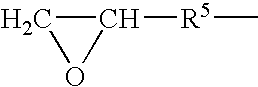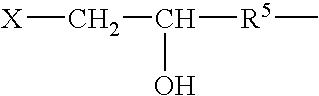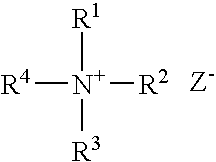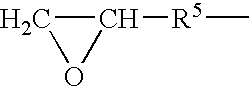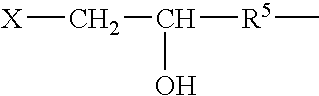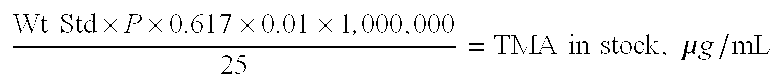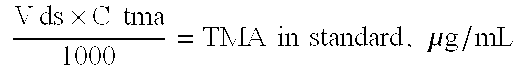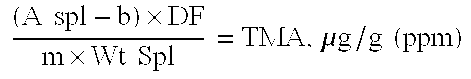Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
407 results about "Galactomannan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
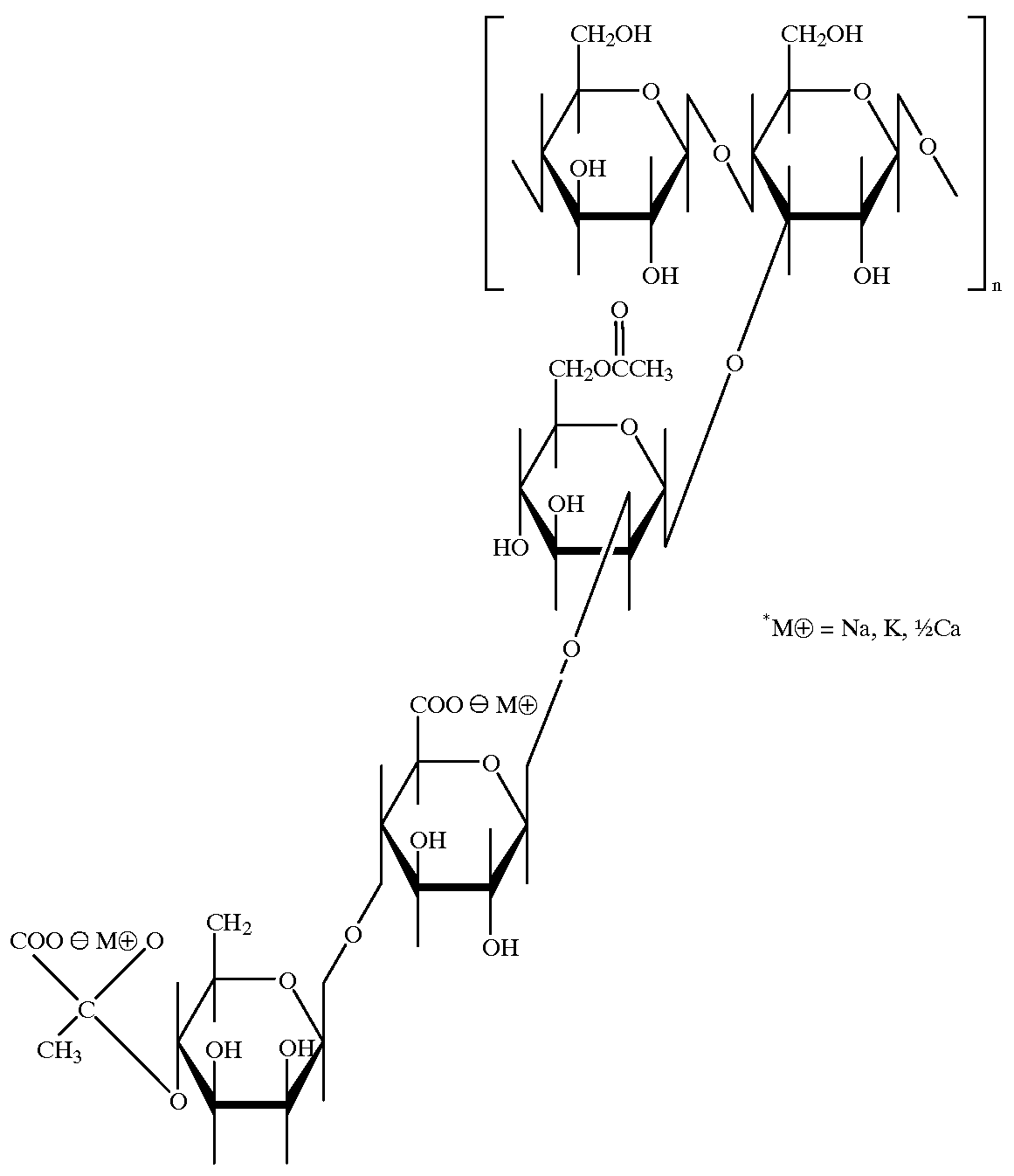
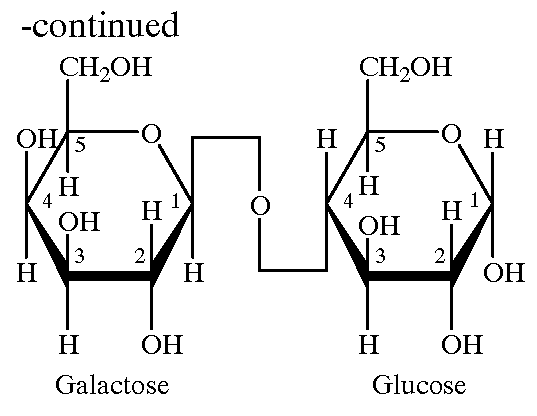
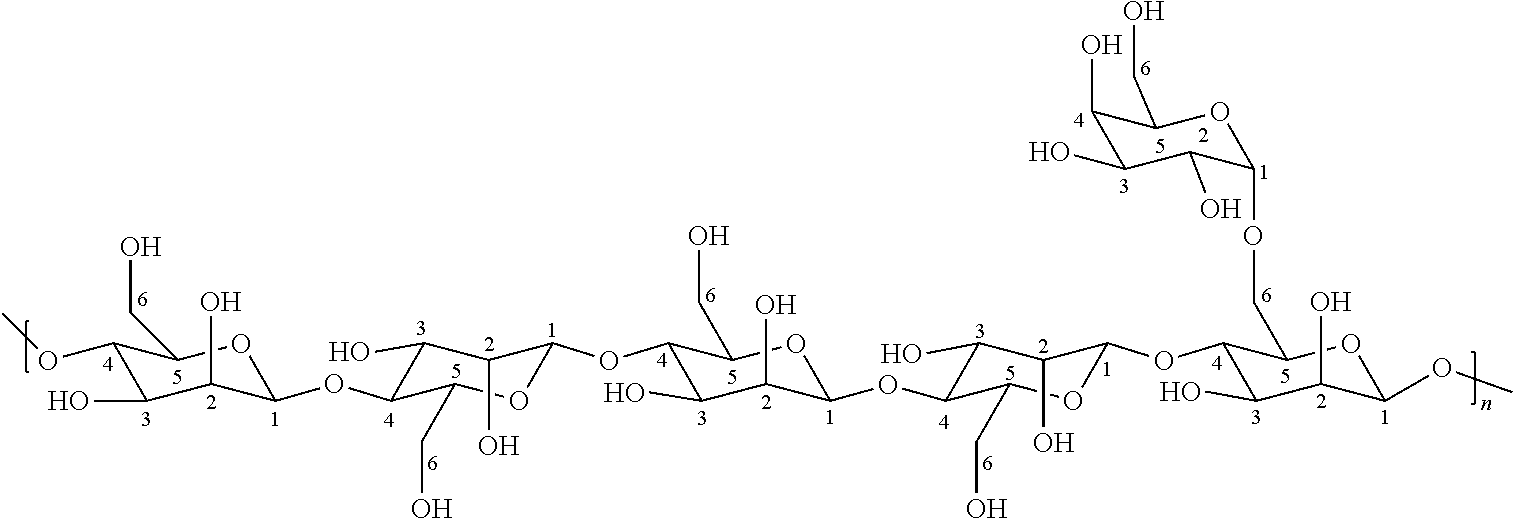
Galactomannans are polysaccharides consisting of a mannose backbone with galactose side groups (more specifically, a (1-4)-linked beta-D-mannopyranose backbone with branchpoints from their 6-positions linked to alpha-D-galactose, (i.e. 1-6-linked alpha-D-galactopyranose).
