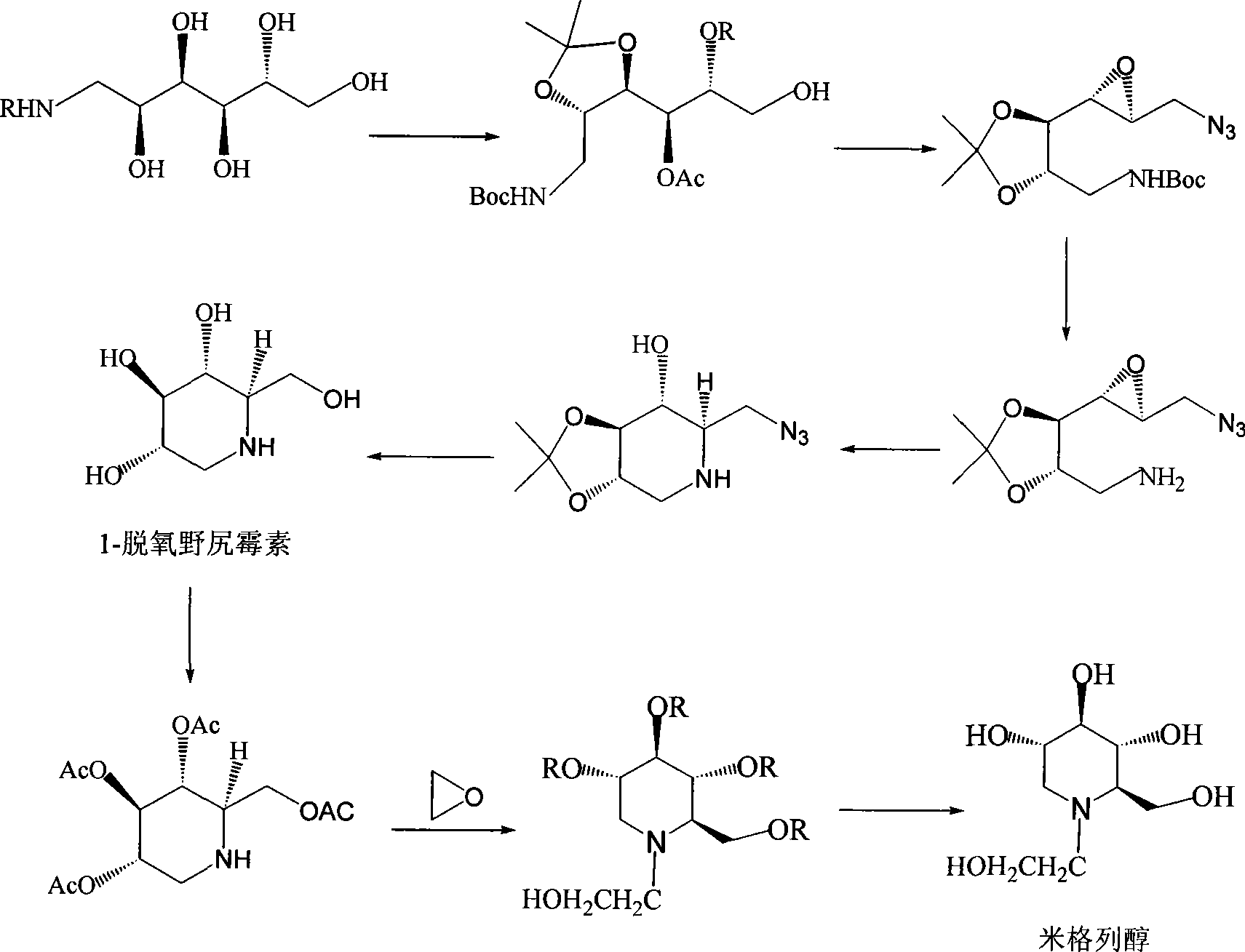
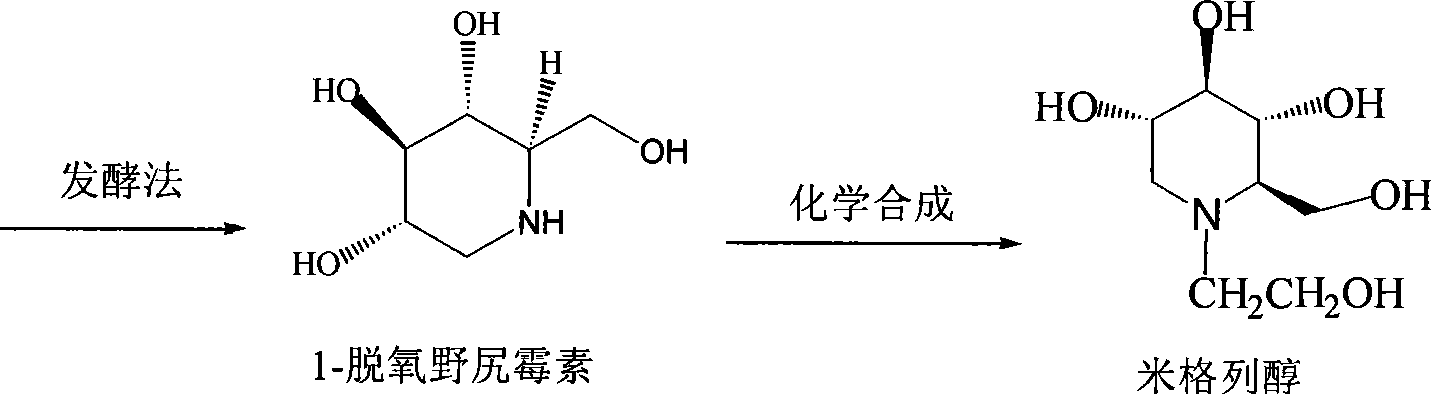
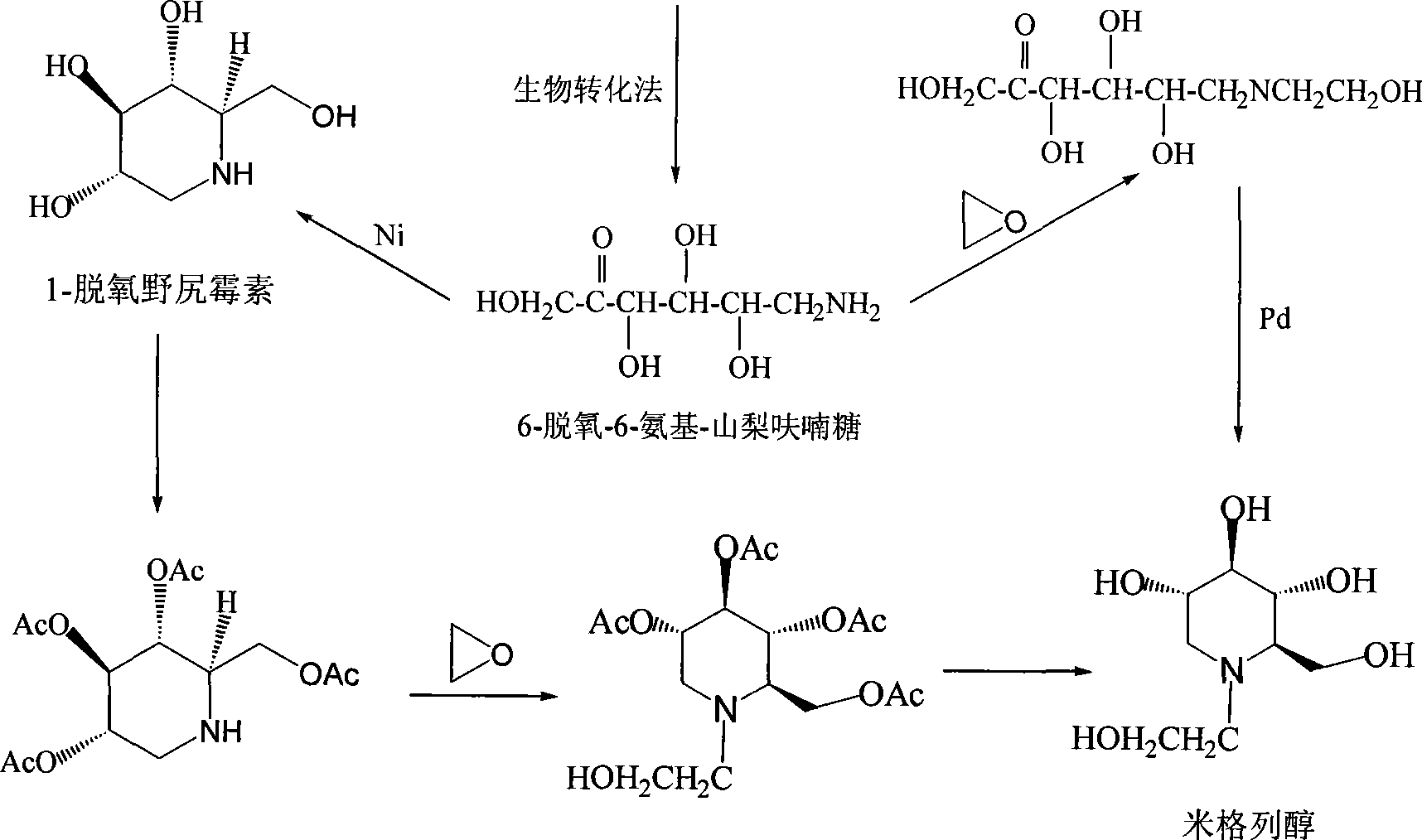
High-purity miglitol production process
A miglitol and high-purity technology, which is applied in the production field of high-purity miglitol, can solve the problems of high bacterial culture cost and biotransformation cost, inability to adapt to large-scale industrial production, and large bacterial loss.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] In a 100L fermenter, according to the following formula D-sorbitol 6.0%; yeast extract 2.4%; KH 2 PO 44.8% is made into culture medium, inserts Miglitol engineering strain seed with the culture medium of 10% inoculum by volume ratio, and be 1:1 (vol:vol) in ventilation rate, stirring speed is 300rpm, 28 ℃ of temperature Under the condition, carry out 24-28 hour fermentation culture.
