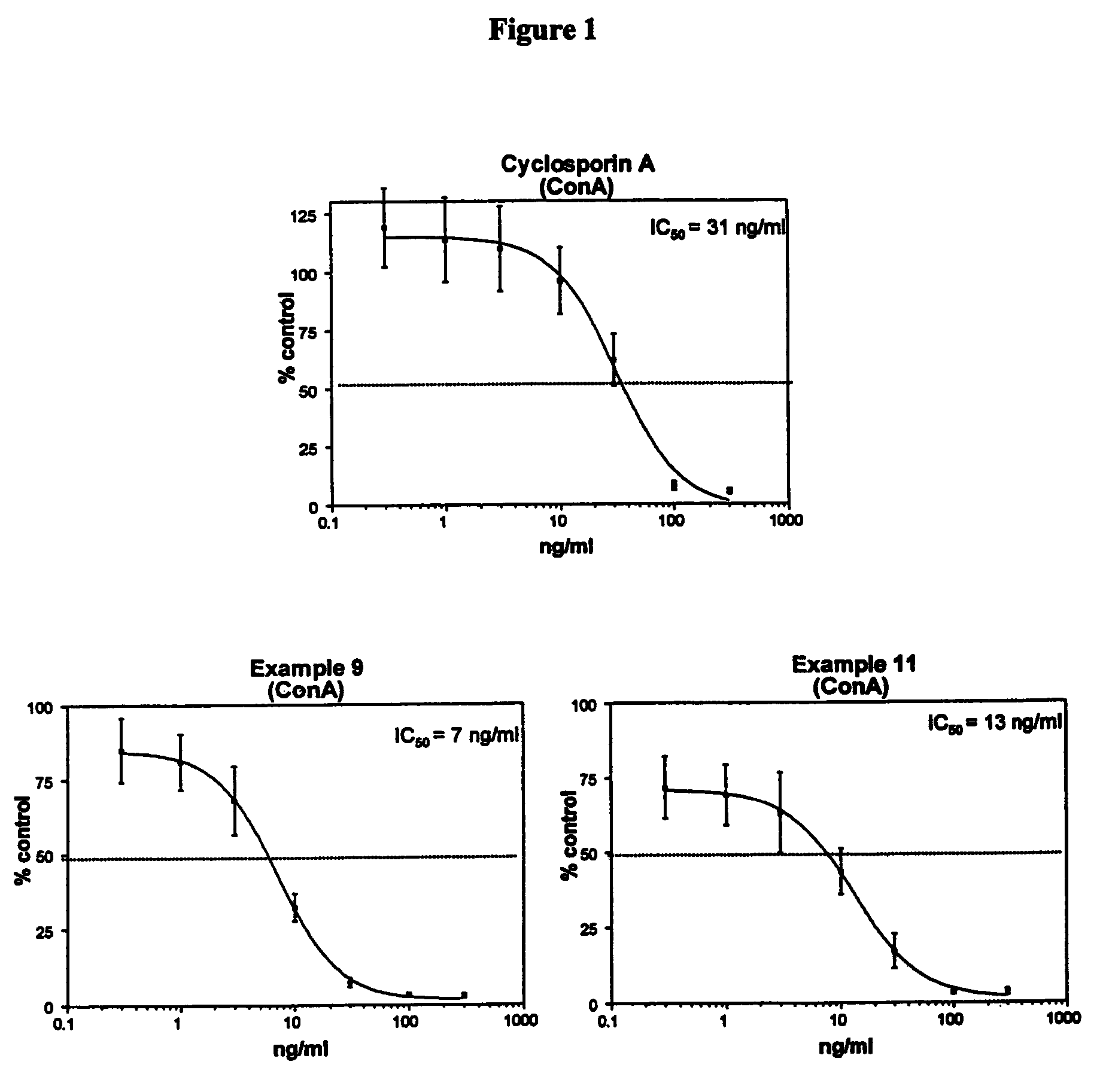
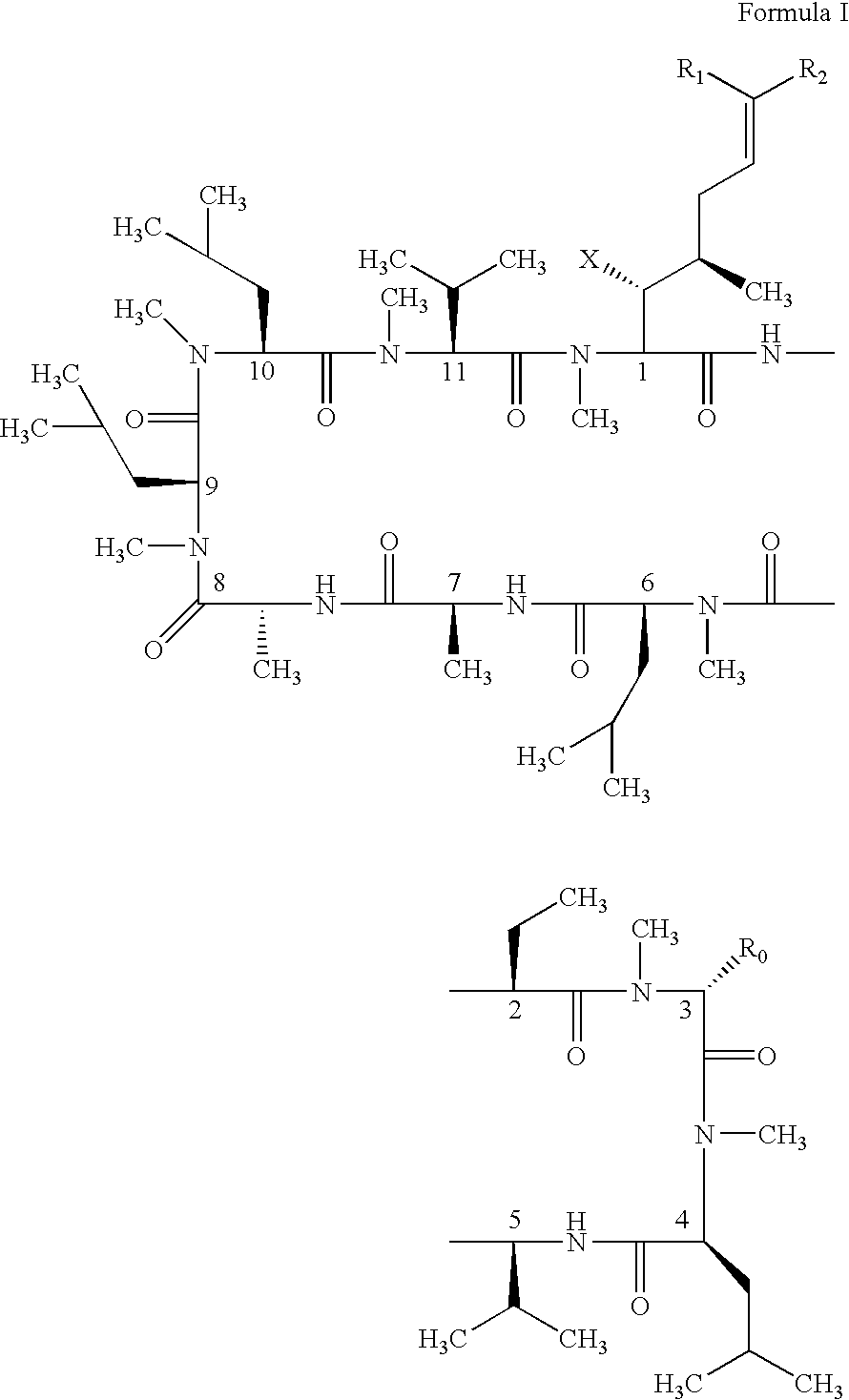
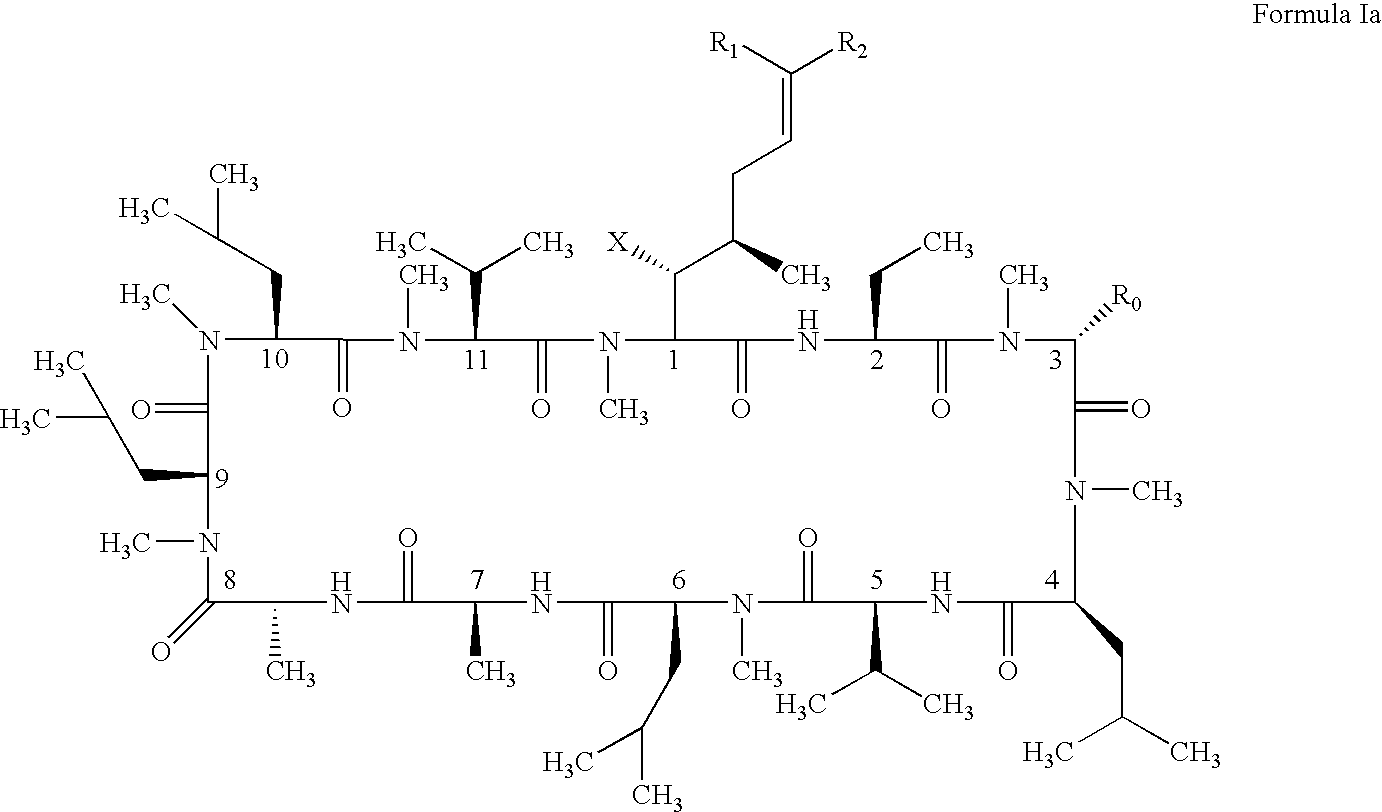
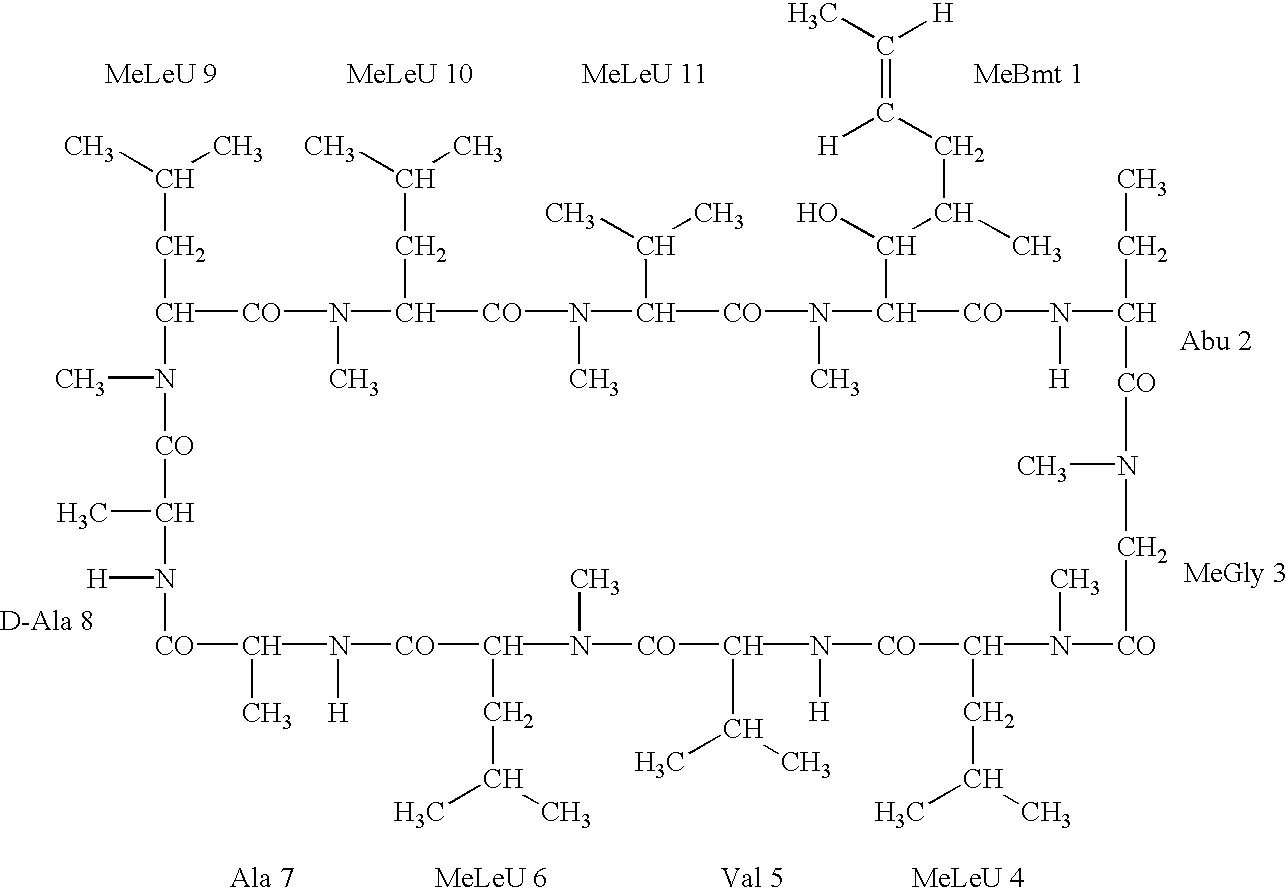
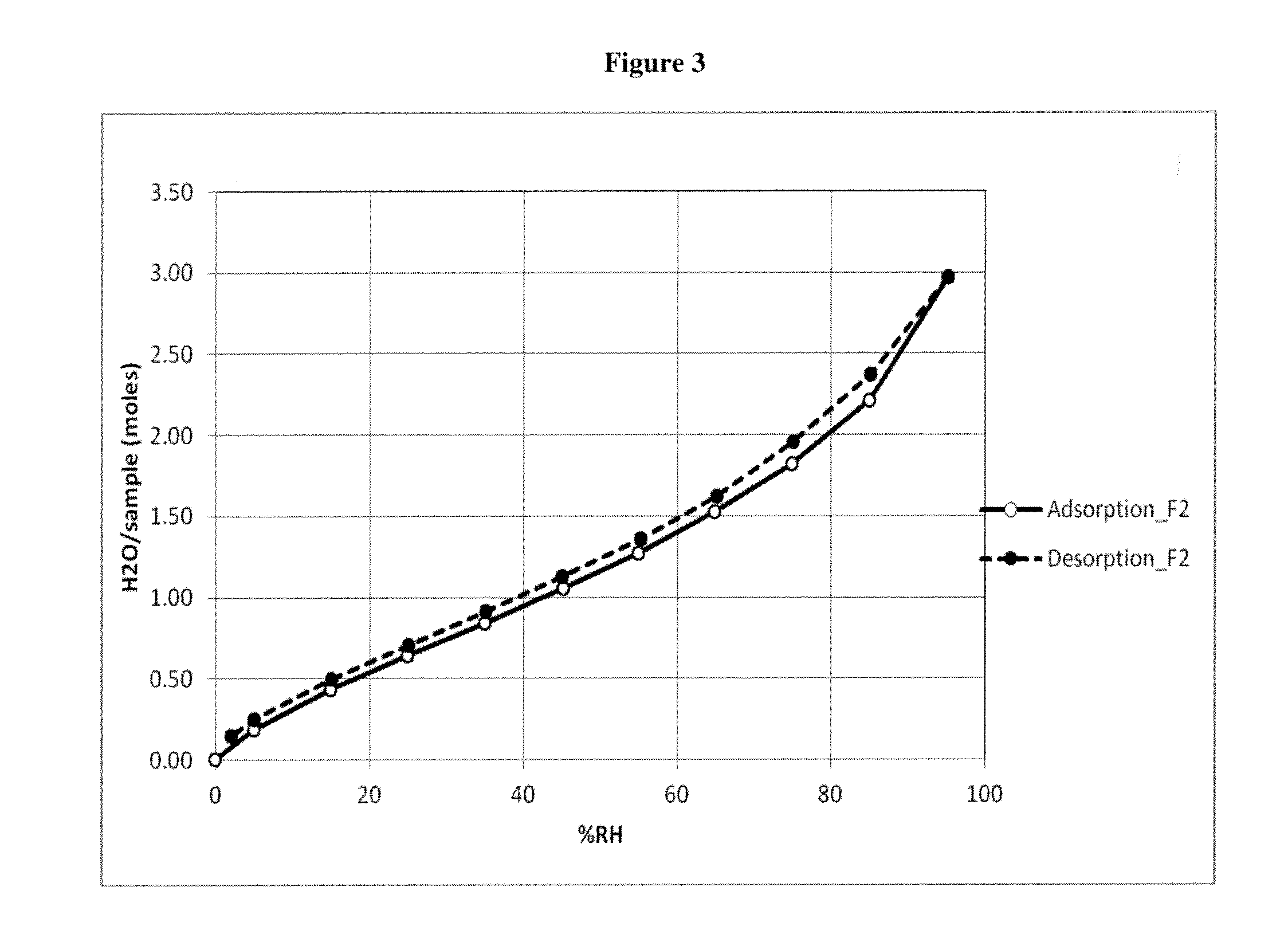
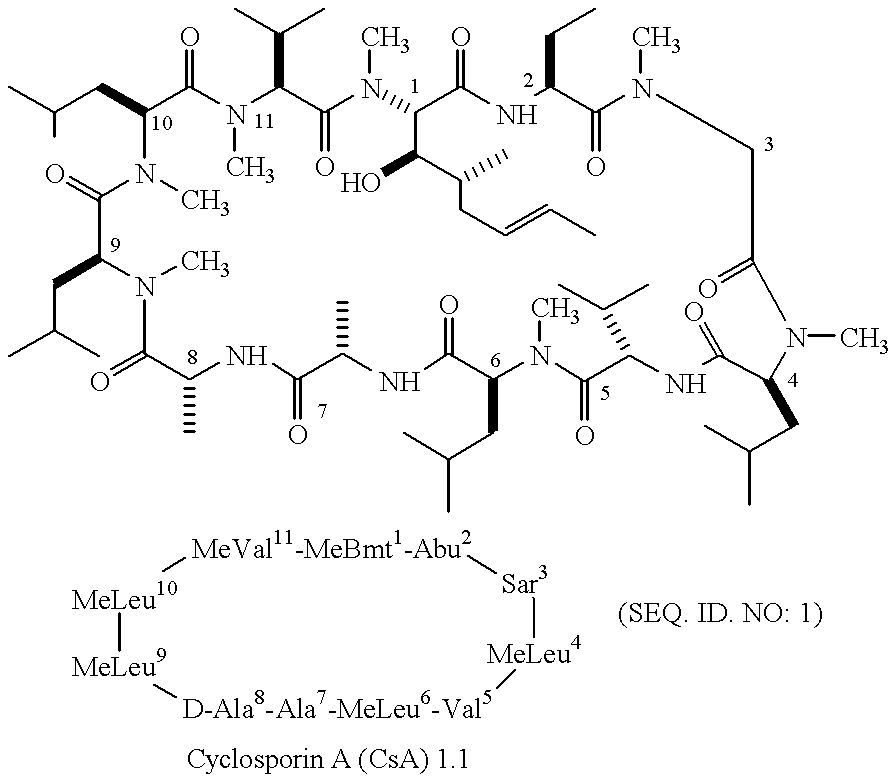
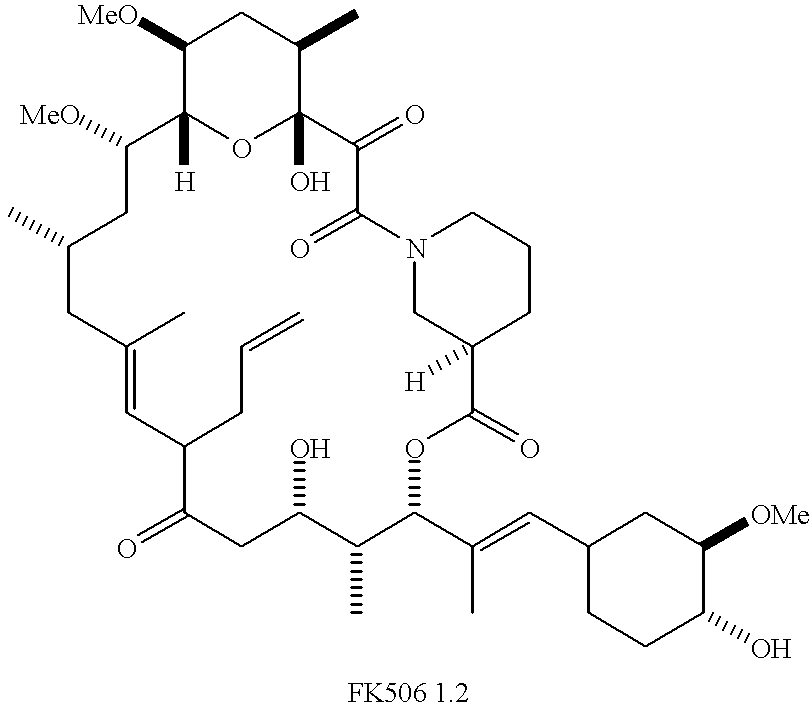
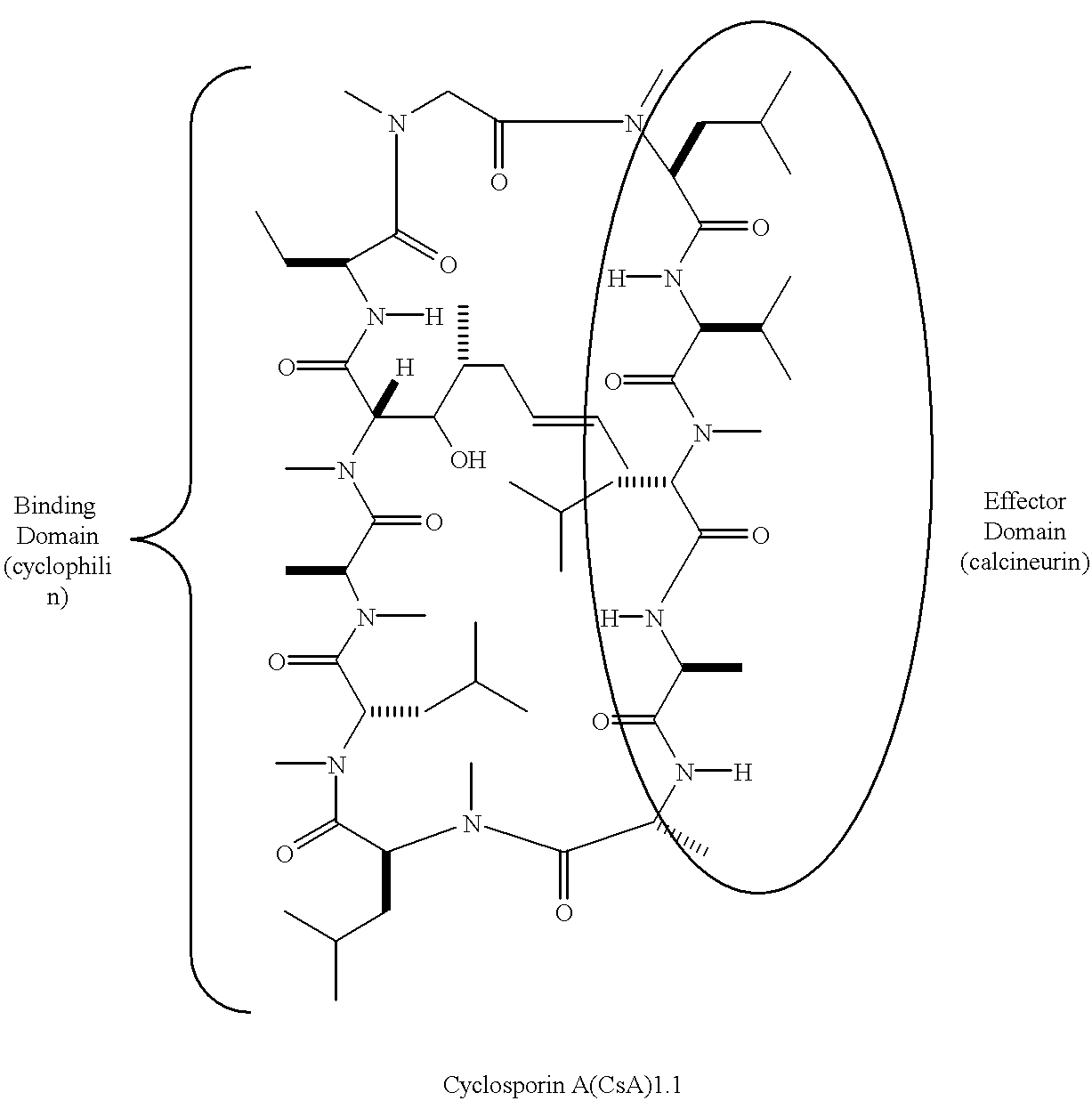
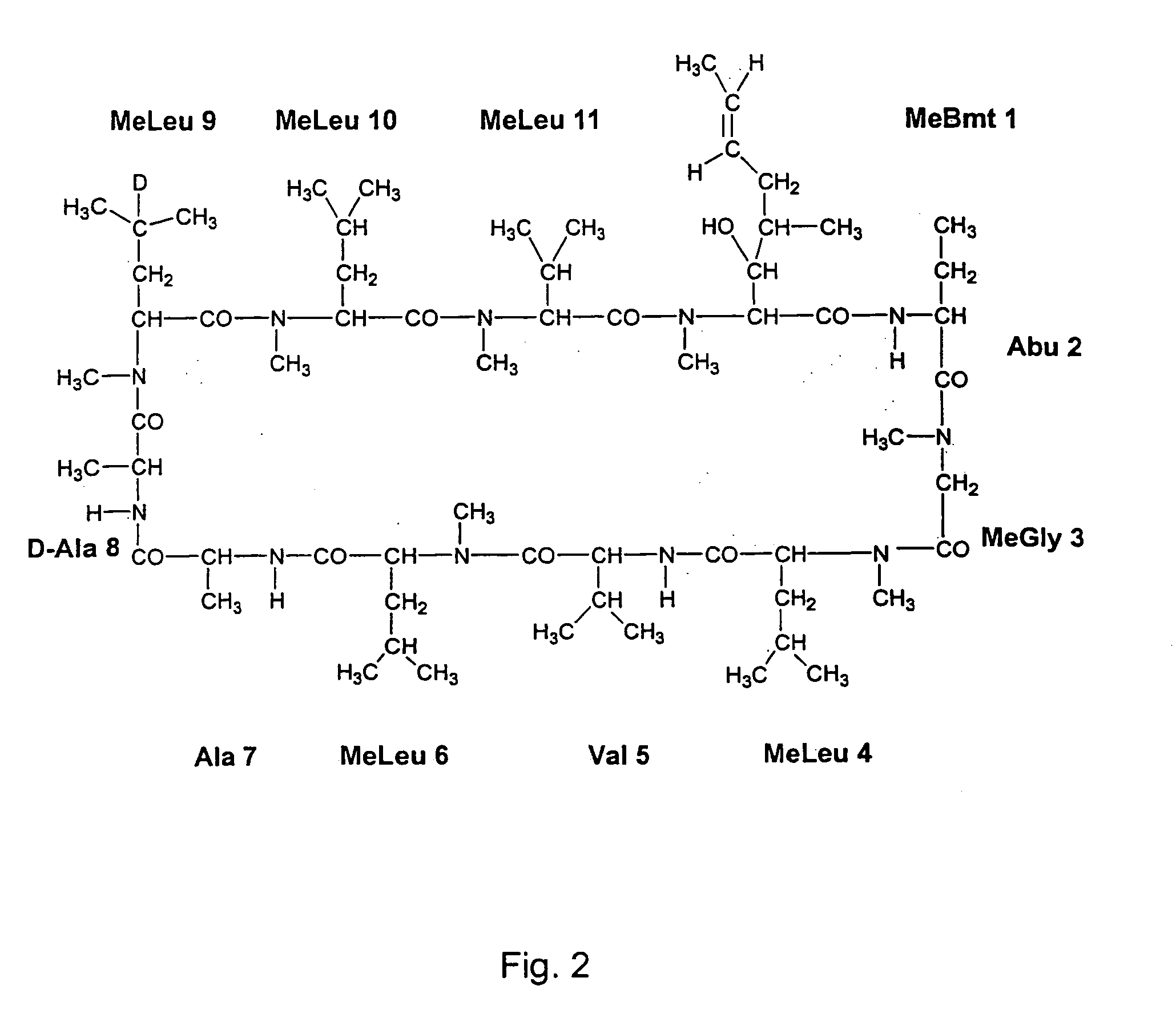
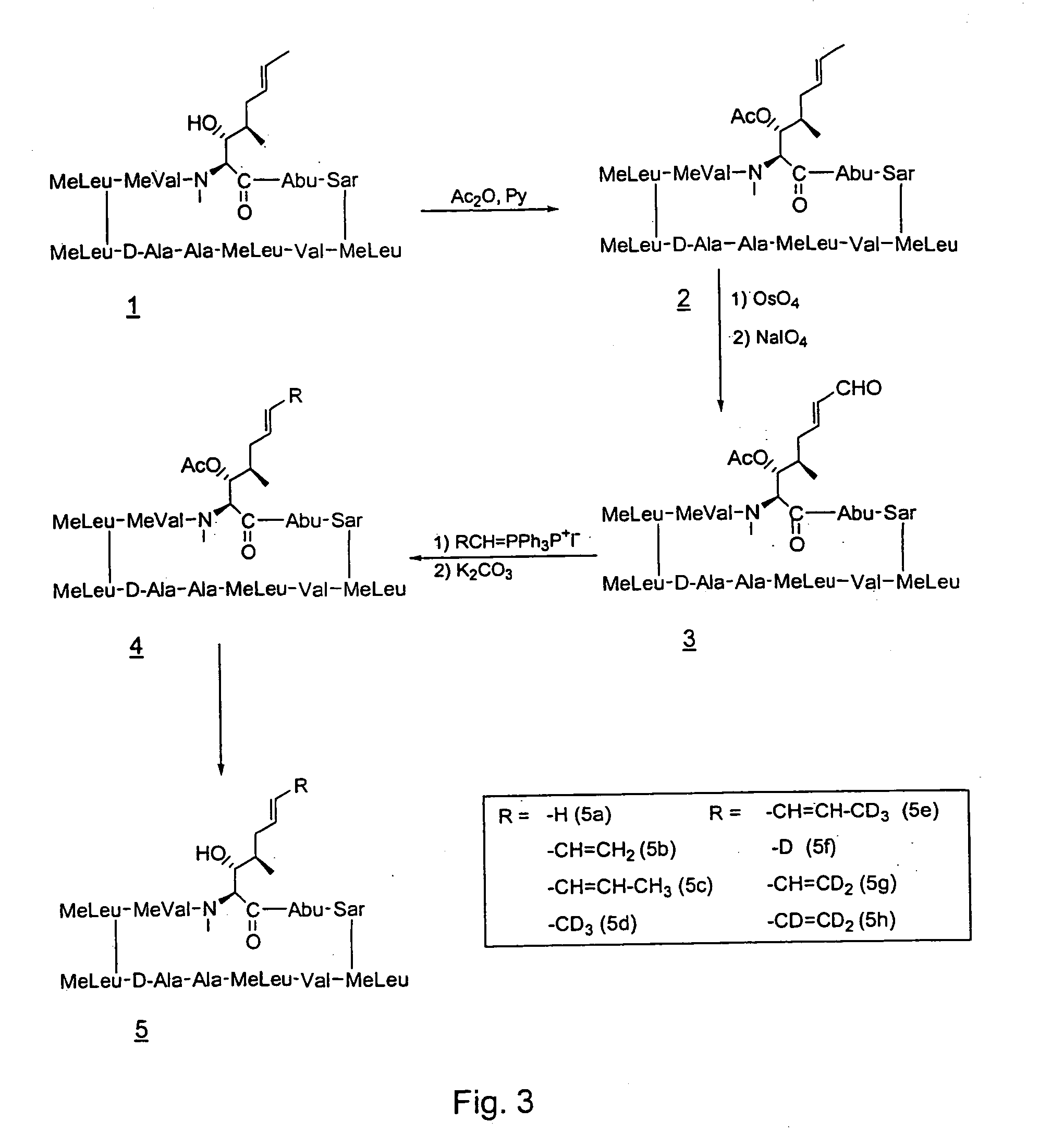
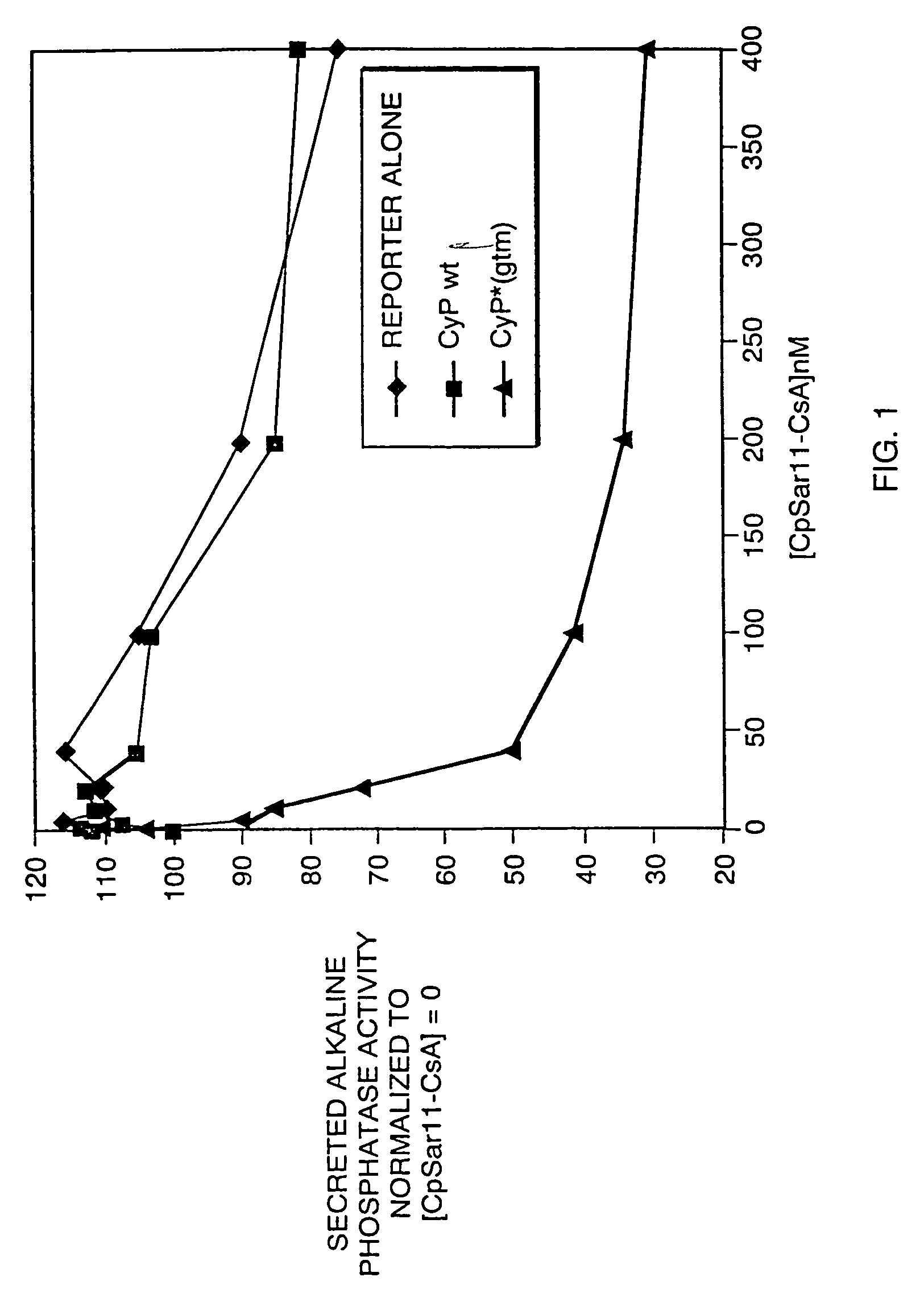
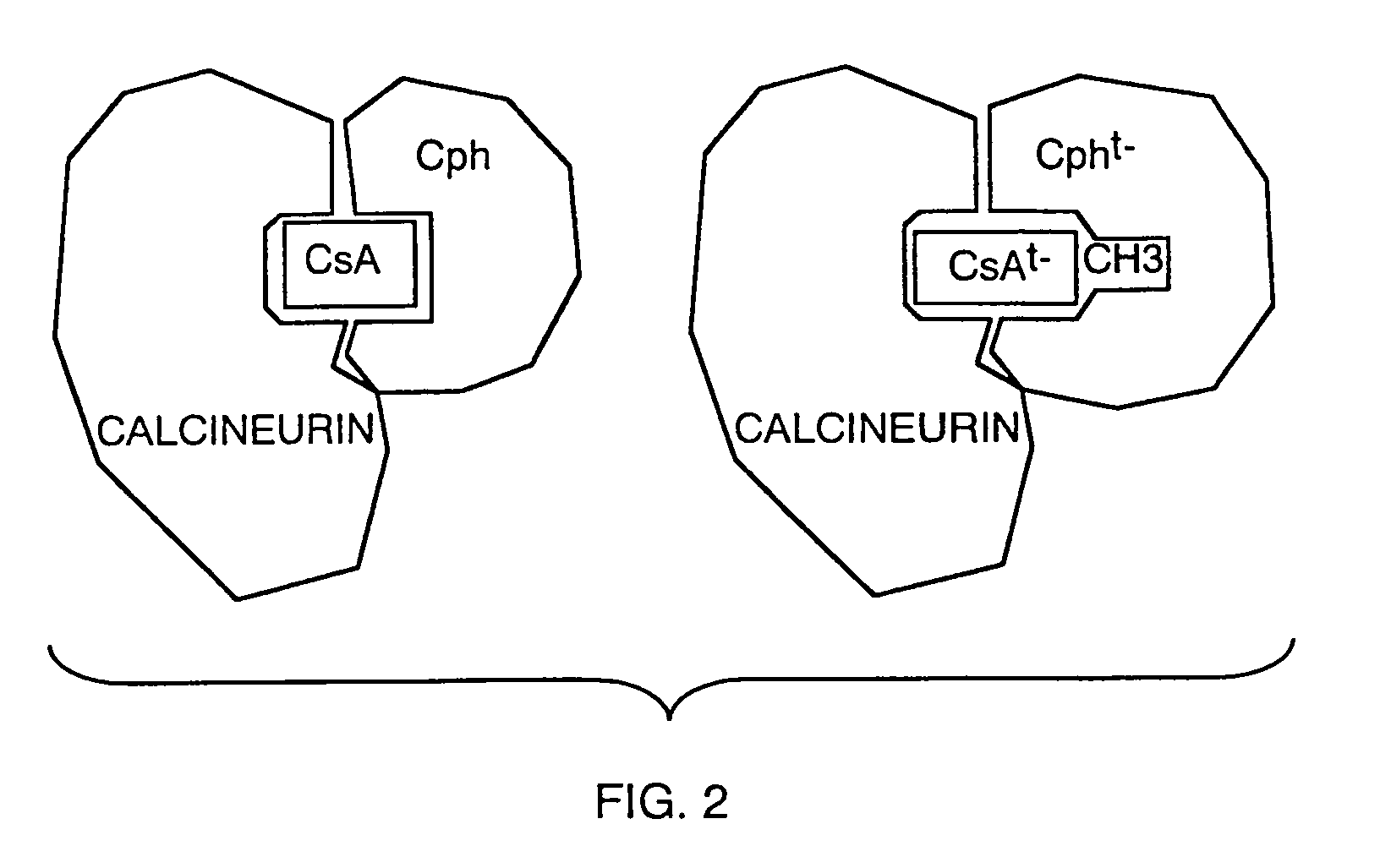
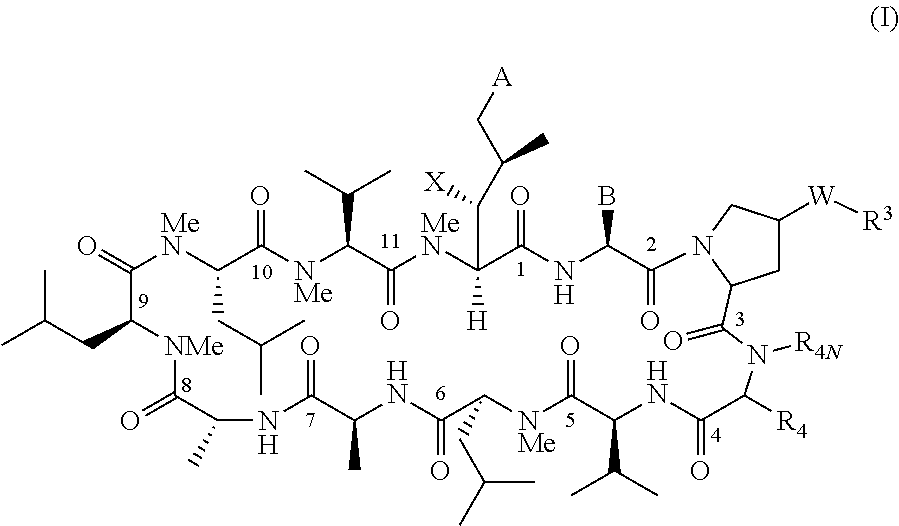
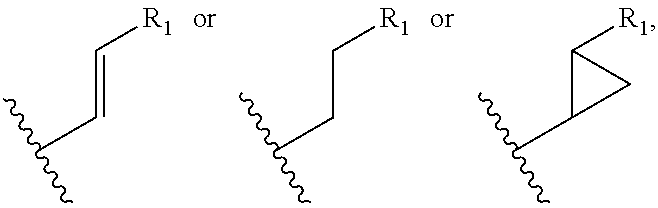
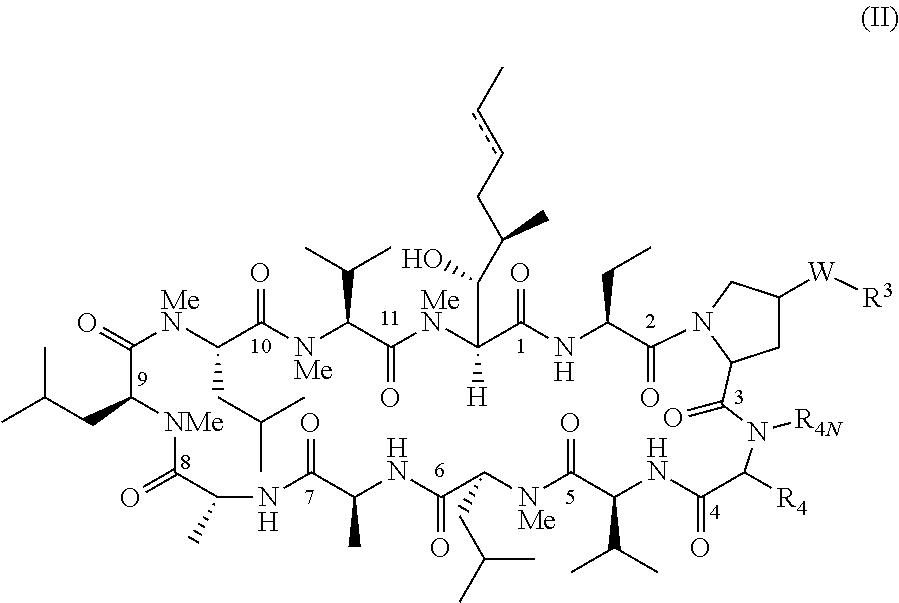
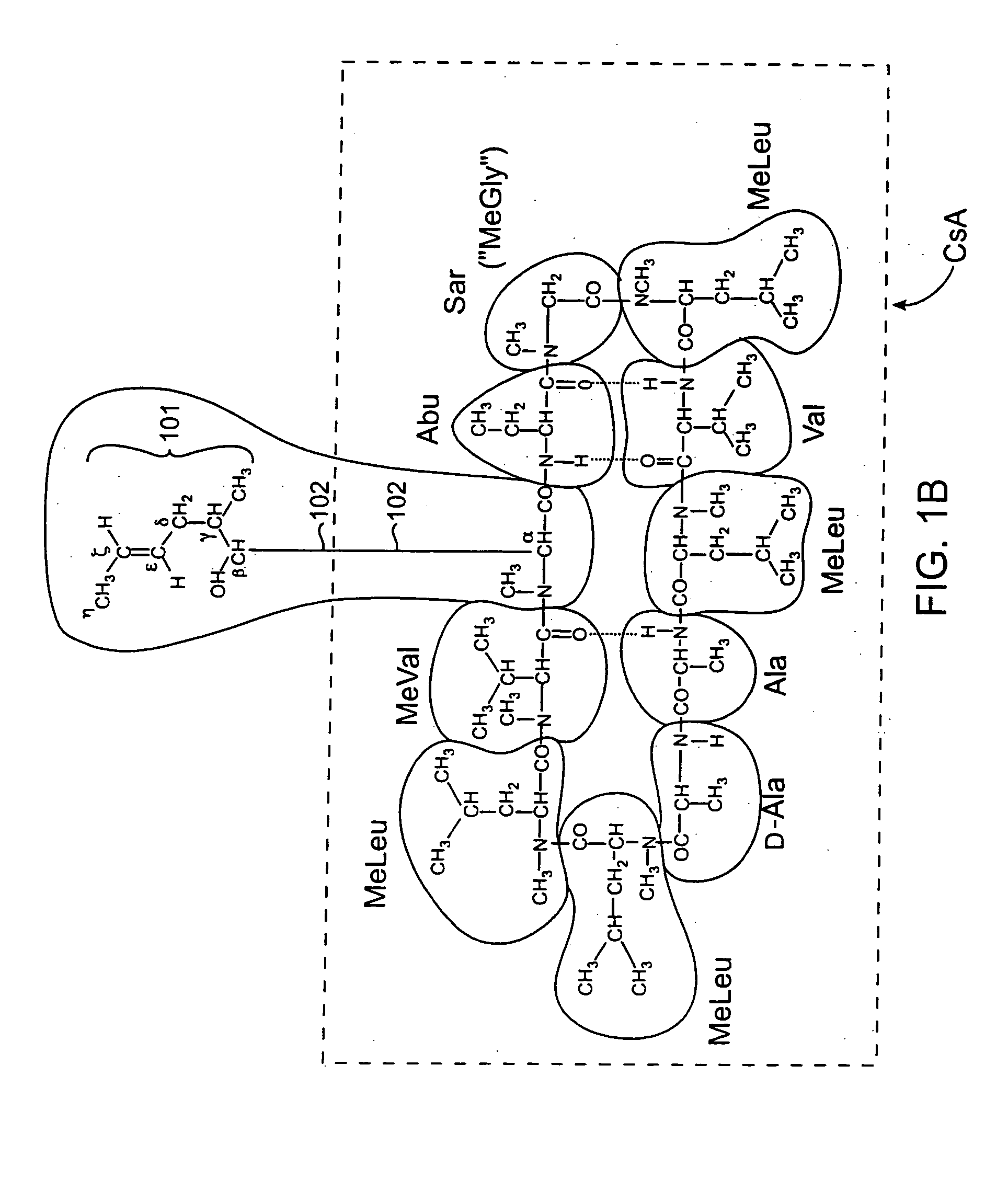
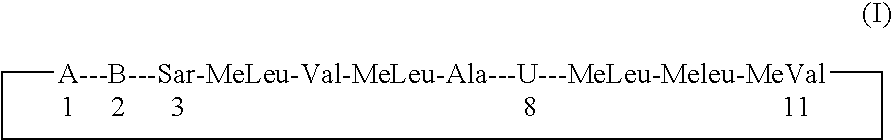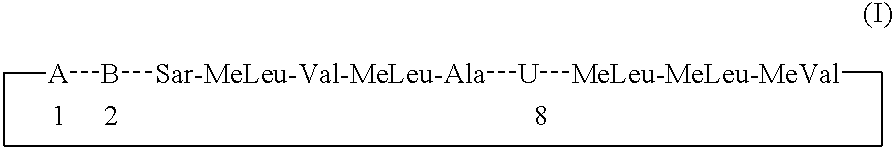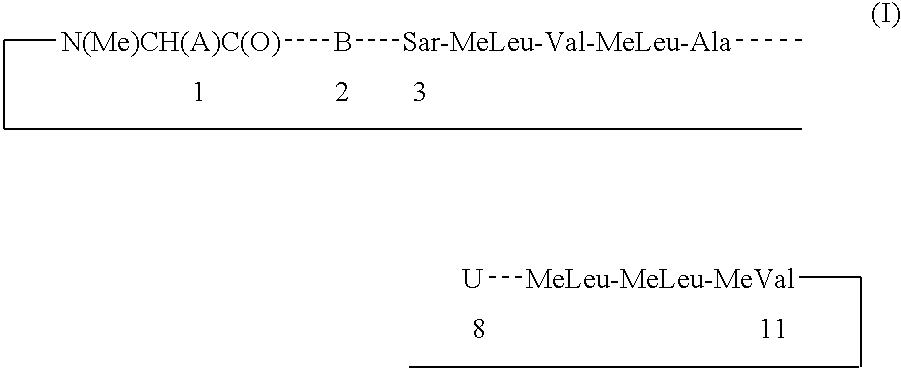Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
