crystallization are performed to the intermediate of the miglitol obtained in the Stage b to obtain the high-purity miglitol. The adoption of the method can improve the production efficiency of the miglitol to the utmost extent, thereby realizing the industrialization of the high-purity miglitol.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Process for producing miglitol key intermediate
ActiveCN101492380AMild reaction conditionsShort reaction timeOrganic compound preparationChemical recyclingMiglitolSolvent
The invention relates to a preparation method of a drug used for diabetes, namely Miglitol intermediate N-hydroxyethyl glucosamine. The preparation method is characterized in that glucose and ethanolamine are used as initial raw materials; with solvent, active nickel catalyst and amine compounds are used as catalysts for the catalytic hydrogenation; the reaction pressure and the temperature are controlled for the reaction; when the reaction is finished, N-hydroxyethyl glucosamine white crystals are obtained after post-processing and refining; the yield is larger than 90 percent, and the purity is larger than or equal to 98.5 percent (HPLC detection). The preparation method solves the problems of the prior art of high cost, harsh reaction conditions and complex preparing process caused by the adoption of dear metals (catalysts such as platinum, palladium-charcoal, etc.) with high catalytic activity for the catalytic hydrogenation.
Owner:LUNAN PHARMA GROUP CORPORATION
Production of miglitol
InactiveCN101029321AActivity declines slowlyShort conversion cycleMicroorganism based processesFermentationMiglitolAlcohol
