Artificial tears including carnosine and preparation method thereof
A technology of artificial tears and carnosine, which can be applied to medical preparations containing active ingredients, pharmaceutical formulations, organic active ingredients, etc., can solve problems such as cataract product listing, achieve the stability of artificial tears, simplify pH adjustment and osmotic pressure adjustment, The effect of the preparation method is simple and convenient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
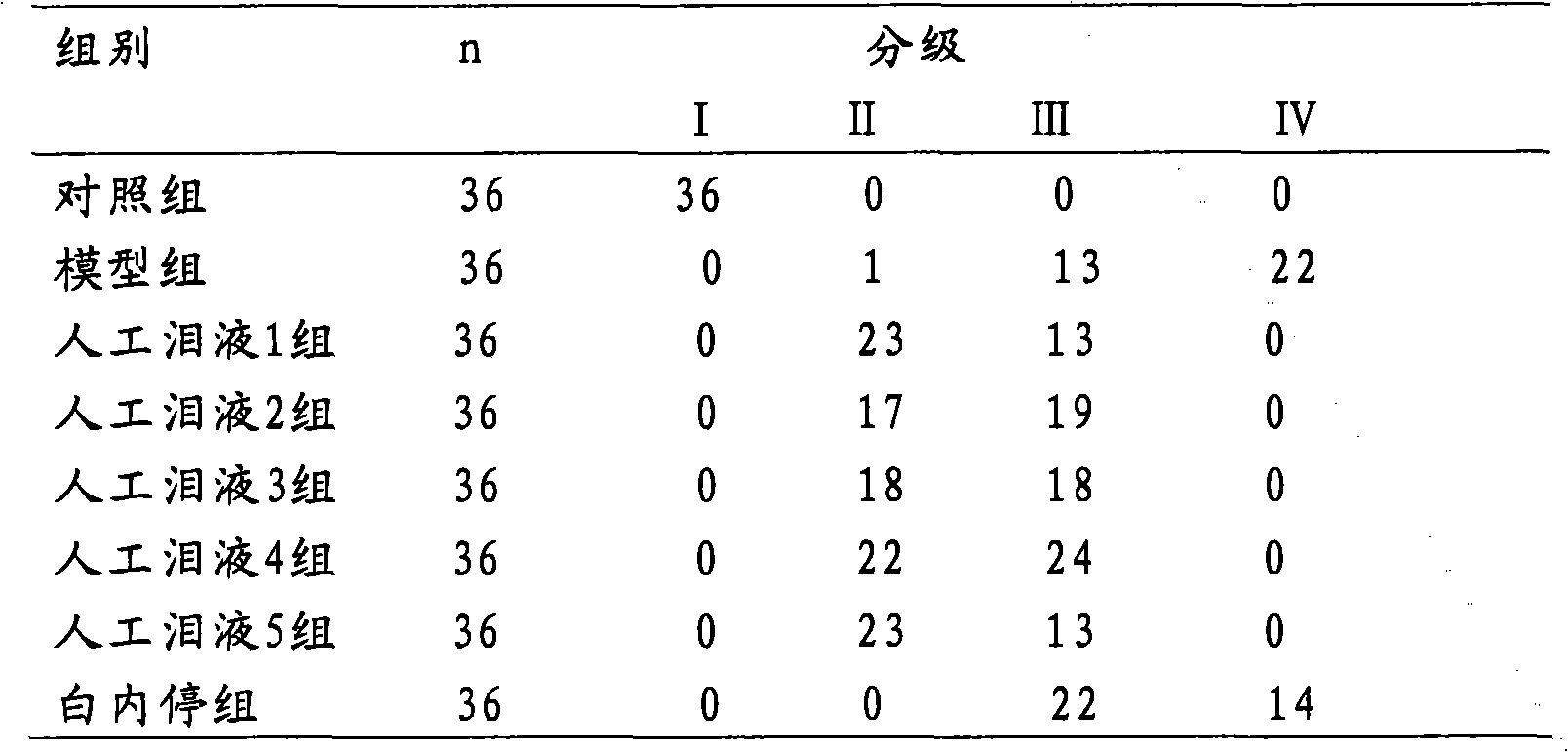
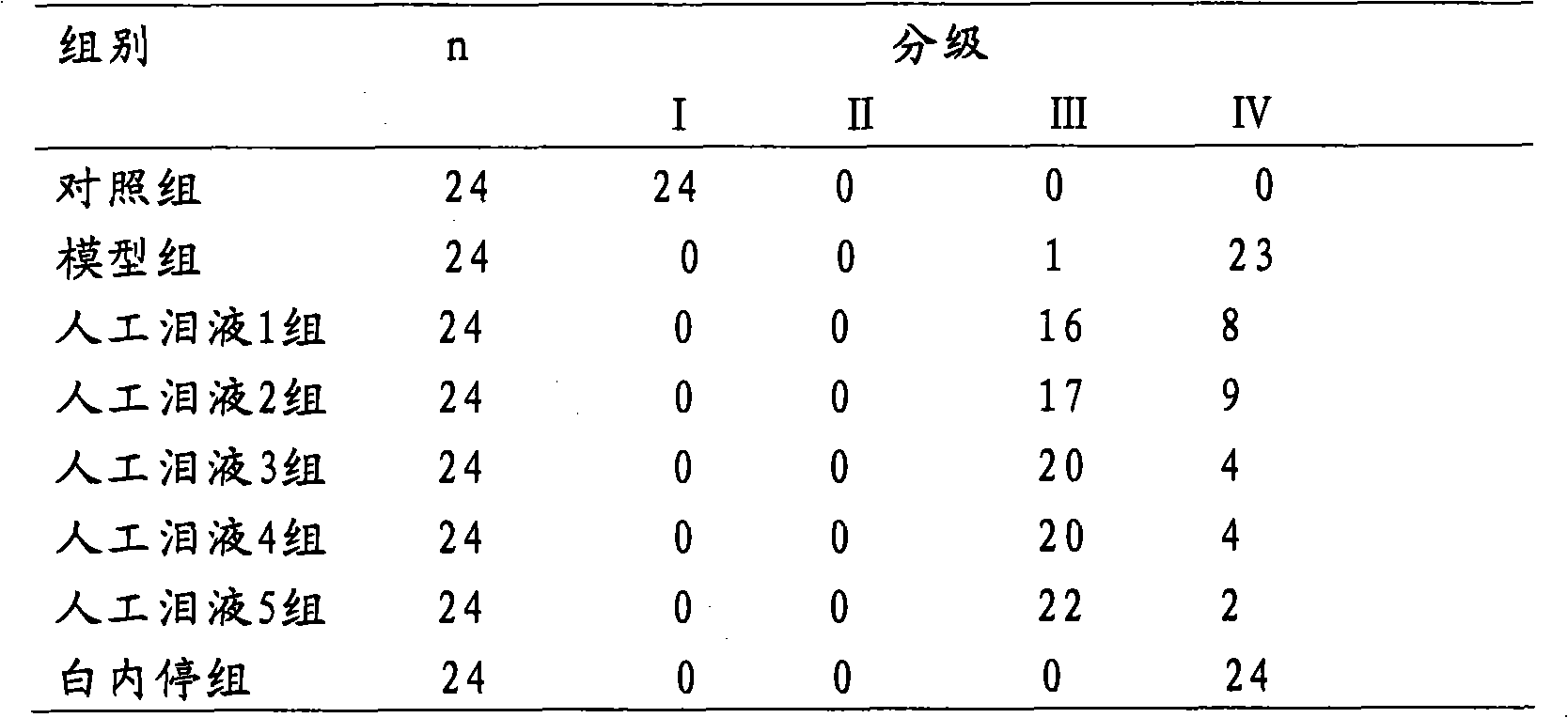
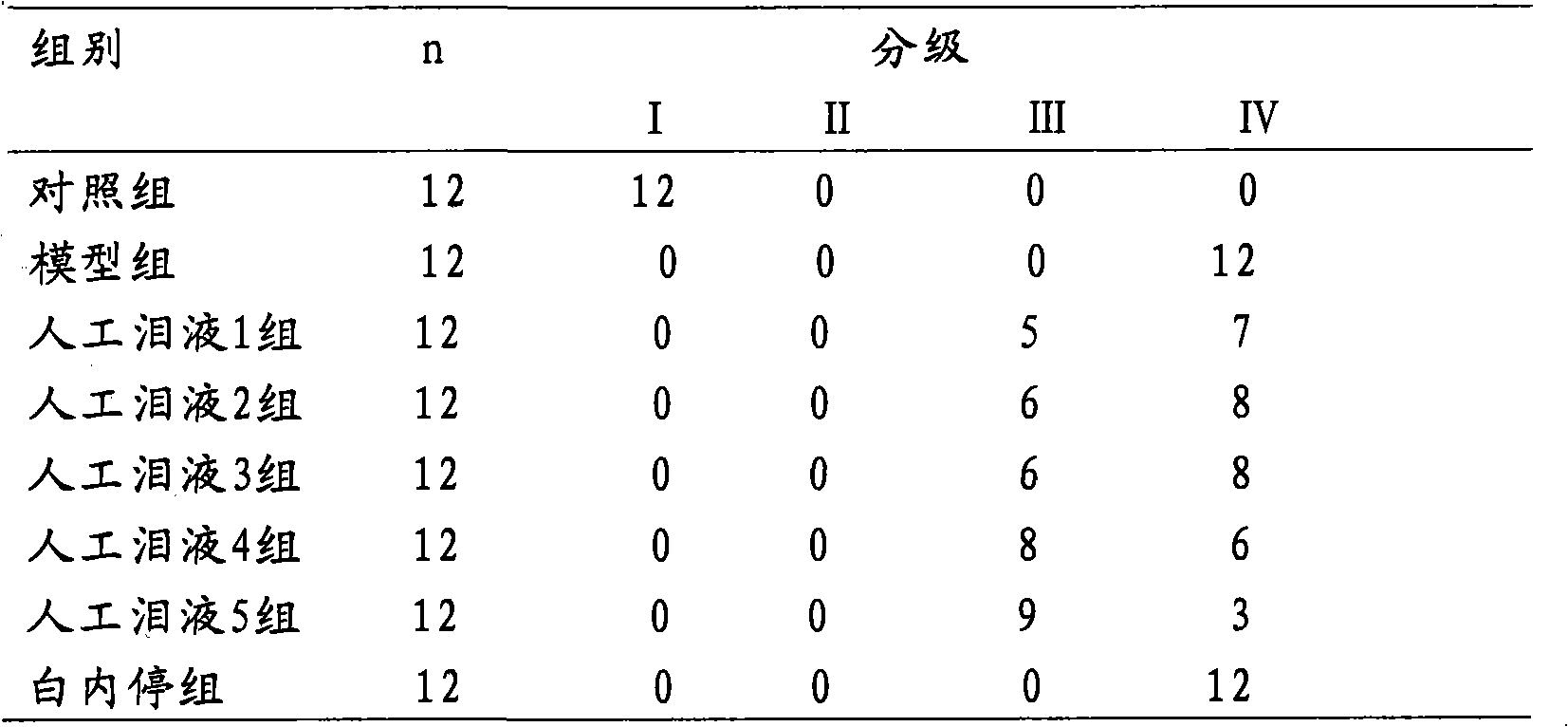
Image
Examples
Embodiment 1
[0064] Example 1 Artificial tears 1 with a carnosine concentration of 0.2% by weight and its preparation method
[0065] Table 1 Prescription of artificial tears 1 with a carnosine concentration of 0.2% (weight)
[0066] Components (per 1000g)
Content
2.0g
0.2g
8.5g
0.1g
0.15g
Glucose
1.0g
0.5g
Replenish water for injection to
1000g
