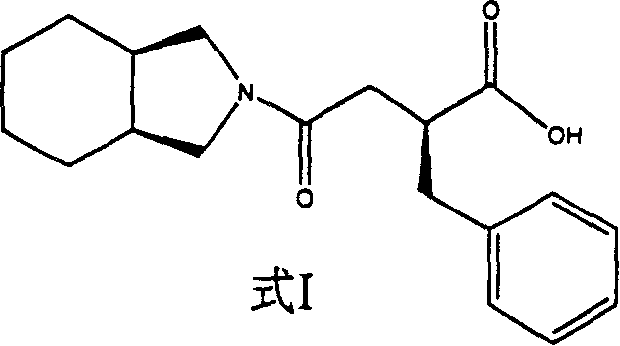
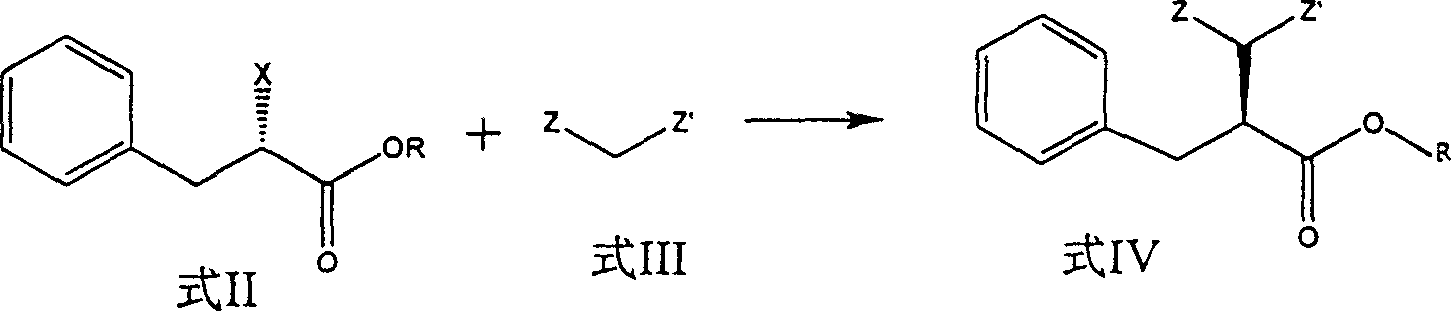Preparation of miglitol
A technology of benzylsuccinic acid monocarboxylic acid and derivatives, which is applied in the field of preparation of mitiglinide, can solve the problems of unavailability of R-isomer, high cost, low yield and the like, and achieves convenient industrial production, Mild process conditions and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
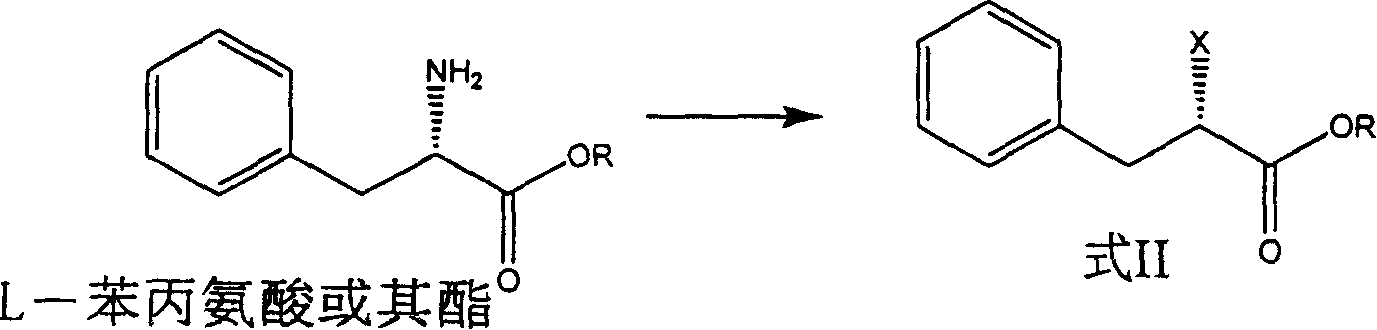
[0015] (S)-2-Chlorophenylpropionic acid
[0016] In a 5000ml reaction bottle, add 3000ml of 6N hydrochloric acid, add 330g (2mol) of L-phenylalanine under ice-salt bath cooling, stir, add 850g of 36% sodium nitrite aqueous solution dropwise below 0°C, and react overnight at room temperature, divide A light yellow organic layer was obtained, and the aqueous layer was extracted with ethyl acetate, combined and dried by adding an appropriate amount of anhydrous sodium sulfate. Ethyl acetate was distilled off under reduced pressure to obtain about 360 g of light yellow residue, [α] D 20 =+4.5° (methanol), yield 97.5% (direct reaction in next step).
[0017] (S)-Ethyl 2-chlorophenylpropionate
[0018] In a 3000ml reaction flask, add 351g (1.90mol) of (S)-2-chloro-3-phenylpropionic acid, 770ml of dichloromethane, 7.8ml of 98% sulfuric acid, add 1000ml of absolute ethanol dropwise under cooling in an ice bath, dropwise Stir the reaction at room temperature for 48 hours, wash with...
Embodiment 2
[0024] (S)-2-Benzylsuccinic acid
[0025] In a 1000ml reaction flask, add 625ml of anhydrous N,N-dimethylformamide, active methylene compound (0.4mol), cool in an ice-salt bath to below 0°C, add 48g (1mol) of 50% sodium hydrogen, and stir (S)-Ethyl 2-halo-3-phenylpropanoate (0.4mol) was added dropwise, and the mixture was kept stirring in an ice-salt bath for 30 minutes, and then stirred at room temperature. Pour the reaction solution into about 500g of crushed ice, extract with ethyl acetate, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure to obtain a yellowish brown residue, add 400ml of acetic acid and 400ml of 37% hydrochloric acid, heat to reflux for reaction, and concentrate under reduced pressure to a small volume, poured into crushed ice and crystallized overnight to obtain a white solid (results are shown in Table 1).
[0026]
[0027] Formula II
Embodiment 3
[0029] (S)-2-Benzylsuccinic anhydride
[0030] In a 1000ml reaction flask, add 62.5g (0.3mol) of (S)-2-benzylsuccinic acid and 220ml (2.33mol) of acetic anhydride, and react at 75-80°C for 1hr. After cooling, add about 415ml of isopropyl ether, put it in the refrigerator to crystallize overnight, and get 39.7g of white solid, mp122-125℃, yield 69.6%, [α] D 20 =+55.6° (ethyl acetate).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com