Preparation method of miglitol sustained release tablet
A technology for miglitol and sustained-release tablets, applied in the field of medicine, can solve the problems of reduced absorption efficiency and bioavailability, undiscovered miglitol sustained-release tablets, tissue damage in the elderly and children, etc., and achieves difficult absorption. , high positioning ability, reducing the effect of degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
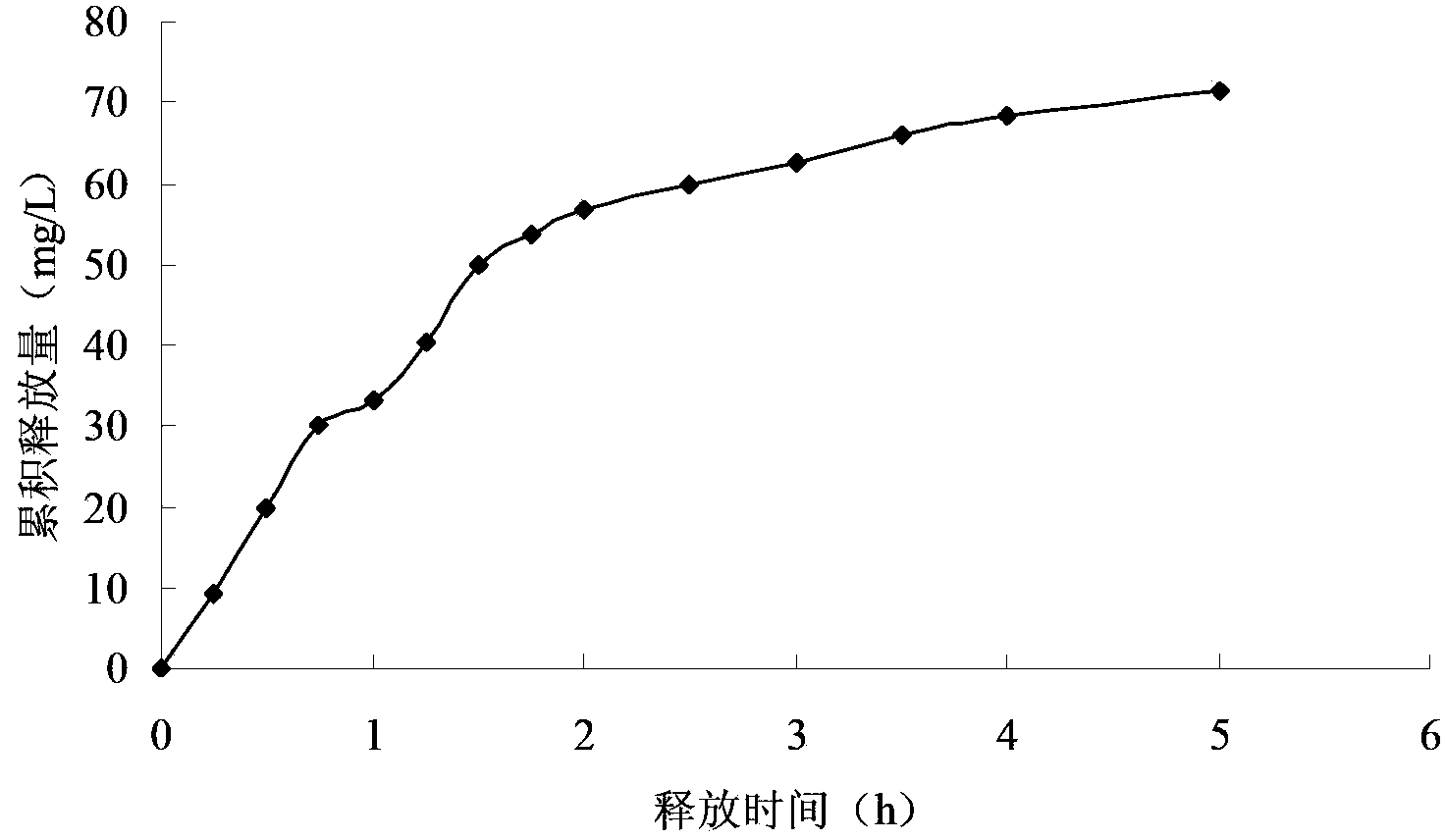
Embodiment 1
[0050] The preparation method of the Miglitol sustained-release tablet of the present embodiment comprises the following steps:
[0051] Step 1, adding miglitol with a mass purity of 99.99%, sodium montmorillonite with a mass purity of 98% and an acidity regulator into deionized water, and stirring to obtain a mixed solution with a pH value of 3; The concentration of Miglitol in the mixed solution is 1.0g / L, and the concentration of Na-montmorillonite is 5.0g / L; The acidity regulator is 8% hydrochloric acid in mass percent concentration;
[0052] Step 2. Stir the mixed solution described in step 1 at a temperature of 40° C. and a stirring rate of 120 r / min for 1.5 h to carry out ion exchange between miglitol and sodium-based montmorillonite, and then ion-exchange the The mixed solution is sequentially filtered, washed until neutral, dried at 50°C for 6 hours, pulverized by a pulverizer, and passed through a 100-mesh sieve to obtain a main material with a particle size not grea...