Cleanup additive
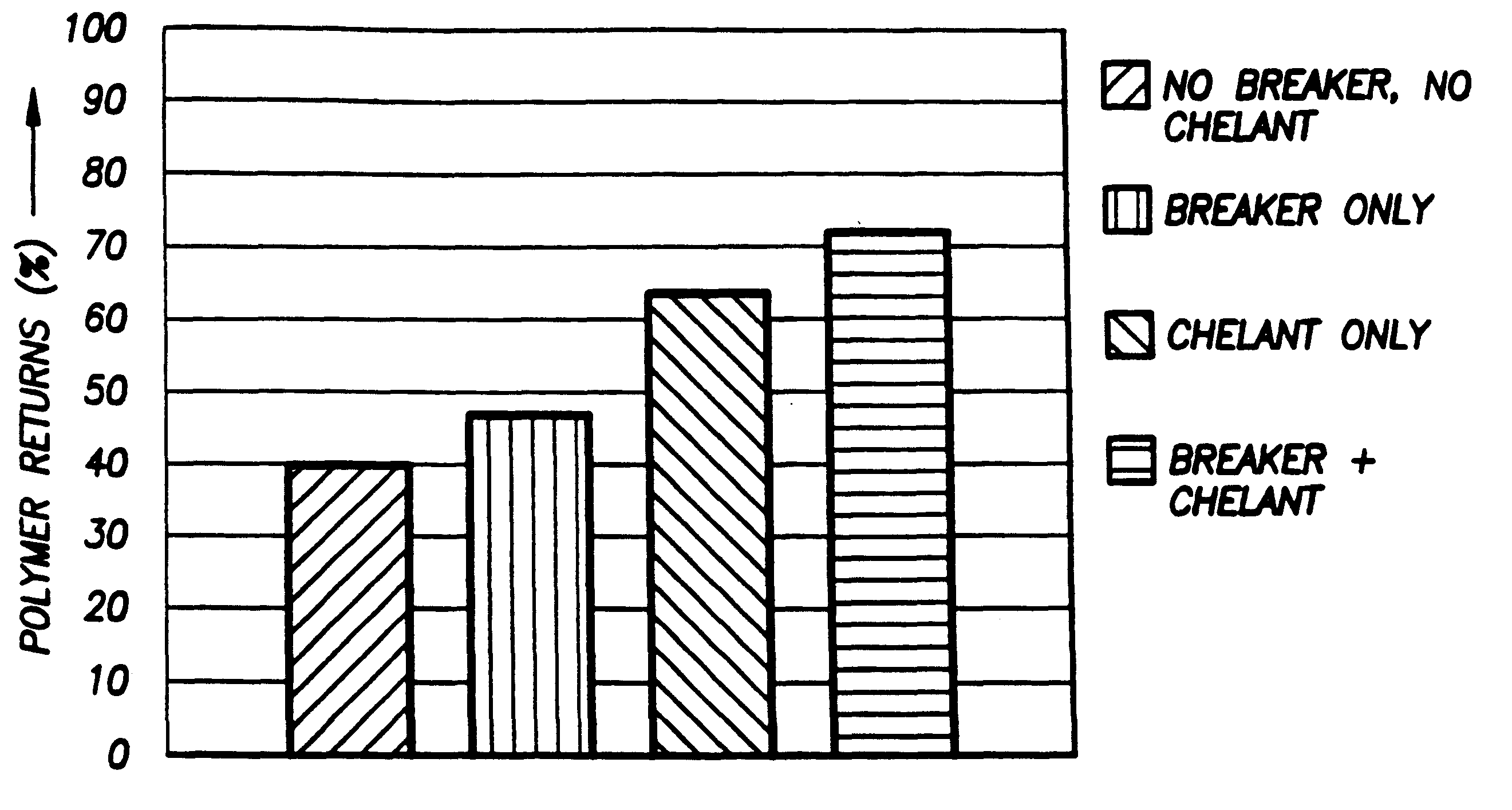
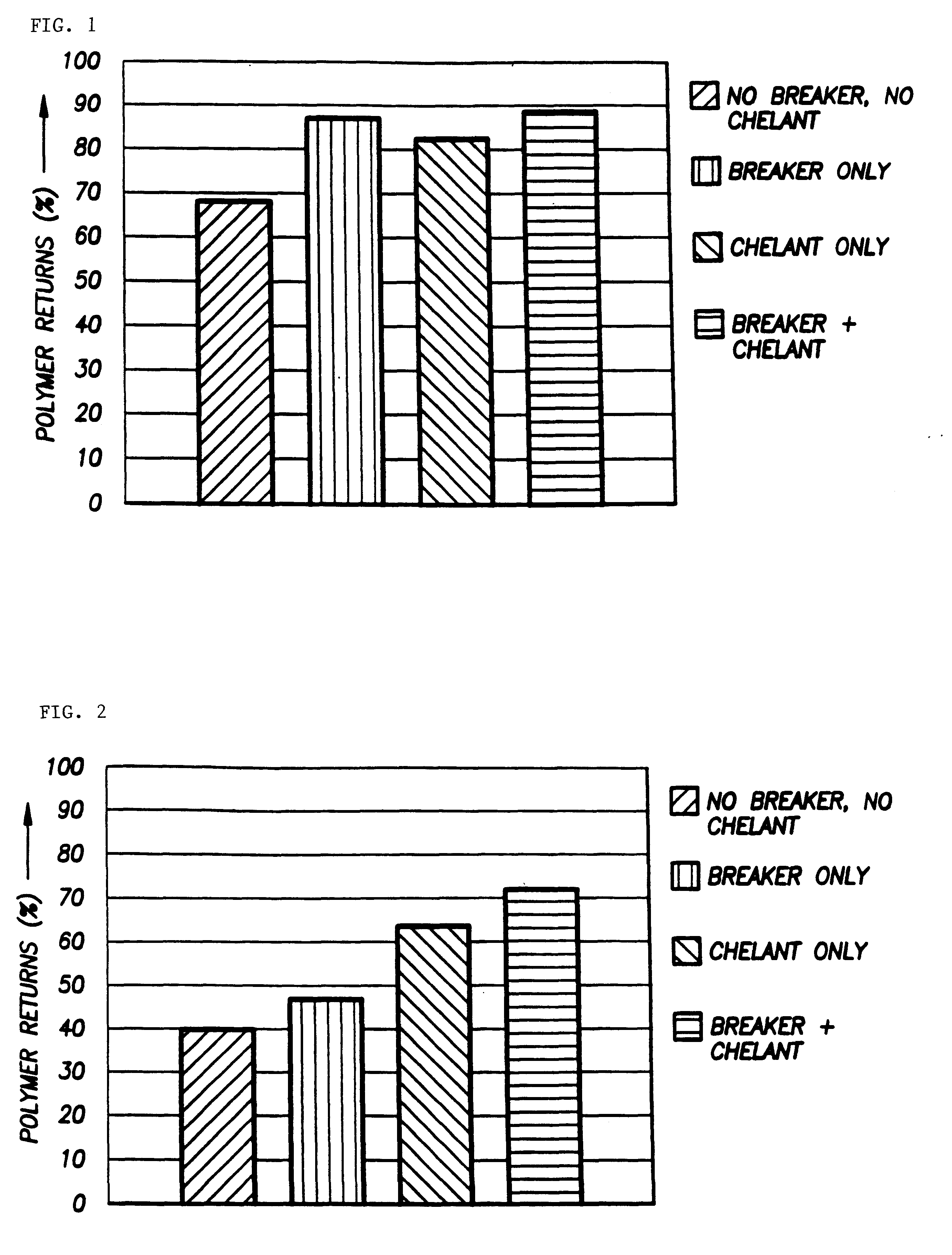
InactiveUS6242390B1Low viscosityEasy to disassembleOther chemical processesFluid removalSolubilityHydraulic fracturing
According to the present invention, a composition and method for hydraulically fracturing a subterranean formation is provided. The composition comprises an aqueous mixture of a hydrated polysaccharide, preferably a galactomannan gum, the hydrated polysaccharide having a plurality of bonding sites; a crosslinking agent for crosslinking the hydrated polysaccharide at the bonding sites at the conditions of the subterranean formation with a polyvalent metal ion to form a polyvalent metal crosslink, thereby increasing the viscosity of the hydrated polysaccharide; and a controlled solubility compound for releasing a chelating agent for controllably breaking the polyvalent metal crosslink and bonding with the polyvalent metal ion released by breaking the crosslink, thereby decreasing the viscosity of the hydrated polysaccharide. The method comprises the steps of injecting the above-described composition into the subterranean formation at fracturing pressures; allowing the controlled solubility compound to begin breaking the polyvalent metal crosslink, thereby reducing the viscosity of the hydrated polysaccharide and yielding a lower viscosity fluid; and removing the lower viscosity fluid from the subterranean formation.
Owner:SCHLUMBERGER TECH CORP
Well treatment fluid compositions and methods for their use
InactiveUS6983801B2High viscosityIncrease volumeFluid removalFlushingAlkaline earth metalCarboxylic acid
Owner:COMBINED SYST +1
Ultrasound coupling medium for use in medical diagnostics
InactiveUS20070087060A1Avoid pollutionSuitable for usePowder deliveryEchographic/ultrasound-imaging preparationsPolyvinyl alcoholPreservative
A composition of an ultrasound coupling medium is provided. The composition comprises at least 90% water, at least one preservative, and at least one base substance, wherein the composition is extensible into a film with a thickness of up to 1 / 10 mm, wherein the composition can withstand a pressure of up to 30 kp without tearing, wherein the composition can adapt exactly to skin surface without causing any significant air pockets, and wherein the composition can be removed from skin with substantially no residue left behind. The at least one base substance may be a galactomannan, a polyvinyl alcohol (PVA), a complex formation of galactomannan and borate ions, or comninations thereof.
Owner:JOKER AG
Personal care composition containing a non-guar galactomannan polymer derivative
Personal care compositions comprise (a) from about from about 5 wt. % to about 35 wt. % of a detersive surfactant; (b) at least about 0.05 wt. % of a galactomannan polymer derivative having a mannose to galactose ratio of greater than 2:1 on a monomer to monomer basis, the galactomannan polymer derivative selected from a cationic galactomannan polymer derivative and an amphoteric galactomannan polymer derivative having a net positive charge, the galactomannan polymer derivative having a molecular weight from about 1,000 to about 10,000,000 and a cationic charge density from about 0.9 meq / g to about 7 meq / g; and (c) at least about 20 wt. % of an aqueous carrier. Methods of treating hair or skin comprise applying the personal care composition as described above to the hair or skin and rinsing the hair or skin.
Owner:THE PROCTER & GAMBLE COMPANY
Coffee plant with reduced alpha-D-galactosidase activity
InactiveUS7238858B2Lower Level RequirementsGalactose branching in the galacto-mannans is increasedOther foreign material introduction processesEnzymesPlant cellD-galactal
The present invention relates to the modification of galactomannans present in the green coffee bean by reducing the endogenous level of α-D-galactosidase activity. In particular, the present invention pertains to a plant cell with reduced α-D-galactosidase activity and to a plant harboring such a plant cell.
Owner:NESTEC SA
Shampoo containing a gel network and a non-guar galactomannan polymer derivative
Shampoo compositions comprise (a) from about 5% to about 50% of one or more detersive surfactants; (b) a dispersed gel network phase comprising: (i) at least about 0.05% of one or more fatty amphiphiles; (ii) at least about 0.01% of one or more secondary surfactants; and (iii) water; (c) at least about 0.05% of a galactomannan polymer derivative with a net positive charge and having a mannose to galactose ratio of greater than 2:1 on a monomer to monomer basis, wherein the galactomannan polymer derivative has: (i) a molecular weight from about 1,000 to about 10,000,000; and (ii) a cationic charge density from about 0.7 meq / g to about 7 meq / g; and (d) at least about 20% of an aqueous carrier; all by weight of the shampoo composition.
Owner:THE PROCTER & GAMBLE COMPANY
Shampoo containing a gel network and a non-guar galactomannan polymer derivative
ActiveUS20060269502A1Cosmetic preparationsCationic surface-active compoundsAmphiphileSURFACTANT BLEND
Shampoo compositions comprise (a) from about 5% to about 50% of one or more detersive surfactants; (b) a dispersed gel network phase comprising: (i) at least about 0.05% of one or more fatty amphiphiles; (ii) at least about 0.01% of one or more secondary surfactants; and (iii) water; (c) at least about 0.05% of a galactomannan polymer derivative with a net positive charge and having a mannose to galactose ratio of greater than 2 : 1 on a monomer to monomer basis, wherein the galactomannan polymer derivative has: (i) a molecular weight from about 1,000 to about 10,000,000; and (ii) a cationic charge density from about 0.7 meq / g to about 7 meq / g; and (d) at least about 20% of an aqueous carrier; all by weight of the shampoo composition.