Embodiment 2-5
[0070] After a large number of bacterial strains have been cultivated by the above method, the above fermentation liquid is put into a microfiltration circulation tank, and the ceramic microfiltration membrane is used to control the temperature conditions of 0°C, 10°C, 25°C, and 55°C, and the inflow pressure of 0 to 4.0bar and 0 ~3.5 bar outflow pressure. Adding purified water to top wash three times during this period can effectively remove impurities while maintaining the activity of the strain, and obtain highly active and relatively pure mycelia 1, 2, 3, and 4.
Embodiment 6-9
[0072] After a large amount of bacterial strains 1, 2, 3, and 4 are obtained by utilizing the above-mentioned method in the present invention, they are dropped into an aqueous solution dissolved with N-(2-hydroxyethyl)-glucosamine in a 100L conversion tank to carry out biotransformation at pH6.0 , the ventilation rate is 1:1.5 (vol:vol), the stirring speed is 400rpm, and the temperature is 28°C, the conversion formula is determined as follows: N-(2-hydroxyethyl)-glucosamine 6.0kg, MgSO 4 ·7H 2 O2.05kg, oxidize N-(2-hydroxyethyl)-glucosamine to: 6-(2-hydroxyethyl)-amino-6-deoxy-α- L-sorbofuranose to obtain transformation solutions 1, 2, 3, and 4.
[0073] The above-mentioned transformation solutions 1-4 were respectively passed through France Delta’s ceramic membrane microfiltration to remove bacteria. During microfiltration, the temperature conditions were 0°C, 10°C, 25°C, 55°C, the inflow pressure was 0-4.0 bar and the pressure was 0-3.5 bar. bar outflow pressure, to obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com