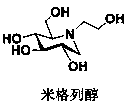
Preparation method for miglitol
A technology of miglitol and alcoholic solvent, applied in the field of compound preparation, can solve the problems of cumbersome steps, high synthesis cost, difficult purification and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a 1L autoclave, add 400 ml 6-deoxy-6-hydroxyethylamino-α-L-sorbose cell resting solution (containing 6-deoxy-6-hydroxyethylamino-α-L-sorbose 40 g), 40 ml ethanol, 1.6 g 10% Pd / C (water content 50%), replace the air with hydrogen, maintain the pressure at 1~1.5 MPa, and react at 25 °C for 7 hours, N 2 Palladium carbon was removed by pressure filtration (for the next batch of reactions), concentrated, ethanol crystallized, suction filtered, washed, and vacuum-dried to obtain 34 g of miglitol crystals, with a yield of 92% and a purity of 99.1% (HPLC detection).
[0022] The spectrum characterization of miglitol crystals: 1 H NMR (600 MHz, D 2 O- d 6 ) δ: 3.81 (ddd, J 1 = 2.4Hz, J 2 = 14.4Hz, J 3 = 15.6 Hz, 2H), 3.71~3.64 (m, 2H), 3.49~3.45 (m, 1H), 3.30(t, J = 9 Hz, 1H), 3.20 (t, J = 9 Hz, 1H), 3.03~3.00 (m, 1H), 2.90~2.86 (m,1H), 2.68~2.64 (m, 1H), 2.31~2.24 (m, 2H); 13 C NMR (100MHz, D 2 O- d 6 ) δ: 78.23,69.92, 68.70, 65.55, 57.86, 57.56, 56.04, 52.7...
Embodiment 2
[0024] In a 1L autoclave, add 400 ml 6-deoxy-6-hydroxyethylamino-α-L-sorbose cell resting solution (containing 6-deoxy-6-hydroxyethylamino-α-L-sorbose 40 g), 200 ml methanol, 4 g Raney nickel, replace air with hydrogen, maintain pressure 1~2 MPa, temperature 27°C, react for 8 hours, N 2 Press filtration to remove Raney nickel (for the next batch of reactions), concentration, ethanol crystallization, suction filtration, and vacuum drying to obtain 35 g of miglitol crystals with a yield of 94% and a purity of 99.0% (by HPLC).
Embodiment 3
[0026] In a 1L autoclave, add 400 ml 6-deoxy-6-hydroxyethylamino-α-L-sorbose cell resting solution (containing 6-deoxy-6-hydroxyethylamino-α-L-sorbose 40 g), 200 ml isopropanol, 4 g 10% platinum carbon (water content 50%), replace air with hydrogen, maintain pressure 1.5~2 MPa, temperature 27 °C, react for 8 hours, N 2 Press filtration to remove platinum carbon (for the next batch of reactions), concentration, ethanol crystallization, suction filtration, and vacuum drying to obtain 35 g of miglitol crystals with a yield of 94% and a purity of 99.0% (HPLC detection).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com