Owner:THE PROCTER & GAMBLE COMPANY
Hair conditioning composition containing a non-guar galactomannan polymer derivative
A hair conditioning composition comprising:a) from about 0.01 wt. % to about 10 wt. % of a non-guar galactomannan polymer derivative having a mannose to galactose ratio of greater than 2:1 on a monomer to monomer basis, said non-guar galactomannan polymer derivative selected from the group consisting of a cationic non-guar galactomannan polymer derivative and an amphoteric non-guar galactomannan polymer derivative having a net positive charge;i. wherein said non-guar galactomannan polymer derivative has a molecular weight from about 1,000 to about 10,000,000; andii. wherein said non-guar galactomannan polymer derivative has a cationic charge density from about 0.7 meq / g to about 7 meq / g;b) a conditioning agent selected from the group consisting of cationic surfactants, cationic polymers, nonvolatile silicones, nonvolatile hydrocarbons, saturated C14 to C22 straight chain fatty alcohols, nonvolatile hydrocarbon esters, and mixtures thereof; andc) wherein said hair conditioning composition is substantially free of an anionic surfactant.
Owner:THE PROCTER & GAMBLE COMPANY
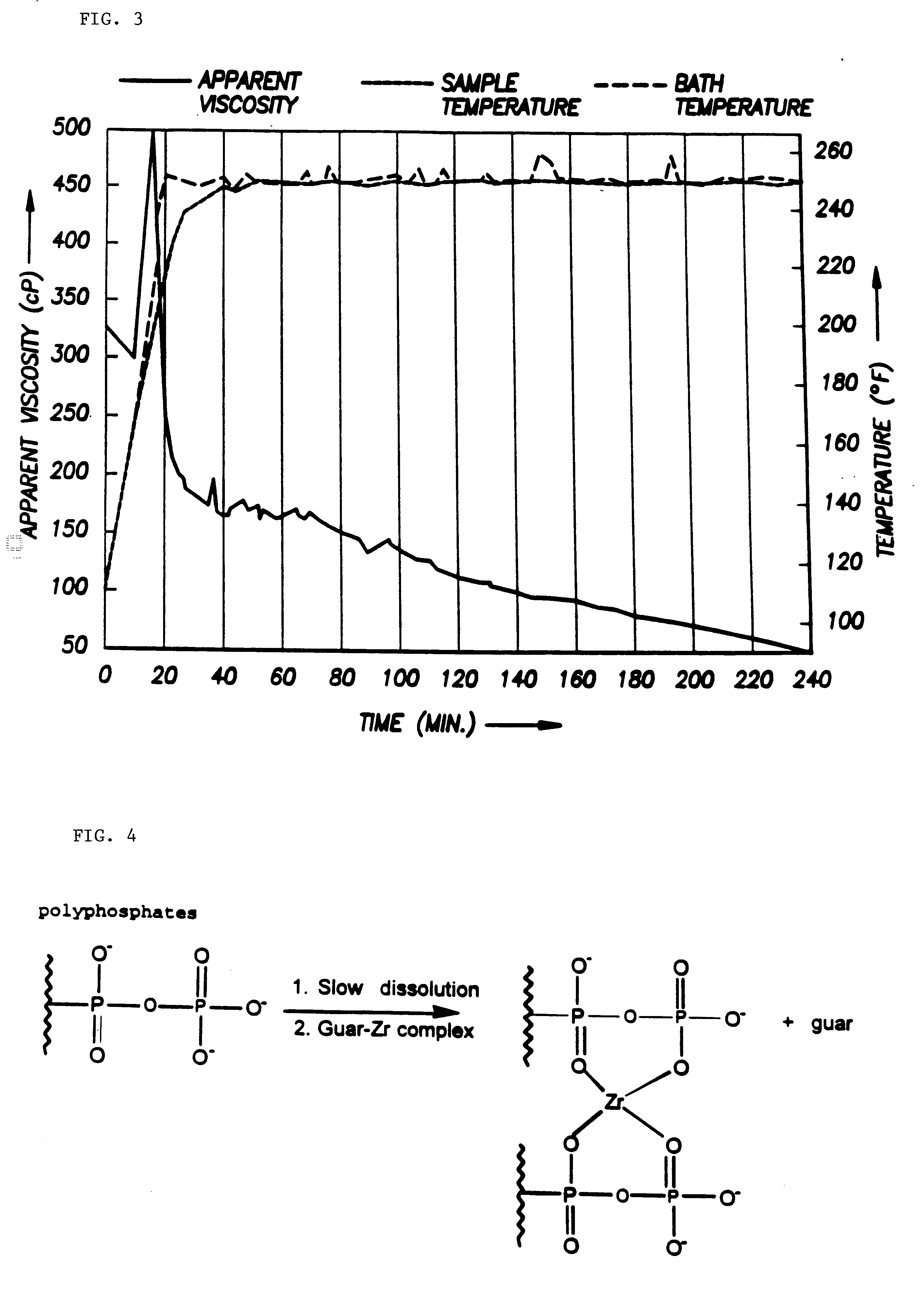
Galactomannan based well treating fluids
ActiveUS20050272612A1Low polymer contentHigh molecular weightFluid removalFlushingPolymer scienceFracturing fluid
A well fracturing fluid is shown which includes an aqueous base fluid, a hydratable polymer, such as a guar gum, and a suitable crosslinking agent for crosslinking the hydratable polymer to form a polymer gel. The hydratable polymer has a higher molecular weight which is achieved by improvements in the processing of the guar split. The higher molecular weight polymer provides improved performance in well fracturing operations.
Owner:BAKER HUGHES INC
Polymeric artificial tear system
