Ciclosporin medicament composition for injection administration
A technology of cyclosporine and composition, which is applied in the field of pharmaceutical compositions for injection and administration, and achieves the effects of convenient industrial production, good stability and high convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
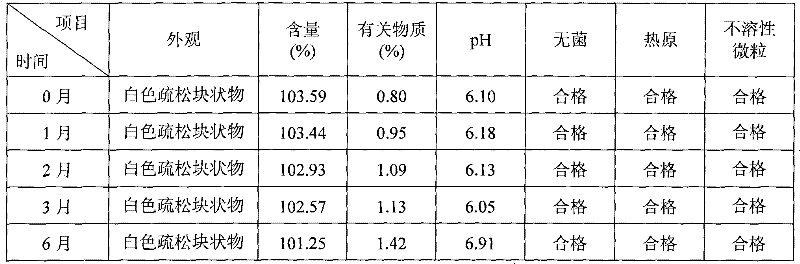
[0039] Example 1: Cyclosporine 250g, Solutol HS 15 3500g, pH value natural, dilute to 5L with 75% (w / w) ethanol aqueous solution for injection.
Embodiment 2
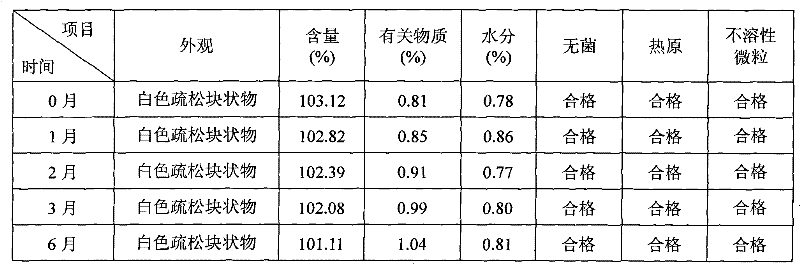
[0040] Example 2: Cyclosporin 250g, Solutol HS 15 7500g, pH value natural, dilute to 10L with 75% (w / w) ethanol aqueous solution for injection.
Embodiment 3
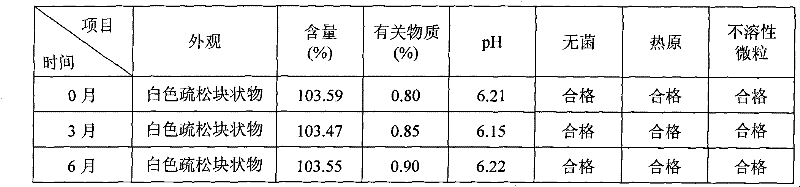
[0041] Example 3: 250g of cyclosporine, 1250g of Solutol HS 15, 200g of propylene glycol, natural pH, and dilute to 10L with 75% (w / w) ethanol aqueous solution for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com