Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
128 results about "Artificial tears" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Artificial tears are lubricant eye drops used to treat the dryness and irritation associated with deficient tear production in keratoconjunctivitis sicca (dry eyes). They are also used to moisten contact lenses and in eye examinations.
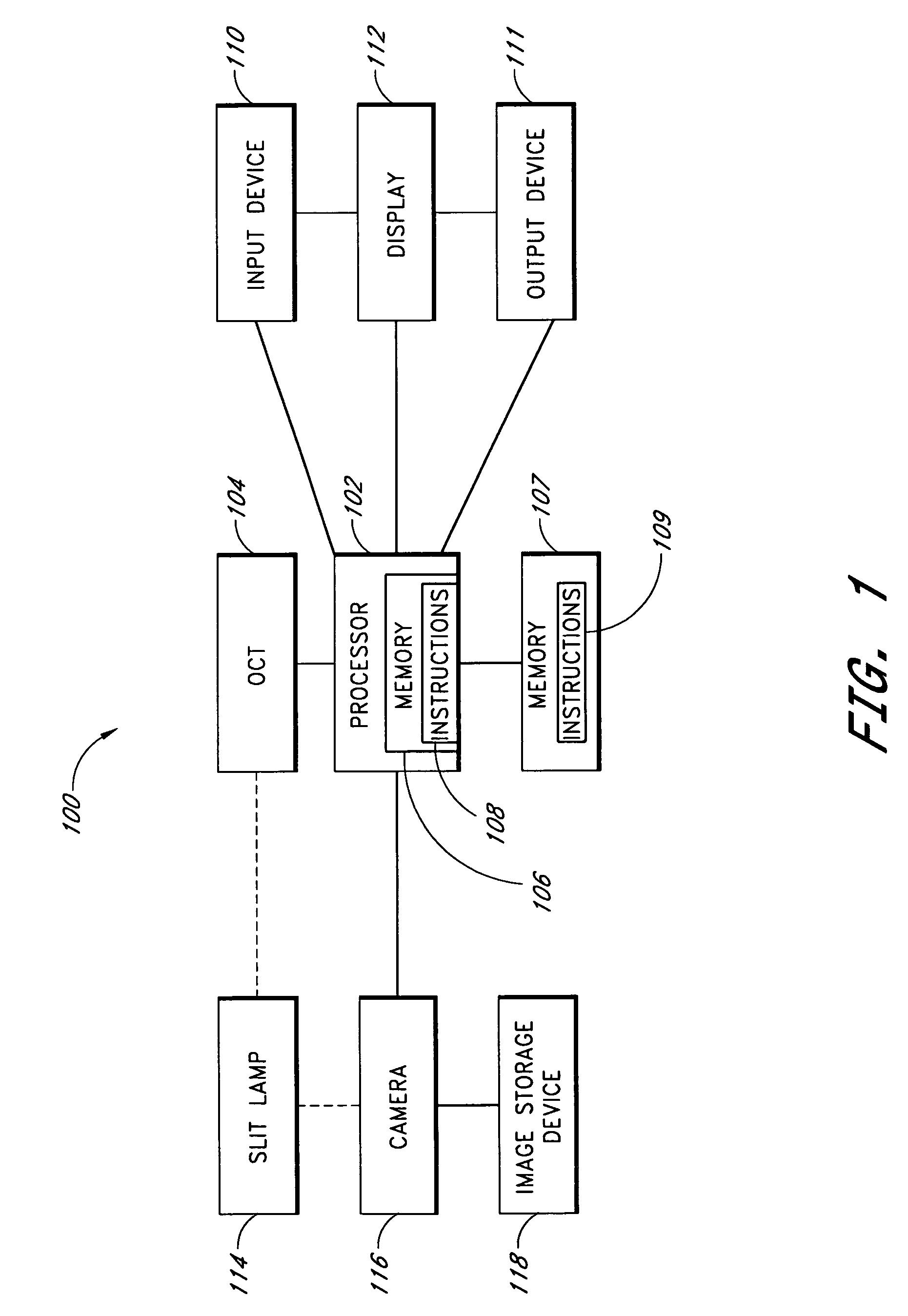
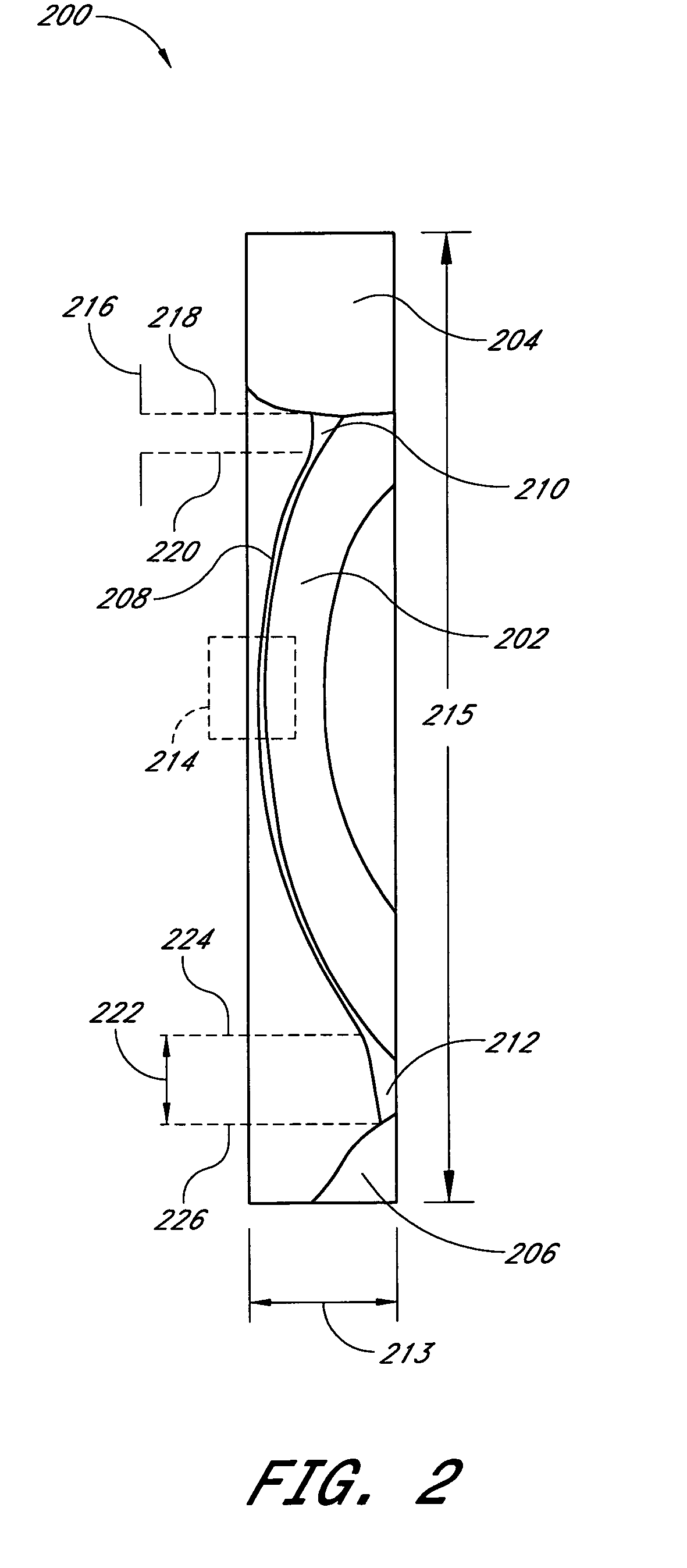
Tear dynamics measured with optical coherence tomography
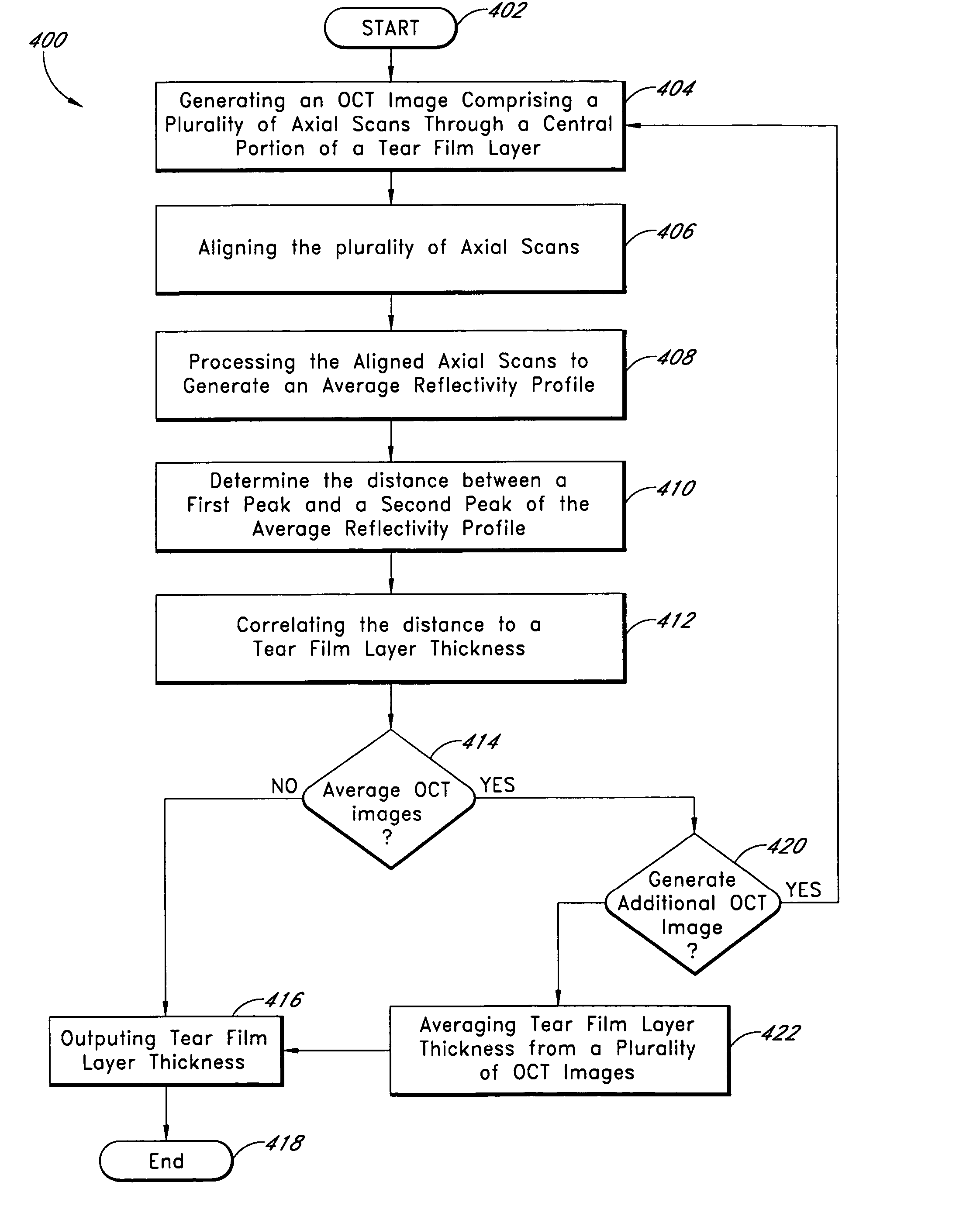
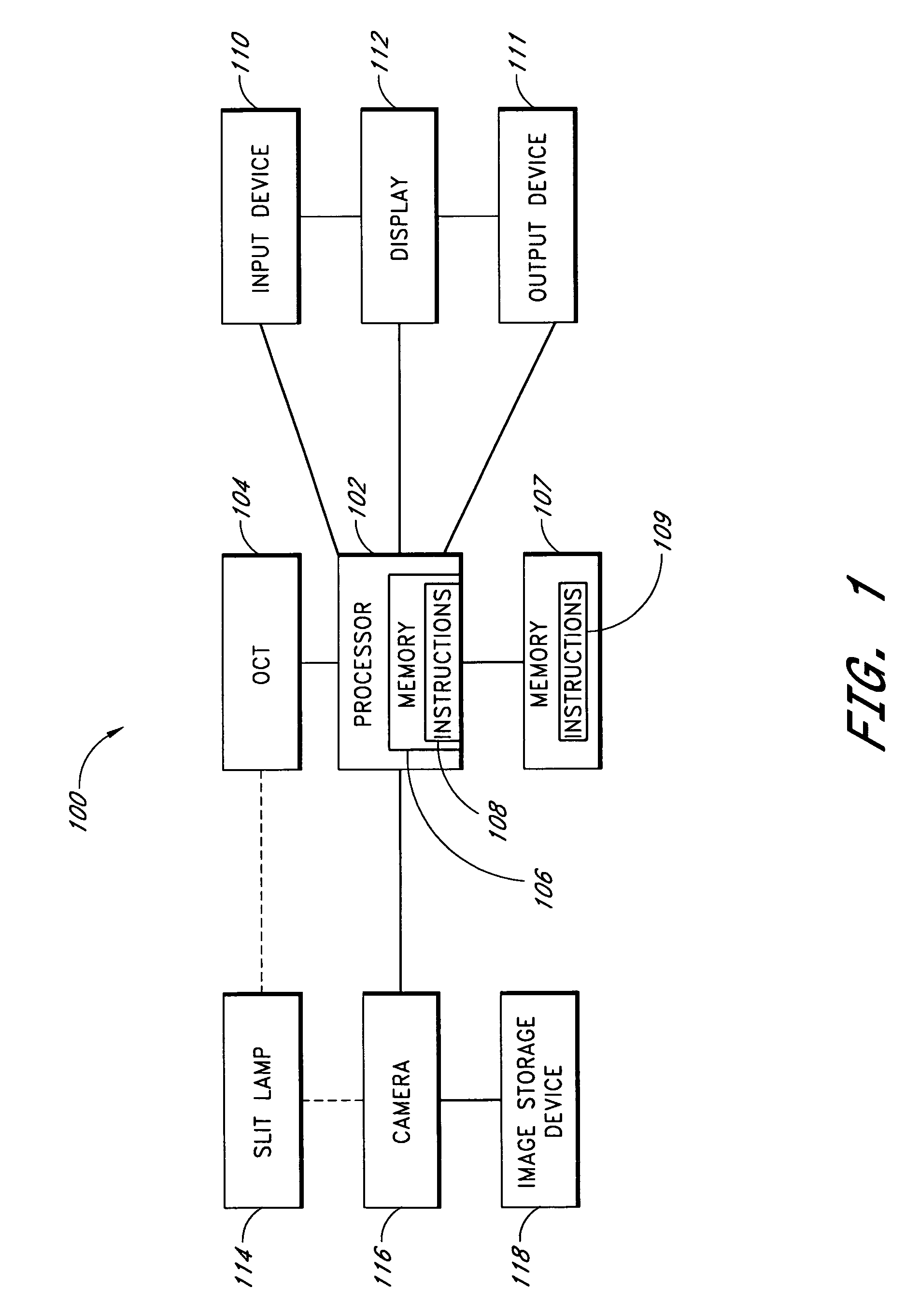
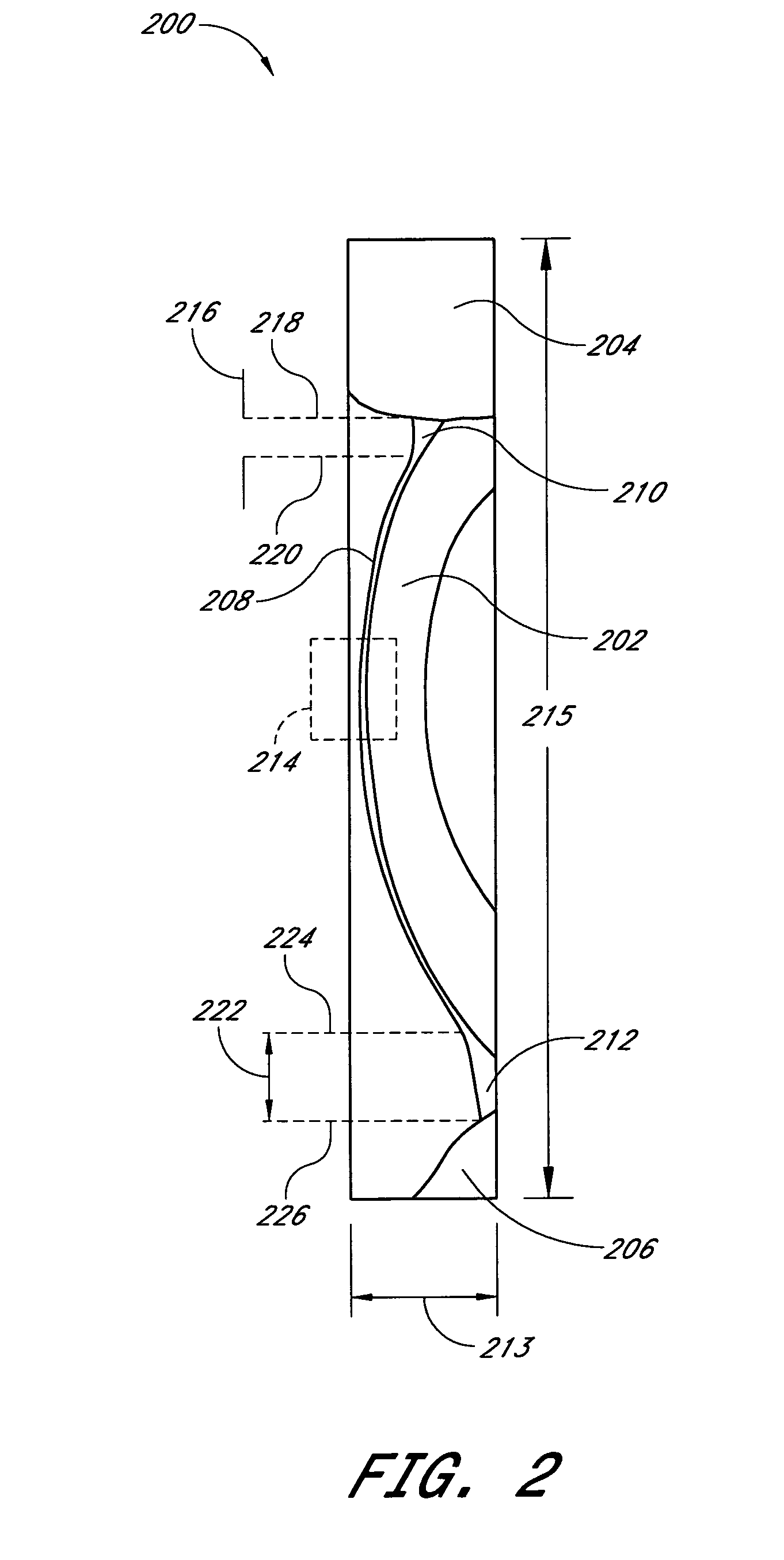
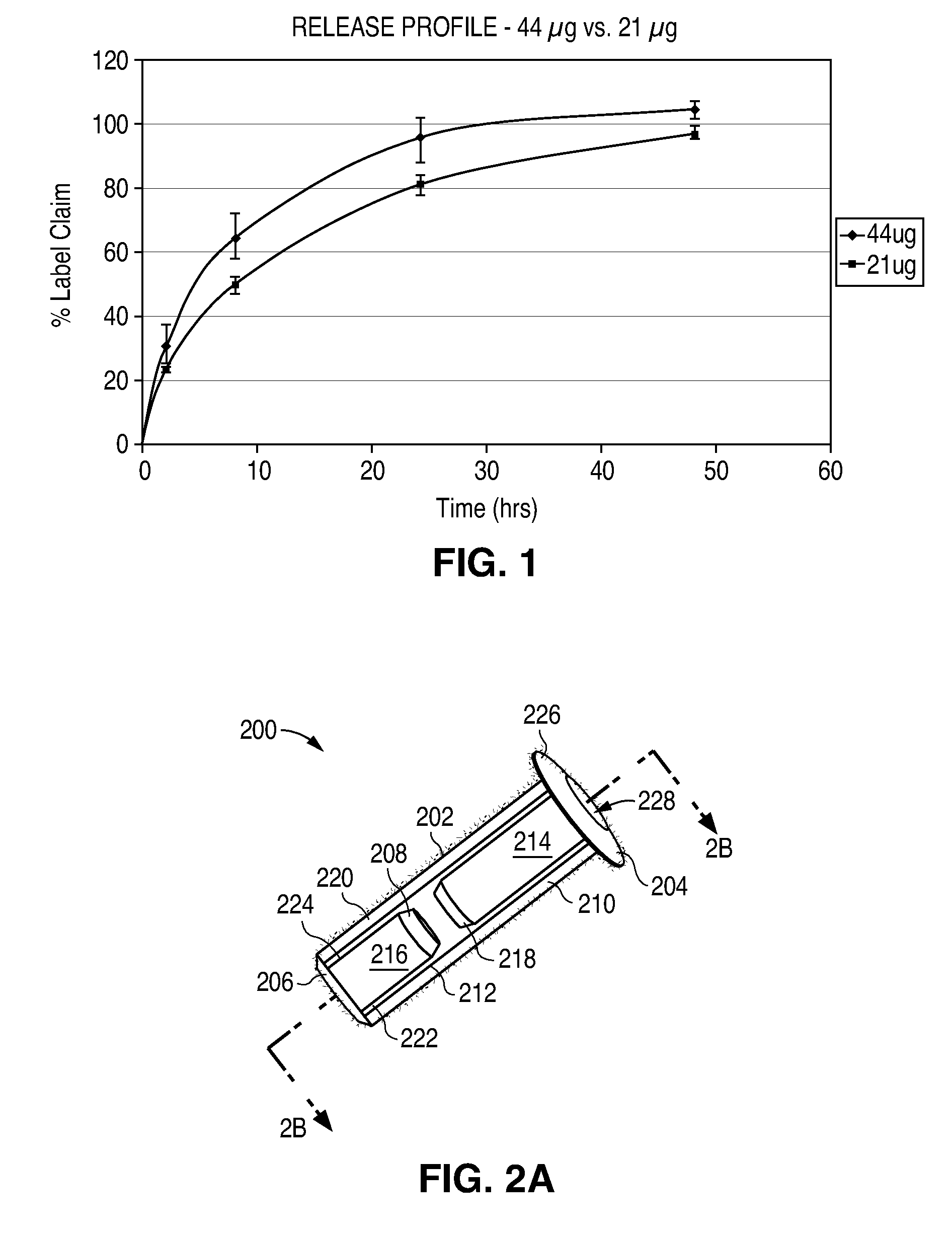
A system and method are provided for measuring the thickness of a tear film layer and the heights of tear menisci around upper and lower eyelids of an eye. A plurality of images are acquired between consecutive blinks the eye using optical coherence tomography (OCT). The images depict the tear film layer and tear menisci as distinct from the cornea of the eye. In an embodiment, a plurality of reflectivity profiles from an OCT image are aligned and averaged. The difference between a first peak and a second peak of the average reflectivity profile is measured to determine the thickness of the tear film layer. The heights of the upper meniscus and the lower meniscus can also be measured from the same OCT image. In an embodiment, thickness measurements from the plurality of OCT images are combined. The measurements can be used to diagnose tear disorders, analyze treatments of dry eye and evaluate artificial tears.
