Taxol double-layer soft capsule oral preparation medicament
An oral preparation, paclitaxel technology, applied in the field of medicine, can solve the problems of reducing bioavailability, unable to take continuous medication, affecting the curative effect, etc., to achieve the effect of adjusting the dosage and time, solving the problems of toxicity and curative effect, and easy to take the dosage and time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
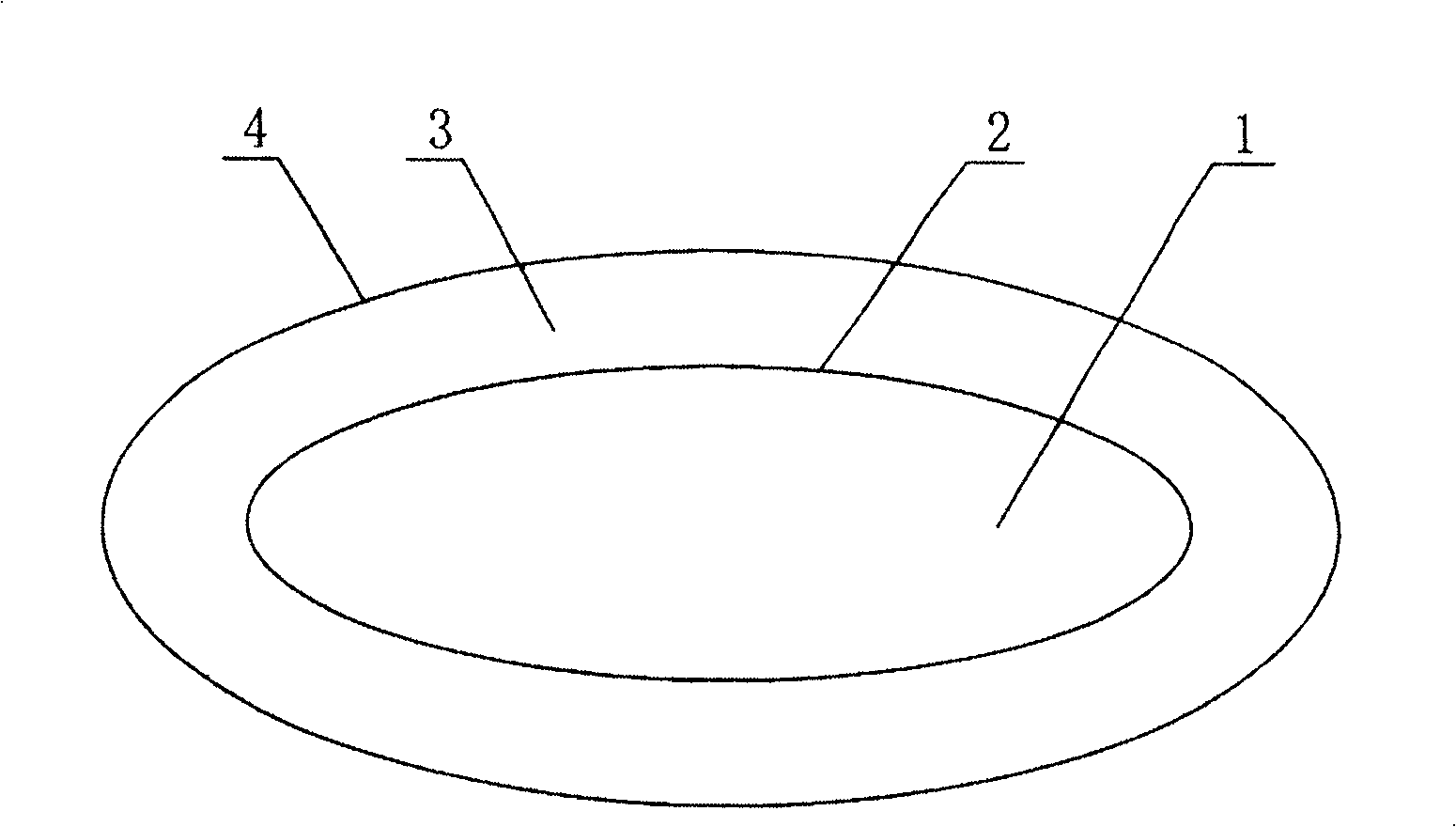
[0027] Paclitaxel-based double-layer soft capsule oral preparation drug, the inner layer is paclitaxel anticancer drug soft capsule, and the outer layer is cyclosporin A semi-solid capsule; figure 1 As shown, from inside to outside are paclitaxel anticancer drug 1, inner soft capsule 2, cyclosporin A semi-solid 3 and outer capsule 4.