141results about "Cyclosporins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
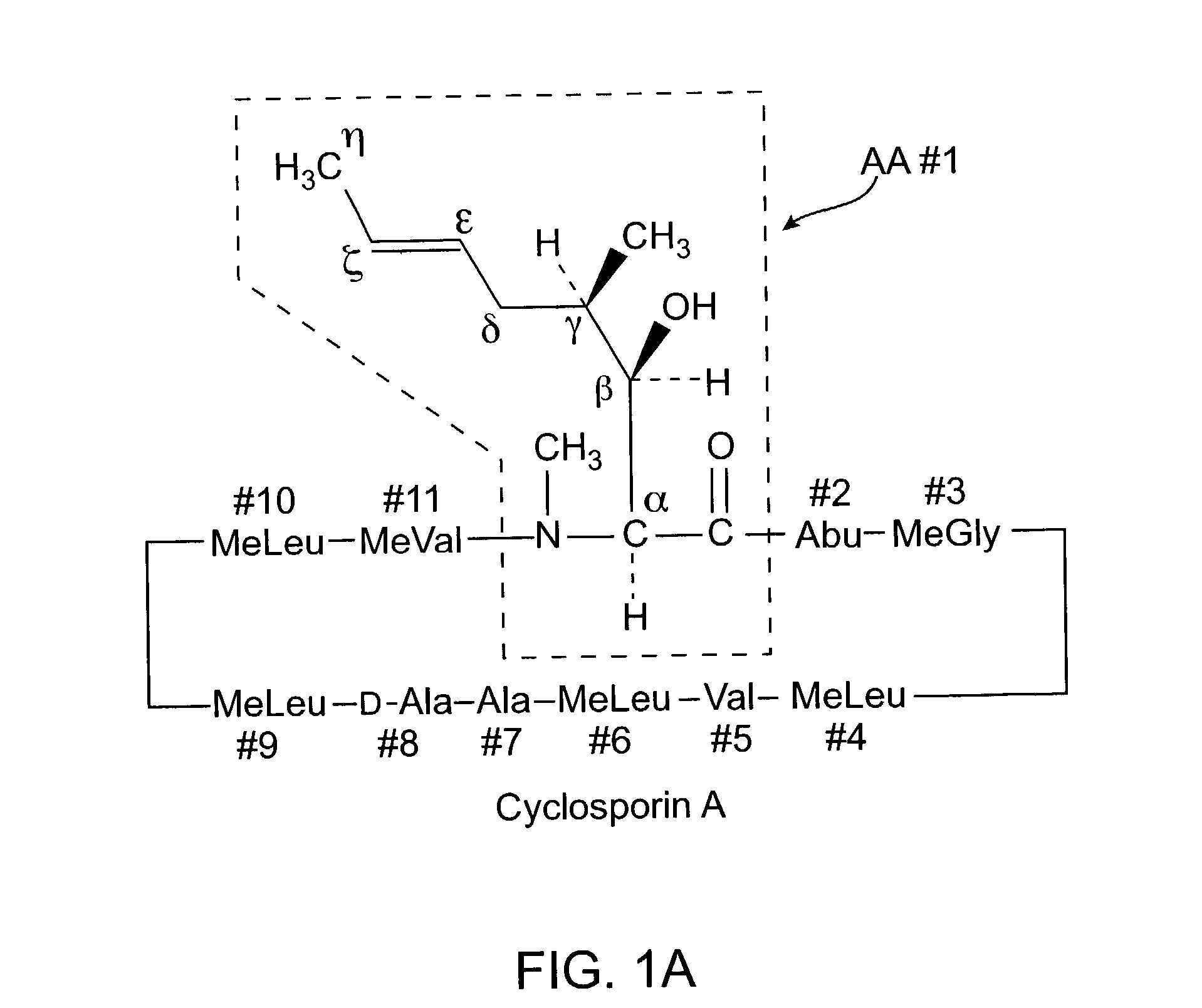
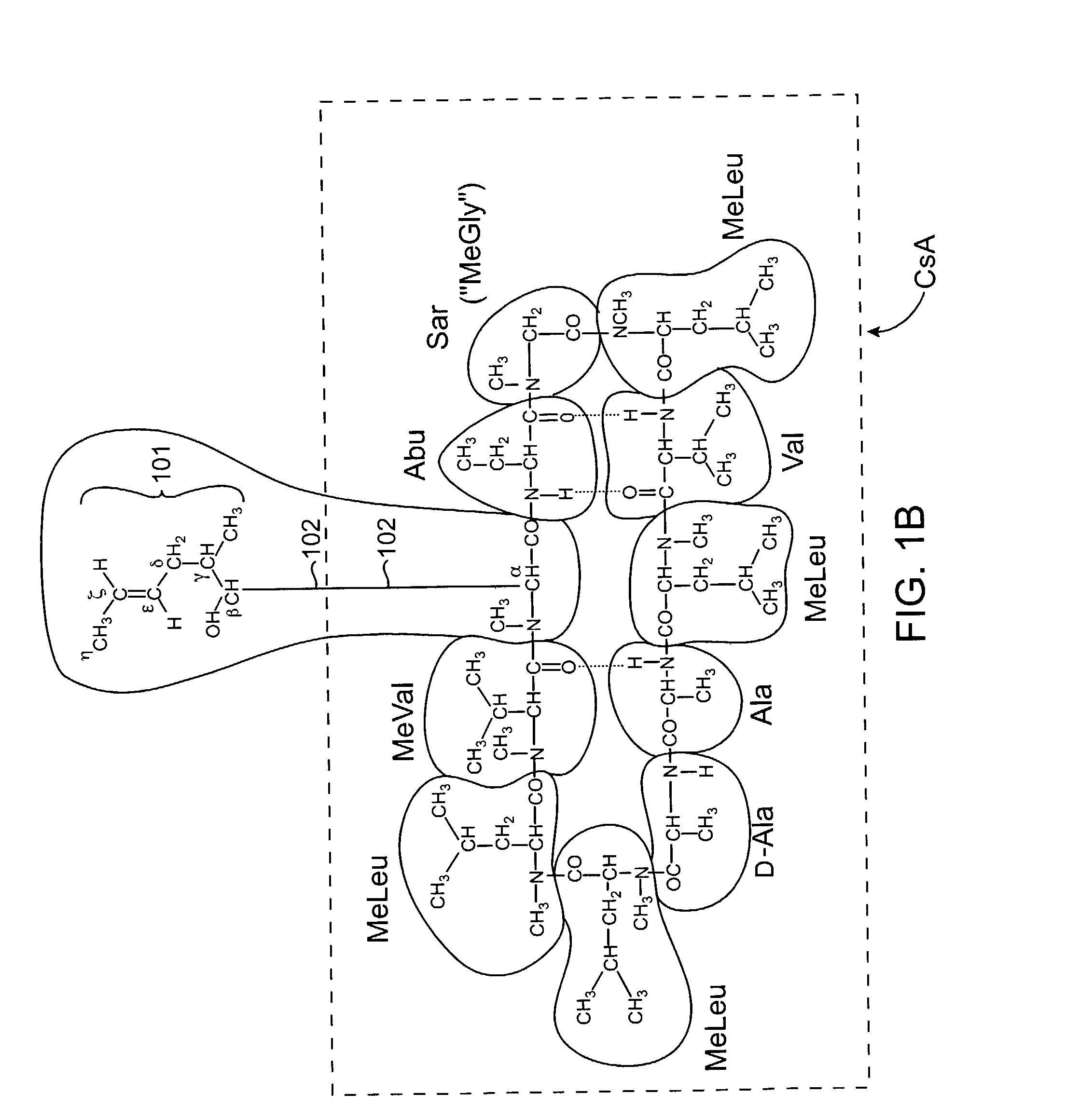
Cyclosporin analogues and their pharmaceutical uses
Owner:ALBANY MOLECULAR RESEARCH INC
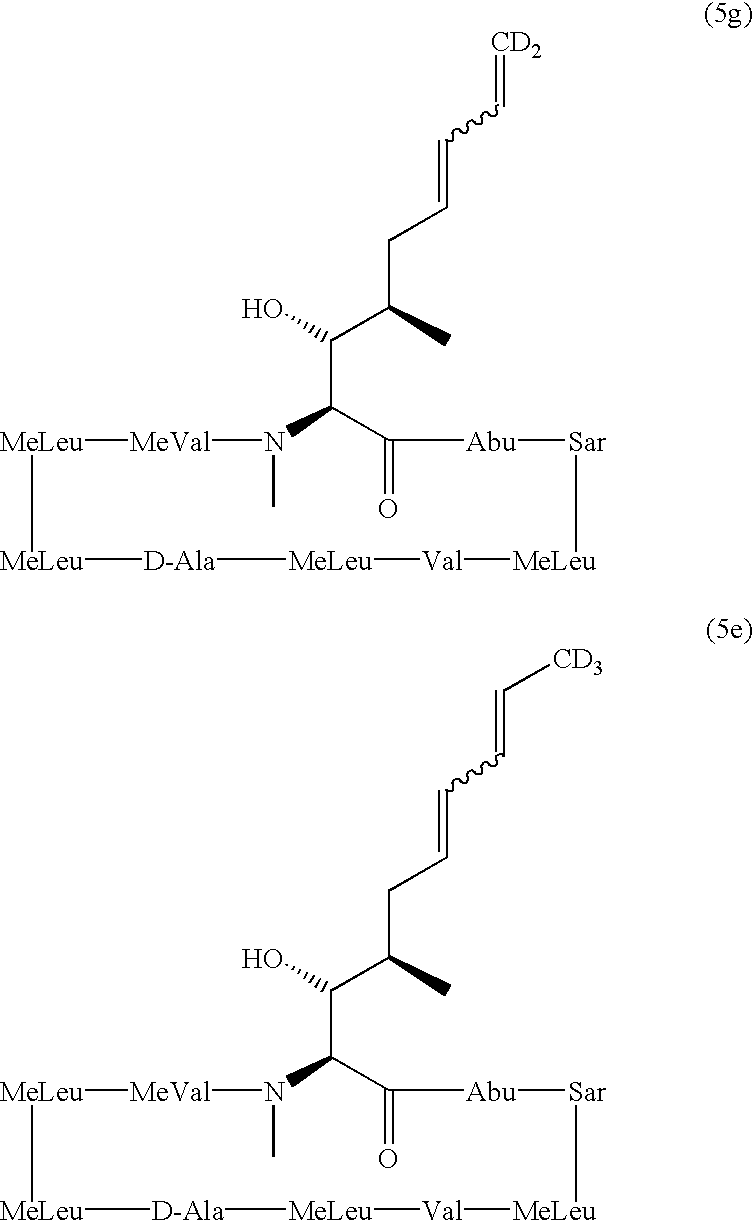
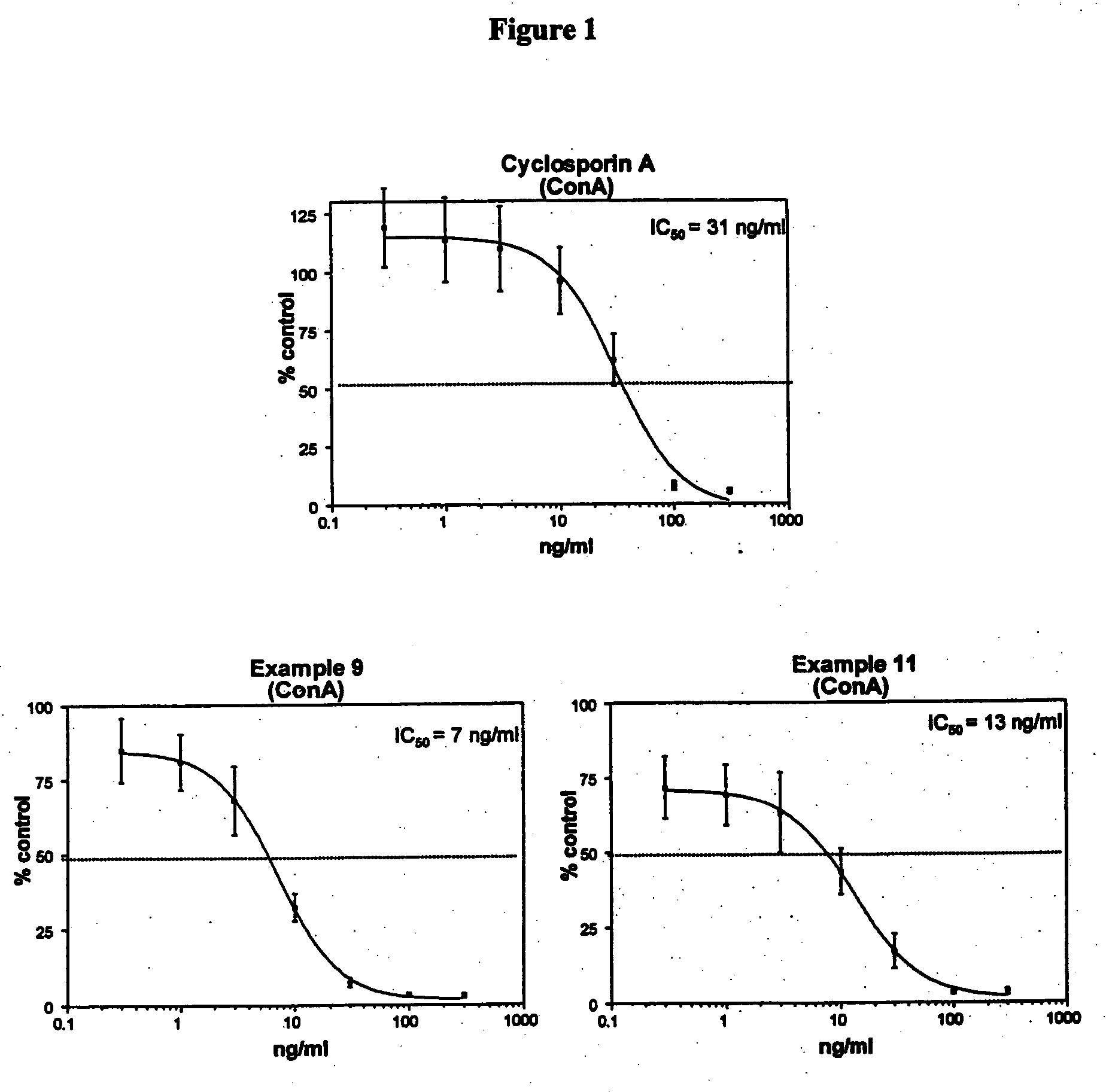
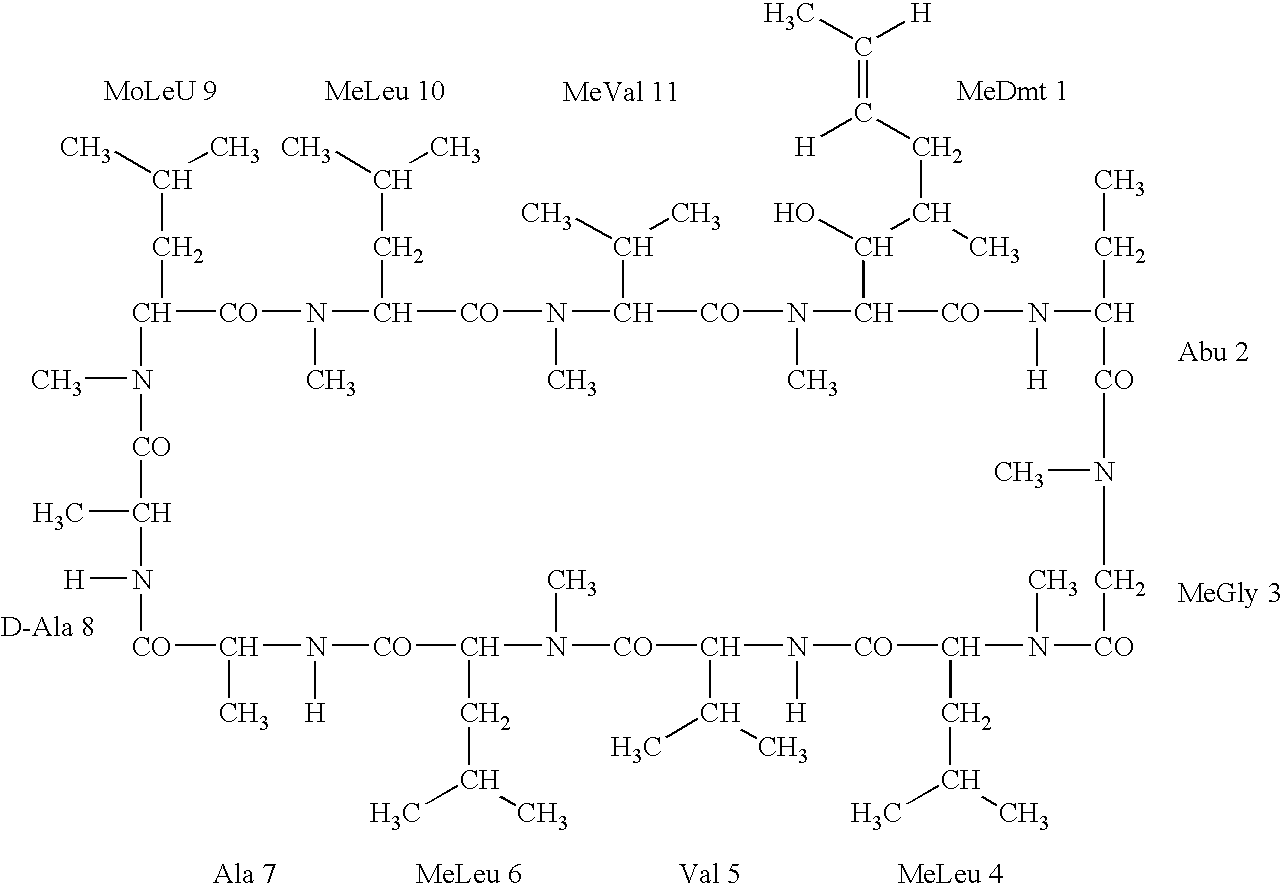
Deuterated cyclosporine analogs and their use as immunomodulating agents
InactiveUS6605593B1Improve practicalityAltered physicochemical and pharmacokinetic propertyNervous disorderAntipyreticCyclosporinsImmunomodulating Agent
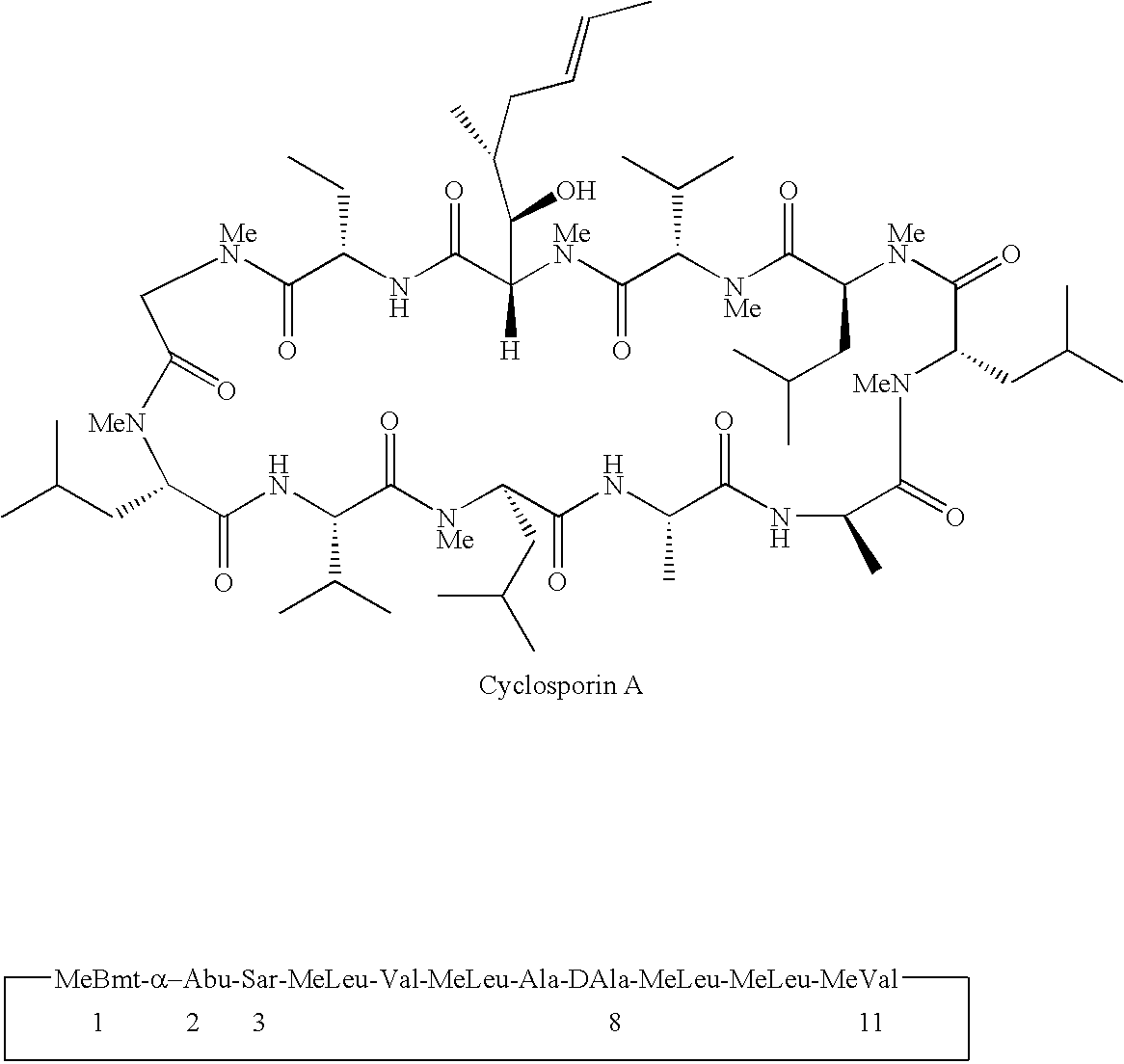
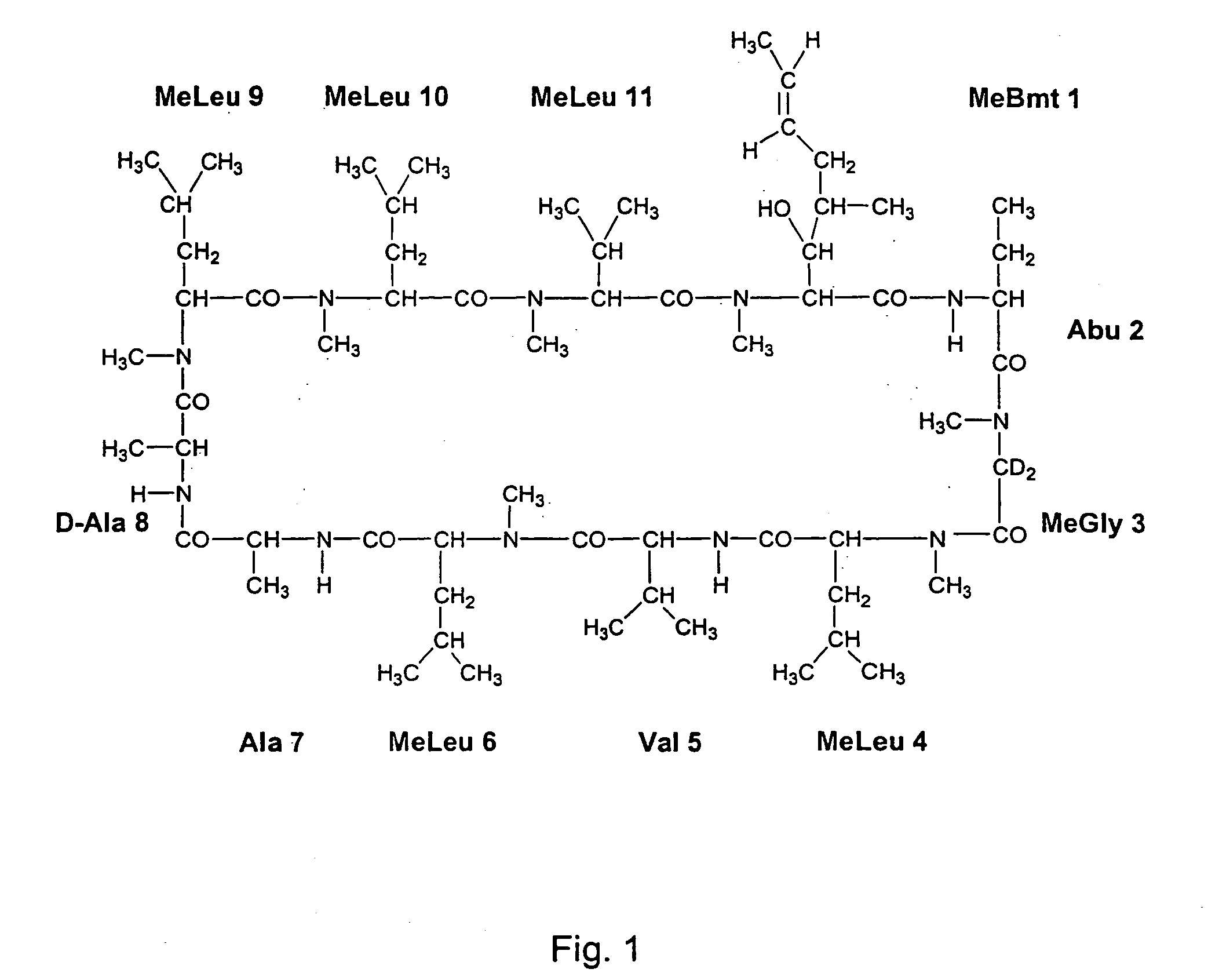