Owner:UNIVERSITY OF ROCHESTER
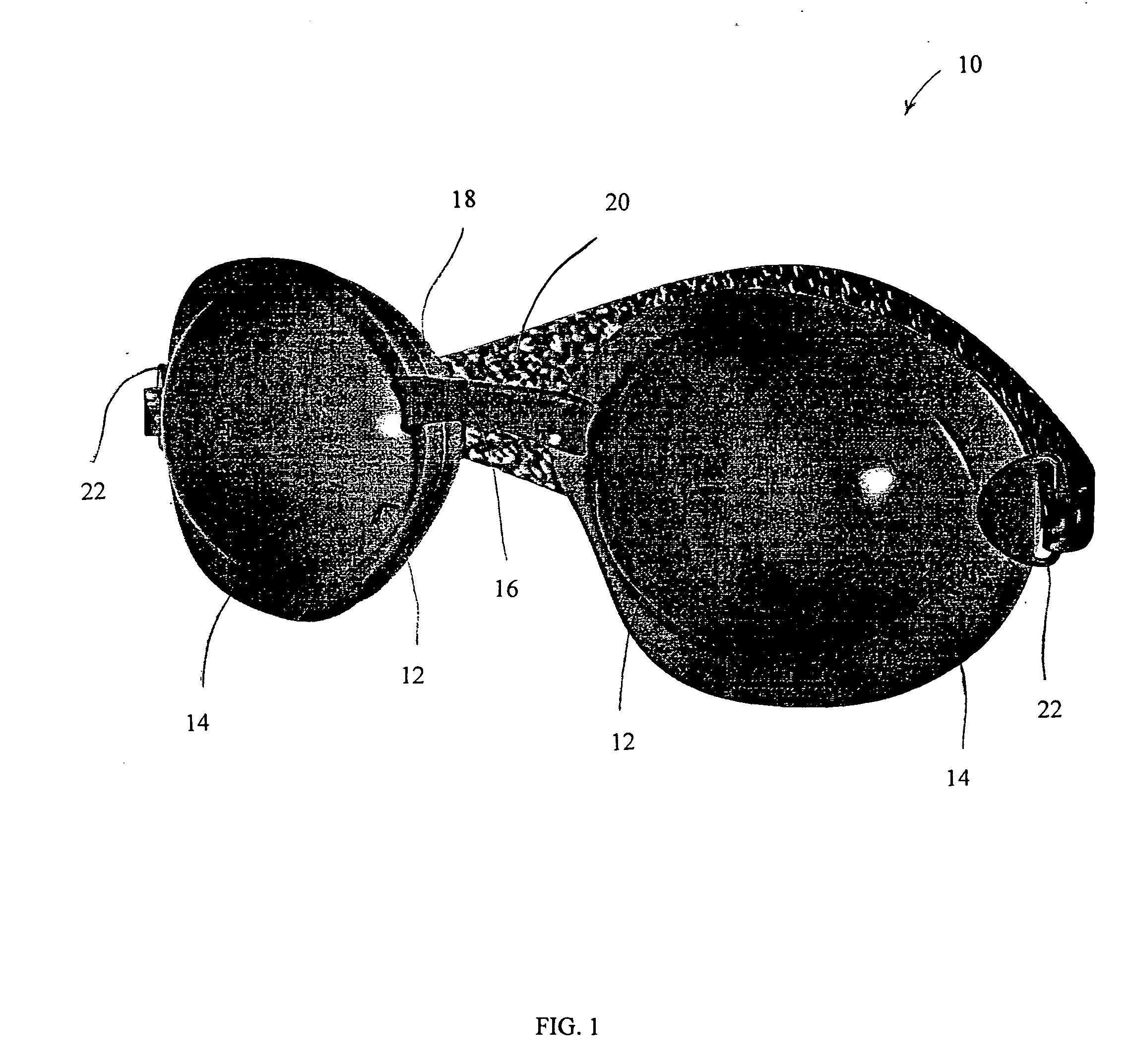
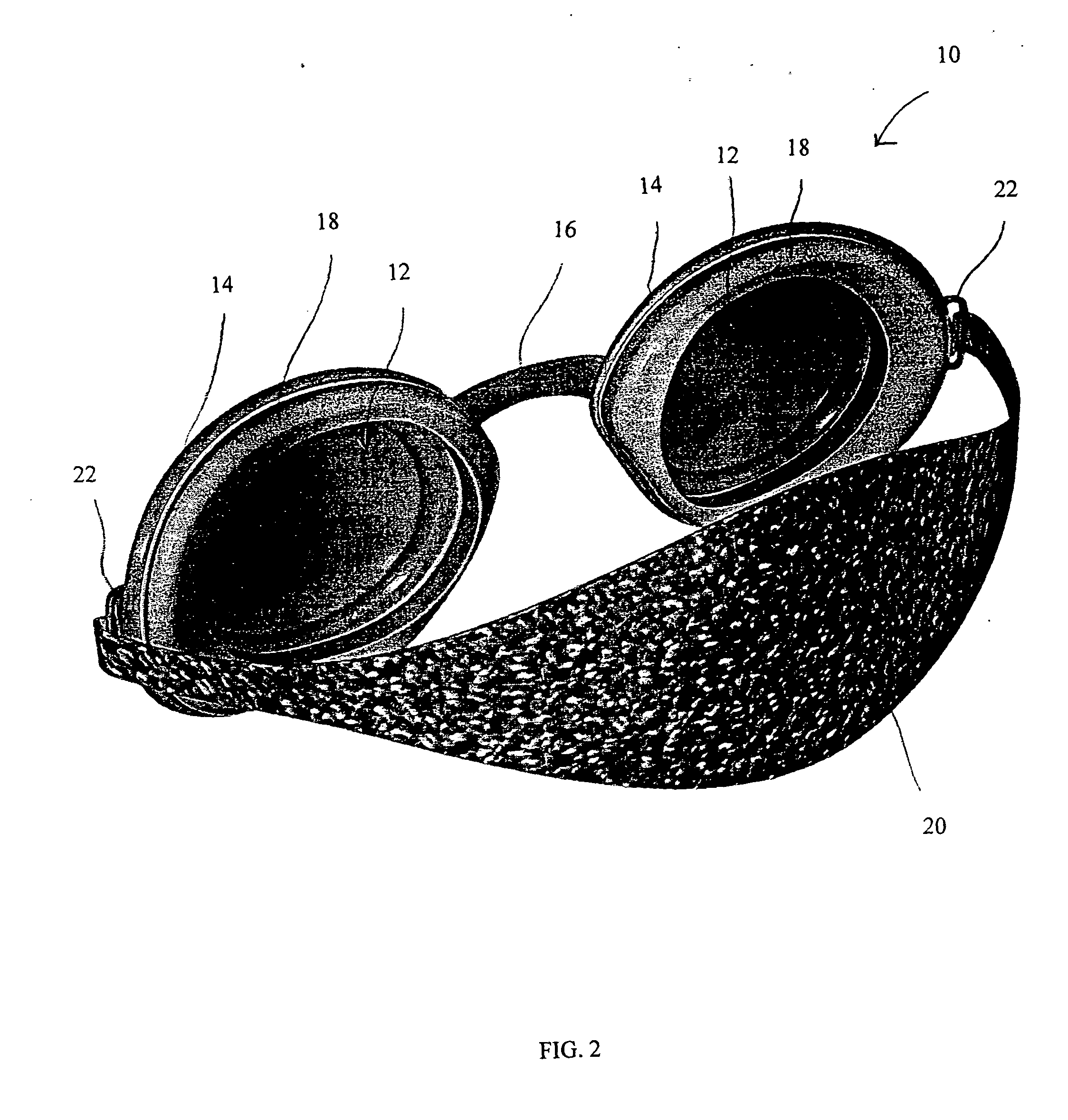
Apparatus, system and method for treating dry eye conditions and promoting healthy eyes
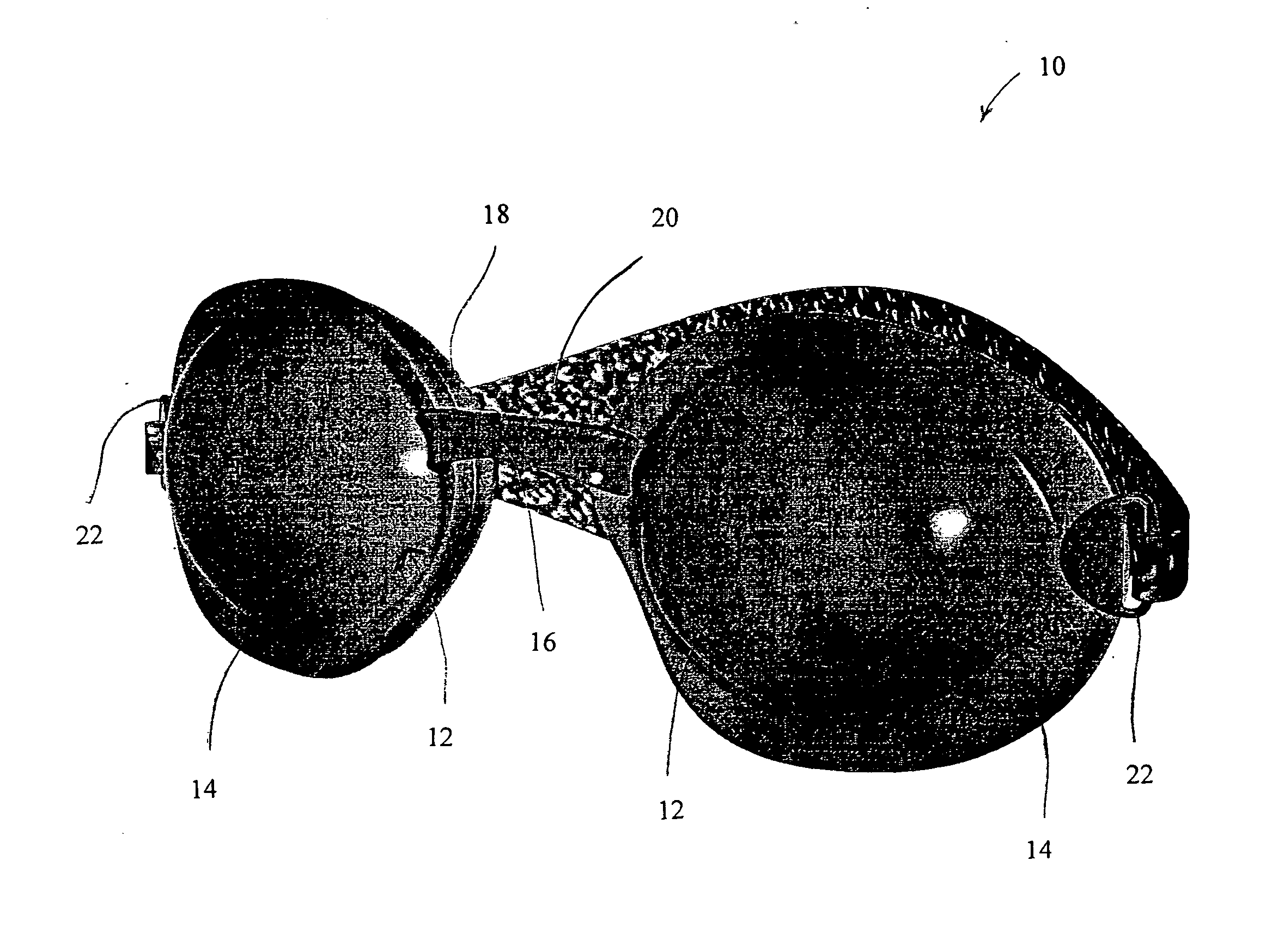
An apparatus and system, and methods for using such apparatus and system, is provided for preserving the eye's natural and artificial tears. The apparatus comprises two soft, pliable eyecups, which each include and curved lens and contoured frame, connected by a soft, pliable bridge. The lenses are maintained within a pliable, and contoured frame that is designed and constructed to encircle the orbital bone of the eye sockets, creating a custom fit and effective seal over each eye. Attached to each frame is a gasket that further seals the apparatus over the eyes and provides additional comfort to the wearer. A contoured strap, which is attached to each frame, maintains the position of the apparatus on the wearer.