Production of migneicohol is carried out by oxidizing for raw material, preparing 1-ethoxy-amino-1-deoxy-D-sorbic alcohol, coating ketogluconic acid oxide bacterium by polymerization ion film to prepare into micro-capsule containing bacterium, adding into 1-ethoxy-amino-1-deoxy-D-sorbic alcohol solution and micro-capsule containing bacterium into fermentor successively, agitating at 28-31degree, agitating, inducing into gas 1 / 1(V / V), converting for 23-28hrs, adjusting pH to 5.0-6.0, filtering out micro-capsule containing bacterium to obtain 1-ethoxy-amino-1-deoxy-D-sorbic alcohol filtrate, adding catalyst into filtrate, hydrogenating, refining for filtrate by resin, concentrating, and crystallizing to obtain the final product.
Owner:四川维奥制药有限公司
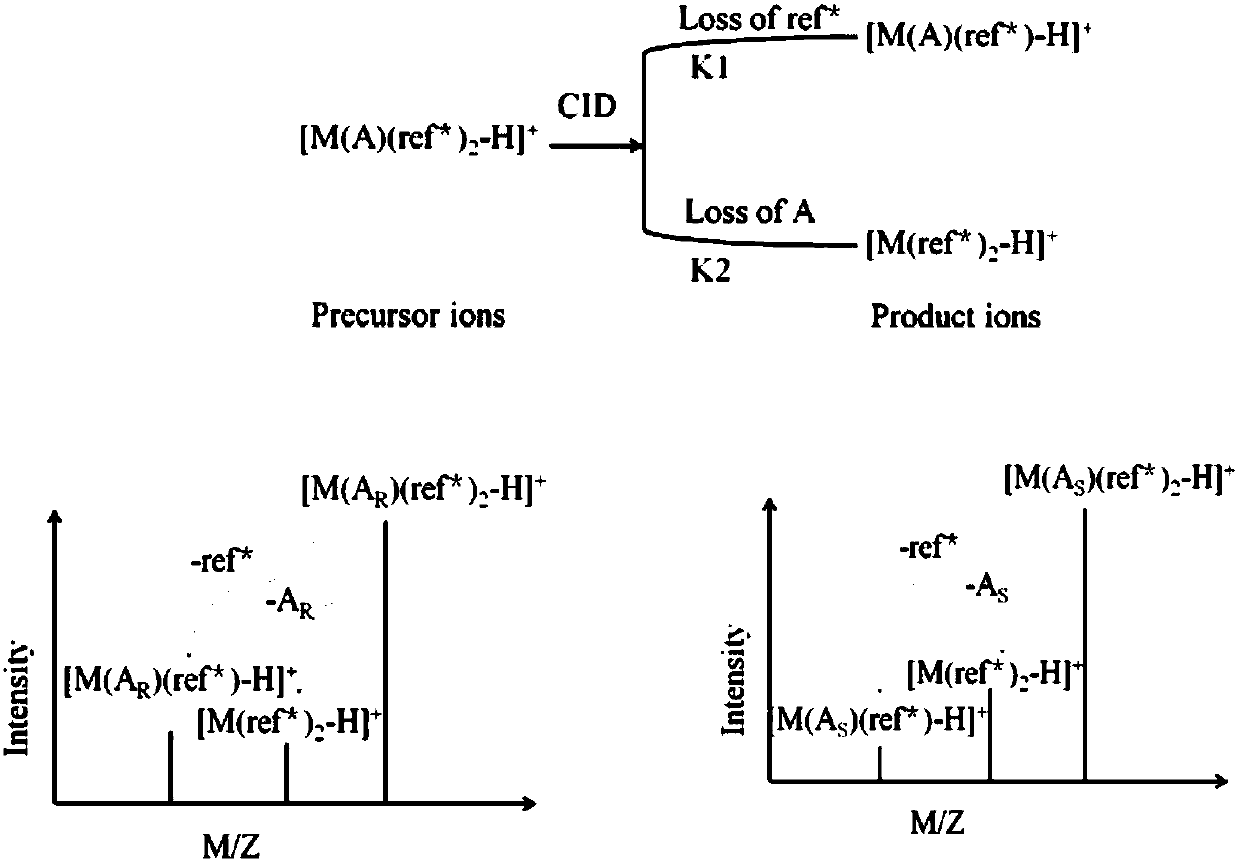
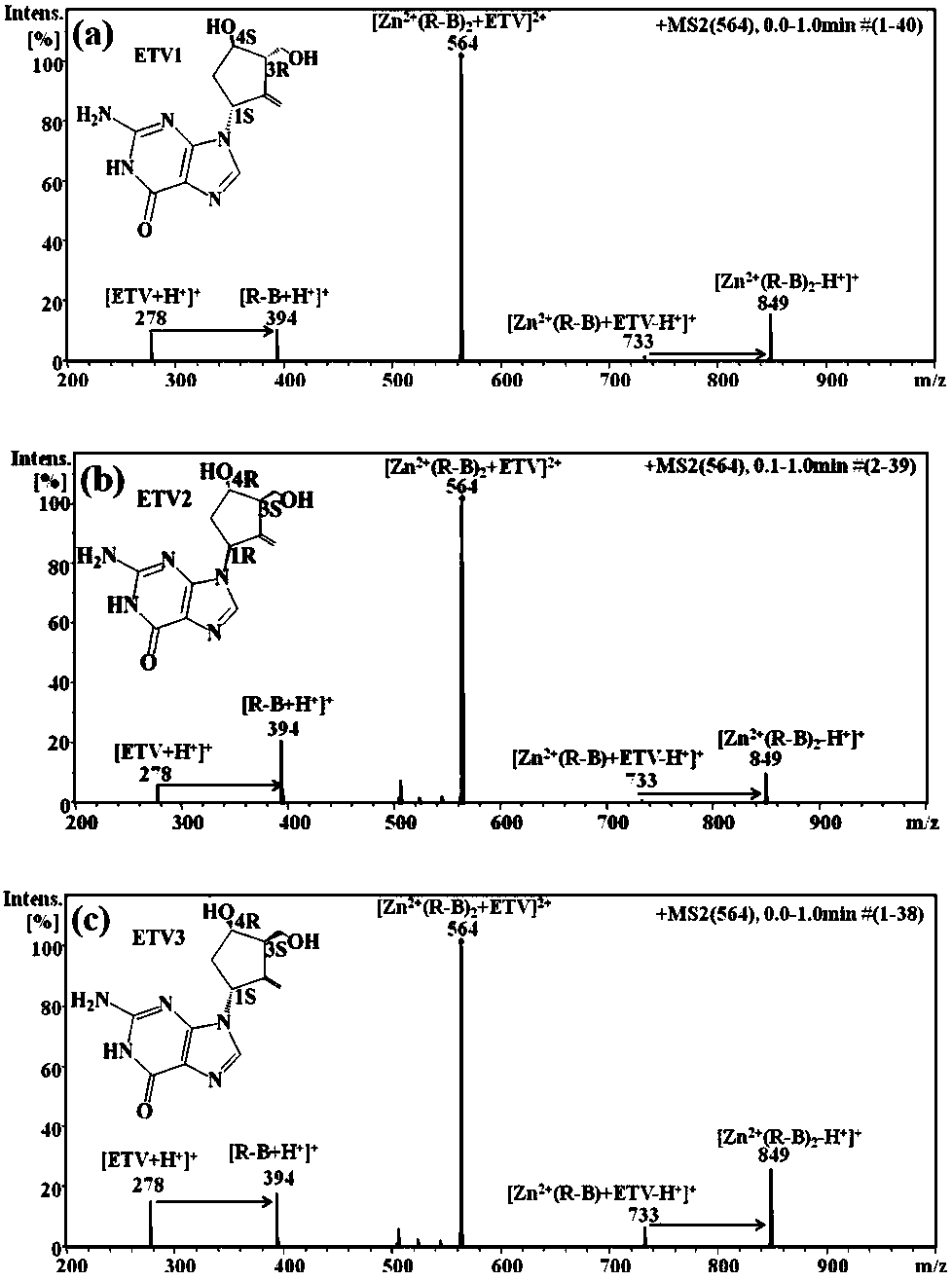
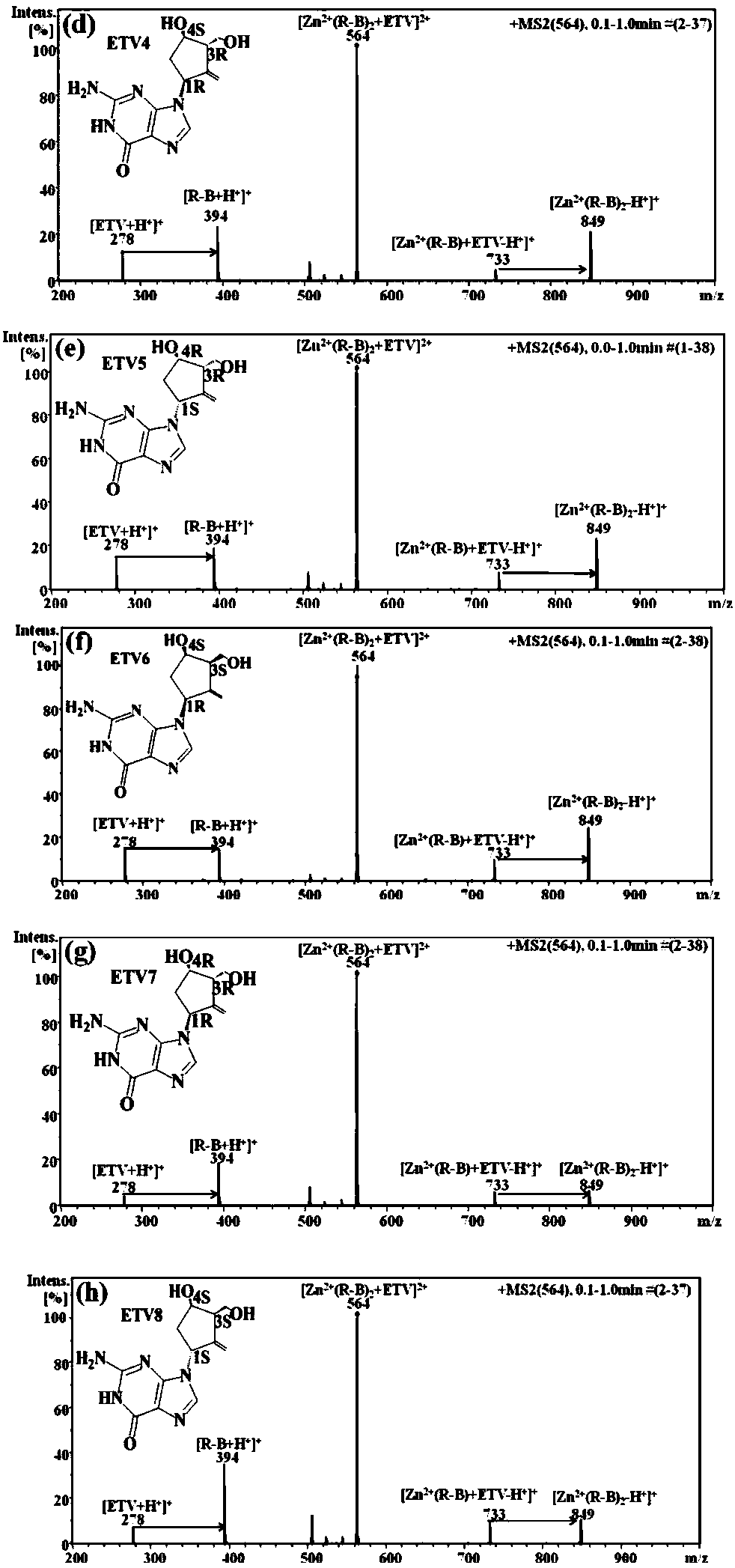
Distinguishing and determining method of entecavir chiral isomers
ActiveCN107764891AImprove separation efficiencyFast wayPreparing sample for investigationMaterial analysis by electric/magnetic meansMiglitolAlkaline earth metal
The invention discloses a distinguishing and determining method of entecavir chiral isomers. The method comprises the steps of adopting a chiral ligand, metal ions and the eight entecavir chiral isomers to form complexes with different stable forms; utilizing a mass-spectrography method for recognizing and distinguishing the complexes, wherein the chiral ligand comprises R-besifloxacin, beta-cyclodextrin, migltol or cefotaxime; the metal ions comprise alkaline-earth metals or transient state metals; the alkaline-earth metals comprise Mg and Ca; the transient state metals comprise Ni, Cu, Co, Zn and Mn. The method is fast, simple and convenient, overcomes the defects that a traditional HPLC method needs a specific chiral stationary phase, solvent gradient eluting consumed time is longer, and an enantiomeric separation degree is insufficient, and can be used for quickly distinguishing the eight entecavir chiral isomers and quantitatively determining.