[0028] The preparation method of paclitaxel double-layer soft capsule oral preparation medicine is as follows:
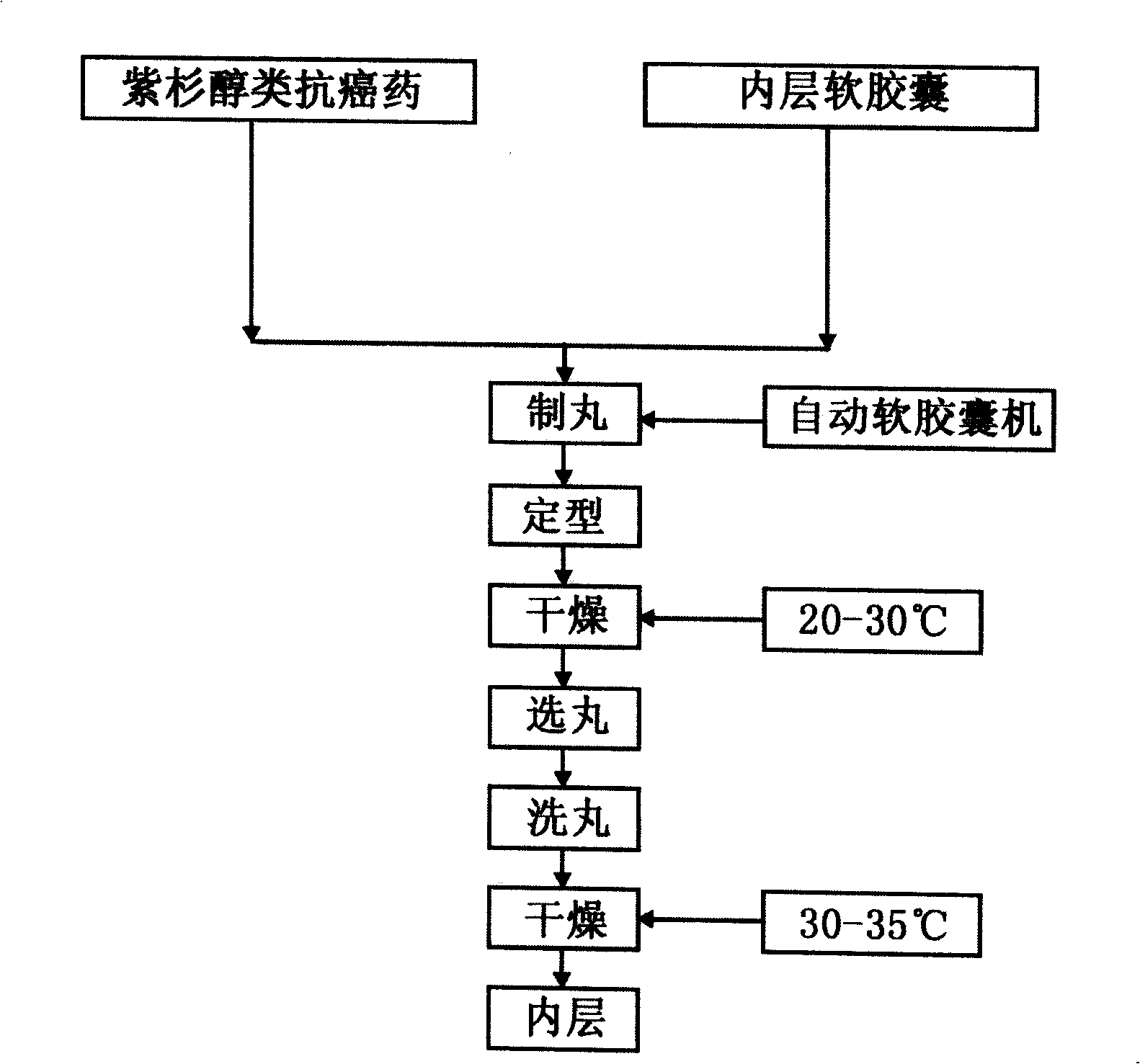
[0029] 1) Preparation of paclitaxel anticancer drugs:
[0030] Dissolve 1.5 grams of paclitaxel in 10 grams of D-α-polyethylene glycol 1000 succinate (TPGS, D-alpha-tocopheryl polyethylene glycol 1000 succinate), add 5 grams of caprylic macrogol glyceride and caprylic macrogol in turn A mixture of alcohol glycerides, 3 grams of Sorbitan monooleate, 0.2 grams of Ascorbylpalmitate, and 1 gram of absolute ethanol. This formulation is blended in an amount of 100 unit doses.
[0031] 2) Wrap paclitaxel anticancer drugs in the inner soft...
Embodiment 2
[0037] Different from Example 1, only the paclitaxel anticancer drug in the inner layer and its preparation method are different:
[0038] Preparation of paclitaxel anticancer drugs: Dissolve 1.5 grams of paclitaxel in 10 grams of D-α-polyethylene glycol 1000 succinate (TPGS, D-alpha-tocopheryl polyethylene glycol 1000 succinate), and then add 3 grams of polyoxyethylene oleic acid in turn Glyceride (LabraWl M 1944CS, polyoxyethylated oleic glycerides), 5 grams of polyethylene glycol 400 (PEG 400: polyethylene glycol 400), 0.2 gram of ascorbyl palmitate (Ascorbylpalmitate) and 1 gram of absolute ethanol. This formulation is mixed in an amount of 100 unit doses.