InactiveUS20090270345A1Facilitate cross-linkingIncreased formationBiocideSenses disorderDiolBoric acid
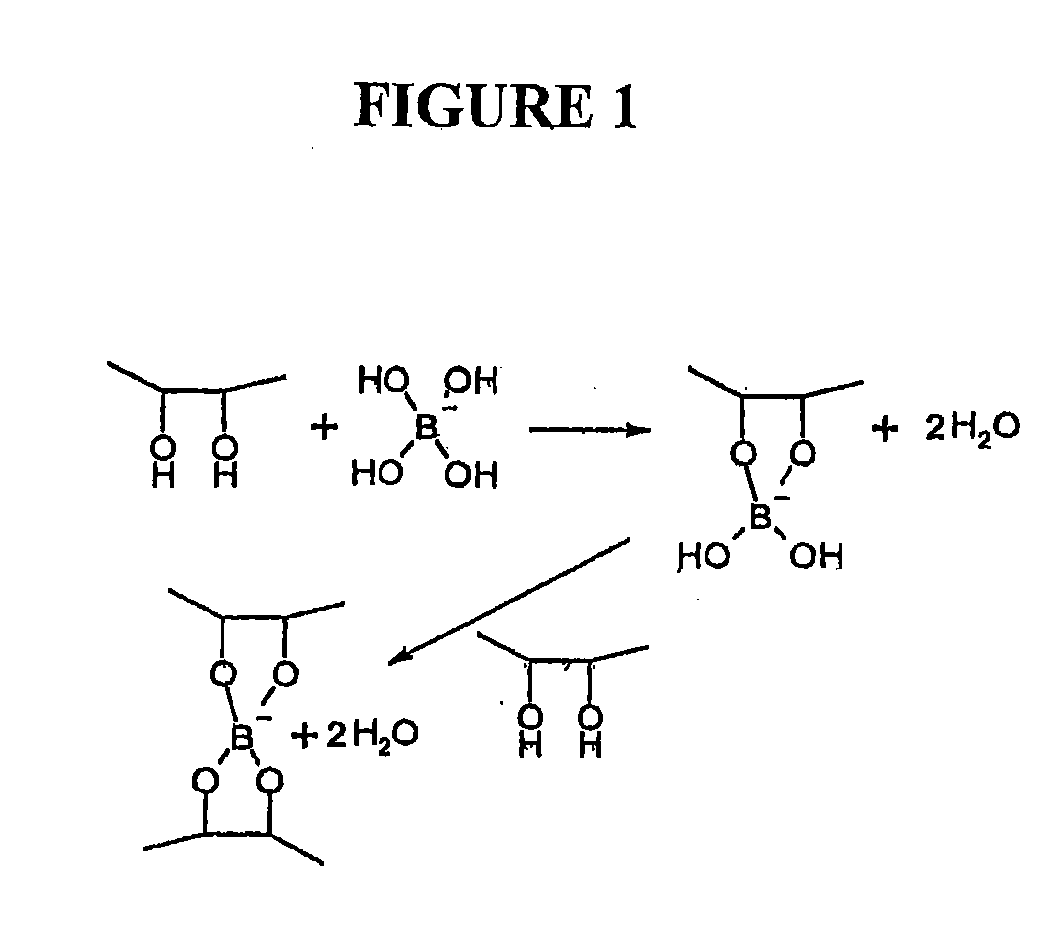
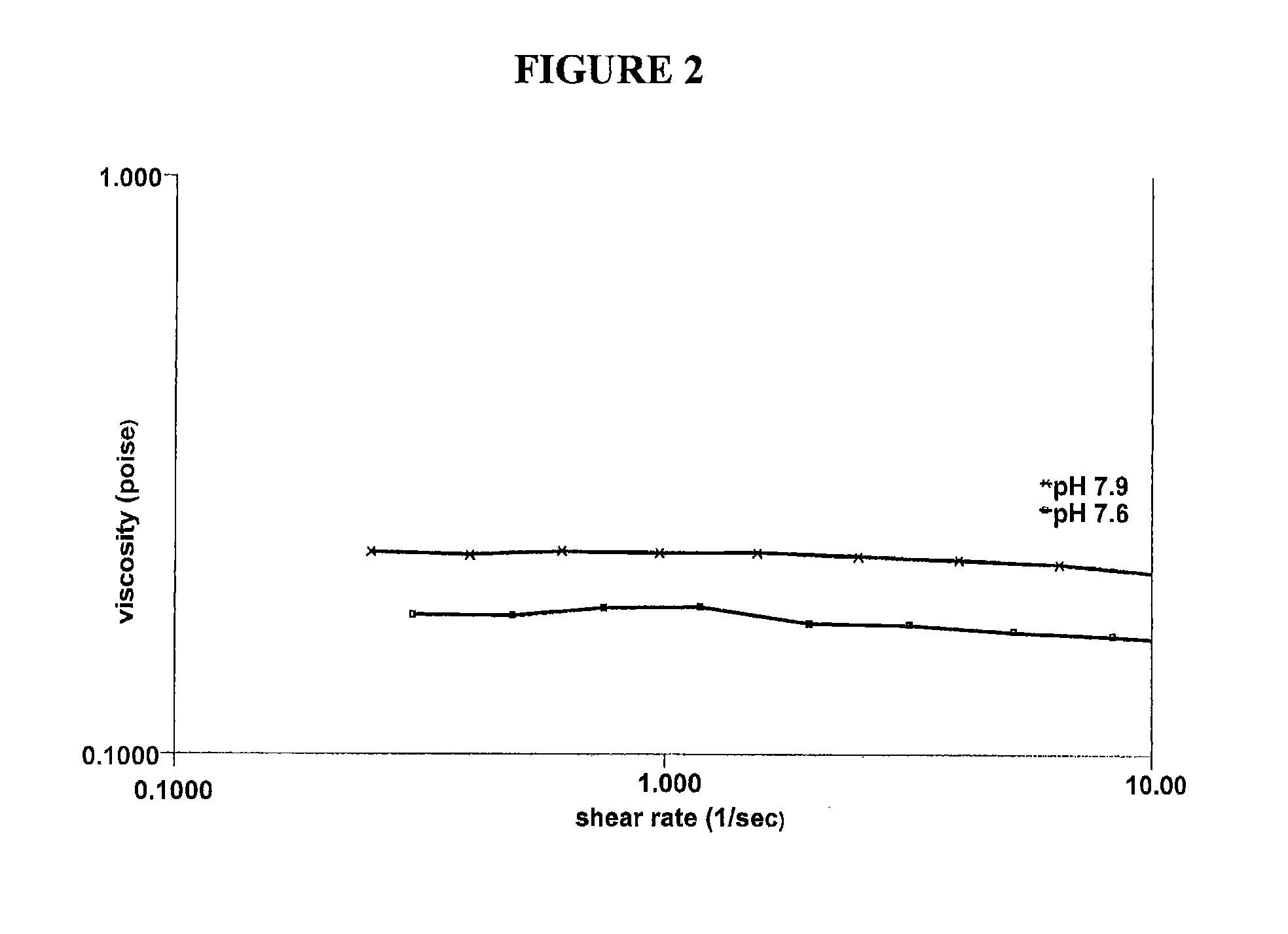
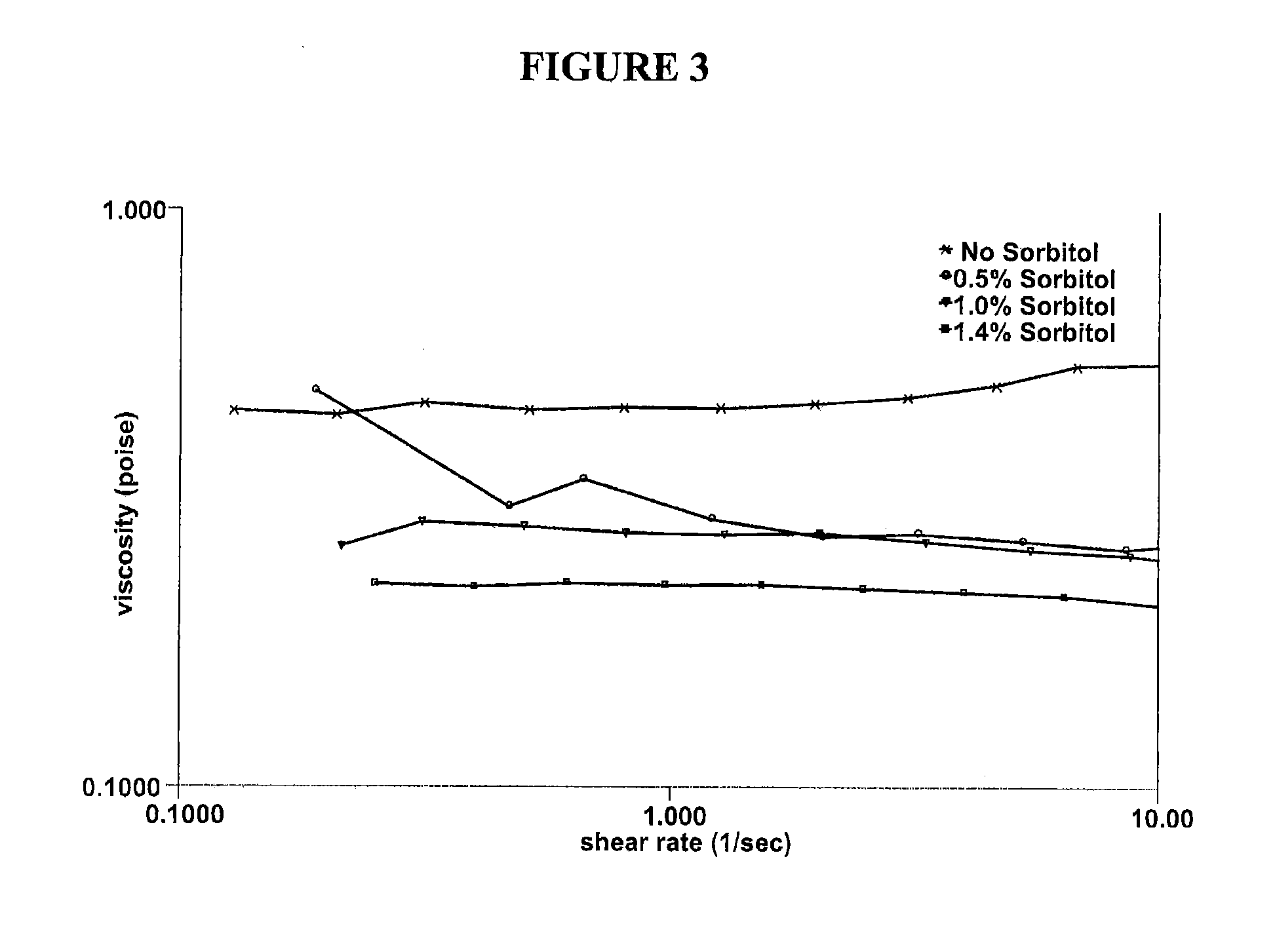
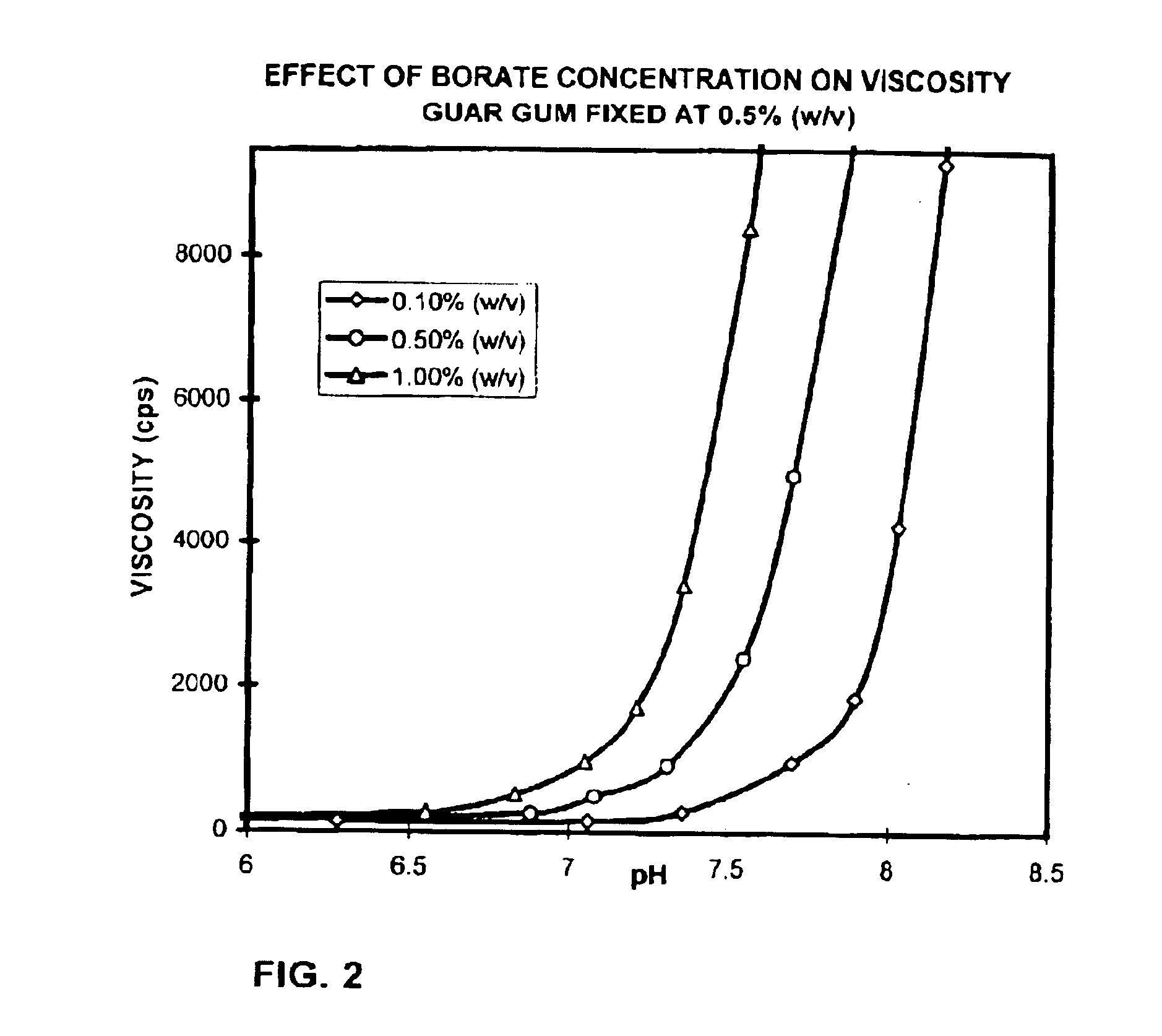
The present invention relates to artificial tear formulations and ophthalmic formulations suitable for drug delivery. The formulations comprise galactomannans such as guar or hydroxypropyl guar and a borate source such as boric acid. The formulations further comprise a cis-diol such as sorbitol that interferes with the cross-linking of galactomannan and borate. Optionally, the formulations are substantially free of divalent cations.
Owner:ALCON RES LTD
Reduced odor in low molecular weight cationic polygalactomannan
InactiveUS20060045861A1Reduce odorLower performance requirementsCosmetic preparationsHair cosmeticsPersonal careMicrobiology
A reduced odor composition is composed of at least one cationic polygalactomannan or a derivative of cationic polygalactomannan having a weight average molecular weight (Mw) having a lower limit of 5,000 and an upper limit of 200,000, a light transmittance in a 10% aqueous solution of greater than 80% at a light wavelength of 600 nm, a protein content of less than 1.0% by weight of polysaccharide, and a trimethylamine content of less than 25 ppm in a 10% aqueous solution of the polymer. This composition is prepared by treating the polymer with reagents that reduce the molecular weight of the polymer, removing the water-insoluble solid material, and removing odorous components, including trimethylamine (TMA) and other amines and low molecular weight components from the aqueous phase to produce a polymer that when used in a functional system such as household care, personal care or pet care products has reduced or no odor at acidic, neutral, or alkaline pH values.