Cyclosporine derivatives are disclosed which possess enhanced efficacy and reduced toxicity over naturally occurring and other presently known cyclosporins and cyclosporine derivatives. The cyclosporine derivatives of the present invention are produced by chemical and isotopic substitution of the cyclosporine A (CsA) molecule by: (1) Chemical substitution and optionally deuterium substitution of amino acid 1; and (2) deuterium substitution at key sites of metabolism of the cyclosporine A molecule such as amino acids 1, 4, 9. Also disclosed are methods of producing the cyclosporine derivatives and method of producing immunosuppression with reduced toxicity with the disclosed cyclosporine derivatives.
Owner:AURINIA PHARMA
Cyclosporin analog formulations
ActiveUS7060672B2Reduced glomerular filtration rateLessen kidney damageOrganic active ingredientsSenses disorderDrugAdverse effect
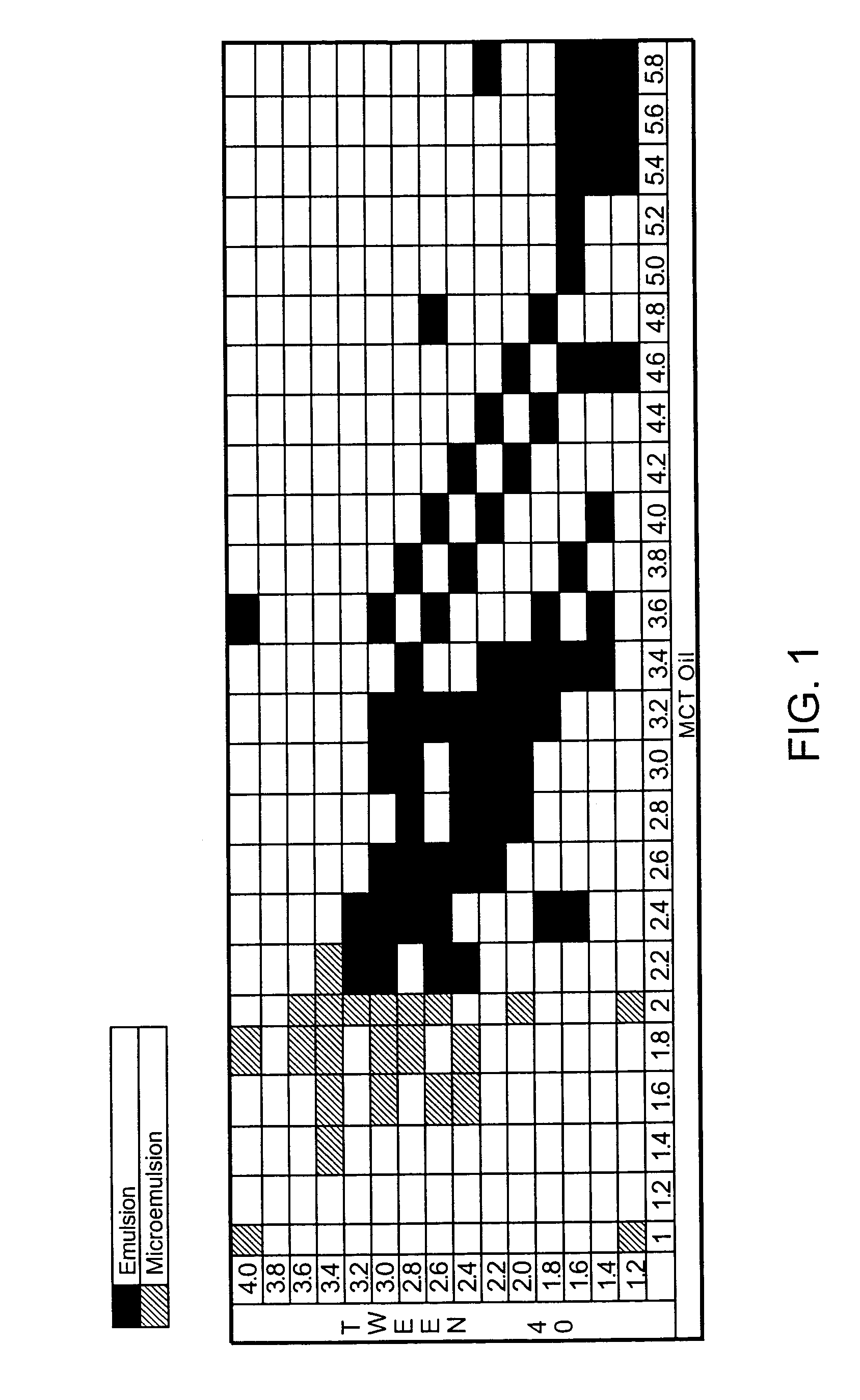
The present invention relates to formulations containing cyclosporin analogs that are structurally similar to cyclosporin A, in particular isomeric mixtures of cyclosporin analogs that are structurally similar to cyclosporin A. The formulations form stable microemulsion preconcentrates and may provide superior drug bioavailability and / or may reduce one or more adverse effects associated with the administration of cyclosporin. Also disclosed are methods for using and preparing the formulations.
Owner:AURINIA PHARMA
Deuterated cyclosporine analogs and their use as immunomodulating agents
InactiveUS20020132763A1Improve practicalityAltered physicochemical and pharmacokinetic propertyNervous disorderAntipyreticCyclosporinsImmunomodulating Agent
Cyclosporine derivatives are disclosed which possess enhanced efficacy and reduced toxicity over naturally occurring and other presently known cyclosporins and cyclosporine derivatives. The cyclosporine derivatives of the present invention are produced by chemical and isotopic substitution of the cyclosporine A (CsA) molecule by: (1) Chemical substitution and optionally deuterium substitution of amino acid 1; and (2) deuterium substitution at key sites of metabolism of the cyclosporine A molecule such as amino acids 1, 4, 9. Also disclosed are methods of producing the cyclosporine derivatives and method of producing immunosuppression with reduced toxicity with the disclosed cyclosporine derivatives.