Embodiment 3
[0040] Preparation of paclitaxel anticancer drugs: dissolve 0.5 g of docetaxel in 3 g of D-α-polyethylene glycol 1000 succinate (TPGS, D-alpha-tocopheryl polyethylene glycol 1000 succinate), and then add 1 g of polyoxyethylene Glyceryl oleate (LabraWl M 1944CS, polyoxyethylated oleic glycerides), 1.5 grams of polyethylene glycol 400 (PEG 400: polyethylene glycol 400), 0.1 gram of ascorbyl palmitate (Ascorbylpalmitate) and 0.4 gram of absolute ethanol. This formulation is mixed in an amount of 100 unit doses.
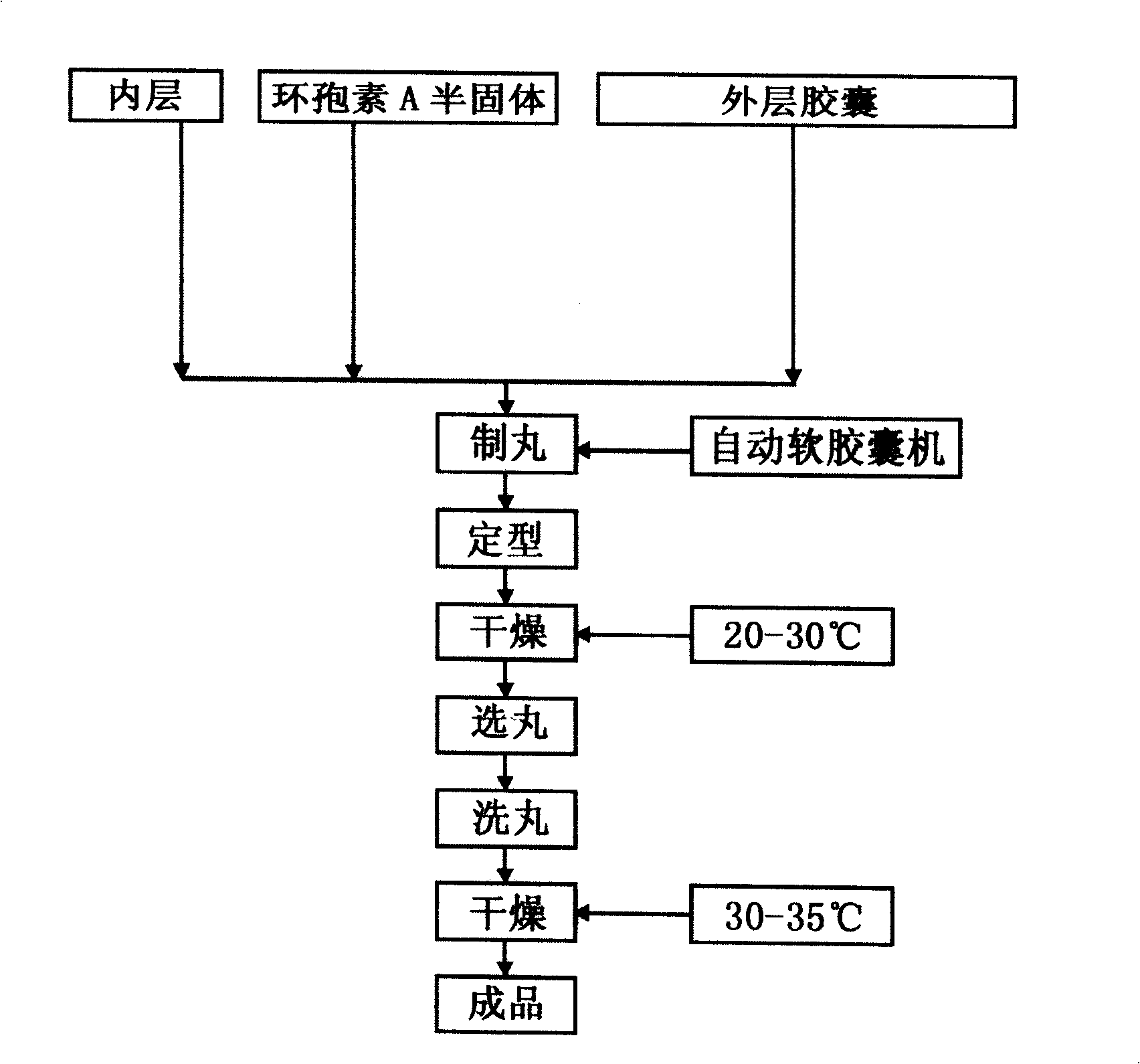
[0041] Preparation of cyclosporine A semi-solid: Mix 7 grams of polyoxyethylene castor oil diglyceride, 7 grams of polyoxyethylene, 7 grams of sorbitan glyceride, 7 grams of poloxamer, and 12 grams of polyethylene glycol-4000, Heat to 60-70°C to dissolve. Add 10 g of cyclosporine A and mix well to dissolve. Cool to room temperature and solidify into a semi-solid. This formulation is in the amount of 100 unit doses.
[0042] The preparation process of the inner layer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com