Owner:HERCULES INC
Purified galactomannan as an improved pharmaceutical excipient
InactiveUS6063402AExcellent hardness propertiesOrganic active ingredientsBiocideProtein materialsImpurity
Disclosed is a substantially anhydrous, powdered, galactomannan composition consisting essentially of a galactomannan hydrocolloid exhibiting about 50% to about 90% by weight of anhydromannose residues and about 10% to about 50% by weight anhydrogalactose residues; less than about 1% by weight of protein material and less than about 3% of other nonaqueous impurities. This material is useful for preparing pharmaceutical compositions both in the substantially anhydrous form but preferably in an anhydrated form which includes about 5-15% by weight water. The pharmaceutical compositions comprise a therapeutically effective amount of a drug, the hydrated powered gallactomannan composition and optionally other pharmaceutically-acceptable excipients. When the hydrated powdered purified glactomannan of the invention is used to form a tablet, one sees improved hardness in the tablet formed. The pharmaceutical composition of the invention is particularly valuable for delivering a therapeutically effective drug to the colon without significant release of the drug in the upper GI tract after oral administration of the composition. Unique means to prepare the purified galactomannan in large quantities is provided.
Owner:AMARIN DEV
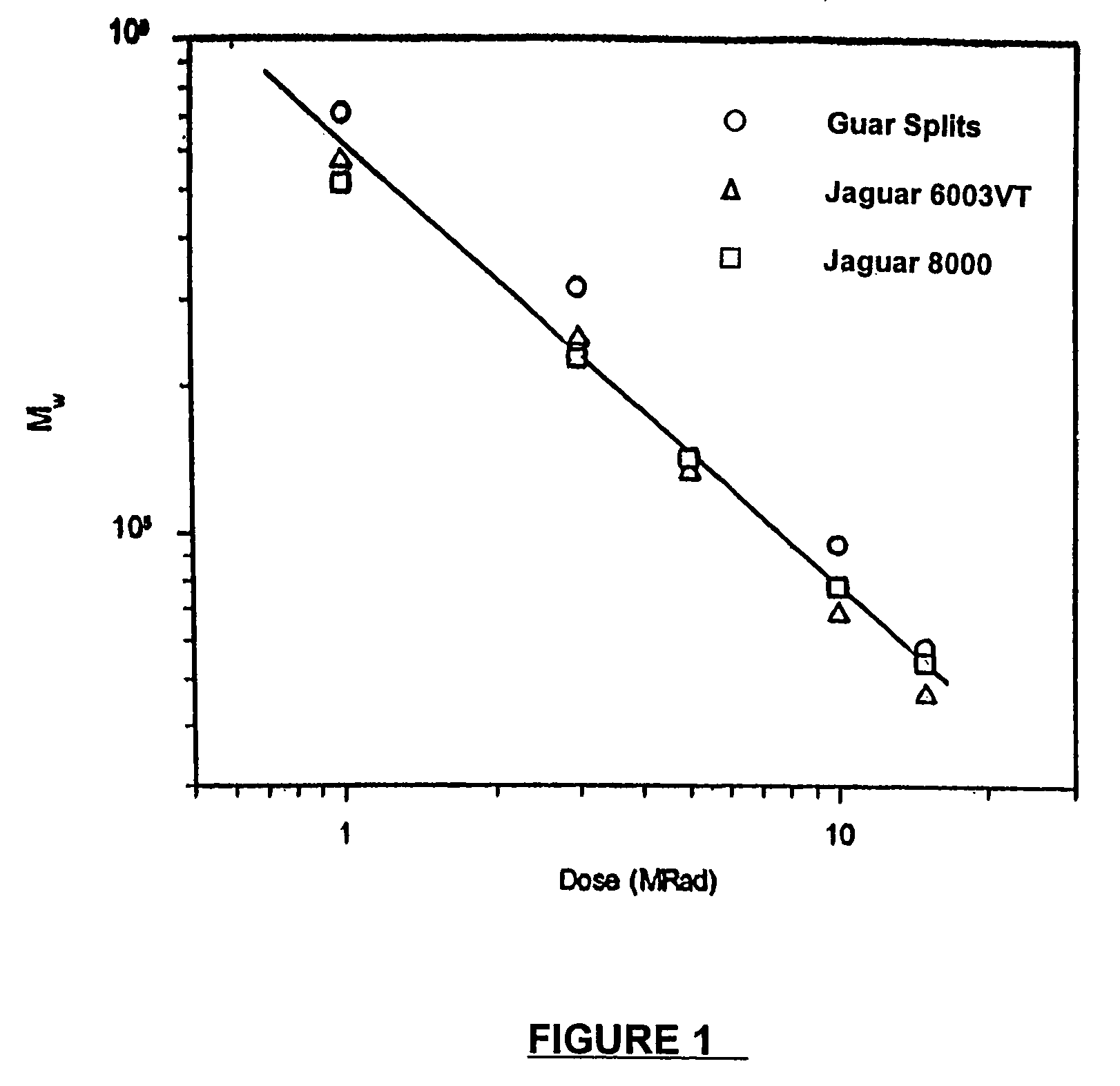
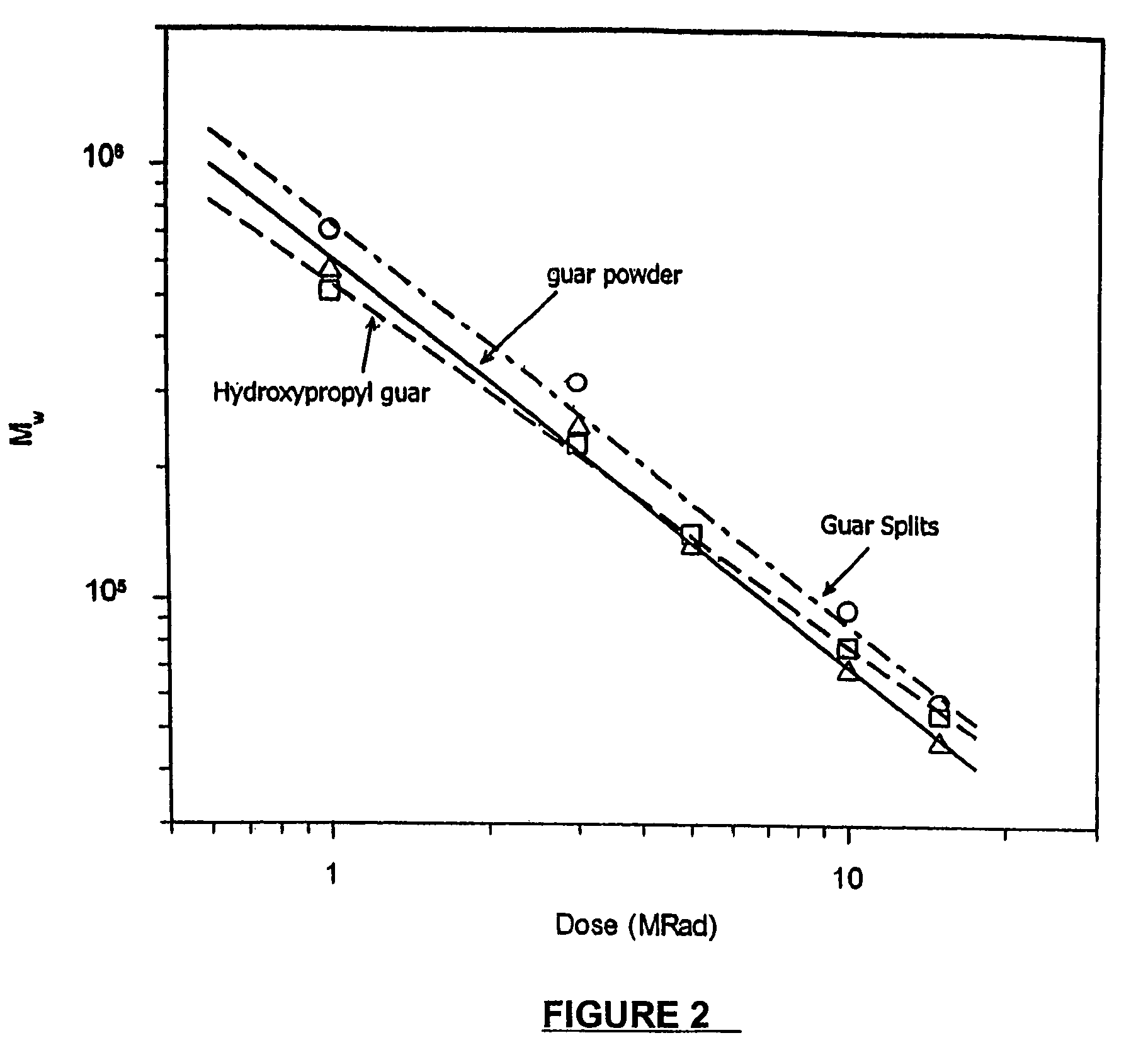
