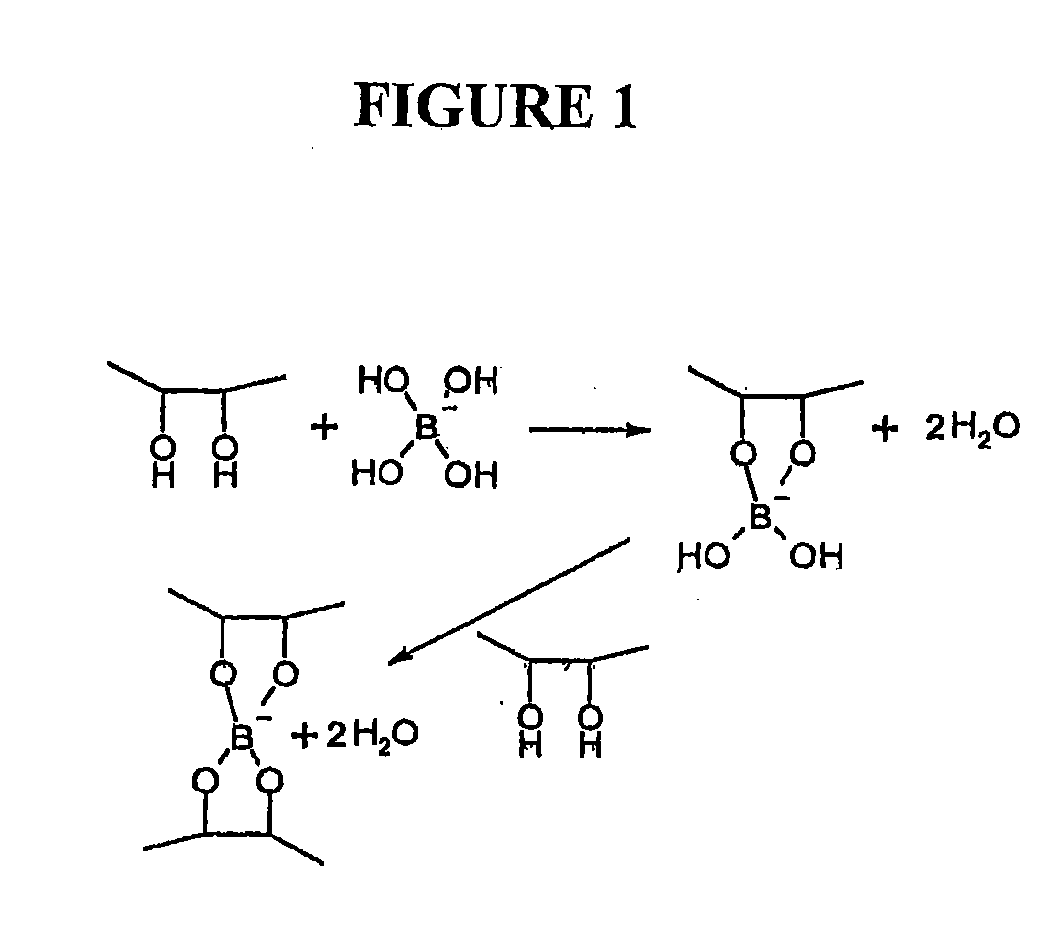
Polymeric artificial tear system
a technology of artificial tear and polymer, which is applied in the field of artificial tear formulations and formulations, can solve problems such as interfering with cross-linking, and achieve the effects of strengthening the cross-linking of galactomannan and borate, enhancing the formation of a structured galactomannan-borate polymer network, and enhancing the formation of a structured network of galactomannan-bora
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0042]
Ingredient% w / vHydroxypropyl Guar0.16 to 0.19Boric Acid0.7Sorbitol1.4Polyethylene Glycol0.4Propylene Glycol0.3Potassium Chloride0.12Sodium Chloride0.1Polyquaternium-10.001 + 10% excess2-Amino-2-methylpropanol0.57Sodium Hydroxide / Hydrochloric Acidq.s. pH 7.9Purified Waterq.s. 100%
example 2
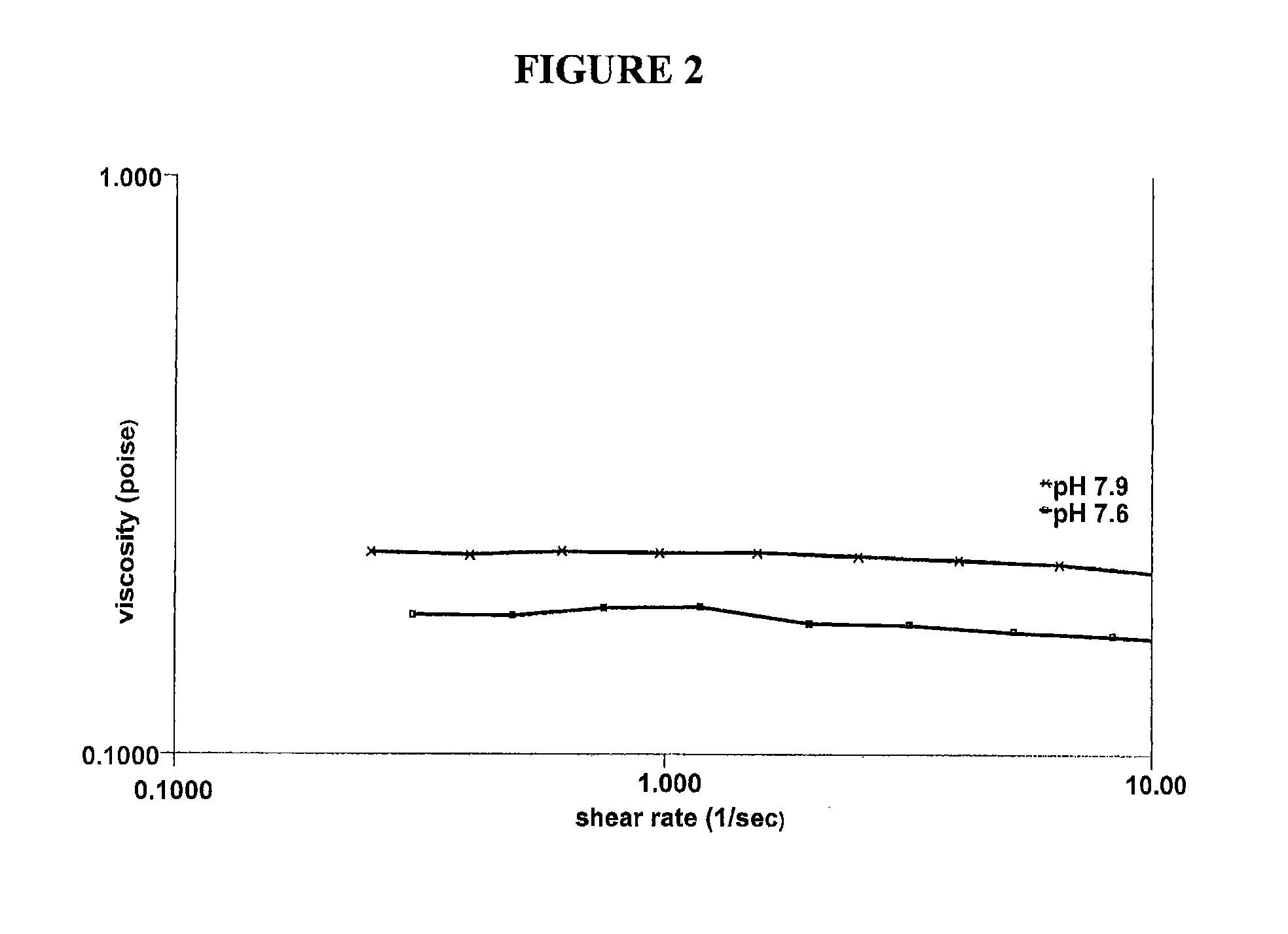
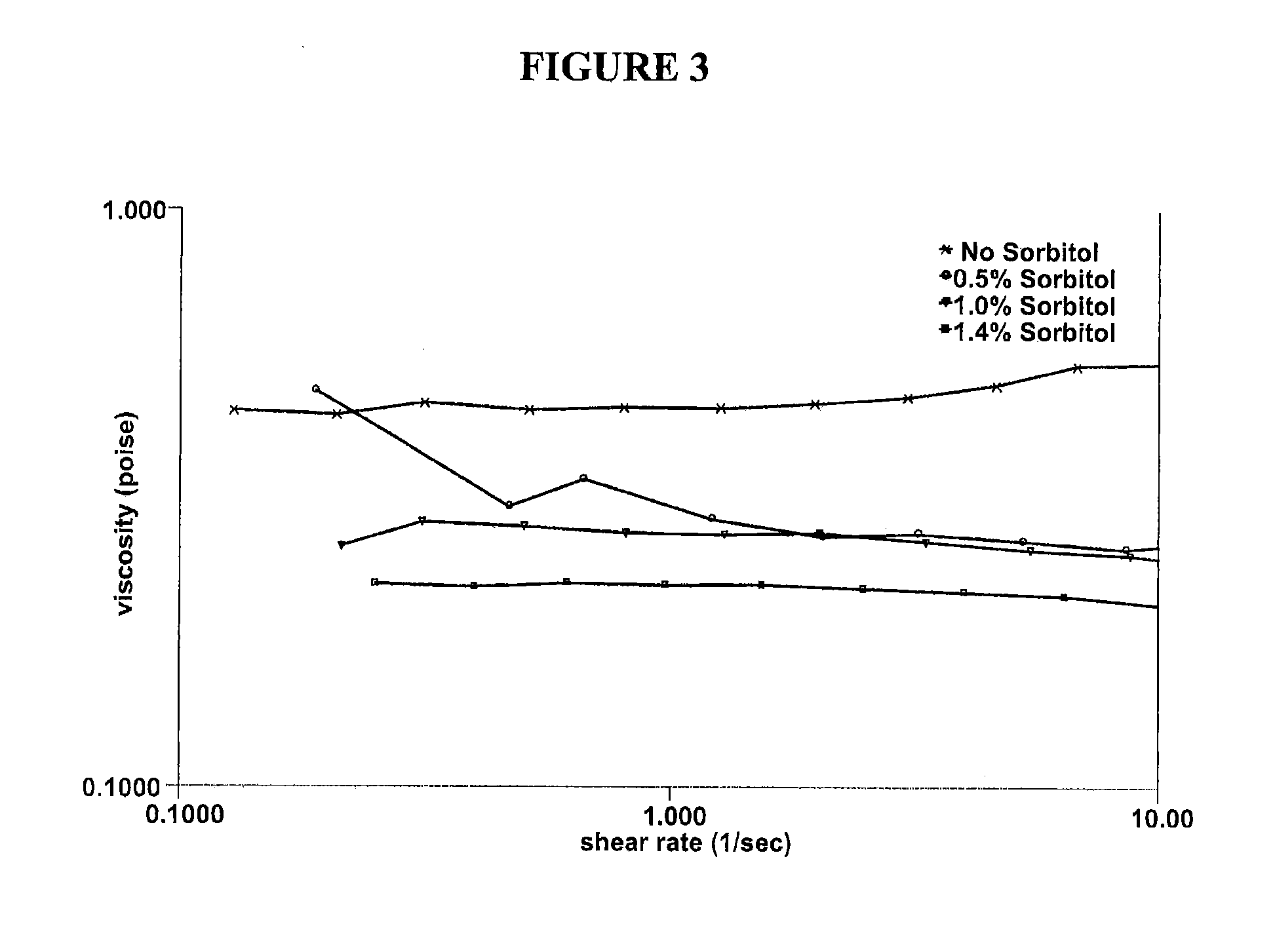