Owner:EYE ECO INC
Methods and devices for measuring tear film and diagnosing tear disorders
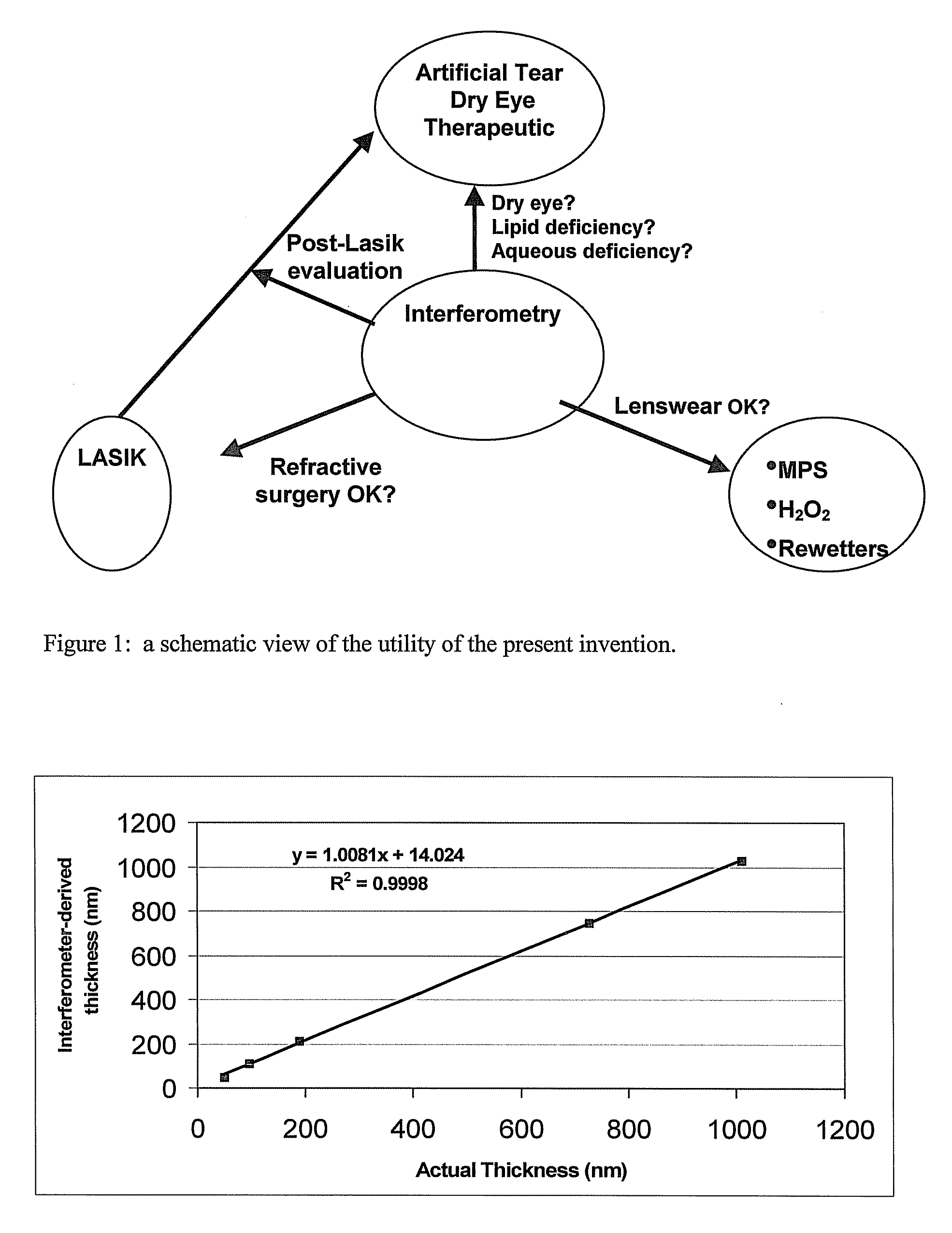
Methods and devices measure eye blinks and tear film lipid and aqueous layer thickness before and following ophthalmic formula application onto the ocular surface, especially wherein the ophthalmic formula is an artificial tear. The methods and devices are suitable for dry eye diagnosis. The methods and devices are suitable for use to evaluate ophthalmic formula effects on the tear film and to use such information to diagnose ophthalmic formula treatment of ocular disease conditions such as dry eye in the absence of contact lens wear or post-surgical eye drop treatment and diagnosis. The methods and devices are also suitable for use in the optimization of ophthalmic drug dosage forms and sustained drug release.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Artificial tear replacement solution
InactiveUS7001607B1Reduce wearGood film formingHalogenated hydrocarbon active ingredientsSenses disorderConjunctivaConjunctival sac
A tear replacement solution that contains at least one water-soluble fluorosurfactant, water and a non-polar component, preferably in gel form, and a method for the external treatment for the eye of an mammal by applying the tear replacement solution to the eye, preferably by placing in the conjunctival sac.
Owner:PHARMPUR
Sustained release delivery of one or more agents
InactiveUS20100209477A1Improve permeabilityReduce the amount requiredBiocideOrganic active ingredientsCross-linkExcipient
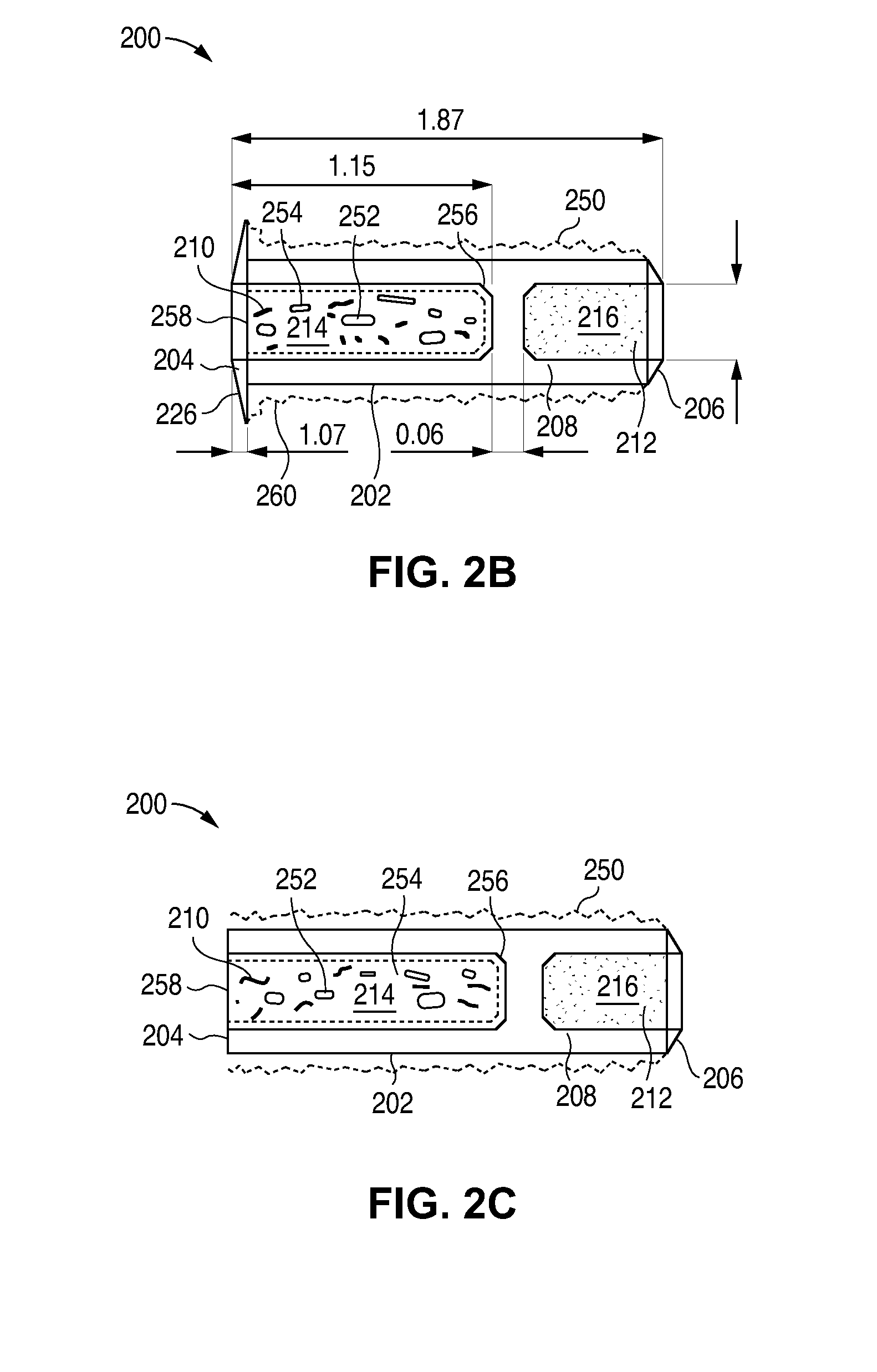
The lacrimal implant delivery systems and methods described herein provide for controlled release of a therapeutic agent for the treatment of disease, including the treatment of glaucoma, ocular hypertension, or elevated intraocular pressure with latanoprost or other anti-glaucoma agents. Treatment of disease, including glaucoma, ocular hypertension, or elevated intraocular pressure with latanoprost or other anti-glaucoma agent in conjunction with penetration enhancer, such as benzalkonium chloride, and / or artificial tears is also provided. Also provided are implants containing a drug core emplacable in a punctum adjacent to an eye of a patient for controlled release of a therapeutic agent such as latanoprost for the treatment of glaucoma, the drug core containing a polymer such as cross-linked silicone, a therapeutic agent, and an excipient, wherein the excipient can increase the rate of release of the agent from the drug core, or can increase the drug loading in the core without loss of desirable homogeneity of the agent within the core, or can improve retention of the agent in the eye or in tear fluid, or can increase corneal penetration of the agent into the eye.