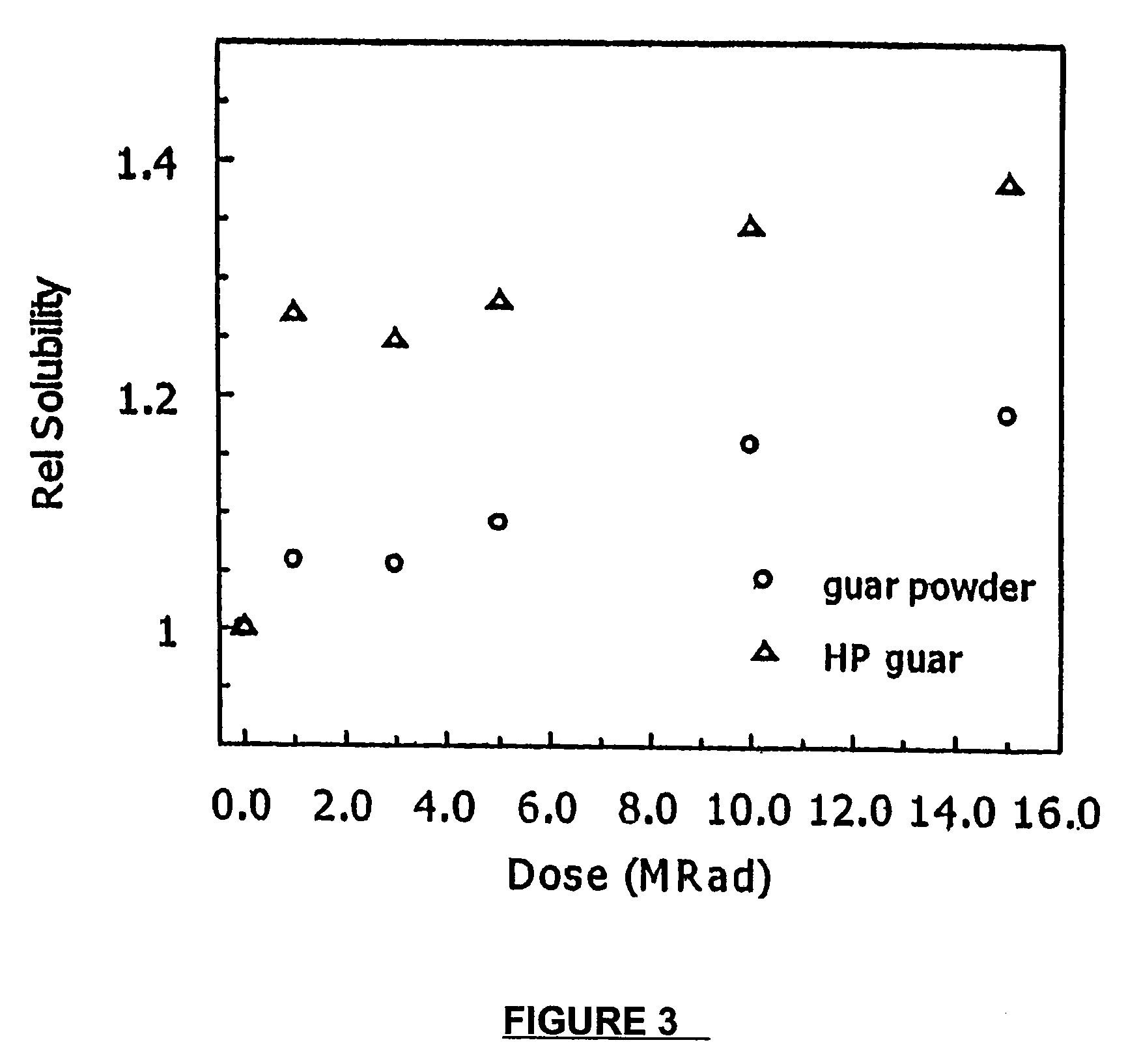
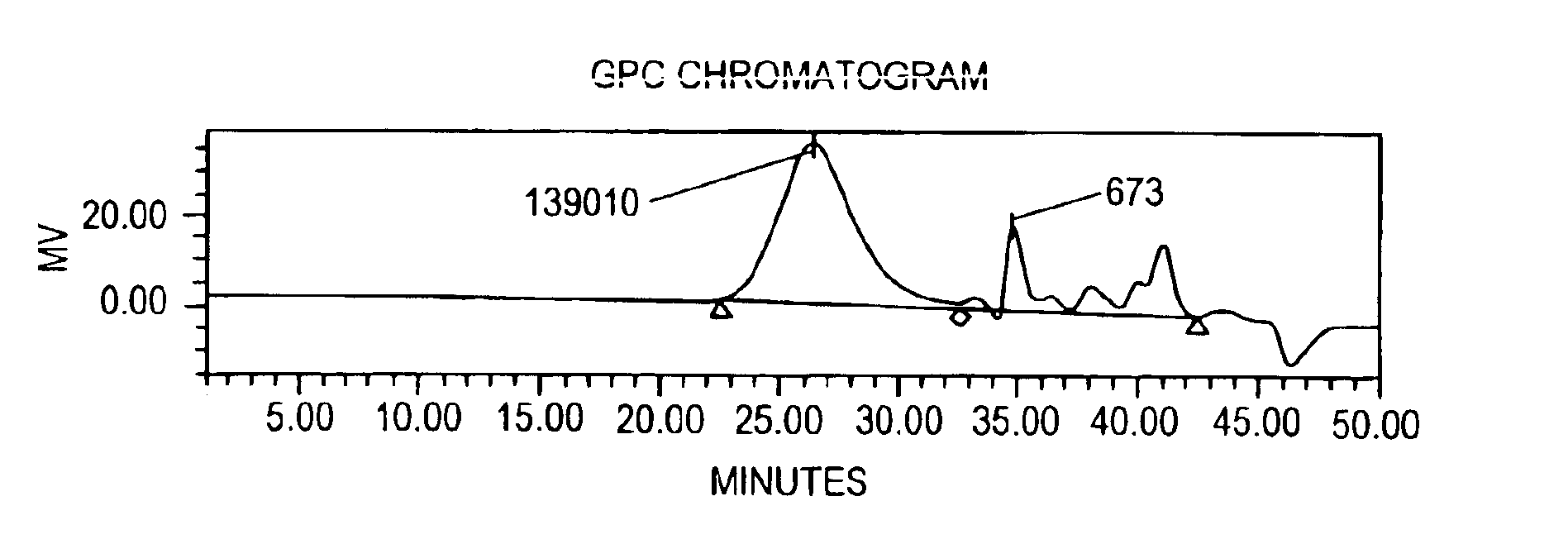
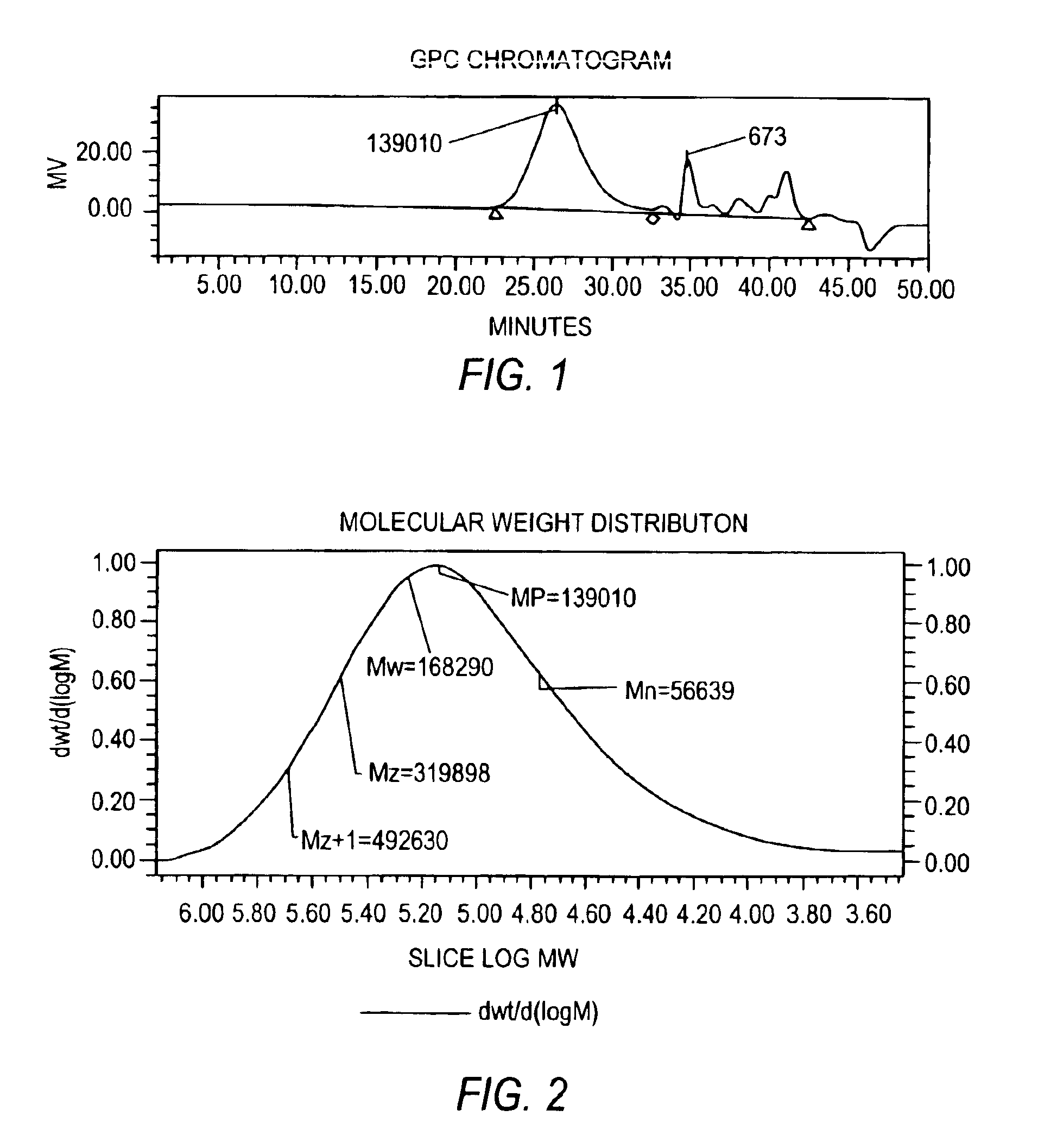
Molecular weight reduction of polysaccharides by electron beams
ActiveUS7259192B2Promote recoveryEasy to useOther chemical processesFibre treatmentHigh energyOil production
A method of depolymerizing galactomannan-type polysaccharide polymers and xanthan, preferably galactomannans, to a pre-selected lower molecular weight by irradiation with high energy electron beams. The preferred galactomannans for treatment according to this method are guar gum, guar splits and hydroxypropyl guar. In a preferred embodiment the guar gum is depolymerized preferably to a molecular weight of about 150,000 Daltons to about 200,000 Daltons. The depolymerized guar has a polydispersity of less than about 3.0 and is useful in oil well fracturing to enhance oil production.
Owner:RHODIA OPERATIONS SAS
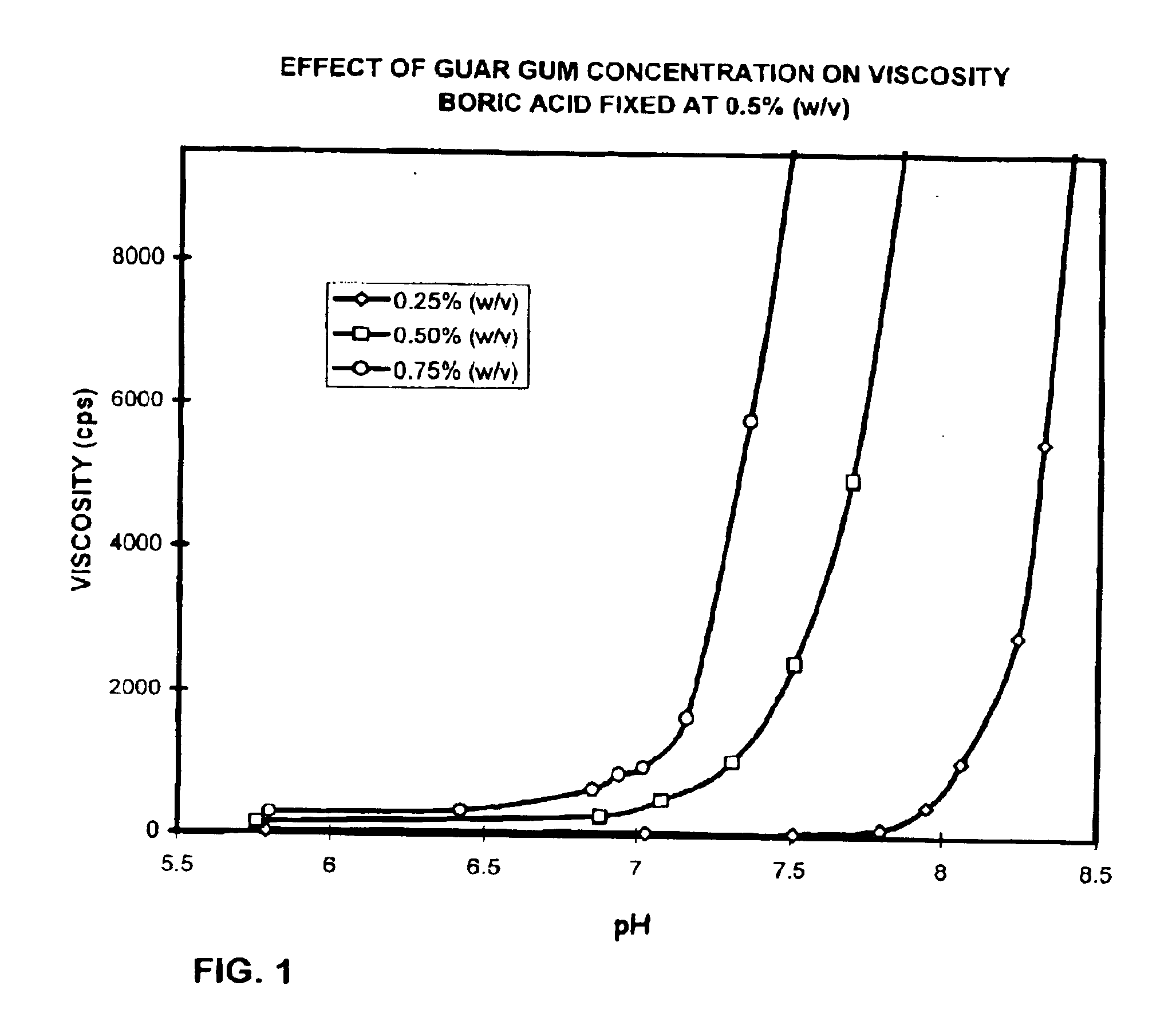
Ophthalmic compositions containing galactomannan polymers and borate
InactiveUS6838449B2Clear visionMinimizes possible irritation of eyeAntibacterial agentsCosmetic preparationsOphthalmologyPolymer
The present invention is directed to ophthalmic compositions containing a gelling amount of a combination of galactomannan polysaccharides and borates. The compositions gel or partially gel upon administration to the eye. The present invention also discloses methods of topical ophthalmic administration of the compositions to the eye.
