Treatment process of cyclosporine eye gel
A treatment process, the technology of cyclosporine, applied in the field of medicine, can solve the problems of no commercial production value and feasibility, easy clogging of filter membranes, difficulty in ensuring production efficiency, etc., achieve good industrial application prospects, reduce product impurities, The effect of improving productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
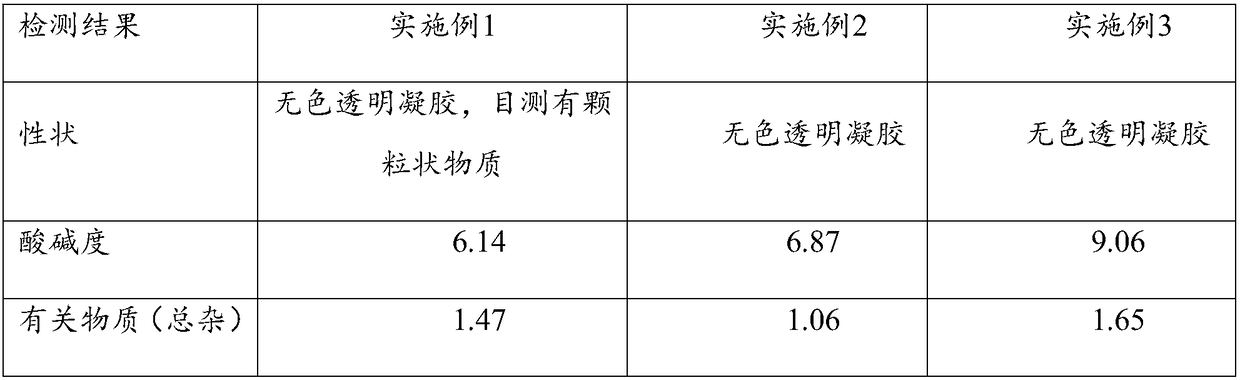
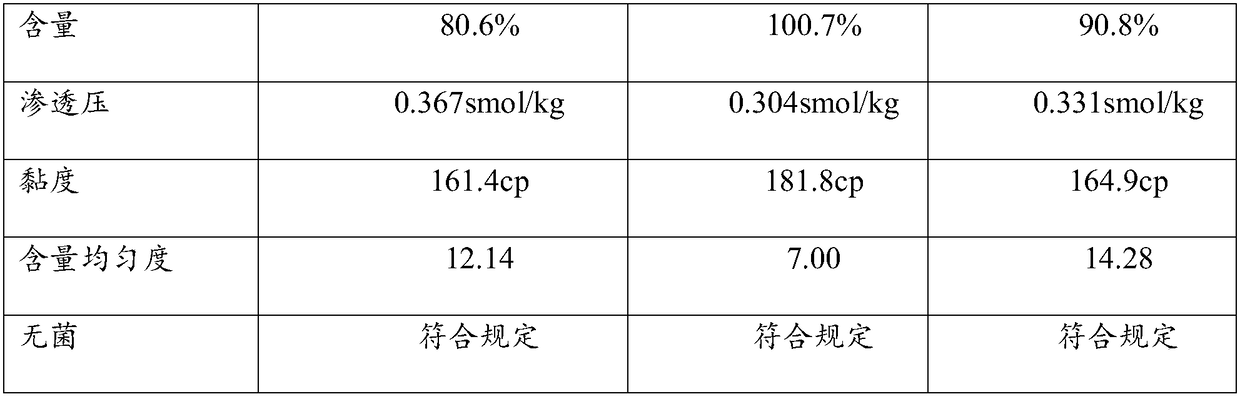
Embodiment 1
[0031] Cyclosporin A eye gel process
[0032] 1. Preparation of Carbomer 981 matrix:
[0033] (1) Add an appropriate amount of water for injection into the liquid preparation tank, add the prescribed amount of Carbomer 981, stir and homogenize;
[0034] (2) Add an appropriate amount of water for injection into the liquid distribution tank, stir and homogenize;
[0035] (3) The matrix swells at rest, and the swelling time is 12 hours;
[0036] (4) Open the equipment, stir and homogenize;
[0037] (5) 115°C, sterilize the matrix with damp heat for 80 minutes, stir and circulate at the same time, cool the matrix to below 40°C, and vacuumize to remove air bubbles;
[0038] 2. Adjustment of pH: Use sodium hydroxide solution to adjust the pH of the matrix to 6.0, and continue to stir for an appropriate time;
[0039] 3. Cyclosporin A main drug solution and sterilizing filter are added to the liquid preparation tank:
[0040] (1) Take the castor oil polyoxyl ester (35) of the pr...
Embodiment 2
[0048] Cyclosporin A eye gel process
[0049] 1. Preparation of Carbomer 981 matrix:
[0050] (1) Add an appropriate amount of water for injection into the liquid preparation tank, add the prescribed amount of Carbomer 981, stir and homogenize;
[0051] (2) Add an appropriate amount of water for injection into the liquid distribution tank, stir and homogenize;
[0052] (3) The matrix swells at rest, and the swelling time is 19 hours;
[0053] (4) Open the equipment, stir and homogenize;
[0054] (5) 117°C, sterilize the matrix with moist heat for 31 minutes, stir and circulate at the same time, cool the matrix to below 40°C, and vacuumize to remove air bubbles;
[0055] 2. Adjustment of pH: Use sodium hydroxide solution to adjust the pH of the matrix to 7.0, and continue to stir for several minutes;
[0056] 3. Cyclosporin A main drug solution and sterilizing filter are added to the liquid preparation tank:
[0057] (1) Take the castor oil polyoxyl ester (35) of the presc...
Embodiment 3
[0065] Cyclosporin A eye gel process
[0066] 1. Preparation of Carbomer 981 matrix:
[0067] (1) Add an appropriate amount of water for injection into the liquid preparation tank, add the prescribed amount of Carbomer 981, stir and homogenize;
[0068] (2) Add an appropriate amount of water for injection into the liquid distribution tank, stir and homogenize;
[0069] (3) The matrix is left to swell, and the swelling time is 24 hours;
[0070] (4) Open the equipment, stir and homogenize;
[0071] (5) 121°C, sterilize the matrix with damp heat for 20 minutes, stir and circulate at the same time, cool the matrix to a temperature below 40°C, and vacuumize to remove air bubbles;
[0072] 2. Adjustment of pH: Use sodium hydroxide solution to adjust the pH of the matrix to 9.0, and continue to stir for several minutes;
[0073] 3. Cyclosporin A main drug solution and sterilizing filter are added to the liquid preparation tank:
[0074] (1) Take the castor oil polyoxyl ester ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com