Owner:NAICKER SALVARAJ +2
Novel cyclosporin analogues and their pharmaceutical uses
Owner:ALBANY MOLECULAR RESEARCH INC
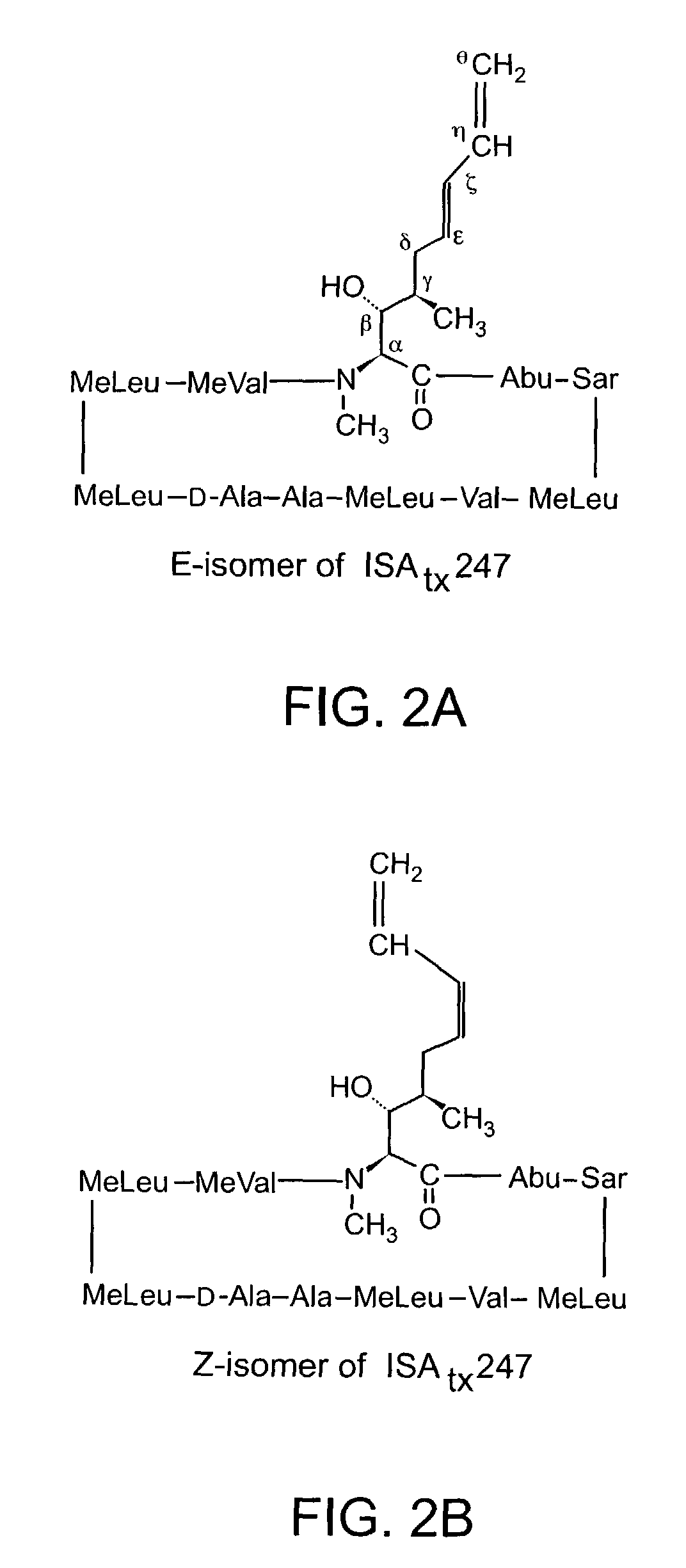
Cyclosporine analogue mixtures and their use as immunomodulating agents
ActiveUS6998385B2Potent immunosuppressantImprove effectivenessSenses disorderNervous disorderCyclosporinsImmunomodulating Agent
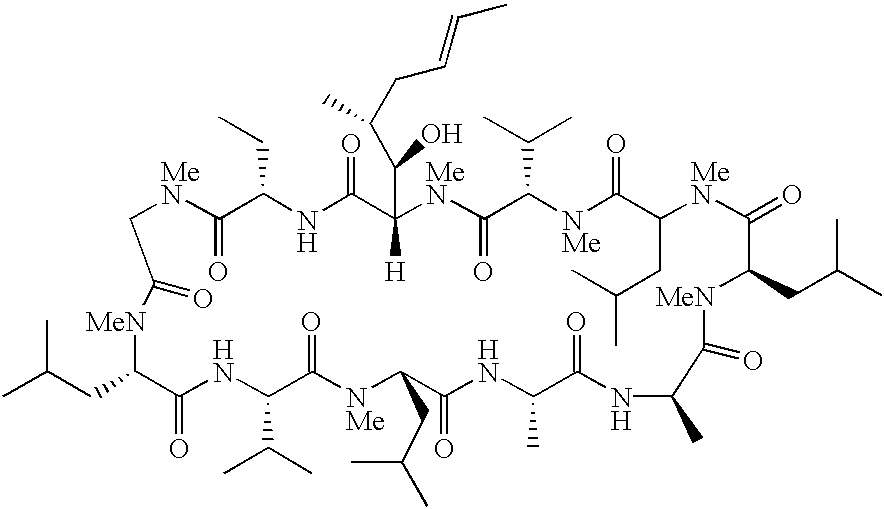
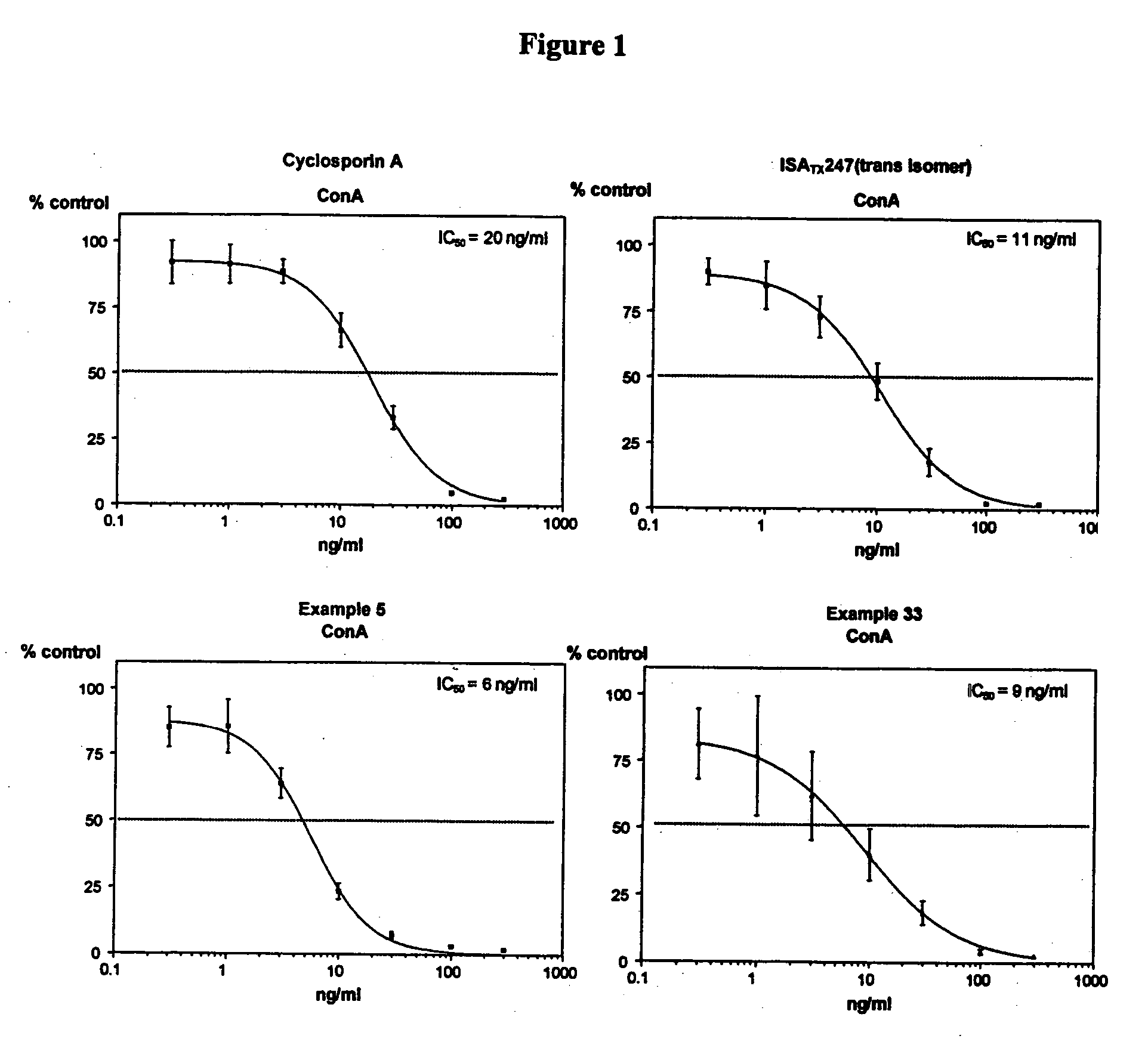
The invention is directed to isomeric mixtures of cyclosporine analogues that are structurally similar to cyclosporine A. The mixtures possess enhanced efficacy and reduced toxicity over the individual isomers and over naturally occurring and other presently known cyclosporines and cyclosporine derivatives. Embodiments of the present invention are directed toward cis and trans-isomers of cyclosporin A analogs referred to as ISATX247, and derivatives thereof. Mixtures of ISATX247 isomers exhibit a combination of enhanced potency and reduced toxicity over the naturally occurring and presently known cyclosporins. ISATX247 isomers and alkylated, arylated, and deuterated derivatives are synthesized