Owner:ALCON RES LTD
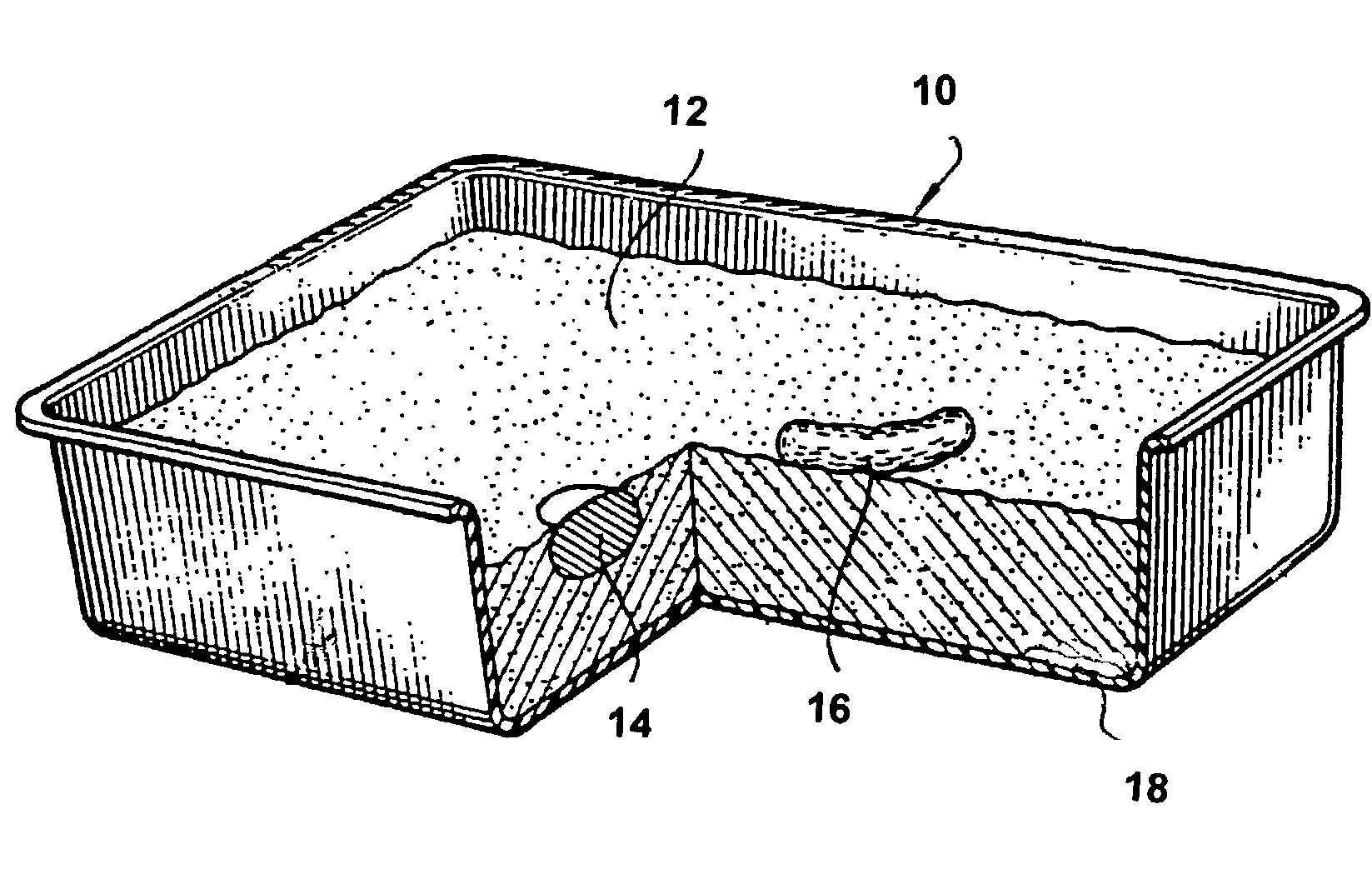
Silica gel based animal litter
A litter composition comprising a substantially particulate primary absorbent material and a binding agent, the binding agent comprising approximately 0.01%-40% of the litter composition. In one embodiment, the primary absorbent material comprises silica gel and the binding agent comprises a galactomannan. In additional embodiments, the litter composition also includes at least one of the following components: fixing agent, colorant agent, anti-bacterial agent, fragrance and / or supplemental absorbent material.
Owner:THE CLOROX CO
Superabsorbent particles containing carboxyalkyl cellulose
ActiveUS20080081191A1Synthetic resin layered productsCellulosic plastic layered productsCelluloseGlucomannan
Particles comprising a combination of a carboxyalkyl cellulose and a galactomannan polymer or a glucomannan polymer, wherein the particles comprise a plurality of non-permanent metal crosslinks.
Owner:INT PAPER CO
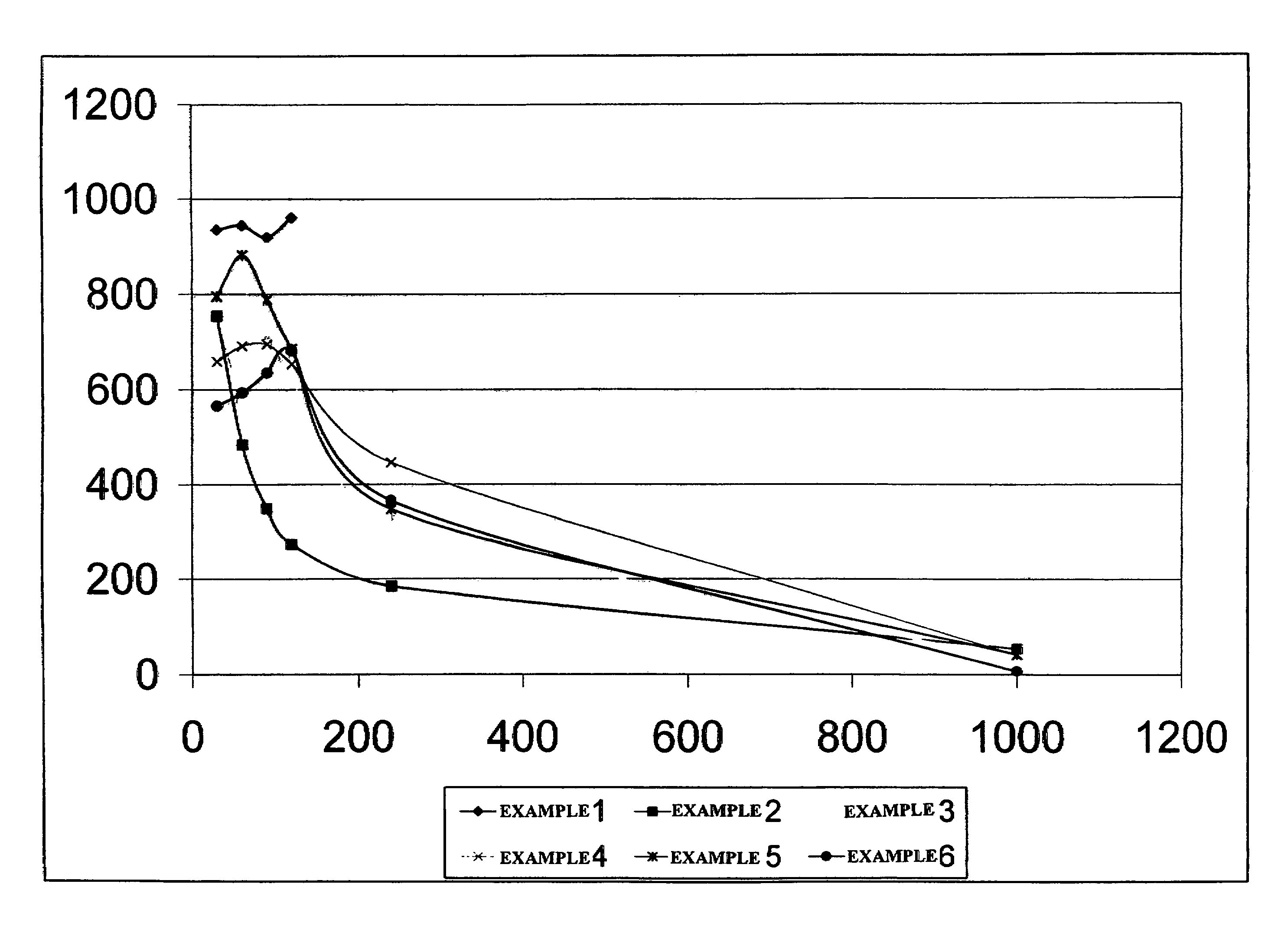
Galactomannan compositions and methods for making and using same
The present invention relates to a method for depolymerizing galactomannan and derivatives thereof. The present invention relates to compositions comprising galactomannan and derivatives thereof prepared according to the methods of this invention and uses for the compositions. The present invention also relates to compositions comprising hydroxypropylgalactomannan having a specific polydispersity index, weight average molecular weight and viscosity in solution.