Owner:HANGZHOU LEADING PHARMATECH CO LTD +1
Method for rapidly screening mutant strain for synthesizing miglitol key intermediate, and strain thereof
ActiveCN108441491AIncrease screening volumeEasy to operateBacteriaMutant preparationMiglitolMicrobiology
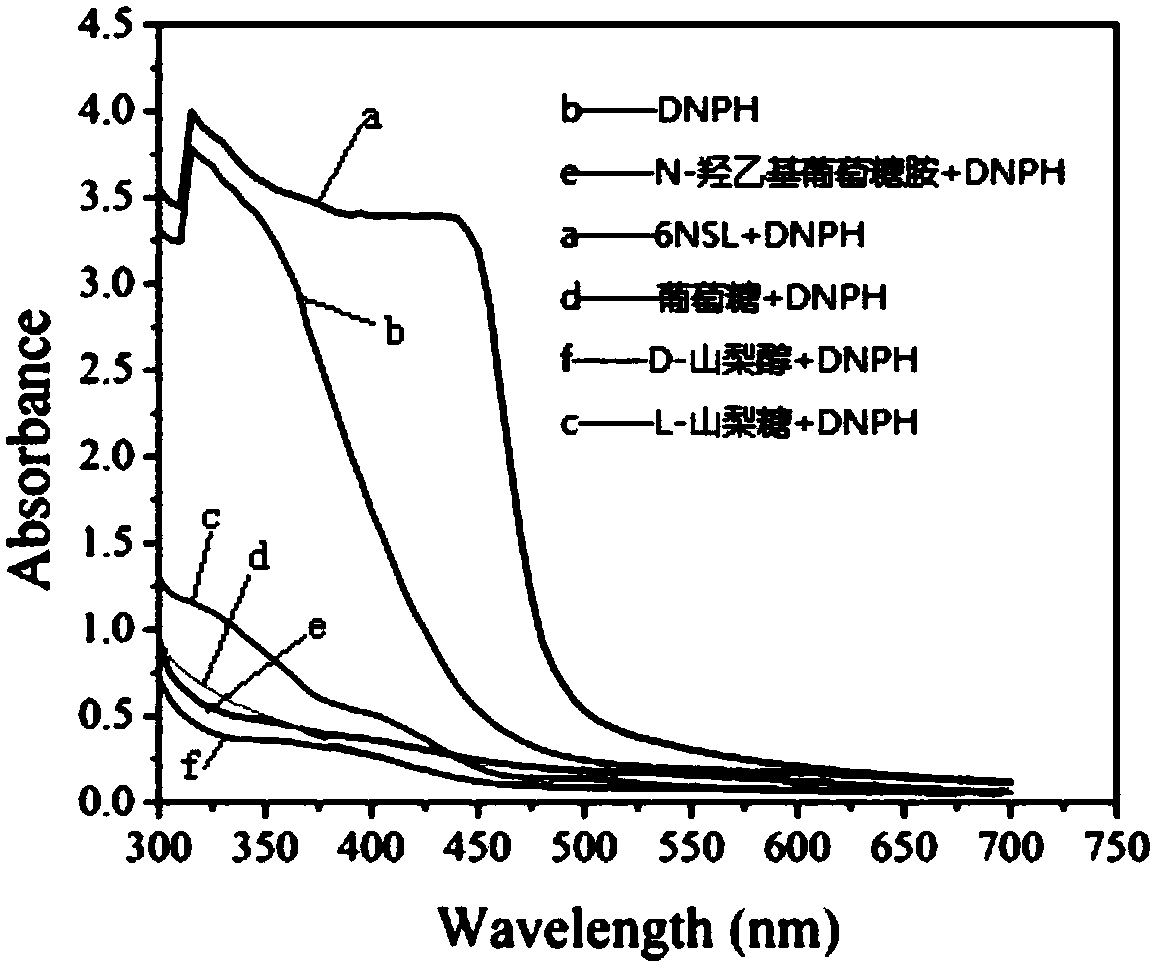
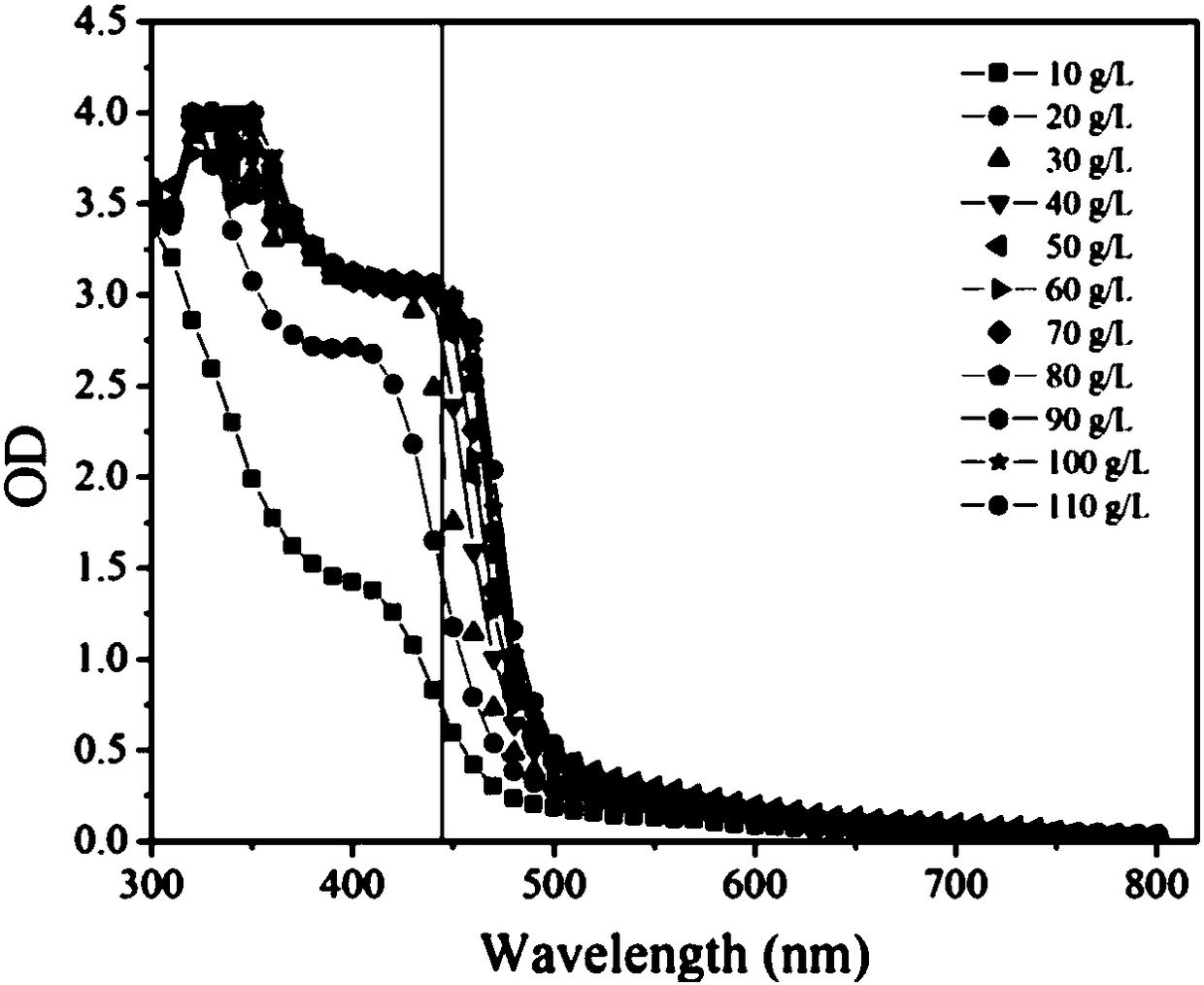
The invention provides a method for rapidly and quantitatively detecting a mutant strain for a miglitol key intermediate, and the strain thereof. The method comprises the following steps: performing fermentation culture on a mutagenized strain obtained by mutagenizing wild Gluconobacter oxydans, taking obtained fermentation-cultured mutated strain wet cells into a substrate reaction solution, completing a conversion reaction at 15 DEG C, centrifuging the obtained reaction solution, measuring the content of 6NSL in the obtained supernatant, and performing screening according to the level of the6NSL content to obtain the high-vitality mutated strain for synthesizing the miglitol key intermediate. The detection method has the characteristics of simplicity, fastness, high specificity and goodrepeatability, and can be further applied to the screening of the high-vitality Gluconobacter oxydans and the effective acceleration of the breeding process, and the screened mutated strain has a significantly improved catalysis effect on the conversion synthesis of the miglitol key intermediate 6NSL, and is suitable for industrial large-scale production of miglitol.
Owner:ZHEJIANG UNIV OF TECH +1
Pharmaceutical composition for improving complications of high-fat and high-sugar diet and application thereof
ActiveCN104840962AOvercoming hypoglycemic effect is not obviousOvercoming large doseMetabolism disorderDigestive systemMiglitolSide effect
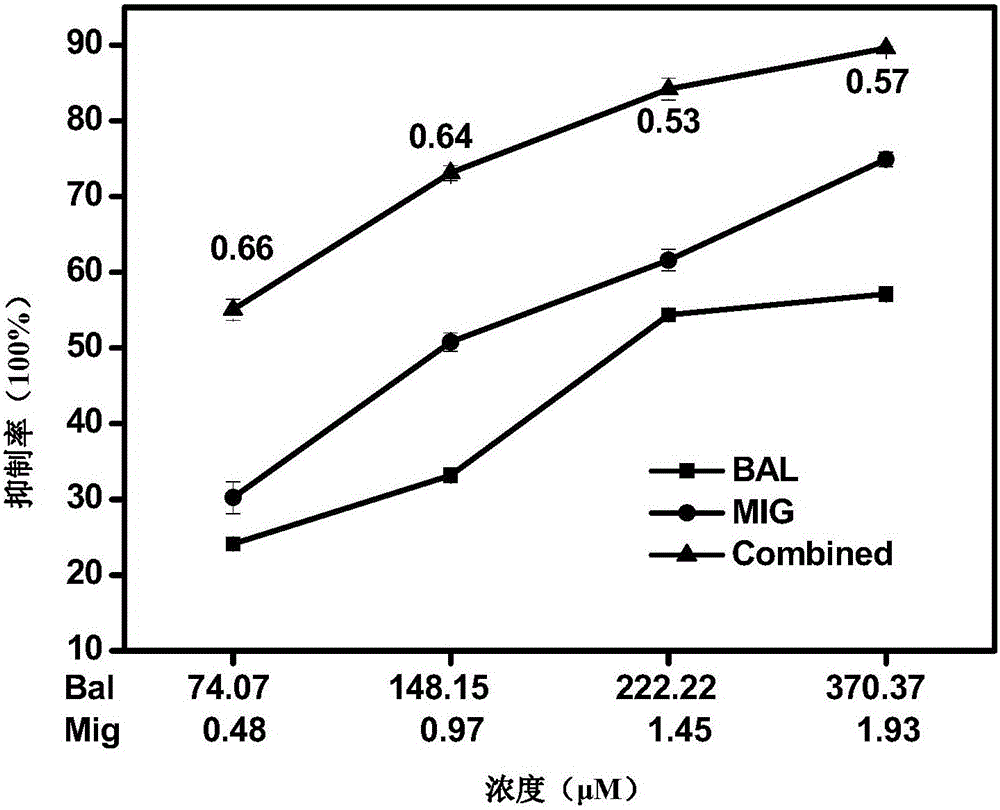
The invention provides a pharmaceutical composition for improving complications of high-fat and high-sugar diet. The pharmaceutical composition comprises flavonoid and an alpha-glucosidase inhibitor, wherein the flavonoid is at least one selected from the group consisting of monomer baicalein, monomer chrysin, organic salts of the monomers and inorganic salts of the monomers, and the alpha-glucosidase inhibitor is one selected from the group consisting of monomer acarbose, monomer voglibose, monomer migltol, organic salts of the monomers and inorganic salts of the monomers. The composition provided by the invention can effectively reduce the dosage of the alpha-glucosidase inhibitor; desired effects can be obtained by mixing the flavonoid with the alpha-glucosidase inhibitor with a dosage 0.01 to 0.75 time of a normal dosage; the disadvantages of a great dosage and great side-effects in individual usage of the alpha-glucosidase inhibitor are overcome; the disadvantages of unobvious hypoglycemic effect, a great dosage, a long administration period and the like in individual usage of the flavonoid are overcome; and the disadvantages of complex components and difficult quality control of a traditional Chinese medicine compound drug are overcome.