by stereoselective pathways where the particular conditions of a reaction determine the degree of stereoselectivity. The ratio of isomers in a mixture may range from about 10 to 90 percent by weight of the (E)-isomer to about 90 to 10 percent by weight of the (Z)-isomer, based on the total weight of the mixture.
Owner:AURINIA PHARMA
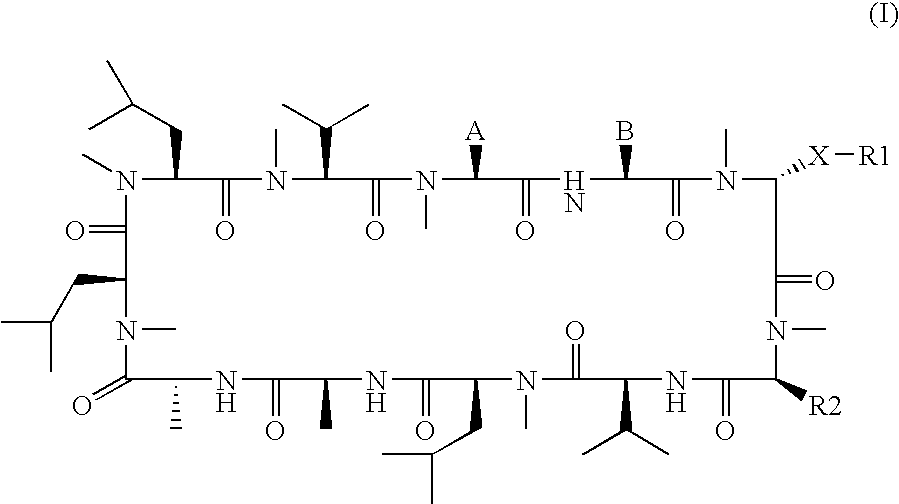
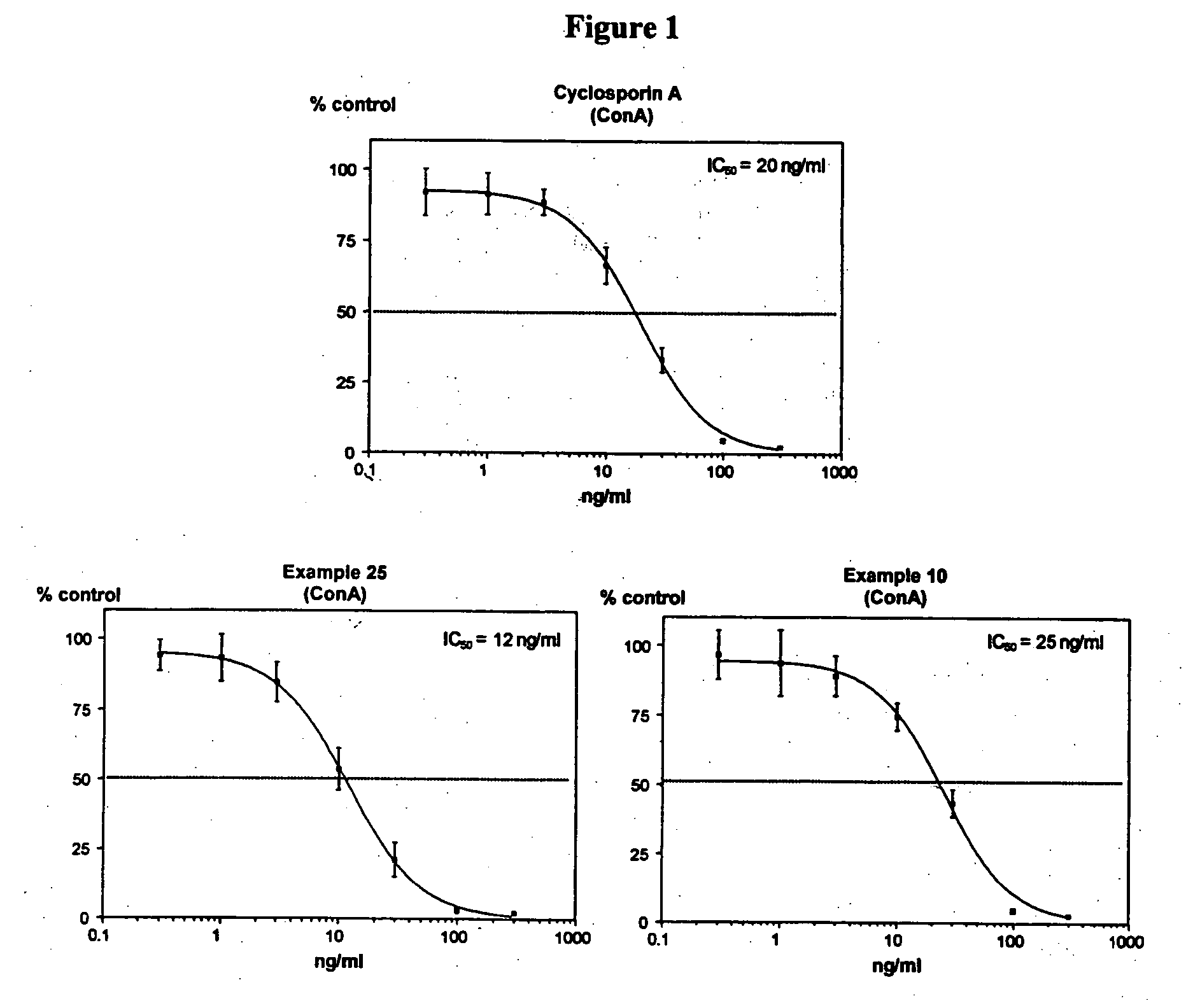
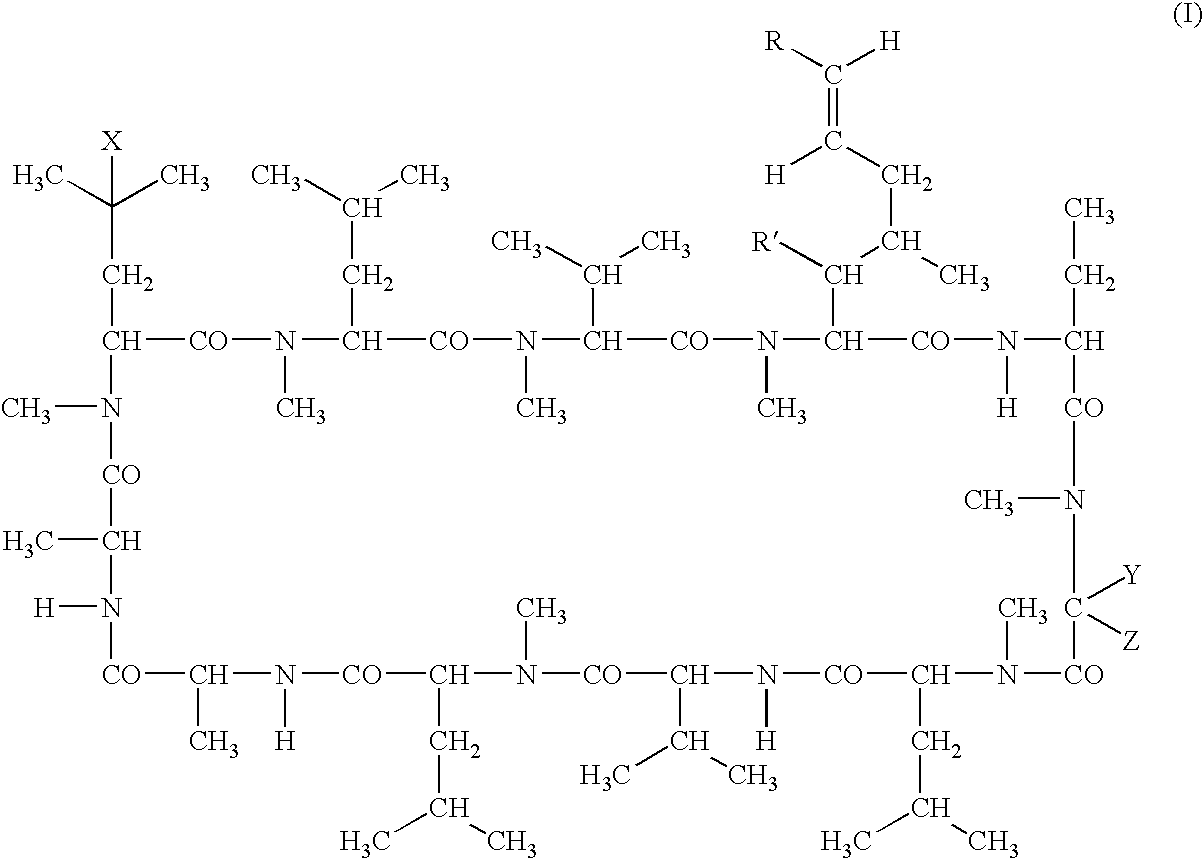
Cyclosporins for the treatment of immune disorders
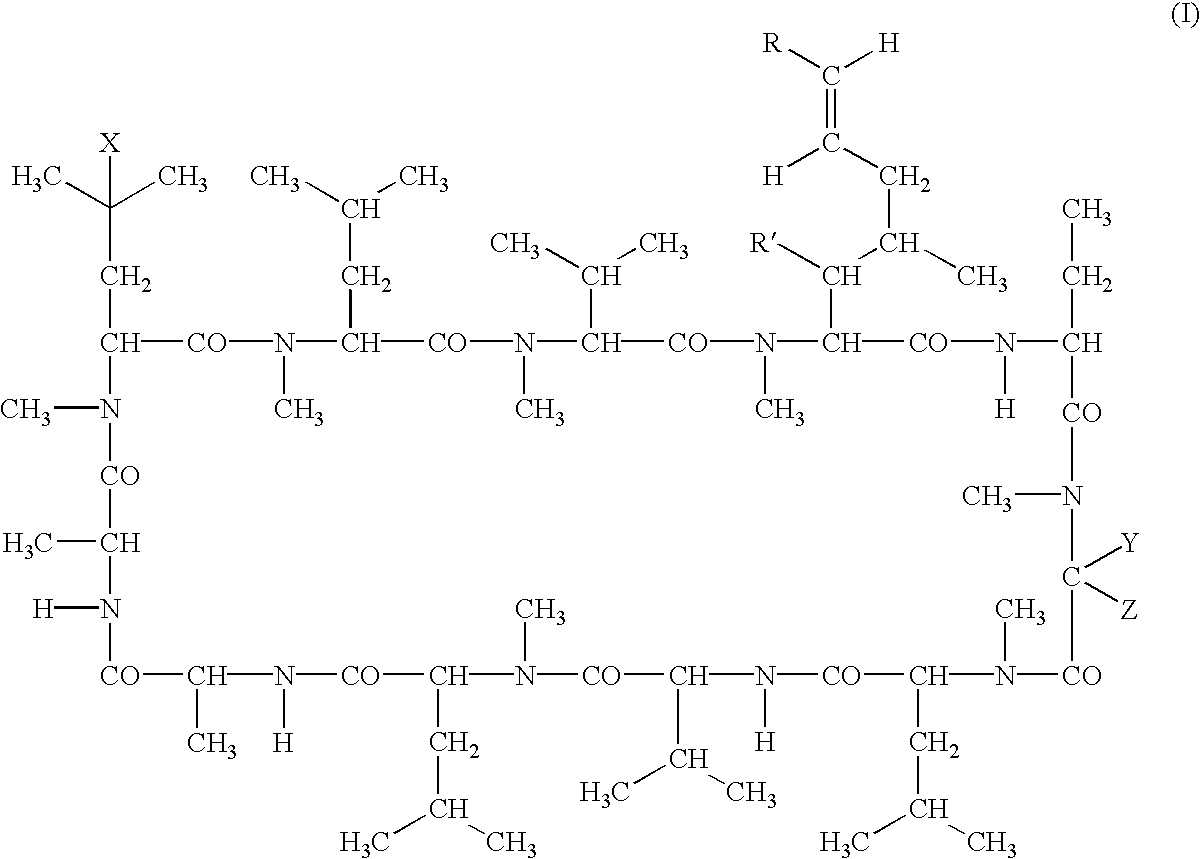
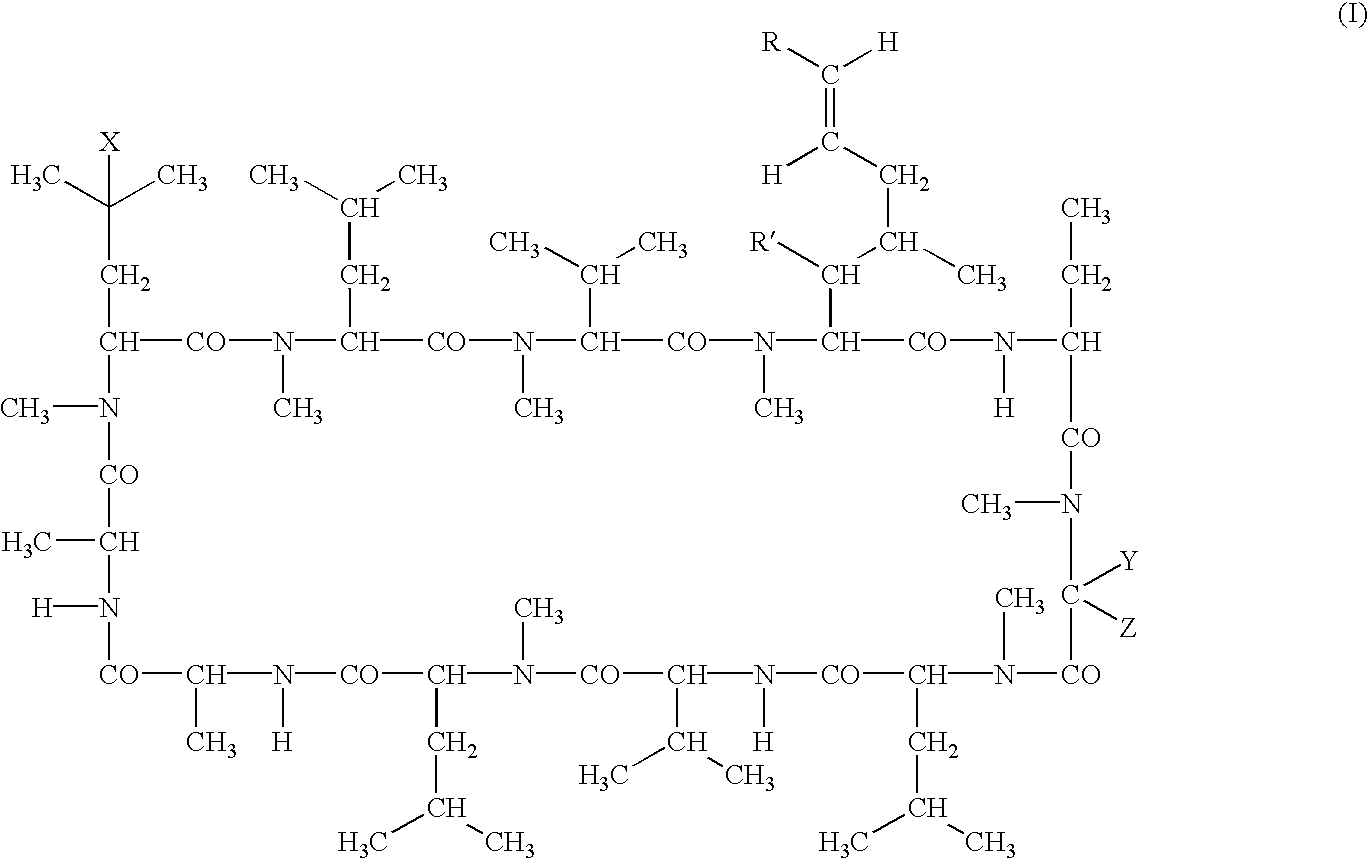
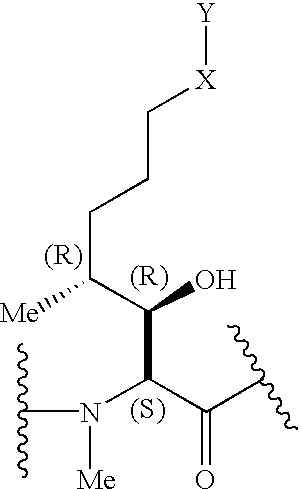
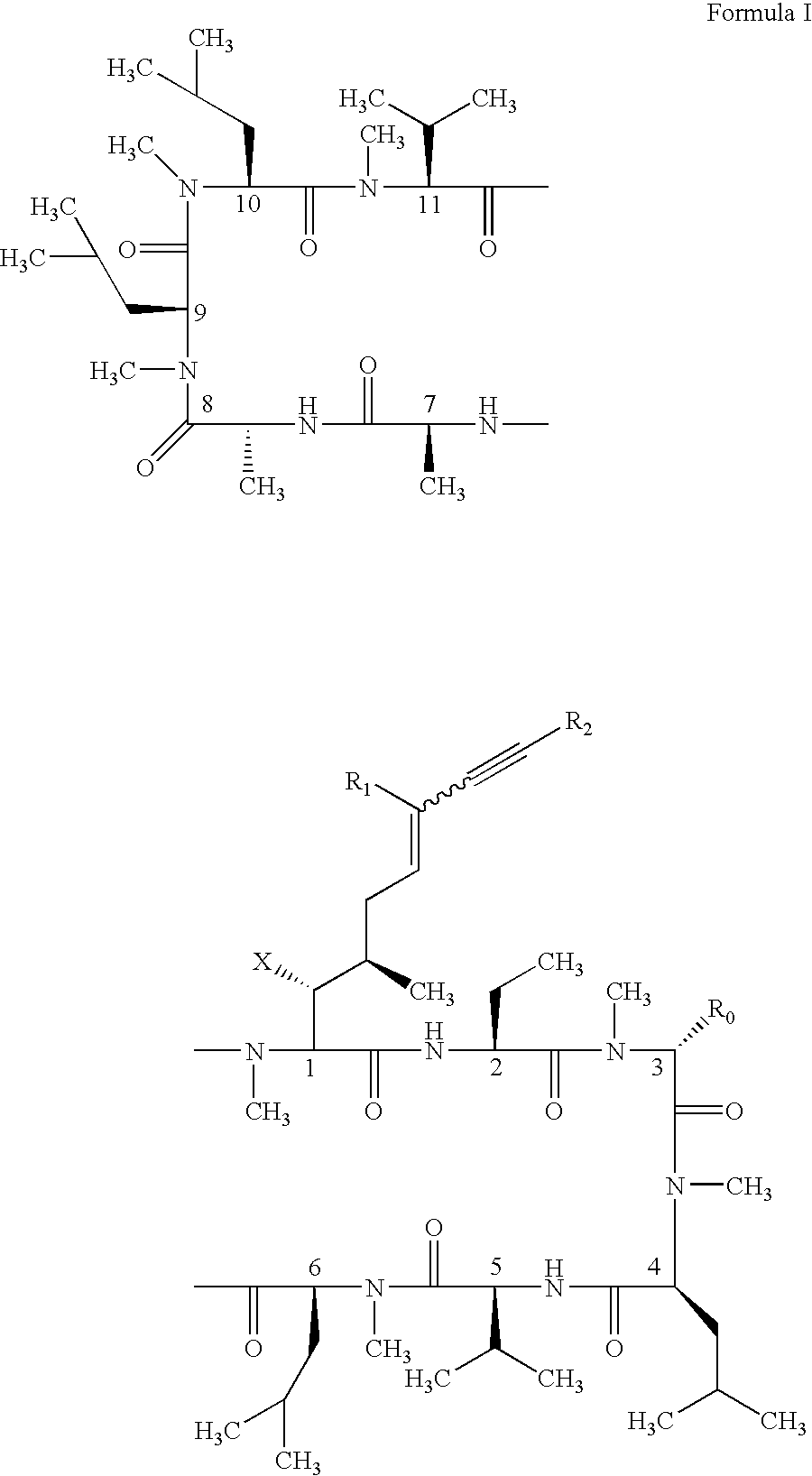
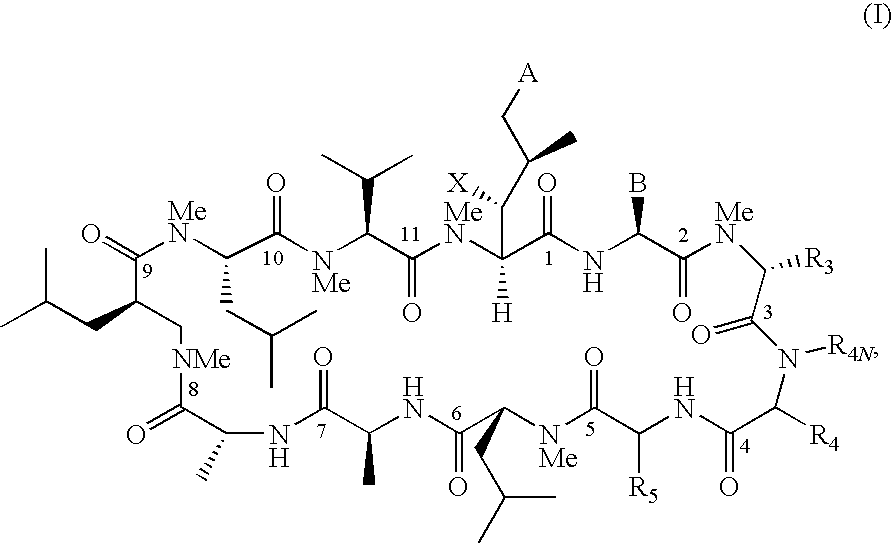
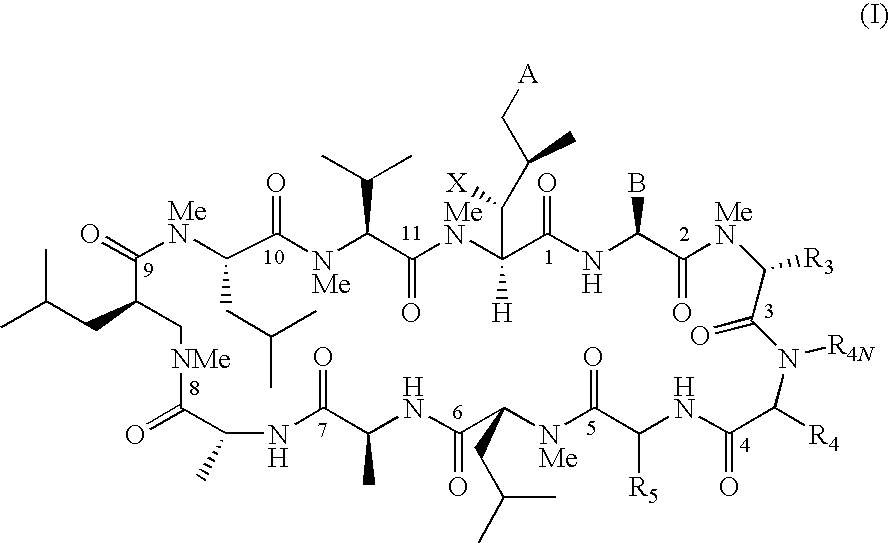
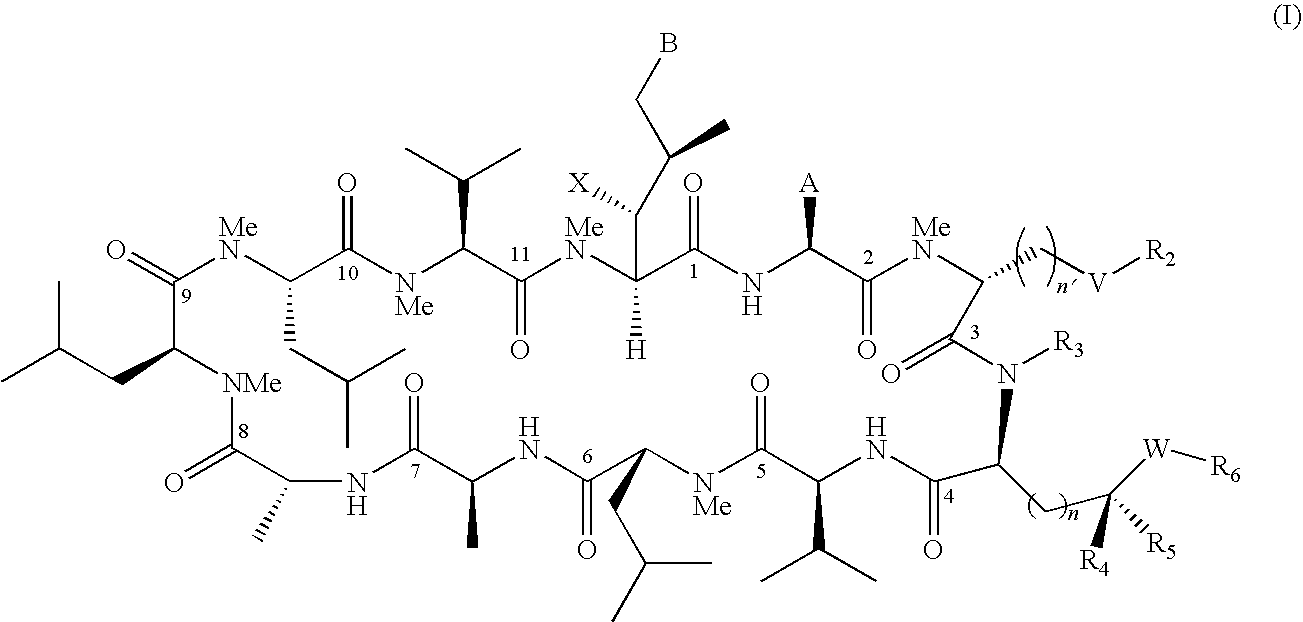
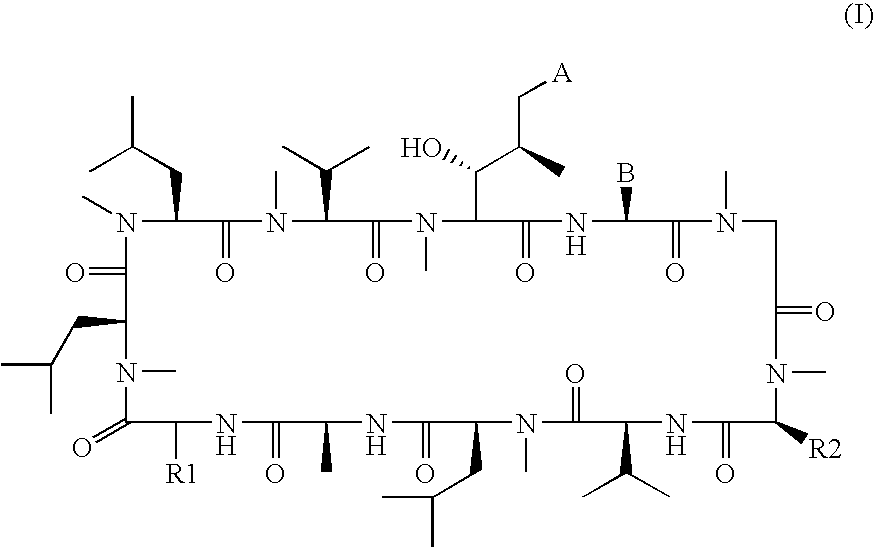
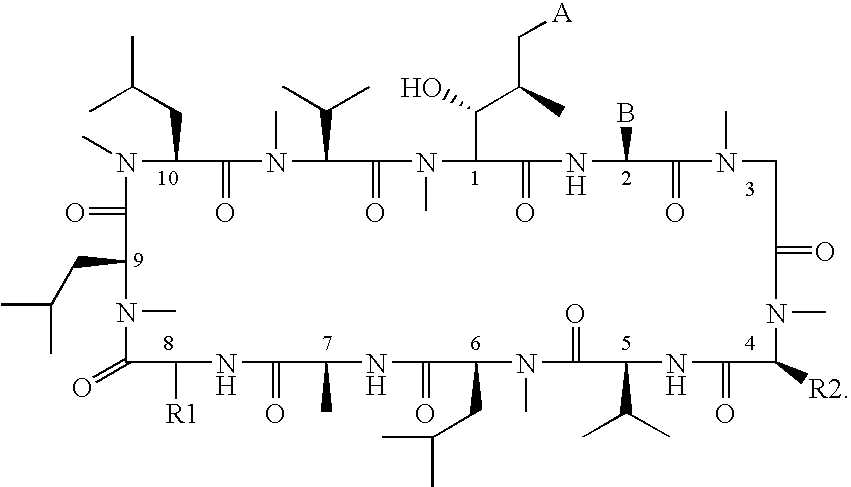
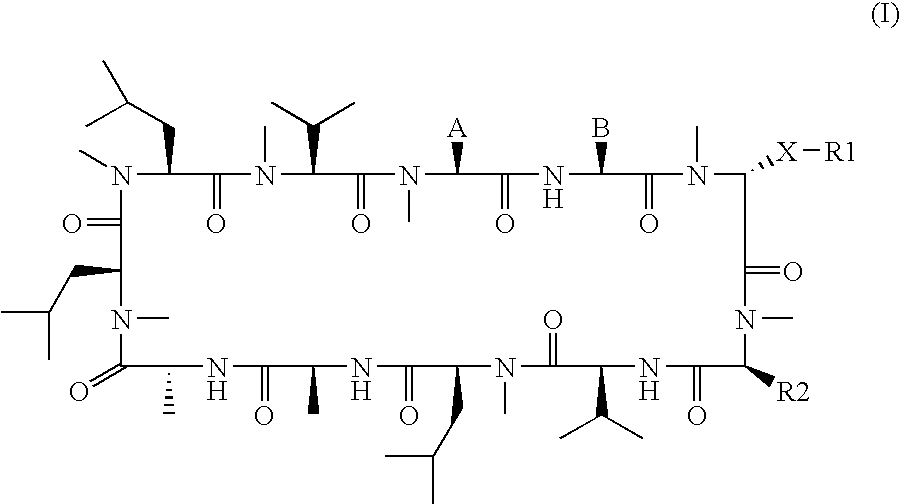
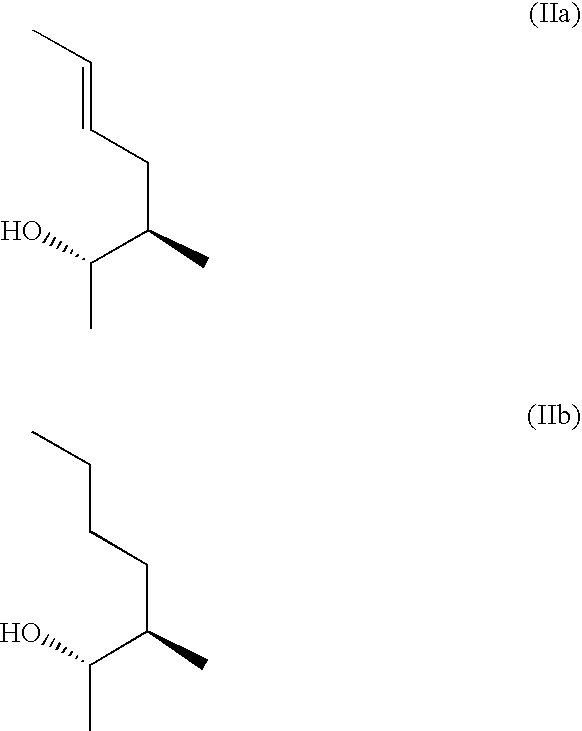
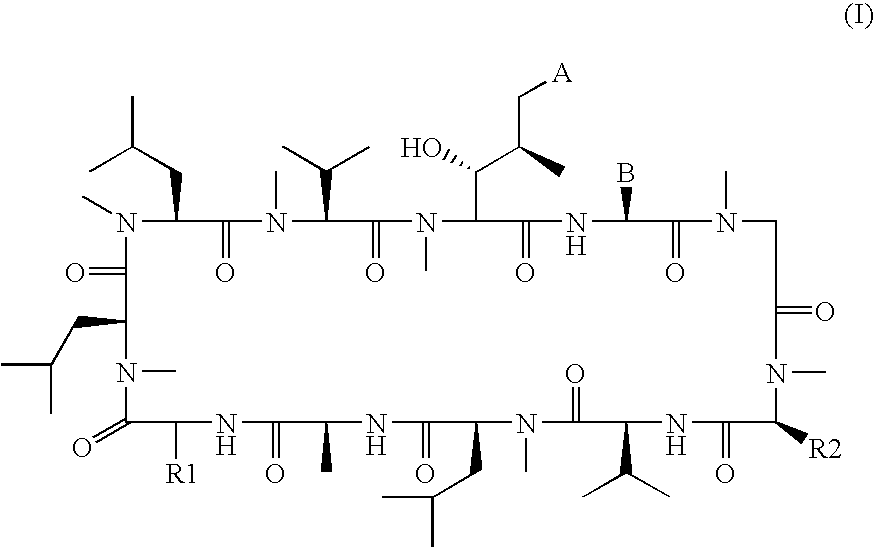
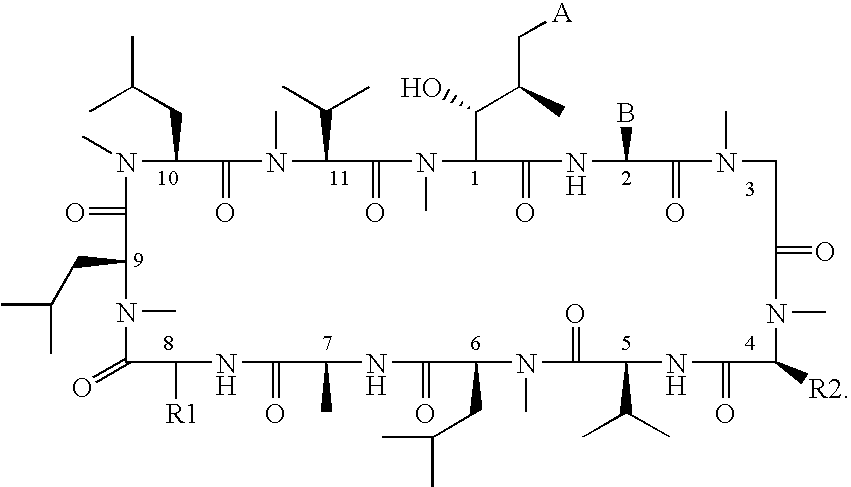