Owner:ENERGY SOLUTIONS (US) LLC
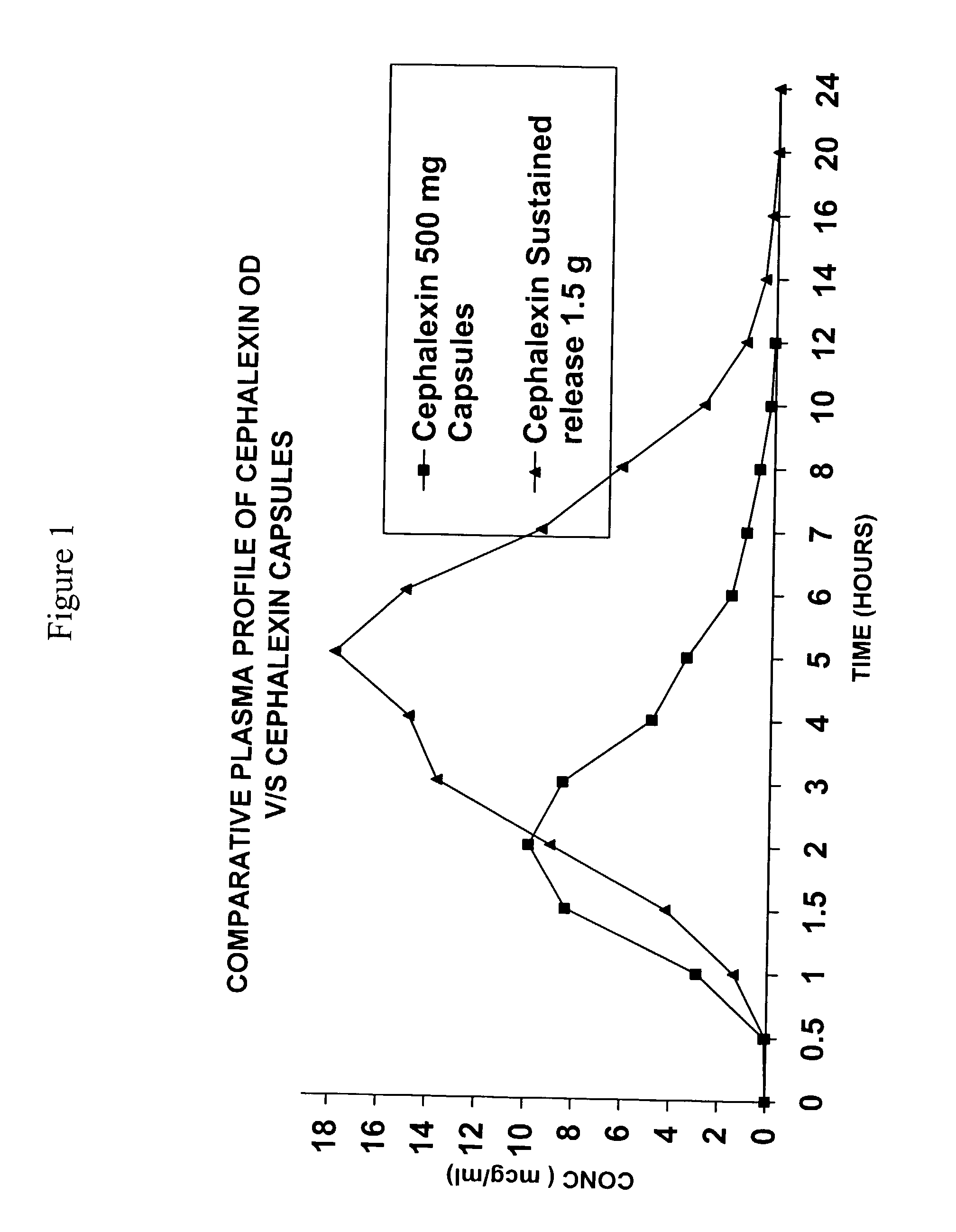
Sustained release pharmaceutical composition of a cephalosporin antibiotic
InactiveUS20040033262A1Sustained releaseMaintain good propertiesBiocideOrganic active ingredientsCombinatorial chemistryBULK ACTIVE INGREDIENT
This invention relates to a sustained release pharmaceutical composition comprising at least a cephalosporin antibiotic, a mixture of polymers and other pharmaceutically acceptable excipients; in the composition, polymers are selected from mixture of galactomannans and neutral swellable polymers, which releases the active ingredient in a predetermined manner.
Owner:ORCHID HEALTH CARE A DIV OF ORCHID CHEM & PHARMA
Mixed polymer superabsorbent fibers containing cellulose
InactiveUS20080082065A1Conjugated cellulose/protein artificial filamentsBaby linensPolymer compositesGalactomannan
Owner:WEYERHAEUSER NR CO
Composition and method for reducing the colonization of animal intestines by salmonella and other bacterial pathogens
InactiveUS6126961AReducing human pathogen colonizationSafe and economical reductionBiocideAnimal feeding stuffIntestinal structureBacteroides
An animal feed composition for reducing colonization of animal intestines by Salmonella and other bacterial pathogens, includes a polysaccharide containing cis-hydroxy sugar units or a derivative thereof, or the monosaccharide ribose or rhamnose, or a derivative thereof, and an animal feed. The polysaccharide cis-hydroxy sugar units may be one or more of mannose, a mannose derivative, galactose, a galactose derivative, galactomannans, galactosamine, fucose and arabinose. The polysaccharides may be incorporated into an animal feed in the form of a food gum or other biolpolymer.
Owner:AURUM CERTUS
Mixed polymer superabsorbent fibers
Owner:WEYERHAEUSER CO
Functional systems with reduced odor cationic polygalactomannan
InactiveUS20060046943A1Reduce body malodorCosmetic preparationsHair cosmeticsPersonal careLower limit
A reduced odor composition is composed of a functional system such as household care, personal care or pet care products and at least one cationic polygalactomannan or a derivative of cationic polygalactomannan having a cationic degree of substitution (DS) lower limit of about 0.01 and an upper limit of about 3.0 and weight average molecular weight (Mw) having a lower limit of 200,000 and an upper limit of 2,000,000 a concentration with a lower limit of 0.005 wt % and an upper limit of 10 wt %, and a trimethylamine content of less than 25 ppm, wherein the composition, at an alkaline pH, has no discernible amine odor.
Owner:HERCULES INC
Mixed polymer superabsorbent fibers
A mixed polymer composite fiber including a carboxyalkyl cellulose and a galactomannan polymer or glucomannan polymer.
Owner:WEYERHAEUSER CO
Cassia Derivatives
InactiveUS20140127149A1Enhance the imageBetter sensory profileOrganic active ingredientsCosmetic preparationsHydrogen atomD-galactal
This invention relates to a cationically and amphiphilically modified polygalactomannan having repeating units with an average D-mannosyl to D-galactosyl residue ratio of at least 5 to 1 and to compositions containing same. A portion of the hydrogen atoms of hydroxyl groups situated on the mannosyl and galactosyl residues of the galactomannan are replaced with an amphiphilic and a cationic substituent.
Owner:LUBRIZOL ADVANCED MATERIALS INC
Fibrous superabsorbent composite containing cellulose
InactiveUS20080081165A1Natural cellulose pulp/paperPulp properties modificationPolymer scienceCellulose fiber
A fibrous composite, comprising cellulose fibers treated with a first galactomannan polymer or a first glucomannan polymer, the treated fibers having particles attached thereto, the particles comprising carboxyalkyl cellulose, a second galactomannan polymer or a second glucomannan polymer, and a plurality of non-permanent metal crosslinks.
Owner:WEYERHAEUSER NR CO
Shampoo containing a gel network and a non-guar galactomannan polymer derivative
Shampoo compositions comprise (a) from about 5% to about 50% of one or more detersive surfactants; (b) a dispersed gel network phase comprising: (i) at least about 0.05% of one or more fatty amphiphiles; (ii) at least about 0.01% of one or more secondary surfactants; and (iii) water; (c) at least about 0.05% of a galactomannan polymer derivative with a net positive charge and having a mannose to galactose ratio of greater than 2:1 on a monomer to monomer basis, wherein the galactomannan polymer derivative has: (i) a molecular weight from about 1,000 to about 10,000,000; and (ii) a cationic charge density from about 0.7 meq / g to about 7 meq / g; and (d) at least about 20% of an aqueous carrier; all by weight of the shampoo composition.
Owner:THE PROCTER & GAMBLE COMPANY
Methods for the preparation of superabsorbent particles containing carboxyalkyl cellulose
ActiveUS20080081843A1Other chemical processesSynthetic resin layered productsCellulosePolymer science
A method for making particles containing carboxyalkyl cellulose, comprising blending a carboxyalkyl cellulose and a galactomannan polymer or a glucomannan polymer in water to provide an aqueous solution; treating the aqueous solution with a crosslinking agent to provide a gel; drying the gel to provide a solid; comminuting the solid to provide a plurality of particles.
Owner:INT PAPER CO
Methods for the preparation of mixed polymer superabsorbent fibers containing cellulose
A method for making mixed polymer composite fibers containing cellulose fibers in which cellulose fibers are dispersed in an aqueous solution comprising a carboxyalkyl cellulose and a galactomannan polymer or a glucomannan polymer in water to provide an aqueous fiber dispersion; the aqueous dispersion treated with a first crosslinking agent to provide a gel; the gel mixed with a water-miscible solvent to provide composite fibers; and the composite fibers treated with a second crosslinking agent to provide crosslinked fibers.
Owner:INT PAPER CO
High DS cationic polygalactomannan for skincare products
InactiveUS20060073110A1Provide protectionCosmetic preparationsHair cosmeticsDegree of substitutionBULK ACTIVE INGREDIENT
A skin care composition is provided with a) from about 1 to about 90 wt % of a surfactant, b) at least about 0.05 wt % of a cationic polymer wherein the cationic polymer has a mean average molecular weight (Mw) from about 2,000 to about 10,000,000 Dalton, and the cationic polymer has a cationic degree of substitution (DS) greater than 0.25 to about 3.0, and c) at least one skin care active ingredient, wherein the skin care composition provides at least one of the functions of cleansing, protection, moisturizing, firming, conditioning, occlusive barrier, emolliency, depositing, and anti-wrinkling to the skin.
Owner:HERCULES INC
Thickener composition for dysphagia patients
InactiveUS20090074940A1Improved profileReduce consumptionOrganic active ingredientsFood ingredient as thickening agentMethyl celluloseGlucomannan
The invention relates to thickening compositions for thickening nutritional products to make the nutritional product suitable for consumption by dysphagia patients, said thickening composition comprising starch, xanthan gum and / or methylcellulose and galactomannan and / or glucomannan.
Owner:NV NUTRICIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com