[0043]Various solutions containing hydroxypropyl guar, borate and sorbitol were characterized in vitro to explore how the use of a cis-diol such as sorbitol can modify the bulk rheology, interfacial rheology and lubrication properties of hydroxypropyl guar-borate formulations. The experiments conducted show the effect of sorbitol in pH 7.9 formulations comprising a galactomannan-borate cross-linking system, and simulate the introduction of such formulations into the eye at a lower pH (7.6). Hydroxypropyl guar (Mn=3×106 g / mol, polydispersity ratio (PD)=2-3) was used in the preparation of the artificial tear solution (ATDS) used in these experiments. The ATDS used for these experiments is the formulation of Example 1 above, pH adjusted to 7.9 or 7.6, and with varying concentrations of sorbitol.
[0044]Bulk rheology experiments were conducted using a controlled stress rheometer (AR 2000ex, TA Instruments, Inc.). The measurement system was a 40 mm acrylic 2° cone and plate with a sample v...
example 3
[0057]The ATDS formulation of Example 1 was compared in two in vivo studies against saline control solution (Unisol®) and the carboxymethylcellulose / glycerin formulation Optive®.
Retention Time Studies
[0058]An important measure of a lubricant eye drop is the assessment of ocular surface retention or swell time. The mean retention time of a galactomannan-borate solution of the present invention (the ATDS formulation of Example 1) was compared to a saline control solution (Unisol®) using a fluorophotometric technique. Briefly, a fluorescein labeled dextran tracer of approximately 70 kD (Molecular Probes, Eugene, Oregon) was added to each test formulation at a concentration of 0.1 w / v %. A scanning fluorophotometer (Ocumetrics, Mountain View, Calif.) was used to monitor signal decay corresponding to elimination of the formulations. 25 dry eye patients were studied, and measurements were taken roughly every two minutes after application of the test formulation.
[0059]The results of the st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| osmolality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com