Owner:MATI THERAPEUTICS
Methods and devices for measuring tear film and diagnosing tear disorders
Methods and devices measure eye blinks and tear film lipid and aqueous layer thickness before and following ophthalmic formula application onto the ocular surface, especially wherein the ophthalmic formula is an artificial tear. The methods and devices are suitable for dry eye diagnosis. The methods and devices are suitable for use to evaluate ophthalmic formula effects on the tear film and to use such information to diagnose ophthalmic formula treatment of ocular disease conditions such as dry eye in the absence of contact lens wear or post-surgical eye drop treatment and diagnosis. The methods and devices are also suitable for use in the optimization of ophthalmic drug dosage forms and sustained drug release.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Lubricant for the ocular surface
InactiveUS20050202097A1Easy to spreadProcess stabilityBiocideSenses disorderEye/ear dropsBlurred vision
A formulation has been developed for treatment of the symptoms of dry eye which incorporates the natural product jojoba wax, or components thereof, to enhance the spreading of the artificial tear and eyedrop as well as stabilize the eyedrop. The improved performance of the jojoba wax supplemented tear relieves irritation and discomfort as well as sharpens the blurred vision.
Owner:MELBJ HLDG
Tear dynamics measured with optical coherence tomography
A system and method are provided for measuring the thickness of a tear film layer and the heights of tear menisci around upper and lower eyelids of an eye. A plurality of images are acquired between consecutive blinks the eye using optical coherence tomography (OCT). The images depict the tear film layer and tear menisci as distinct from the cornea of the eye. In an embodiment, a plurality of reflectivity profiles from an OCT image are aligned and averaged. The difference between a first peak and a second peak of the average reflectivity profile is measured to determine the thickness of the tear film layer. The heights of the upper meniscus and the lower meniscus can also be measured from the same OCT image. In an embodiment, thickness measurements from the plurality of OCT images are combined. The measurements can be used to diagnose tear disorders, analyze treatments of dry eye and evaluate artificial tears.
Owner:UNIVERSITY OF ROCHESTER
Treatment for Meibomian Gland Dysfunction or Obstruction
ActiveUS20110124725A1Easy to spreadStabilize the tear filmBiocideSenses disorderIrritationBlurred vision
A jojoba formulation has been developed for administration to the meibomian gland, for treatment of the symptoms of dry eye, and / or for drug delivery to the meibomian gland. The formulation incorporates the natural product jojoba wax, or components thereof, to enhance the spreading of the artificial tear as well as stabilize the tear film. The jojoba wax tear relieves irritation and discomfort as well as sharpens the blurred vision. Jojoba, because of its close chemical and physical properties to meibomian gland secretions, is effective upon topical application to penetrate the lid margin to reach the gland tissues where it may exert a therapeutic effect with or without an adjunctive agent.
Owner:MGD INNOVATIONS INC
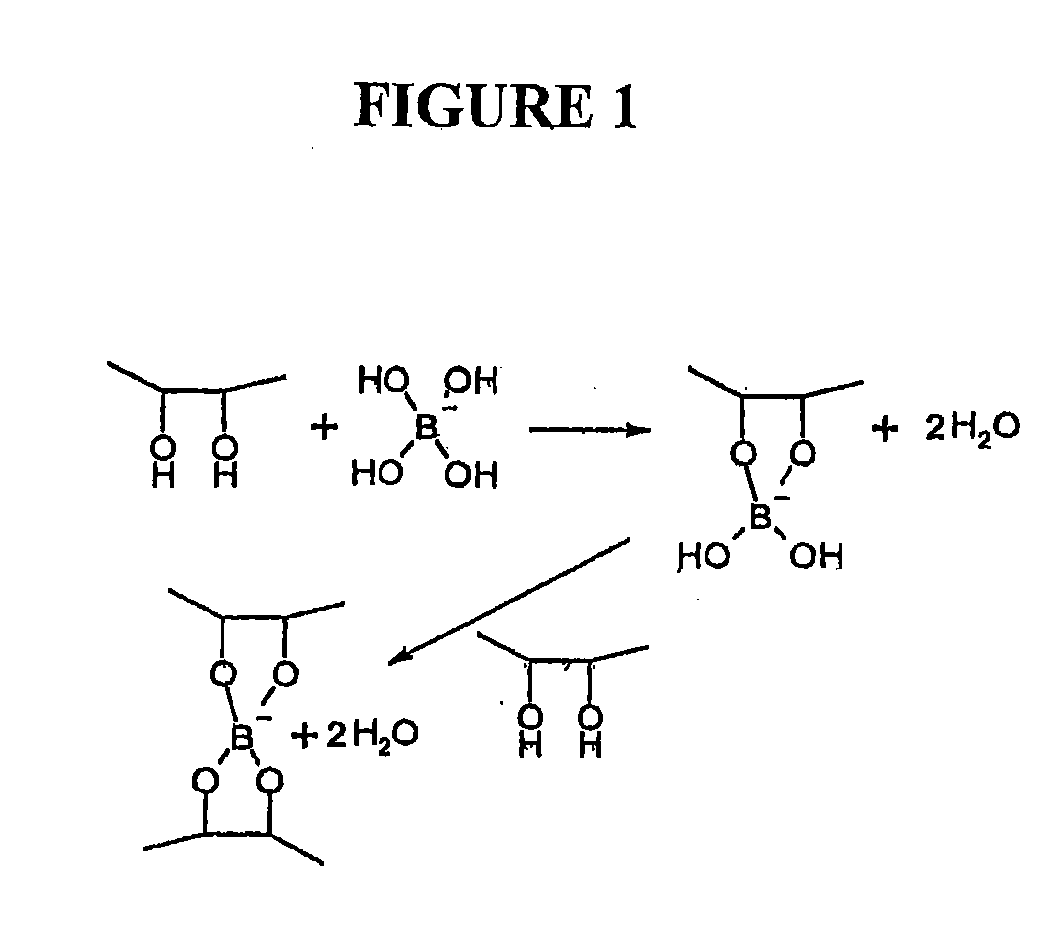
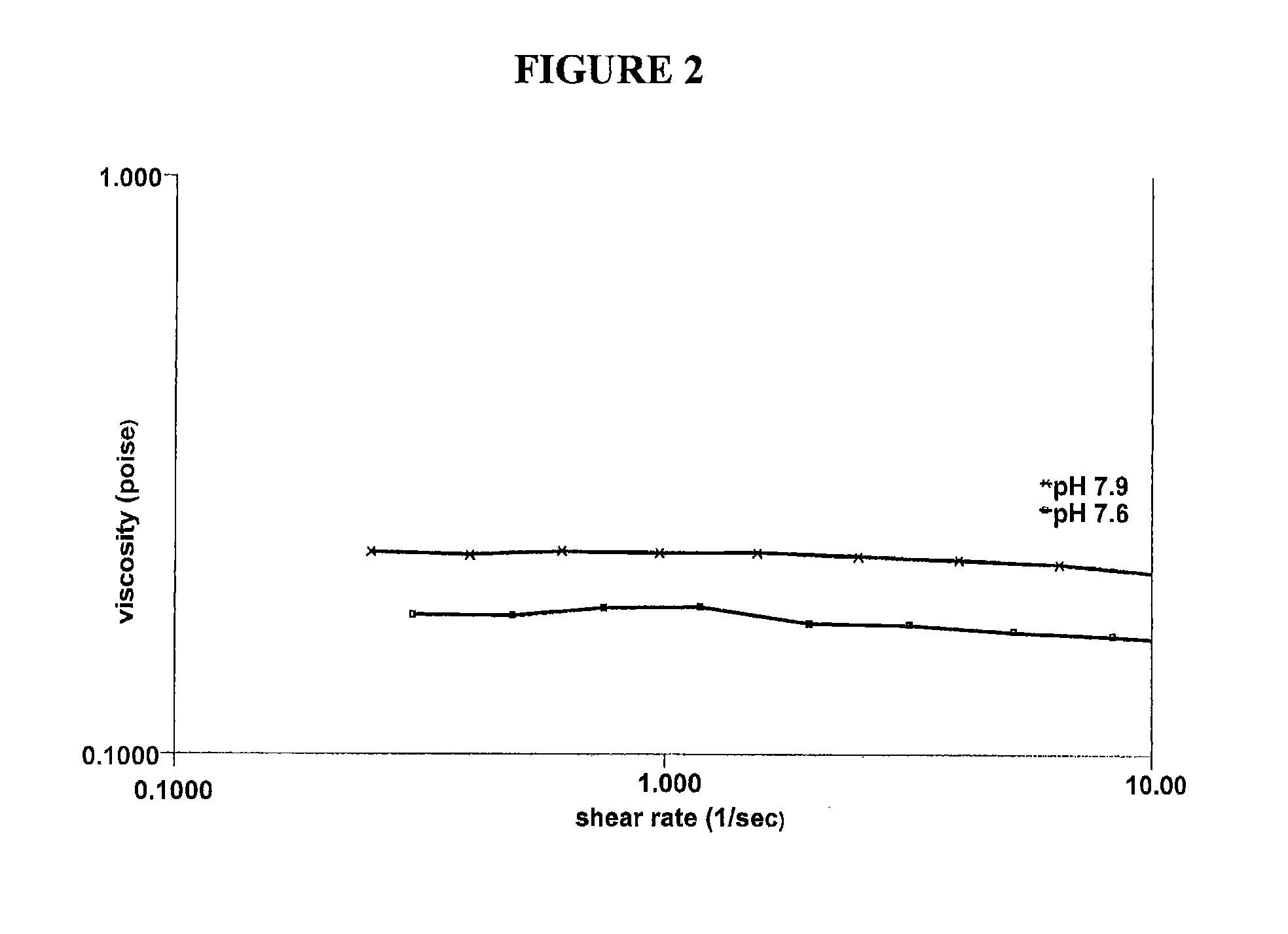
Polymeric artificial tear system
InactiveUS20090270345A1Facilitate cross-linkingIncreased formationBiocideSenses disorderDiolBoric acid
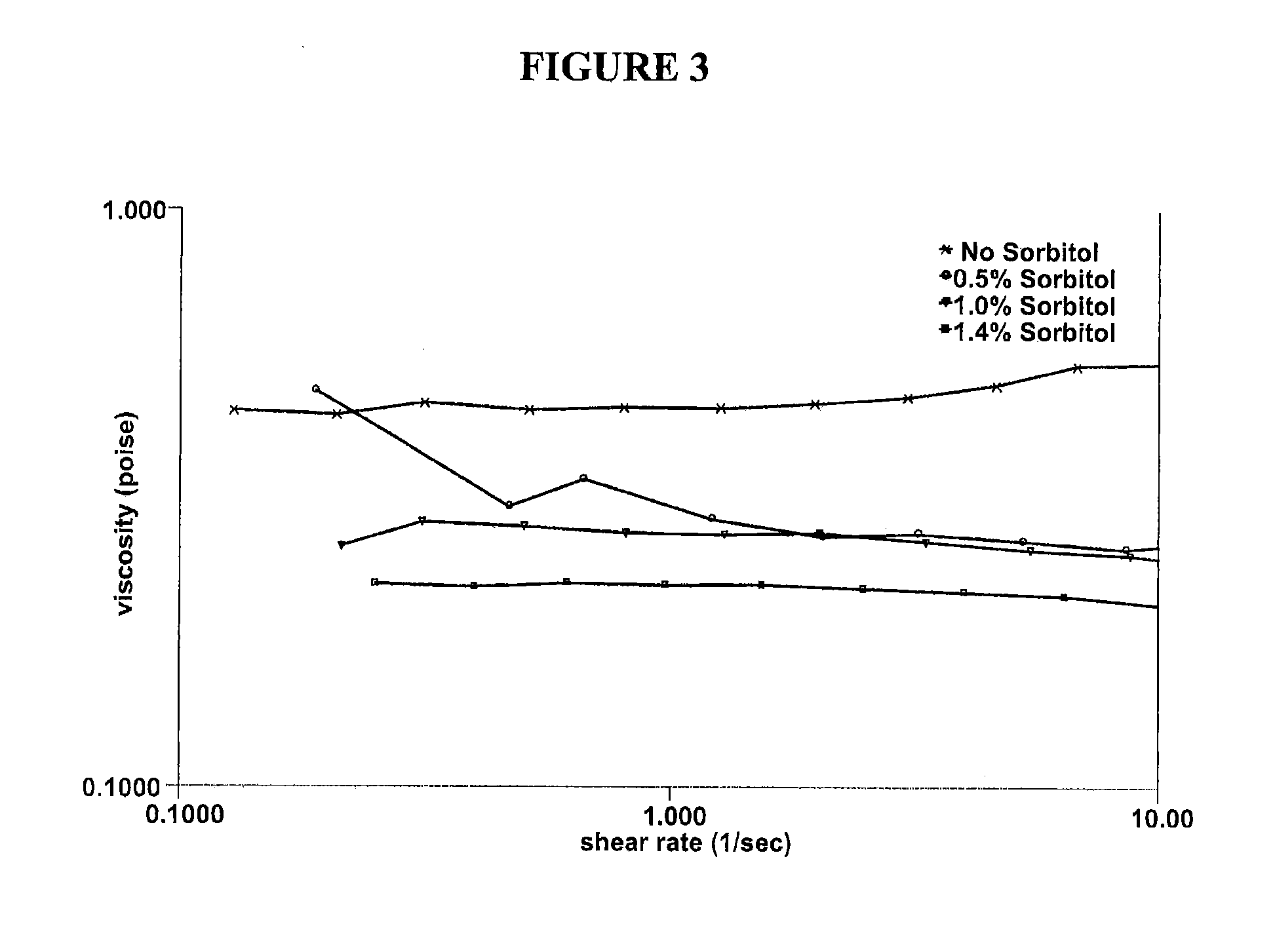
The present invention relates to artificial tear formulations and ophthalmic formulations suitable for drug delivery. The formulations comprise galactomannans such as guar or hydroxypropyl guar and a borate source such as boric acid. The formulations further comprise a cis-diol such as sorbitol that interferes with the cross-linking of galactomannan and borate. Optionally, the formulations are substantially free of divalent cations.