Owner:上海皋鱼医药科技有限公司
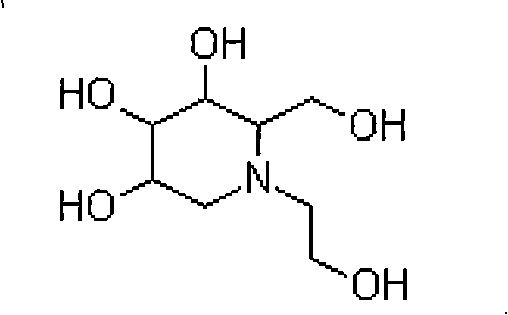
Miglitol crystal and method for preparing same
ActiveCN101289418ALow hygroscopicityEasy to purifyOrganic chemistryMetabolism disorderMiglitolHigh humidity
The invention discloses a crystalline miglitol and a preparation method thereof. The technical contents related to the crystalline miglitol are not recorded in the existing literature. The miglitol crystal of the invention uses Cu-Ka radiation, and diffraction peaks are arranged at 14.1 degrees, 15.9 degrees, 19.5 degrees and 23.5 degrees of a powder X-ray diffraction spectrum which is embodied by a diffraction angle of 2 theta plus or abstract 0.2 degree. The crystalline miglitol has the advantages of low moisture absorption and being easy to purify except in the condition of high humidity; the crystalline miglitol which is excellent in stability is beneficial for the preparation of pharmaceutical preparation and improving bioavailability of drug.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
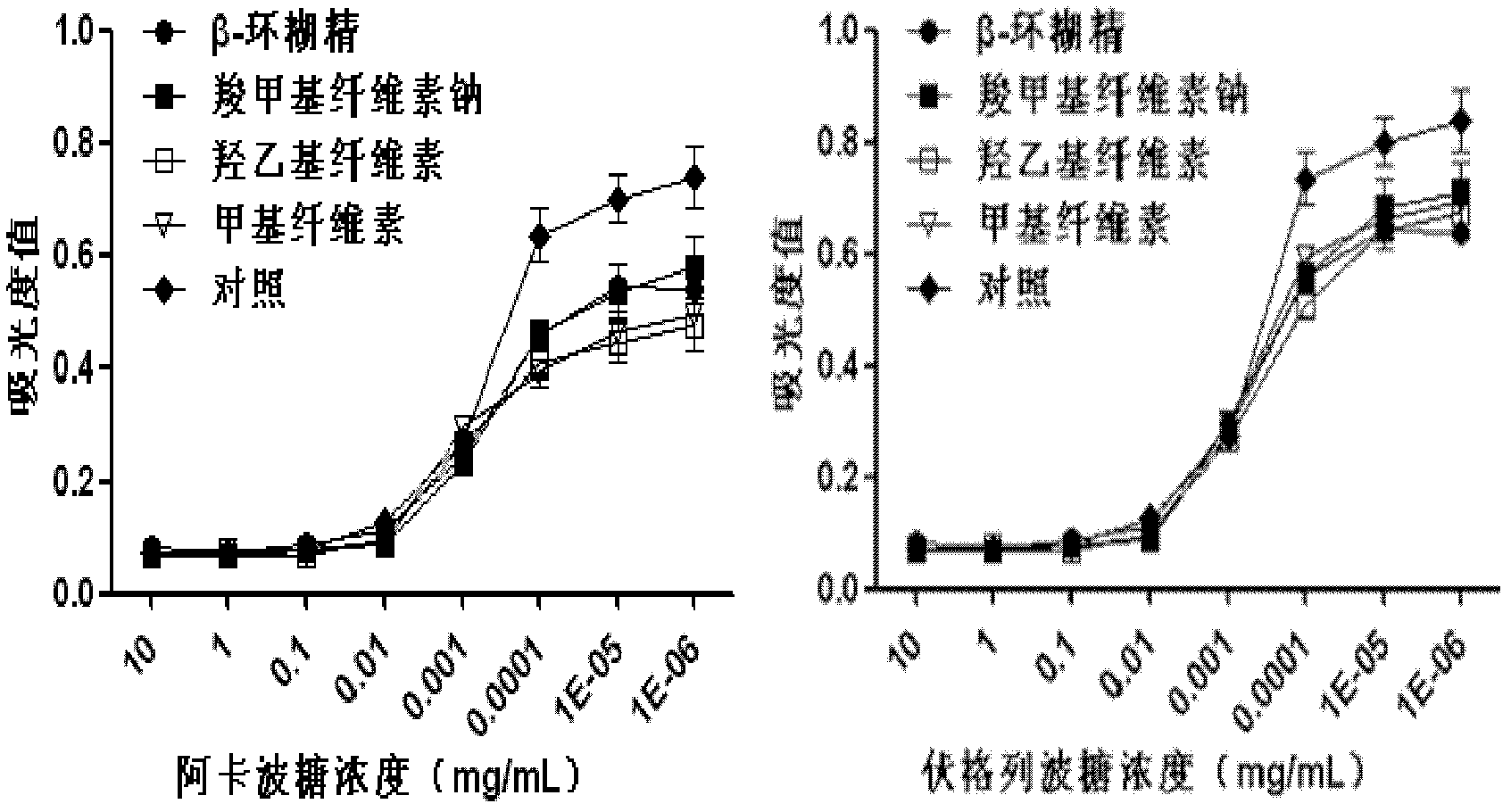
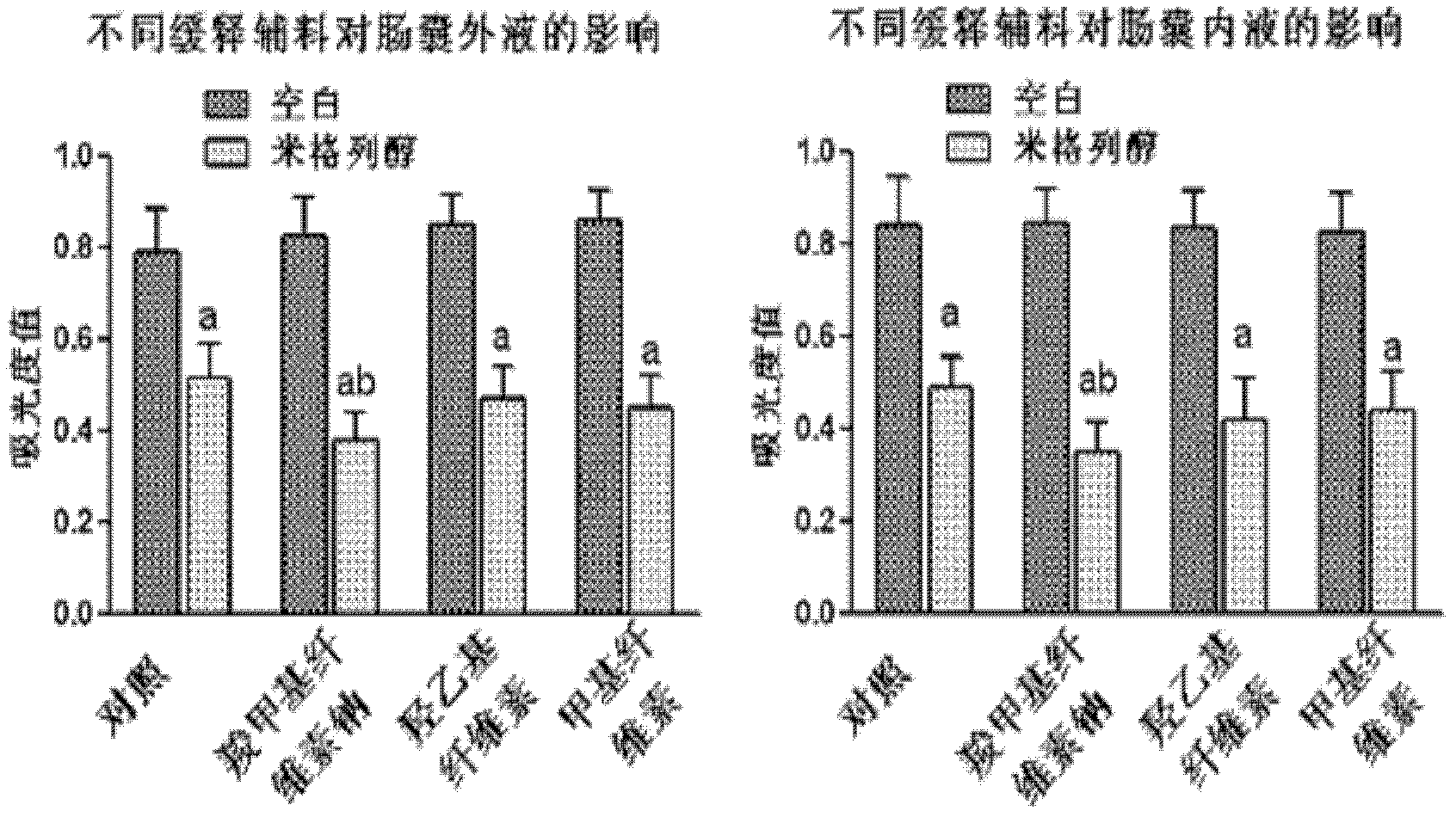
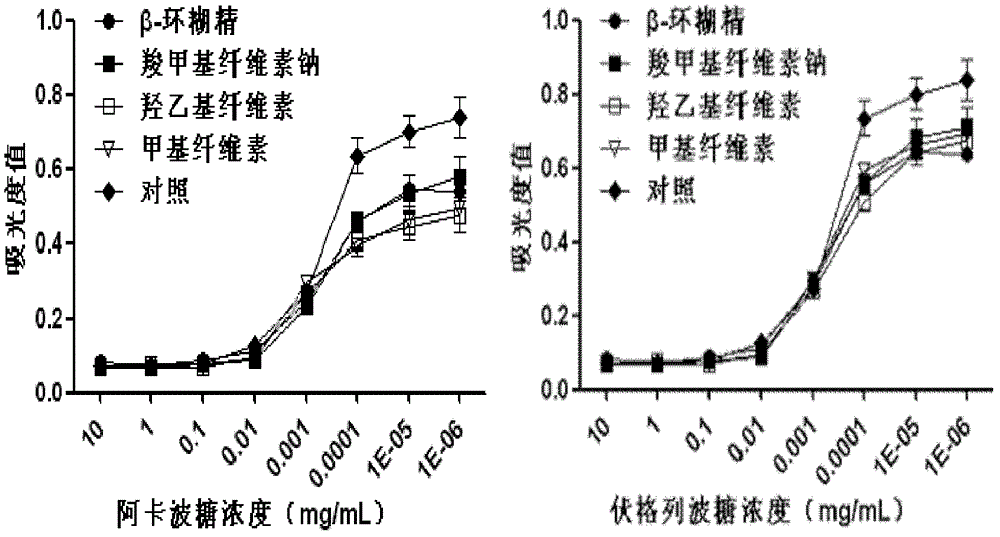
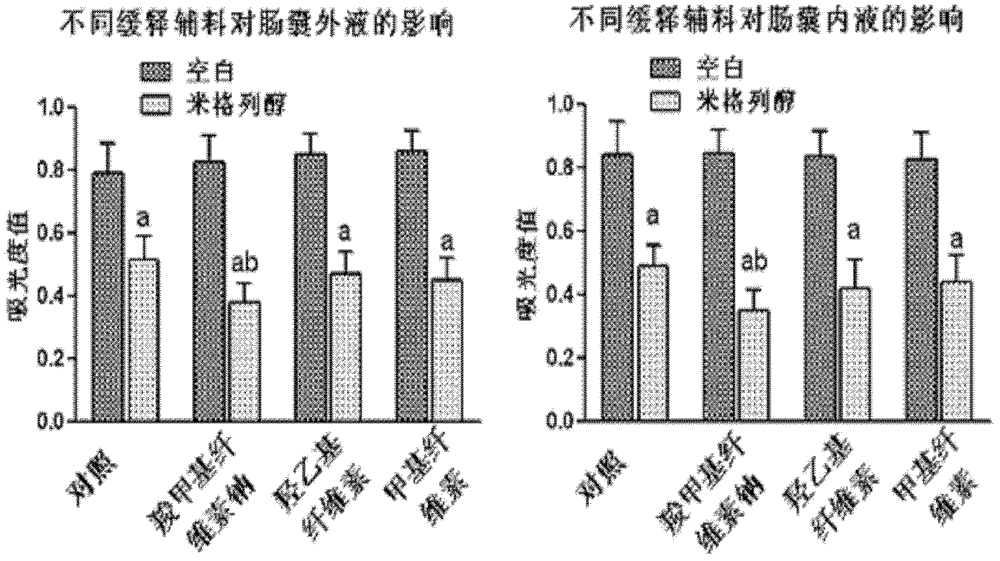
Oral preparation for slowing down absorption of alpha-glycosidase inhibitor and enhancing hypoglycemic drug effect
ActiveCN102210866AReduce absorptionProlong the action timeMetabolism disorderMacromolecular non-active ingredientsDiseaseCellulose
The invention discloses an oral preparation for slowing down the absorption of an alpha-glycosidase inhibitor and enhancing a hypoglycemic drug effect. The preparation is an oral preparation prepared by adding the following adhesive auxiliary materials based on the certain proportion: carboxymethyl cellulose, hydroxyethyl cellulose, methyl cellulose or beta-cyclodextrin and the like, to each alpha-glycosidase inhibitor (acarbose, voglibose, miglitol, 1-deoxynojirimycin and extract of the 1-deoxynojirimycin). Compared with singly taken alpha-glycosidase inhibitors, the adhesives can be used for prolonging the action time of the alpha-glycosidase inhibitor in the intestine, slowing down the absorption of a medicament and glucose in the intestine and further reducing a plasma concentration, thus the hypoglycemic drug effect is improved. The oral preparation disclosed by the invention can be applied to the prevention and therapy of diseases, such as diabetes, obesity and the like.