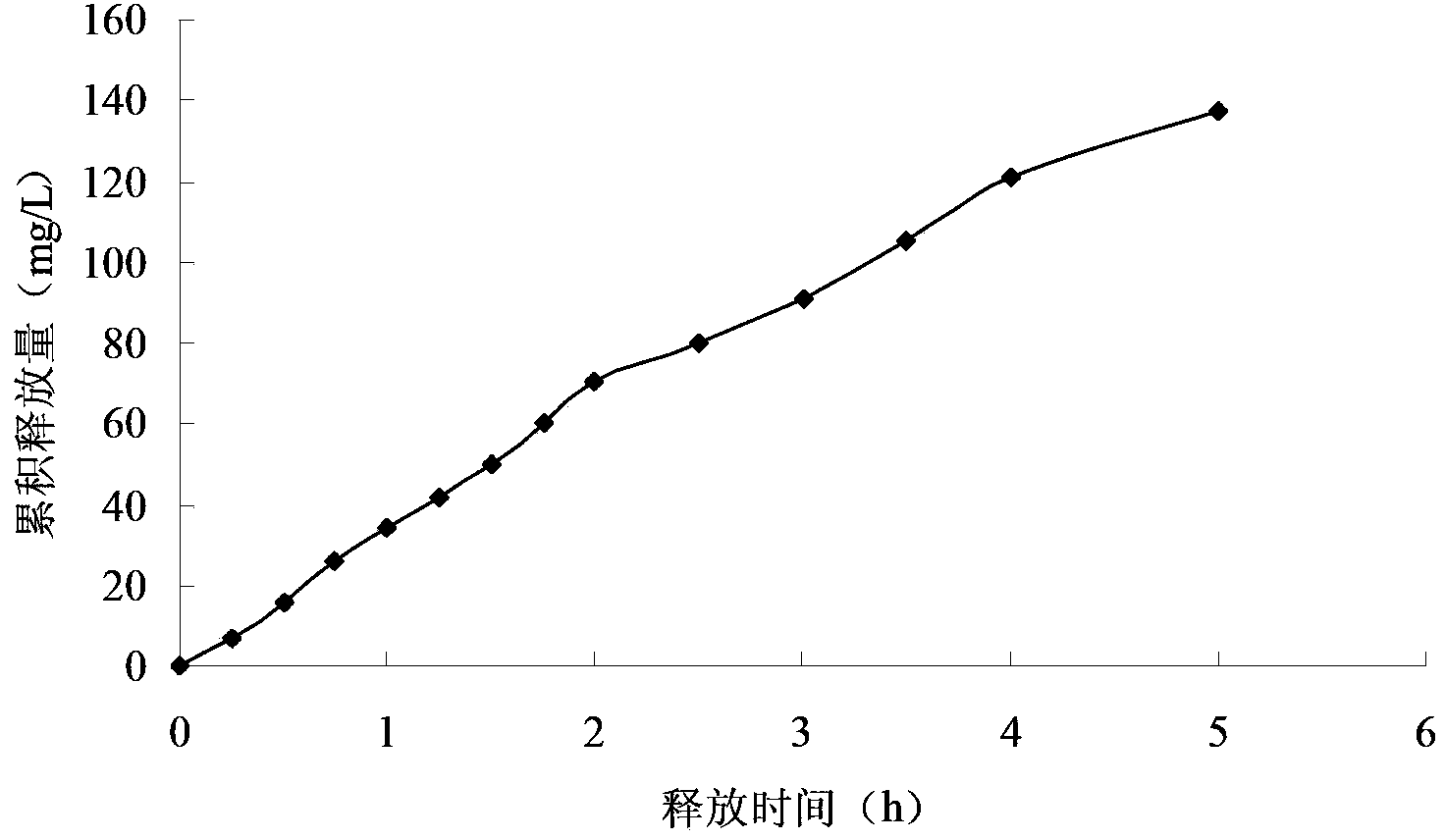
Embodiment 2
[0057] The preparation method of the Miglitol sustained-release tablet of the present embodiment comprises the following steps:
[0058] Step 1, adding miglitol with a mass purity of 99.95%, sodium montmorillonite with a mass purity of 96% and an acidity regulator into deionized water, and stirring to obtain a mixed solution with a pH value of 2; The concentration of Miglitol in the mixed solution is 0.8g / L, and the concentration of Na-montmorillonite is 4.5g / L; Described acidity regulator is the hydrochloric acid that mass percent concentration is 10%;
[0059] Step 2. Stir the mixed solution described in step 1 at a temperature of 50° C. and a stirring rate of 80 r / min for 2 h to carry out ion exchange between miglitol and sodium-based montmorillonite, and then mix the ion-exchanged The solution is sequentially filtered, washed until neutral, dried at 60°C for 5 hours, pulverized by a pulverizer, and passed through a 120-mesh sieve to obtain the main material with a particle...