Owner:ALCON RES LTD
Lubricant for the ocular surface
InactiveUS20120128763A1Easy to spreadStabilize the tear filmOrganic active ingredientsSenses disorderNatural productIrritation
Owner:MELBJ HLDG
Artificial tears and therapeutic uses
Owner:ALLERGAN INC
Ophthalmic Lipophilic and Hydrophilic Drug Delivery Vehicle Formulations
InactiveUS20140378401A1Increase shear forcePhase-low viscosityBiocideSenses disorderSolubilitySide effect
The ophthalmic drug delivery vehicles provide comfort and compliance; drug solubility, residence time and permeability; and reduce side effects. In addition, the delivery vehicle can be slightly modified to provide an artificial tear formulation.
Owner:PS THERAPIES LTD
Treatment for meibomian gland dysfunction or obstruction
ActiveUS8455016B2Easy to spreadStabilize the tear filmBiocideSenses disorderBlurred visionIrritation
A jojoba formulation has been developed for administration to the meibomian gland, for treatment of the symptoms of dry eye, and / or for drug delivery to the meibomian gland. The formulation incorporates the natural product jojoba wax, or components thereof, to enhance the spreading of the artificial tear as well as stabilize the tear film. The jojoba wax tear relieves irritation and discomfort as well as sharpens the blurred vision. Jojoba, because of its close chemical and physical properties to meibomian gland secretions, is effective upon topical application to penetrate the lid margin to reach the gland tissues where it may exert a therapeutic effect with or without an adjunctive agent.
Owner:MGD INNOVATIONS INC
Treatment for Meibomian Gland Dysfunction or Obstruction
ActiveUS20130131171A1Easy to spreadStabilize the tear filmBiocideSenses disorderBlurred visionIrritation
A jojoba formulation has been developed for administration to the meibomian gland, for treatment of the symptoms of dry eye, and / or for drug delivery to the meibomian gland. The formulation incorporates the natural product jojoba wax, or components thereof, to enhance the spreading of the artificial tear as well as stabilize the tear film. The jojoba wax tear relieves irritation and discomfort as well as sharpens the blurred vision. Jojoba, because of its close chemical and physical properties to meibomian gland secretions, is effective upon topical application to penetrate the lid margin to reach the gland tissues where it may exert a therapeutic effect with or without an adjunctive agent.
Owner:MGD INNOVATIONS INC
Artificial tear, contact lens and drug vehicle compositions and methods of use thereof
PendingUS20180098937A1Enhancing contact lens wear timeReduce decreaseOrganic active ingredientsSenses disorderPolyolMedicine
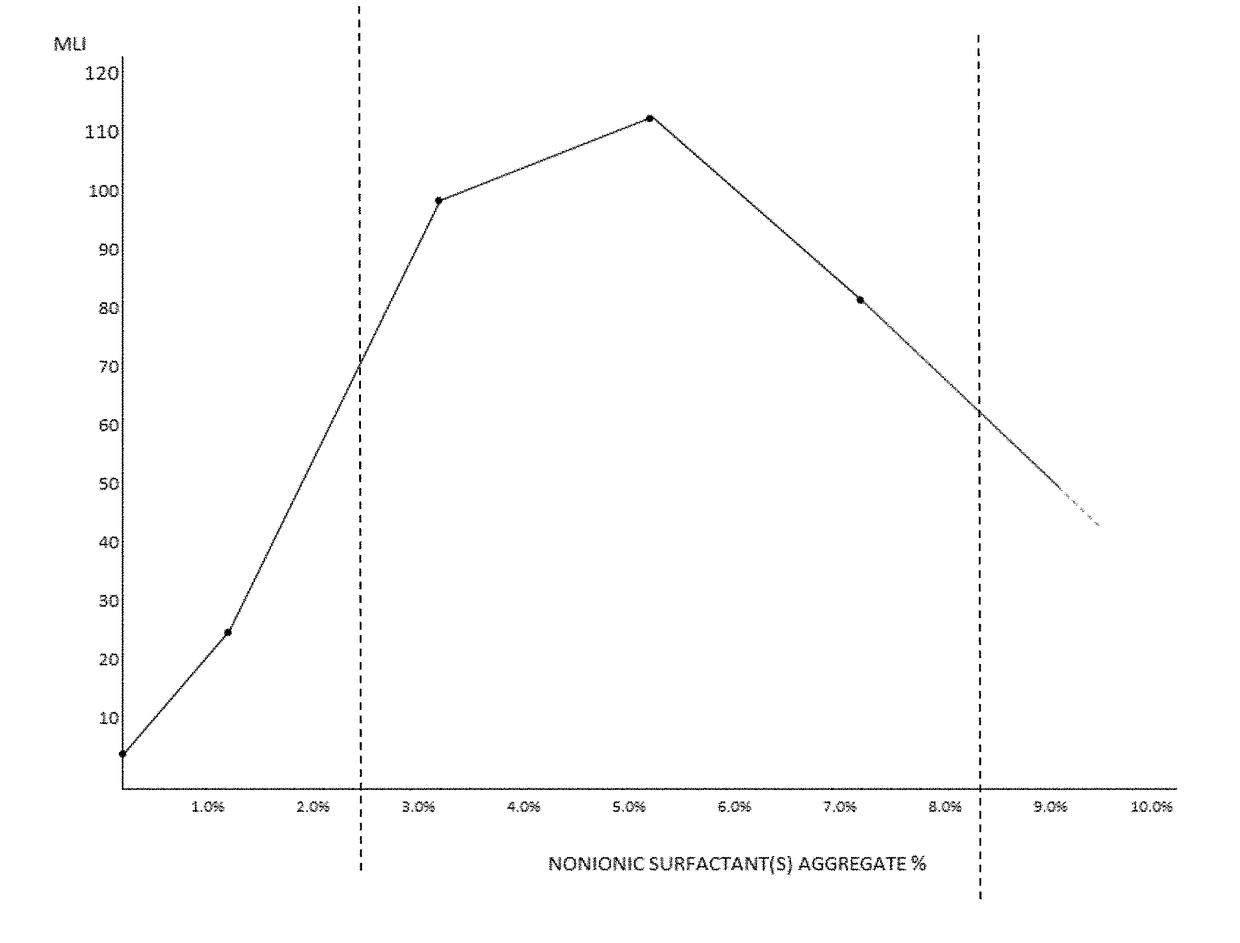
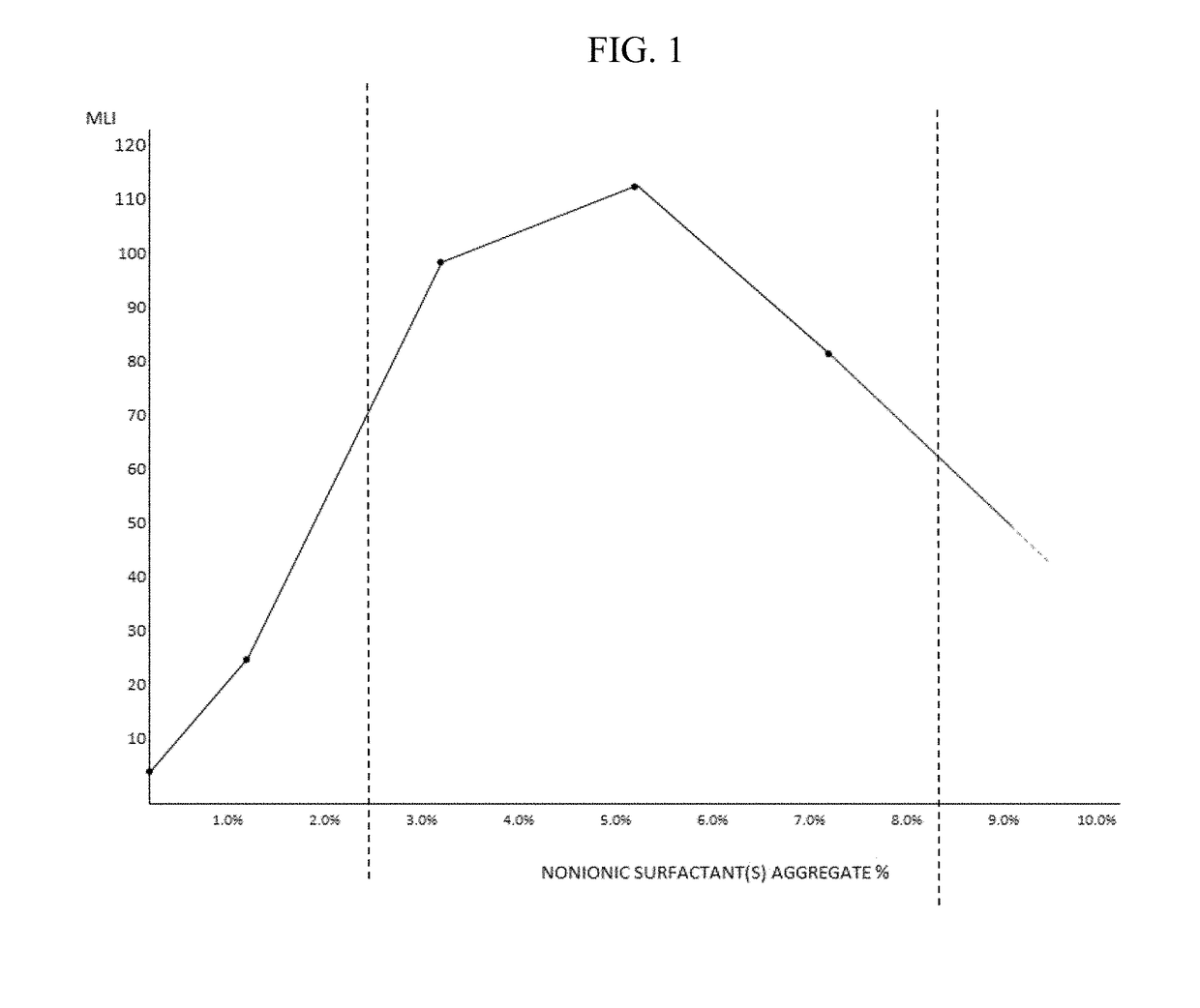
The invention provides artificial tear compositions, artificial tear-gel compositions, contact lens storage compositions, contact lens treatment compositions, ophthalmological drug vehicle compositions and topical drug vehicle compositions comprising one or more nonionic surfactants with one or more non-Newtonian viscosity enhancing excipients and one or more of a polyol and or an electrolyte and methods of their use.