Owner:NANKAI UNIV +1
Method for preparing miglitol midbody N-substituted-1-deoxidization nojirimycin derivative
InactiveCN101270378AReduce lossesLarge amount of processingMicroorganism based processesOn/in organic carrierMiglitolNojirimycin
The invention relates to a preparation method of miglitol intermediate, in particular to a preparation method of a derivative of N-substitution-1-deoxynojirimycin which is the key intermediate of miglitol. Gluoconobacter oxydans or the Gluoconobacter oxydans treated by immobilized cell technology are used repeatedly for transforming the substrate to the derivative of N-substitution-1-deoxynojirimycin which is the miglitol intermediate, the transfer temperature is controlled at 5 to 25 DEG C, pH value is controlled at 4.0 to 6.5 and dissolved oxygen is controlled at 5 to 80 percent (in volume). Adopting the invention can decrease the biotransformation cost, reduce the inter-assay of cell, and be suitable for the large scale industrial production, increase the mechanical strength of the wall of the strain prepared, make the strain not to dissolve during the cultivation in long-term liquid environment, lengthen the service life and have outstanding slow release performance.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Alpha-glycosidase inhibitor and hydroxymethyl glutaryl coenzyme A reductase inhibitor composition for treating diabetes and complications
The invention discloses an alpha-glycosidase inhibitor and hydroxymethyl glutaryl coenzyme A reductase inhibitor composition for treating diabetes and complications. The medicinal composition consists of an alpha-glycosidase inhibitor or a pharmaceutically acceptable salt thereof, a hydroxymethyl glutaryl coenzyme A reductase inhibitor or a pharmaceutically acceptable salt thereof and a medicinal carrier or an excipient, wherein the alpha-glycosidase inhibitor is acarbose, voglibose or miglitol; and the hydroxymethyl glutaryl coenzyme A reductase inhibitor is simvastatin, lovastatin, fluvastatin, pravastatin, pitavastatin, rosuvastatin, atorvastatin, cerivastatin, mevastatin or rosuvastatin. The invention also relates to application of the medicinal composition in treatment of diabetes and prevention and treatment of diabetic complications such as diabetic nephropathy, diabetic hypertension, hyperlipidemia and dyslipidemia.