[0067] Take the prescribed amount of carnosine, sodium bicarbonate, sodium chloride, benzalkonium chloride, potassium chloride, glucose, calcium chloride and dissolve it with appropriate amount of water for injection, add water for injection to the full amount, stir well and pass through 0.45μm, 0.22μm Microporous membrane filtration and filling.
[0068] Thus, artificial tears 1 with a carnosine concentration of 0.2% by weight was prepared.
Embodiment 2
[0069] Example 2 Artificial tears 2 with carnosine concentration of 0.5% by weight and preparation method thereof
[0070] Table 2 The prescription of artificial tears 2 with a carnosine concentration of 0.5% (weight)
[0071] Components (per 1000g)
Content
5.0g
Carbomer
3.0g
[0072] Sodium dihydrogen phosphate
5.0g
0.3g
5.5g
1.0g
Replenish water for injection to
1000g
[0073] Take the prescribed amount of carbomer, add appropriate amount of water for injection to swell, and then sterilize for use; take appropriate amount of water for injection, add the prescribed amount of carnosine, sodium dihydrogen phosphate, ethyl paraben, propylene glycol, sodium sulfite to dissolve, stir well and pass 0.45μm, Filter with 0.22μm microporous filter membrane, add it to carbomer, add water for injection to full volume, stir evenly, and fill to obtain gelatinous artificial tears containing carnosine.
[0074] ...
Embodiment 3
[0075] Example 3 Artificial tears 3 with a carnosine concentration of 1% by weight and its preparation method
[0076] Table 3 The prescription of artificial tears 3 with a carnosine concentration of 1% (weight)
[0077] Components (per 1000g)
Content
Carnosine
10.0g
Hydrochloric acid (1M)
8.0g
Sodium chloride
6.5g
Replenish water for injection to
1000g
[0078] Take the prescription amount of carnosine and sodium chloride to dissolve it with water for injection, add hydrochloric acid to adjust the PH value between 7.0 and 7.5, replenish the water for injection to the full amount, use 0.45μm, 0.22μm microporous membrane for the drug solution in a hundred-level environment Filter, use automatic filling equipment to fill the medicine into a single-dose packaging container to prepare a single-dose artificial tear.
[0079] Thus, artificial tears (daily dose product without preservatives) with a carnosine concentration of 1% by weight were prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com