Owner:PS THERAPIES LTD +1
Artificial tear compositions comprising a combination of nonionic surfactants
The invention provides artificial tear compositions comprising one or more nonionic surfactants with one or more non-Newtonian viscosity enhancing excipients.
Owner:PS THERAPY INC +1
Dry eye treatment
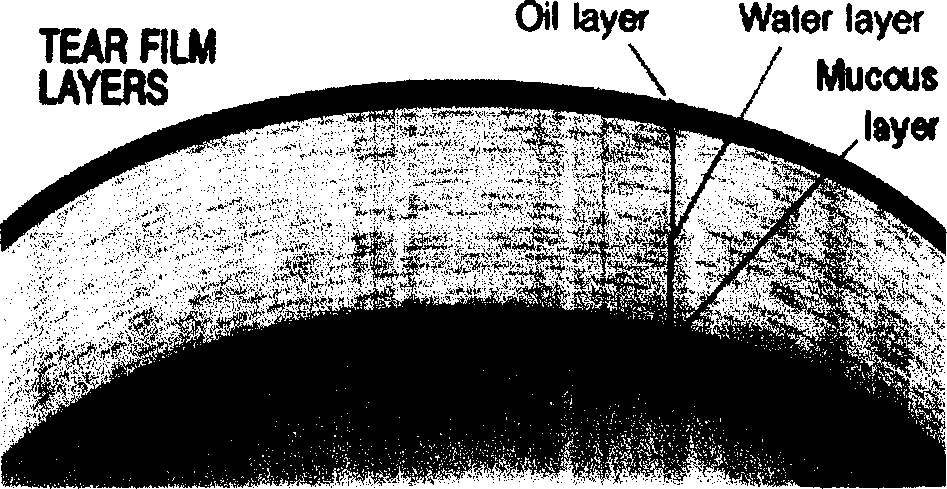
This invention relates to an emulsion composition for the formation of an artificial tear film over the ocular surface of the eye capable of providing mechanical lubrication for the ocular surface while reducing evaporation of fluid therefrom. The emulsion is desirably in the form of a meta stable emulsion and is characterized by the use of a surfactant comprising a combination of a primary and secondary surfactant where the primary surfactant permits formation of the emulsion and the secondary surfactant permits autoclaving of the surfactant. The invention also includes a method for the formation of such an emulsion.
Owner:OCULAR RES OF BOSTON INC
Artificial tear composition adapted to be used with contact lenses
InactiveUS20040028645A1Prevent complexation and other uptakeOrganic detergent compounding agentsLens cleaning compositionsPreservativeSodium citrate
An aqueous artificial tear / rewetting drop solution is described. The solution may be utilized as both an artificial tear for persons wearing contact lenses and as a contact lens rewetting drop. The solution is based on the use of a citric acid / sodium citrate buffer system to prevent binding or other complexation between cationic antimicrobial preservatives and soft contact lenses, thereby making it possible to apply the solution directly to contact lenses while the lenses are being worn.
Owner:ALCON INC
Ophthalmic compositions with improved dessication protection and retention
InactiveUS20130296264A1Improved desiccation protectionImprove retention characteristicsBiocideSenses disorderDiolHyaluronic acid
The present invention relates to artificial tear compositions and ophthalmic compositions suitable for drug delivery. In one embodiment of the present invention, the compositions comprise a galactomannan polymer such as guar or hydroxypropyl guar, hyaluronic acid, and a cis-diol such as sorbitol. In a preferred embodiment, the compositions also comprise a borate compound.
Owner:ALCON RES LTD
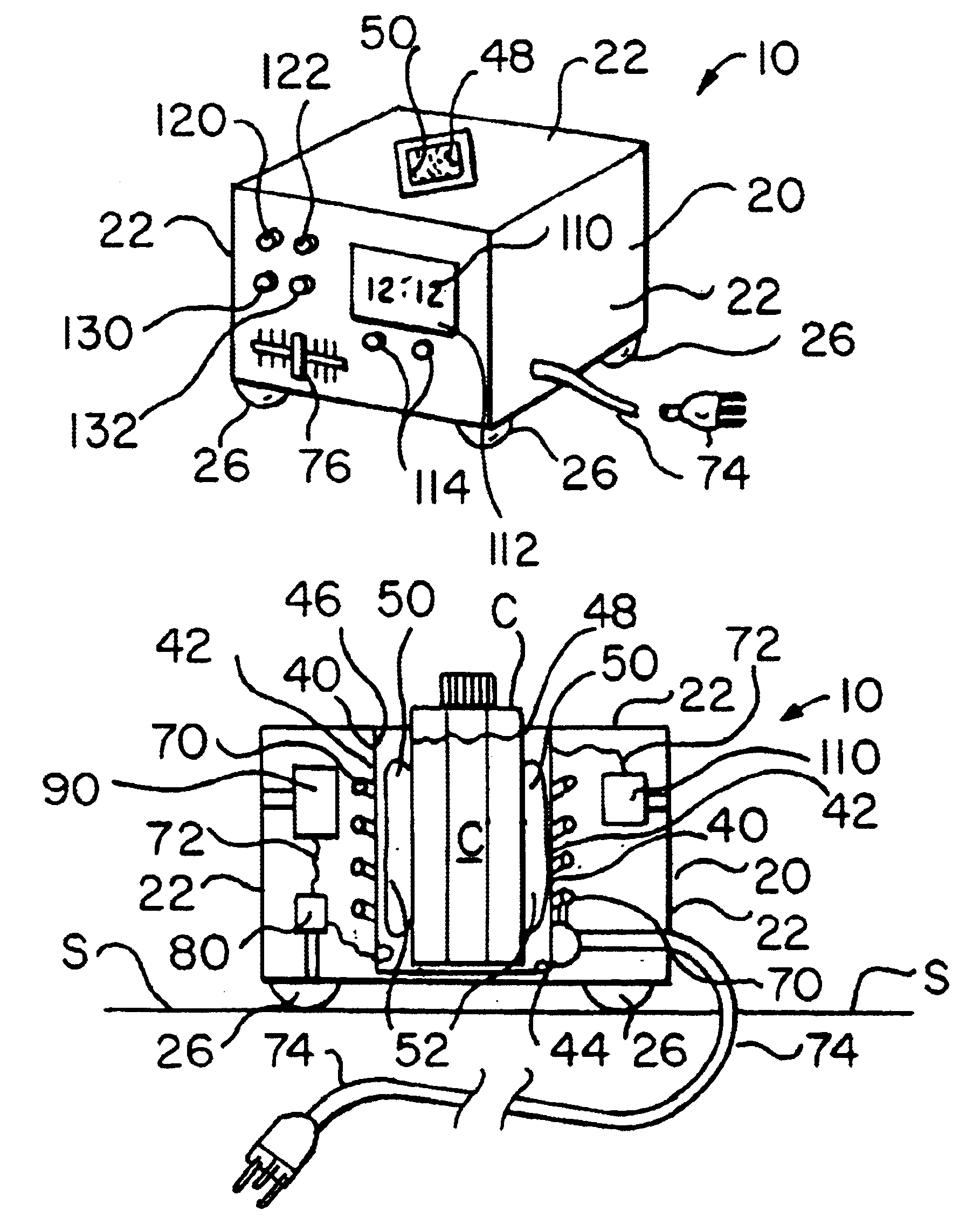
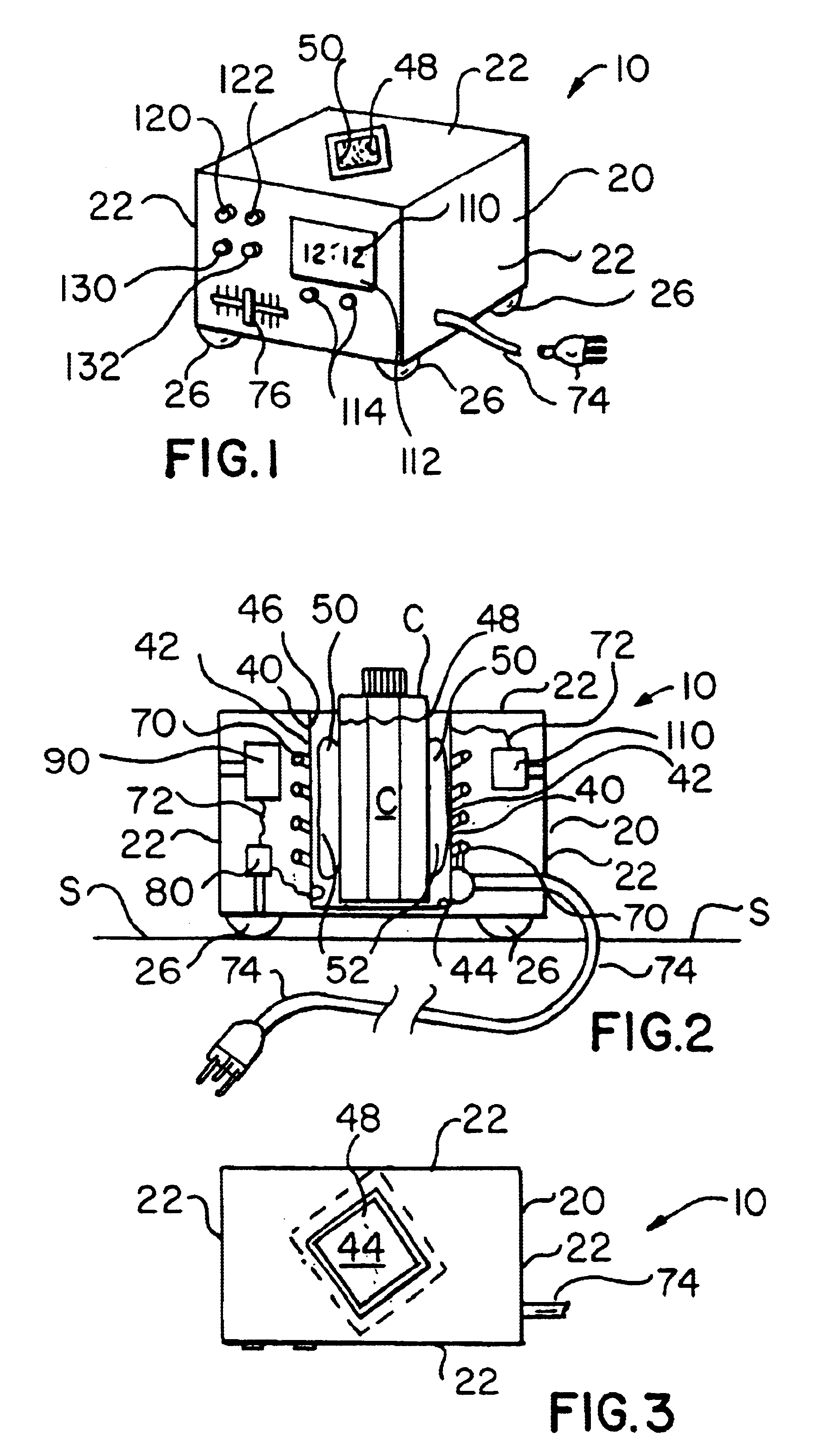
Artificial tears container warming apparatus
InactiveUS6870137B1Maximize heat transfer efficiencyMinimize escapeHolders and dispensersOther accessoriesEngineeringElectric power
A warming apparatus for receiving and heating a product container includes a heating chamber having a chamber side wall and a chamber bottom wall defining a chamber having a chamber interior surface and a chamber port through which a product container is inserted into and removed from the chamber; a heating element adjacent to one of the chamber side wall and the chamber bottom wall, for heating a product container within the chamber; an apparatus circuit electrically connected to the heating element for supplying electric power to the heating element; a flexible chamber liner within the chamber for receiving a product container and deforming to the shape and size of the product container; and a flowable heat transfer substance retained by the chamber liner adjacent to the chamber interior surface for flowing as the chamber liner deforms to the shape and size of a product container.