Owner:乔文龙
Medicine composition containing insulin intensifier and miglitol
ActiveCN101121004BReduce complicationsImprove risk factorsMetabolism disorderPharmaceutical non-active ingredientsMiglitolAcute hyperglycaemia
The present invention relates to an oral blood-sugar-reducing compound medicinal preparation, which consists of an insulin sensitizer, a miglitol and auxiliary materials. Compared with the prior art, the present invention is characterized in that under a circumstance of a same curative effect, a separate dosage of the insulin sensitizer or the miglitol is reduced; at the same time compared with other hypoglycemic drugs, a side effect of the present invention is reduced; the insulin sensitizer and the miglitol have a synergistic effect: the insulin sensitizer and the miglitol respectively takecurative actions towards a patient with hyperglycemia synchronously according to the different pharmacological effects and directly provide the patient or a doctor with a scientific combined medication to improve the curative effect and provide a clinic or the patient with convenience.
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation method for miglitol
InactiveCN107746385ANo pollution in the processHigh reaction yieldOrganic chemistryMiglitolFiltration
The invention discloses a preparation method for miglitol. The method comprises the following steps: selecting 6-deoxy-6-hydroxyethylamino-alpha-L-sorbose as a raw material, adding an alcohol solvent,performing a catalytic hydrogenation reaction under a certain pressure condition in the presence of a hydrogen gas, then performing pressure filtration, performing concentration, performing crystallization, performing vacuum filtration, performing washing, and performing vacuum drying to obtain the miglitol crystal. According to the invention, the reaction time is 7-8h, the temperature is 20-30 DEG C, the yield is greater than 90%, and the purity is greater than 99% (HPLC detection); and the reagents used by the method are green, environmentally friendly, pollution-free, economical and practical, the reaction yield is high, and the method is simple and convenient for operation, and suitable for industrialized mass production.
Owner:ZHEJIANG UNIV OF TECH
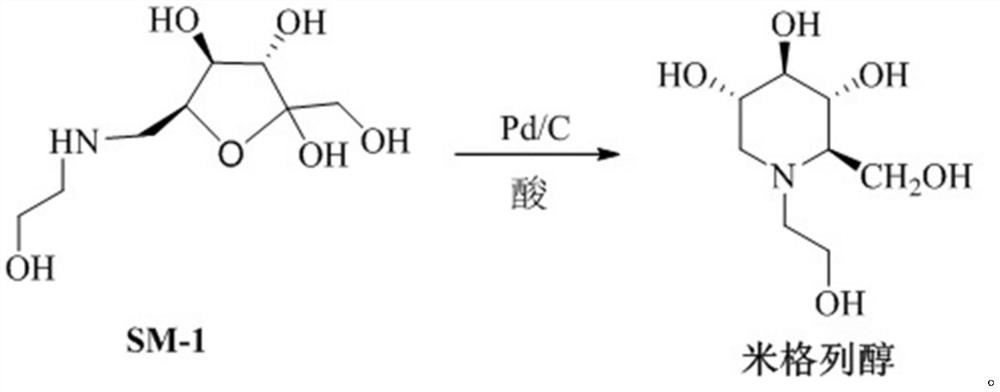
Preparation method of miglitol
ActiveCN112142648AHigh reaction yieldHigh purityOrganic chemistryBulk chemical productionMiglitolOrganic solvent
The invention belongs to the technical field of medicine synthesis, and particularly relates to a preparation method of miglitol. The method comprises the following steps: (1) adding Pd / C and SM-1 into a reaction solvent in a high-pressure reaction kettle, adding an acid, conducting stirring, controlling the temperature and pressure, carrying out hydrogenation reaction, cooling the reaction solution to room temperature after the reaction is finished, conducting filtering, and carrying out reduced pressure concentration until the reaction solution is dry to obtain a solid; and (2) adding an organic solvent into the obtained solid for dissolution, adding a crystallization solvent for crystallization, completely conducting crystallizing, conducting filtering, and carrying out vacuum drying toobtain a target compound miglitol. Compared with the prior art, the invention provides a simple, convenient and efficient method for preparing miglitol, and the whole synthesis method has the advantages of short route, simple operation steps, high reaction yield, high product purity, mild reaction conditions, effective shortening of the production period, and suitableness for industrial scale-upproduction.
Owner:LUNAN PHARMA GROUP CORPORATION
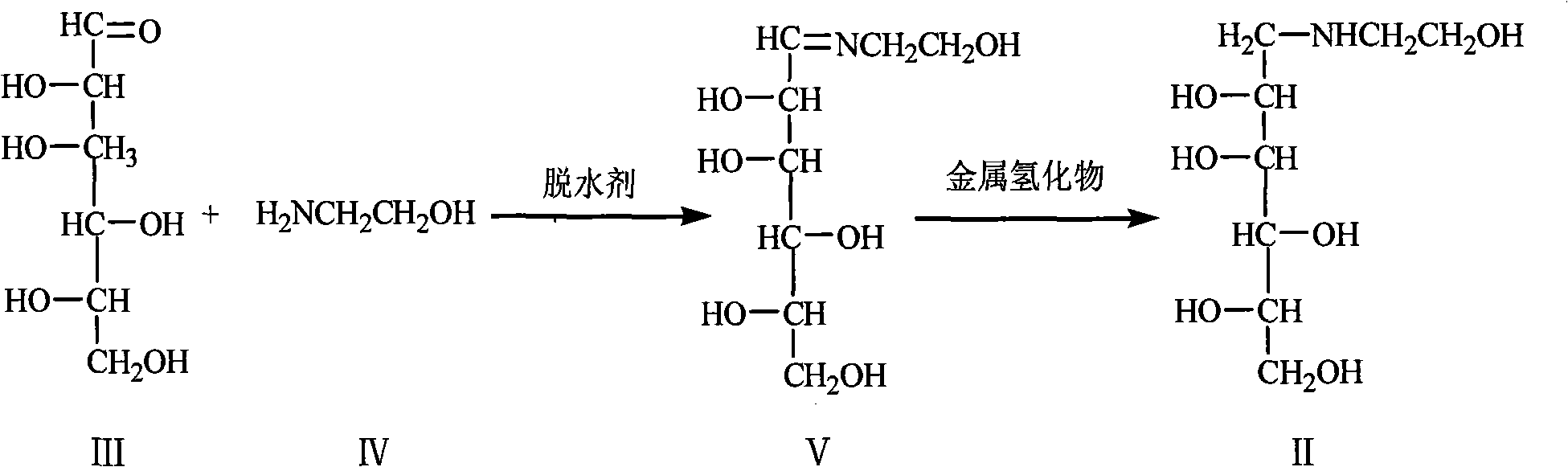
Method for preparing miglitol intermediate N-hydroxyethyl glucosamine
ActiveCN101891777AIncrease costLow yieldSugar derivativesSugar derivatives preparationMiglitolReaction temperature
The invention relates to a method for preparing miglitol intermediate N-hydroxyethyl glucosamine which is used for curing diabetes mellitus. The method is characterized by comprising the following steps: taking glucose and ethanolamine as raw materials, controlling reaction temperature and reaction environment in the presence of a solvent and a proper amount of dehydrating agent, carrying out a reaction with metal hydride, finishing the reaction, and obtaining white crystals of the droxyethyl glucosamine after aftertreatment and refining. Yield coefficient is more than 95% and purity is more than 99% (HPLC detection).
