Preparation process of compound gentamicin film
The invention relates to a gentamicin film and a preparation process technology, which is applied in the field of compound gentamicin film preparation technology, and can solve the problems of easy degradation, unstable content of compound gentamicin film, and inability to fully and stably exert its function.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] 2.2 Preparation of mucilage: take enough purified water and add dilute hydrochloric acid to prepare a dilute hydrochloric acid aqueous solution with a pH value of 4. Dissolve the chitosan with an appropriate amount of dilute hydrochloric acid aqueous solution prepared above; set aside. Add polyvinyl alcohol to the prepared dilute hydrochloric acid aqueous solution, heat it in a water bath at 100°C to dissolve, add the dissolved chitosan and an appropriate amount of glycerin and stir evenly to obtain a glue solution.
[0070] 2.3 Verification of glue
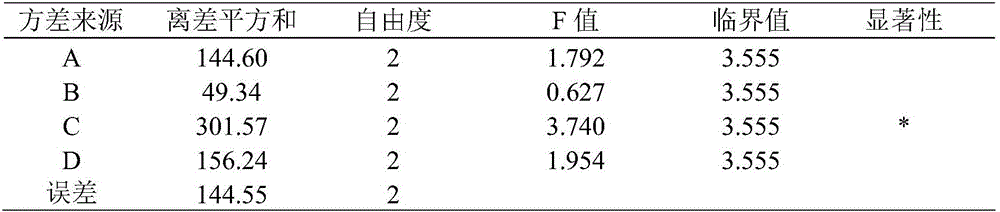
[0071] 2.3.1 Orthogonal test method was used to optimize the glue formula after adding chitosan, and observe its film-forming properties.
[0072] 2.3.2 Prescription screening: On the basis of the single factor test, the amount of glycerin, chitosan and polyvinyl alcohol was selected as the investigation object, and the film-forming time was used as the investigation index, and L 9 (3 4 ) Orthogonal test, using intuitiv...
Embodiment 2
[0099] The second batch of experiments of embodiment 2:
[0100] (1) Weighing and ingredients
[0101] Receive 760 parts of gentamicin sulfate by weight, 2050 parts of tetracaine hydrochloride, 55 parts of dexamethasone acetate, appropriate amount of polyvinyl alcohol (PVA), appropriate amount of chitosan, appropriate amount of sodium bisulfite, appropriate amount of stevia, tartaric acid Appropriate amount, appropriate amount of beautiful blue, appropriate amount of lemon yellow. Check the product name, specification, batch number, and quantity, weigh out each material, and have a special person check it.
[0102] (2) Preparation of tetracaine hydrochloride hydroxypropyl-β-cyclodextrin inclusion compound
[0103] Take tetracaine hydrochloride and hydroxypropyl-β-cyclodextrin according to the weight ratio of 1:2, put the hydroxypropyl-β-cyclodextrin in a beaker, add a certain amount of purified water to make it saturated, and heat to dissolve and cooling; then add half the ...
Embodiment 3
[0116] The third batch of experiments of embodiment 3:
[0117] (1) Weighing and ingredients
[0118] Receive 770 parts of gentamicin sulfate by weight, 2100 parts of tetracaine hydrochloride, 60 parts of dexamethasone acetate, appropriate amount of polyvinyl alcohol (PVA), appropriate amount of chitosan, appropriate amount of sodium bisulfite, appropriate amount of stevia, tartaric acid Appropriate amount, appropriate amount of beautiful blue, appropriate amount of lemon yellow. Check the product name, specification, batch number, and quantity, weigh out each material, and have a special person check it.
[0119] (2) Preparation of tetracaine hydrochloride hydroxypropyl-β-cyclodextrin inclusion compound
[0120] Take tetracaine hydrochloride and hydroxypropyl-β-cyclodextrin according to the weight ratio of 1:2, put the hydroxypropyl-β-cyclodextrin in a beaker, add a certain amount of purified water to make it saturated, and heat to dissolve and cooling; then add half the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com