Owner:CLAPP MICHAEL G
Ophthalmic compositions containing a polysaccharide/borate gelling system
InactiveUS20050129771A1Low degree of branchingAntibacterial agentsPowder deliveryCompound (substance)Diol
Topical ophthalmic compositions that form a gel or partial gel upon application to the eye are described. The compositions are particularly useful as artificial tears and ocular lubricants, but may also be utilized for the topical delivery of pharmaceutically active compounds to the eye. The compositions contain a polysaccharide / borate gelling system. The polysaccharides that may be utilized contain cis-diol groups and have a structure that is predominately linear, with a slight degree of branching.
Owner:ALCON INC
Artificial tear compositions
InactiveUS20140377210A1ViscosityPharmaceutical delivery mechanismSynthetic polymeric active ingredientsMedicineExcipient
The invention provides artificial tear compositions comprising one or more nonionic surfactants with one or more non-Newtonian viscosity enhancing excipients.
Owner:PS THERAPIES LTD
Vitamin A liposome artificial lacrimal eye drops
ActiveCN1850054ASenses disorderHydroxy compound active ingredientsSodium bicarbonateAdditive ingredient
The present invention relates to a vitamin A liposome artificial tears eye drops for preventing and curing xerophthalmia and ophthalmokopia. Its composition includes fat-soluble vitamin A, liposome encapsulating material, vitamin E and artificial tears hydrated liquor, in which the fat-soluble vitamin A is vitamin A palmitate or vitamin A acetate, the liposome encapsulating material is soya bean lecithin and cholesterol, and the composition of artificial tears hydrated liquor includes sodium hydrogen carbonate, sodium chloride, glucose, potassium chloride, calcium chloride and water. Besides, according to requirements one or several kinds of auxiliary components of menthanol, PVP and HPMC, etc. also can be added.
Owner:CHINA RESOURCES SAIKE PHARMA
Method and ophthalmic formulation for eye protection or treatment
An ophthalmic composition for artificial tears useful for treating dry eye symptoms or for general eye protection. The composition includes a physiologically balanced salt solution and one or more anti-oxidative ingredients which collectively confer on said ophthalmic composition a total antioxidant capacity as a FRAP value of greater than 100 μmol / l, preferably greater than 150 μmol / l. The composition also has peak UV absorption in the range of 200-350 nm. Suitable anti-oxidative ingredient includes ascorbate (ascorbic acid), urate (uric acid), cysteine, glutathione, tyrosine, lactoferrin, transferrin, albumin, caeruloplasmin and lacrimal gland peroxidase.
Owner:THE HONG KONG POLYTECHNIC UNIV
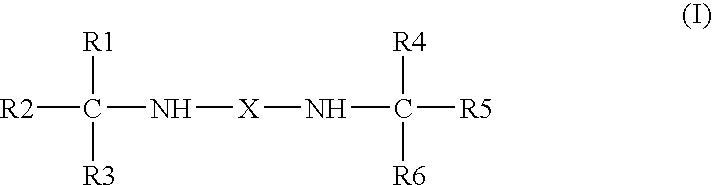
Use of bis-amines to enhance the antimicrobial activity of aqueous compositions
The use of bis-amines to enhance the antimicrobial activity of pharmaceutical compositions is described. The bis-amines are particularly useful for enhancing the antimicrobial activity of aqueous ophthalmic compositions, such as artificial tears or ocular lubricants, and solutions for disinfecting contact lenses.
Owner:ALCON INC
Arificial tears containing trehalose as well as preparation and application method thereof
InactiveCN101181279AEffective treatmentStable physical and chemical propertiesOrganic active ingredientsSenses disorderDiseaseOphthalmic drug
The invention relates to artificial tear containing trehalose and the preparation and application method, which pertains to the technical field of pharmaceutical manufacture. The main component of the invention is the trehalose, the basic composition of the artificial tear is the trehalose, simulating mucin protein and conventional ophthalmic pharmaceutical excipients; the weight ratio of the three is that 0.3 to 97 percent trehalose, 0.3 to 97 percent simulating mucin protein and 2 to 98 percent conventional ophthalmic pharmaceutical excipients. The pH and the osmotic pressure of the artificial tear of the invention are equivalent to physiologic tear, the invention contains rich electrolyte ions, the type and the content of the ions are equivalent to the physiologic tear, and the invention can be used in the treatment of dry eye syndrome and the adjuvant treatment of diseases which are caused by other dry eye syndromes. At the same time, the invention can be used as the drug delivery system of various ophthalmic drugs.
Owner:黑龙江海昌生物技术有限公司 +1
Artificial tears including carnosine and preparation method thereof
InactiveCN102100693AComfortable to useNon-irritatingOrganic active ingredientsSenses disorderMedicineConcentration ratio
The invention relates to artificial tears, in particular to artificial tears including carnosine, concretely, the artificial tears including carnosine comprise the following components and contents in percentage by weight: 0.2-5.0 percent of carnosine, an osmotic pressure regulator, a pH regulator and the balance of water, wherein the osmotic pressure regulator makes the mill-osmotic pressure mol concentration ratio of the artificial tears be between 0.9 and 1.1, and the pH regulator makes the pH value of the artificial tears be between 5.0 and 9.0. The artificial tears including carnosine can be used for treating dry eye syndrome, and also has a function of preventing cataract. The invention also relates to a preparation method of the artificial tears.
Owner:SHENYANG XINGQI PHARM CO LTD
Eye formulation administering system containing lecithin and sodium hyaluronic acid and its preparing method
The invention relates to an eye formulation administering system containing lecithin and sodium hyaluronic acid and its preparing method, wherein the system can directly serve as artificial tears to be applied to the treatment of xerophthalmia and other ophthalmology diseases. The system may also include other active constituents.
Owner:凌沛学
Ophthalmologic external preparation, as well as preparation method and application thereof
InactiveCN103040888APlay a lubricating roleInhibit apoptosisSenses disorderHydroxy compound active ingredientsExperimental researchGoblet cell
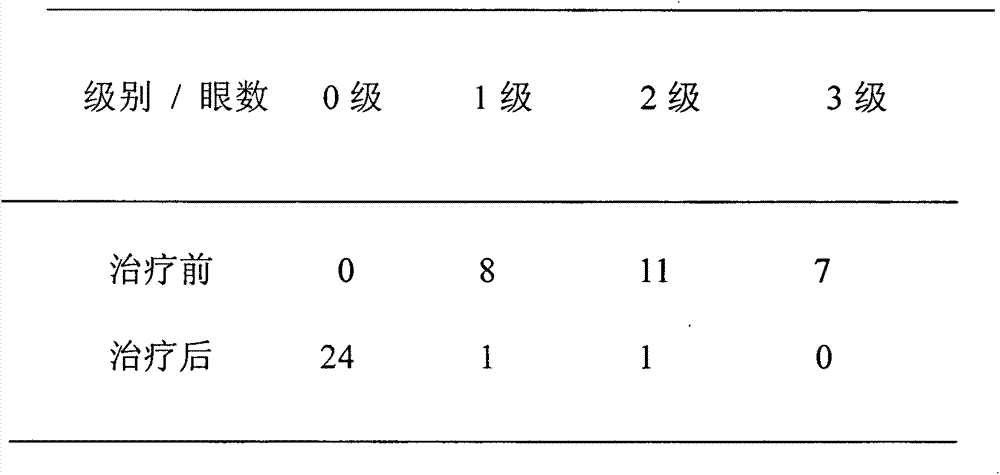
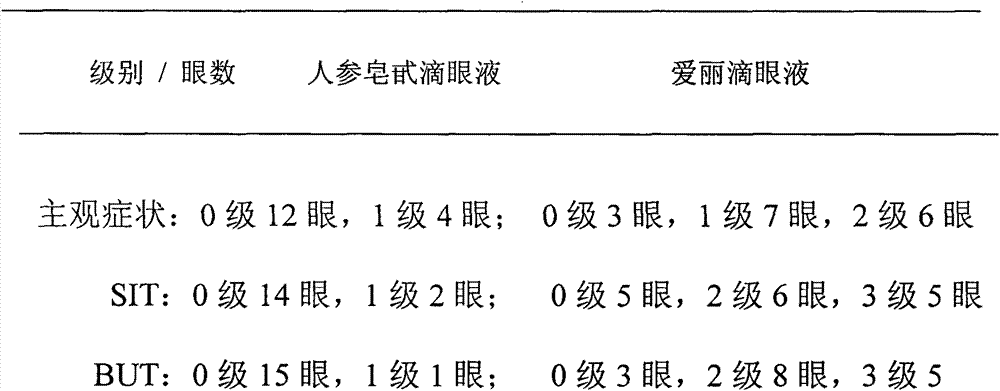
The invention discloses an ophthalmologic external preparation, as well as a preparation method and an application thereof, belongs to the technical field of pharmaceuticals, and relates to a pharmaceutical active substance-ginsenoside which is prepared to form eye drops, oculentum, ophthalmologic gel, ophthalmologic external hydropathic compressing tissues and ophthalmologic external atomized liquid for treating dry eyes. The addition of the ginsenoside is 0.1-5.0 wt%. The ophthalmologic external preparation also is added with one or a plurality of pharmaceutical active substances which are selected from flos lonicerae, radix arnebiae, herba taraxaci, chrysanthemum and borneol. The pharmacodynamical and experimental research of the preparation shows that the ginsenoside has a function of increasing conjunctival goblet cells and effectively treating the dry eyes; and the preparation is safe and effective, is better than conventional single-thickening agent artificial tears and has significant popularization and application values.
Owner:段亚东
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com