Owner:鲁南新时代生物技术有限公司
Melbine/migltol hypoglycemic oral preparation composition and preparation thereof
InactiveCN101756980AEnsure complianceConvenience guaranteedMetabolism disorderHeterocyclic compound active ingredientsMiglitolEnteric-coated granules
The invention provides a melbine / migltol hypoglycemic oral preparation composition and a preparation thereof. The weight ratio of the two main medicaments comprised in the composition is 2:3 to 200:1, preferably, 1:1 to 150:1. Except the main medicaments, the composition also can further comprises normal medicament auxiliary materials, such as an adhesive, a filling agent, a disintegrating agent, a lubricating agent, a flavoring agent, a wetting agent and a glidant. The prepared composition can be prepared into tablets, granules, capsules and sustained-release preparations by the common method, preferably, enteric-coated tablets, enteric-coated granules and enteric-coated capsules. The composition of the invention has the advantages of complementarity of action mechanisms of the main medicaments, multiple target points, good patient compliance and the like. The hypoglycemic oral preparation composition can be used for first-line treatment of type 2 diabetes, or can be used for second-line therapy under the condition that a melbine or sulfonylurea medicament cannot singly and effectively control the blood sugar.
Owner:北京瑞伊人科技发展有限公司 +1
Medicament composition for treating diabetes and complications of diabetes
ActiveCN101584705AConvenient treatmentGood effectMetabolism disorderPill deliveryMiglitolBlood sugar
The invention provides an oral medicament composition for treating diabetes and the complications of diabetes, belonging to the field of medicines. The oral medicament composition for treating diabetes and the complications of diabetes comprises migltol and heparin or low molecular heparin or a medicinal salt of the heparin or the low molecular heparin in the weight ratio of 1:0.1-9. The medicament composition plays a good synergic action on lowering blood sugar, can reduce the complications of diabetes and generate positive effect on the prognosis of a diabetic who takes the oral medicament composition for a long time so that the oral medicament composition for treating diabetes and the complications of diabetes is quite beneficial to the recovery of the diabetic.
Owner:LUNAN HOPE PHARM CO LTD
Medicine composition containing insulin intensifier and miglitol
ActiveCN101121004AReduce complicationsImprove risk factorsMetabolism disorderPill deliveryMiglitolAcute hyperglycaemia
The present invention relates to an oral blood-sugar-reducing compound medicinal preparation, which consists of an insulin sensitizer, a miglitol and auxiliary materials. Compared with the prior art, the present invention is characterized in that under a circumstance of a same curative effect, a separate dosage of the insulin sensitizer or the miglitol is reduced; at the same time compared with other hypoglycemic drugs, a side effect of the present invention is reduced; the insulin sensitizer and the miglitol have a synergistic effect: the insulin sensitizer and the miglitol respectively take curative actions towards a patient with hyperglycemia synchronously according to the different pharmacological effects and directly provide the patient or a doctor with a scientific combined medication to improve the curative effect and provide a clinic or the patient with convenience.
Owner:LUNAN PHARMA GROUP CORPORATION
Transforming bacterium strain and method for catalytically synthesizing miglitol intermediate
ActiveCN110016455AIncreased dehydrogenase activityIncrease vitalityBacteriaMicroorganism based processesMiglitolMicrobiology
The present invention discloses a transforming bacterium strain and a method for catalytically synthesizing a miglitol intermediate. The transforming bacterium strain is obtained by transforming a recombinant expression plasmid pBBR-sldAB into a starting bacterium strain ZJB16009 and then conducing screening, and named as gluconobacter oxydans, has a strain number of pBBR-sldAB and a preservationnumber of CCTCC No.M2019033, is significantly higher in N-hydroxyethylglucosamine dehydrogenase activity than the starting bacterium strain ZJB16009, and also has a space-time yield about 2 times of the starting bacterium strain ZJB16009. The gluconobacter oxydans pBBR-sldAB has relatively high potential application value in catalytic synthesis of a key intermediate 6NSL of the miglitol.
Owner:ZHEJIANG UNIV OF TECH +1
High-purity miglitol production process
ActiveCN101302549BEfficient purificationRealize industrializationOrganic chemistryBacteriaMiglitolDesorption
The invention relates to a method for producing high-purity miglitol. The method comprises the following stages that: Stage a. a miglitol producing strain is obtained through the cultivation of a culture medium consisting of D-sorbierite, a yeast extract and KH2PO4 and microfiltration separation; Stage b. the miglitol producing strain performs biotransformation, microfiltration, hyperfiltration, nanofiltration, and activated carbon decolorization to a substrate to obtain an intermediate of the miglitol; Stage c. hydrogenation reaction, ion fractionating, activated carbon decolorization, desorption, condensation and crystallization are performed to the intermediate of the miglitol obtained in the Stage b to obtain the high-purity miglitol. The adoption of the method can improve the production efficiency of the miglitol to the utmost extent, thereby realizing the industrialization of the high-purity miglitol.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
A kind of preparation method of Miglitol
The invention belongs to the technical field of medicine and particularly relates to a preparation method of migltol. The preparation method comprises the following steps: by taking glucose and ethanol amine as raw materials, under the conditions of hydrogen gas and high pressure, carrying out catalytic hydrogenation to prepare an intermediate hydroxyl 1; then carrying out biological oxidation conversion by using gluconic acid oxidizing bacteria to obtain an intermediate 2; carrying out catalytic hydrogenation under the conditions of hydrogen gas and high pressure to prepare a crude product of migltol; and then purifying, crystallizing and refining the crude product to obtain a finished product.
Owner:四川维奥制药有限公司
Oral preparation for slowing down absorption of alpha-glycosidase inhibitor and enhancing hypoglycemic drug effect
ActiveCN102210866BReduce absorptionProlong the action timeMetabolism disorderMacromolecular non-active ingredientsIntestinal structureDisease
The invention discloses an oral preparation for slowing down the absorption of an alpha-glycosidase inhibitor and enhancing a hypoglycemic drug effect. The preparation is an oral preparation prepared by adding the following adhesive auxiliary materials based on the certain proportion: carboxymethyl cellulose, hydroxyethyl cellulose, methyl cellulose or beta-cyclodextrin and the like, to each alpha-glycosidase inhibitor (acarbose, voglibose, miglitol, 1-deoxynojirimycin and extract of the 1-deoxynojirimycin). Compared with singly taken alpha-glycosidase inhibitors, the adhesives can be used for prolonging the action time of the alpha-glycosidase inhibitor in the intestine, slowing down the absorption of a medicament and glucose in the intestine and further reducing a plasma concentration, thus the hypoglycemic drug effect is improved. The oral preparation disclosed by the invention can be applied to the prevention and therapy of diseases, such as diabetes, obesity and the like.