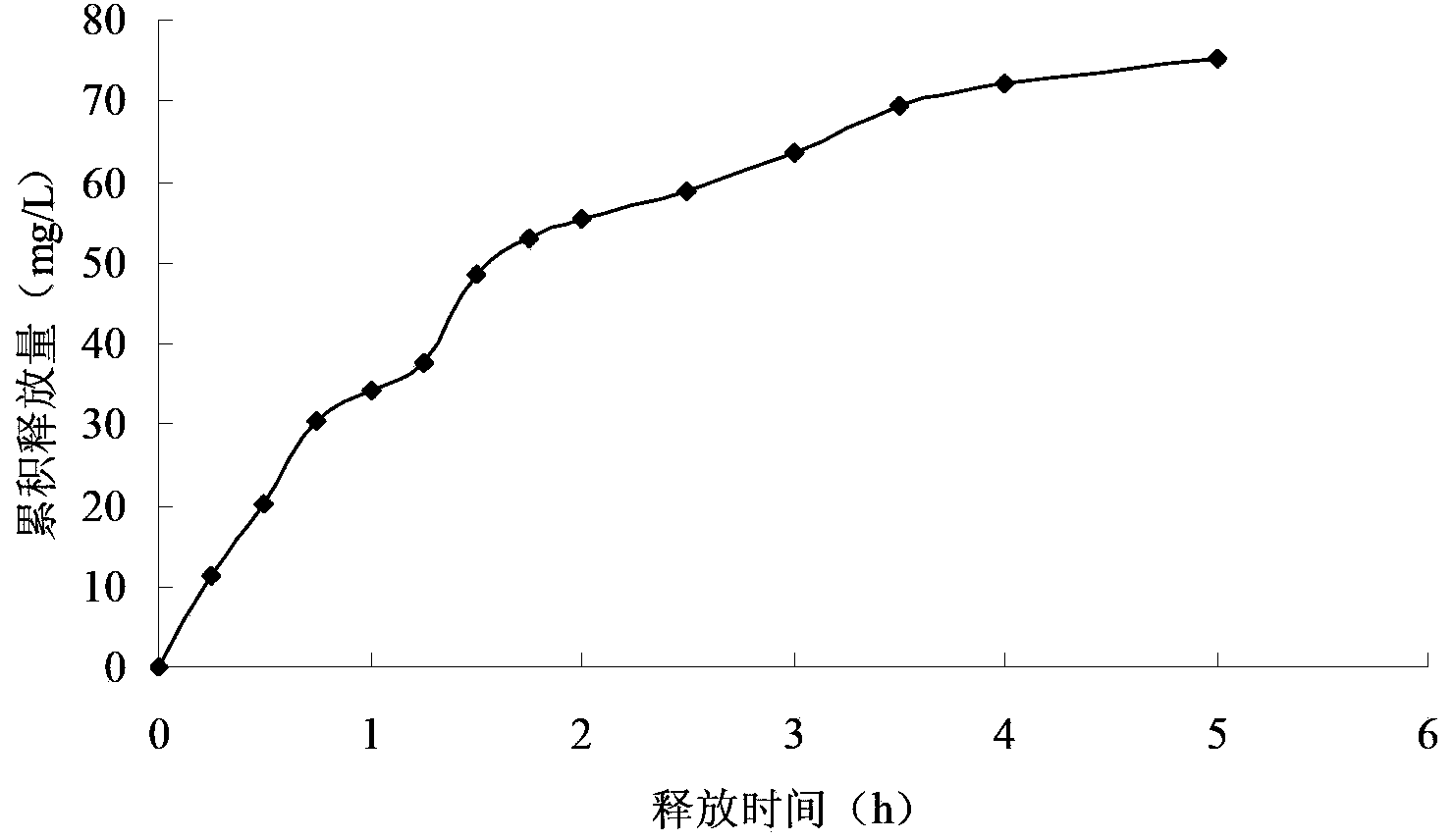
Embodiment 3
[0064] The preparation method of the Miglitol sustained-release tablet of the present embodiment comprises the following steps:
[0065] Step 1, adding Miglitol with a mass purity of 99.9%, sodium montmorillonite with a mass purity of 97% and an acidity regulator into deionized water, and stirring to obtain a mixed solution with a pH value of 4; The concentration of Miglitol in the mixed solution is 0.8g / L, and the concentration of Na-montmorillonite is 4.5g / L; Described acidity regulator is the hydrochloric acid that mass percent concentration is 5%;
[0066] Step 2. Stir the mixed solution described in step 1 at a temperature of 20° C. and a stirring rate of 150 r / min for 2 h to carry out ion exchange between miglitol and sodium montmorillonite, and then mix the ion exchanged solution The solution is sequentially filtered, washed until neutral, dried at 60°C for 5 hours, pulverized by a pulverizer and passed through a 60-mesh sieve to obtain the main material with a particle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com