Owner:NANKAI UNIV +1
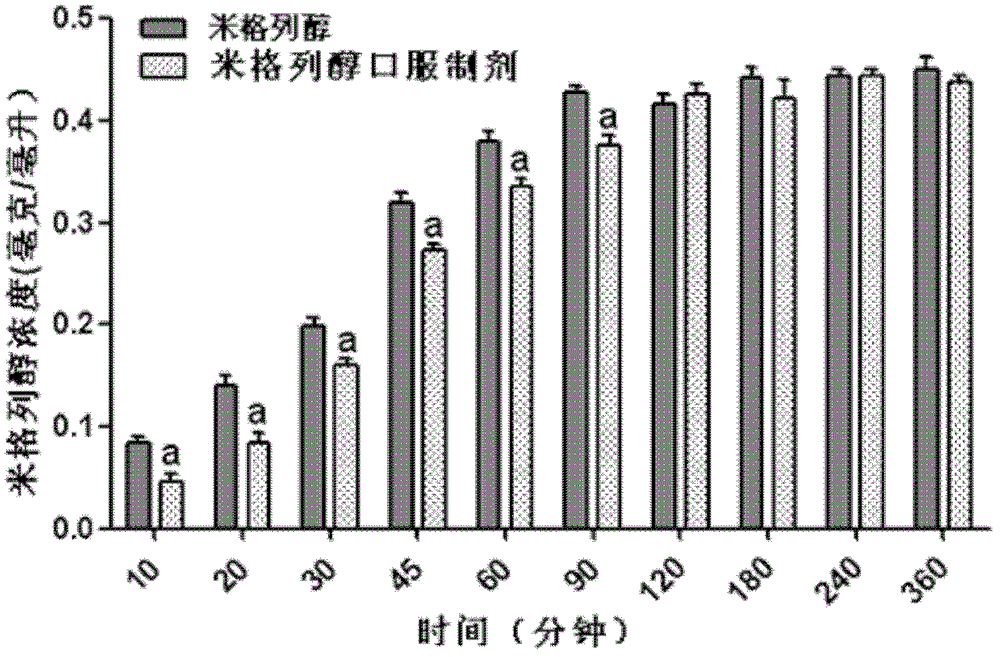
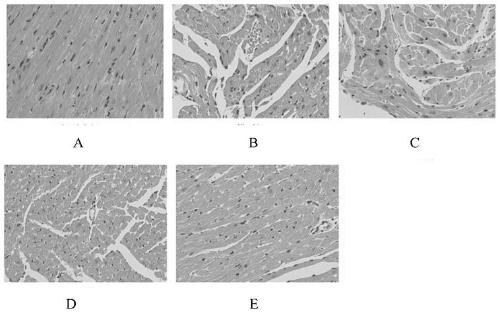
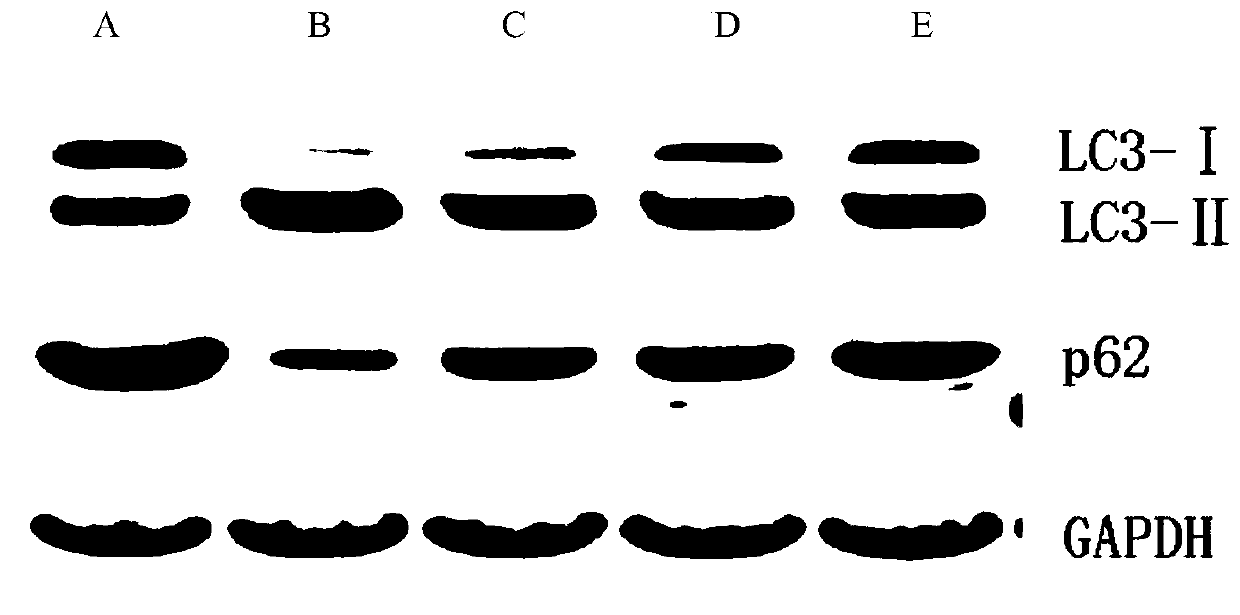
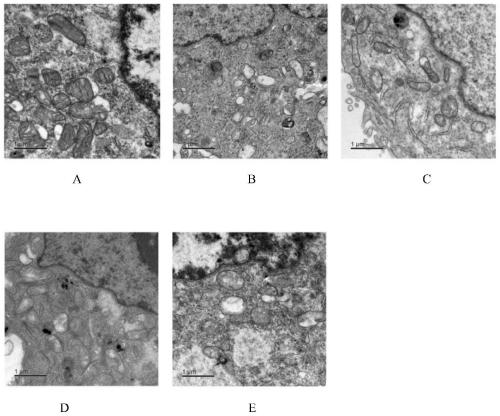
Application of miglitol to preparation of medicine for treating cardiovascular diseases
PendingCN111346091AGood treatment effectSignificant effect in treating heart failureHeterocyclic compound active ingredientsCardiovascular disorderMiglitolPharmaceutical drug
The present invention discloses an application of miglitol to preparation of a medicine for treating cardiovascular diseases, and belongs to the technical field of medicines. The medicine is a medicament prepared from the miglitol as an active ingredient and a pharmaceutically acceptable excipient, and the obtained medicament has the remarkable treatment effect on the cardiovascular diseases caused by myocardial cell autophagy. Pharmacological tests show that the miglitol can obviously improve cardiac function injury and myocardial cell necrosis, improves the expression level of LC3 protein, and is suitable for being widely applied to treatment of the cardiovascular diseases.
Owner:LUNAN PHARMA GROUP CORPORATION
Pharmaceutical composition for treating or preventing obesity and metabolic syndrome
InactiveCN102805744AReduce adverse reactionsThe ingredients of the drug are clearMetabolism disorderHeterocyclic compound active ingredientsMiglitolOrlistat
The invention discloses pharmaceutical composition for treating or preventing obesity and metabolic syndrome and belongs to the field of pharmaceuticals. The pharmaceutical composition comprises active ingredients, namely orlistat and miglitol according to a preferable weight ratio of 1:0.1-100, and a more preferable weight ratio of 1:0.5-10. The pharmaceutical composition can be made into conventional pharmaceutical dosage forms, preferably the solid form. The pharmaceutical composition has evident synergistic effect on treating or preventing obesity and metabolic syndrome, is capable of lowering the risks of cardiovascular diseases and has broad medical application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation method of miglitol sustained release tablet
InactiveCN103070842BHas medicinal propertiesSuitable cation exchange capacityMetabolism disorderInorganic non-active ingredientsSustained Release TabletMiglitol
The invention provides a preparation method of a miglitol sustained release tablet. The preparation method comprises the following steps of: I, adding miglitol, Na-montmorillonite and an acidity regulator into deionized water, and stirring uniformly to obtain a mixed solution; II, stirring the mixed solution, performing ion exchange on the miglitol and the Na-montmorillonite, and filtering, washing, drying, smashing and sieving in sequence to obtain a main material; and III, mixing the main material and auxiliary materials uniformly, and tableting to obtain the miglitol sustained release tablet. A preparation process of the method is simple, special equipment and process are not required, and the method is suitable for large-scale batch production. Specific to the defects of the conventional miglitol medicament administration way, a method for compounding the Na-montmorillonite and the miglitol is adopted, so that the in-vivo release rate of the miglitol is lowered, the utilization ratio of a miglitol medicament is increased remarkably, and the taking compliance of a patient is enhanced greatly.
Owner:XIAN UNIV OF SCI & TECH
Preparation of miglitol
InactiveCN1324010CUnavailable solutionMild process conditionsOrganic chemistryMiglitolAsymmetric hydrogenation
Production of miglina is carried out by providing chiral center by L-phenylalanine. It achieves high efficiency and good optical purity, and to avoid chemical resolution and asymmetric hydrogenation.
Owner:江苏省药物研究所有限公司
A method for rapidly screening mutant strains of key intermediates in the synthesis of miglitol and bacterial strains
ActiveCN108441491BIncrease screening volumeEasy to operateBacteriaMutant preparationMiglitolMicrobiology
The invention provides a method and bacterial strain for rapid quantitative detection of mutant strains of key intermediates of Miglitol. In the method, the mutagenized strains of wild Gluconobacter oxidans are subjected to fermentation and cultivation, and the fermented and cultivated mutant strains are obtained. Add the wet bacterial cells of the mutagenic strain to the substrate reaction solution. After the transformation reaction is complete at 15°C, centrifuge the reaction solution, measure the 6NSL content in the supernatant, and screen to obtain the key intermediate for the synthesis of miglitol according to the level of 6NSL content. highly active mutant strains. The detection method provided by the present invention has the characteristics of simplicity, quickness, high specificity, and good repeatability. The method can be further applied to the screening of high-activity Gluconobacter oxydans, effectively speeding up the breeding process, and transforming the mutant strains obtained through screening into synthetic rice. The catalytic ability of the glitol intermediate 6NSL has been significantly improved, and it is suitable for the industrialized large-scale production of miglitol.
Owner:ZHEJIANG UNIV OF TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com