The present invention relates to methods of treating or preventing an inflammatory or immune disorder in a subject while eliminating or reducing the toxicity associated with the administration of cyclosporin A, comprising systemically administering to said subject a pharmaceutical composition comprising a therapeutically effective amount of at least one compound of Formula (I) or a pharmaceutically acceptable salt, ester or prodrug thereof, in combination with a pharmaceutically acceptable carrier or excipient: in Formula (I), the formula for residue A is:
Owner:ENANTA PHARM INC
Cyclosporins for the treatment of immune disorders
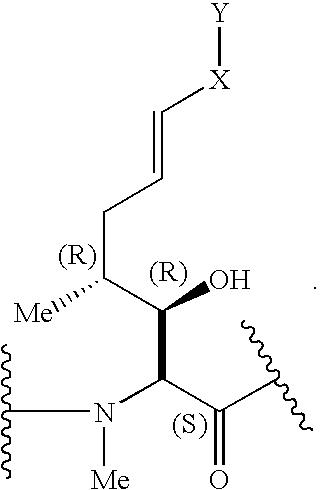
The present invention relates to a cyclosporin analog of the following formula (I) or its pro-drug or pharmaceutically acceptable salt: In formula I, the formula for residue A is: where X and Y are defined according to the claimed invention the present invention also relates to pharmaceutical compositions comprising pro-drugs or pharmaceutically acceptable salts of the compounds of the present invention and the use thereof for treating an inflammatory or immune disorder in a subject need of such treatment.
Owner:ENANTA PHARM INC
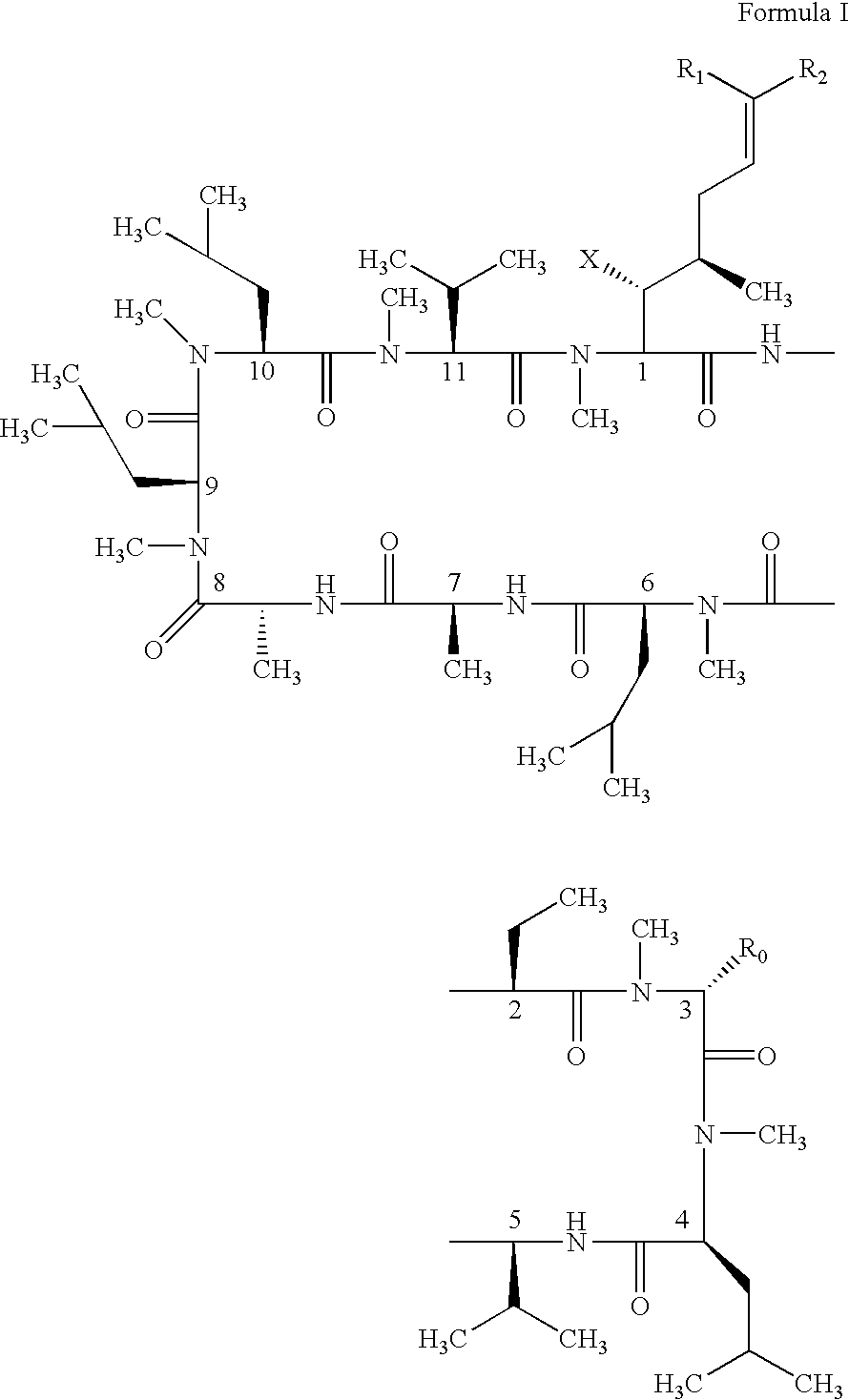
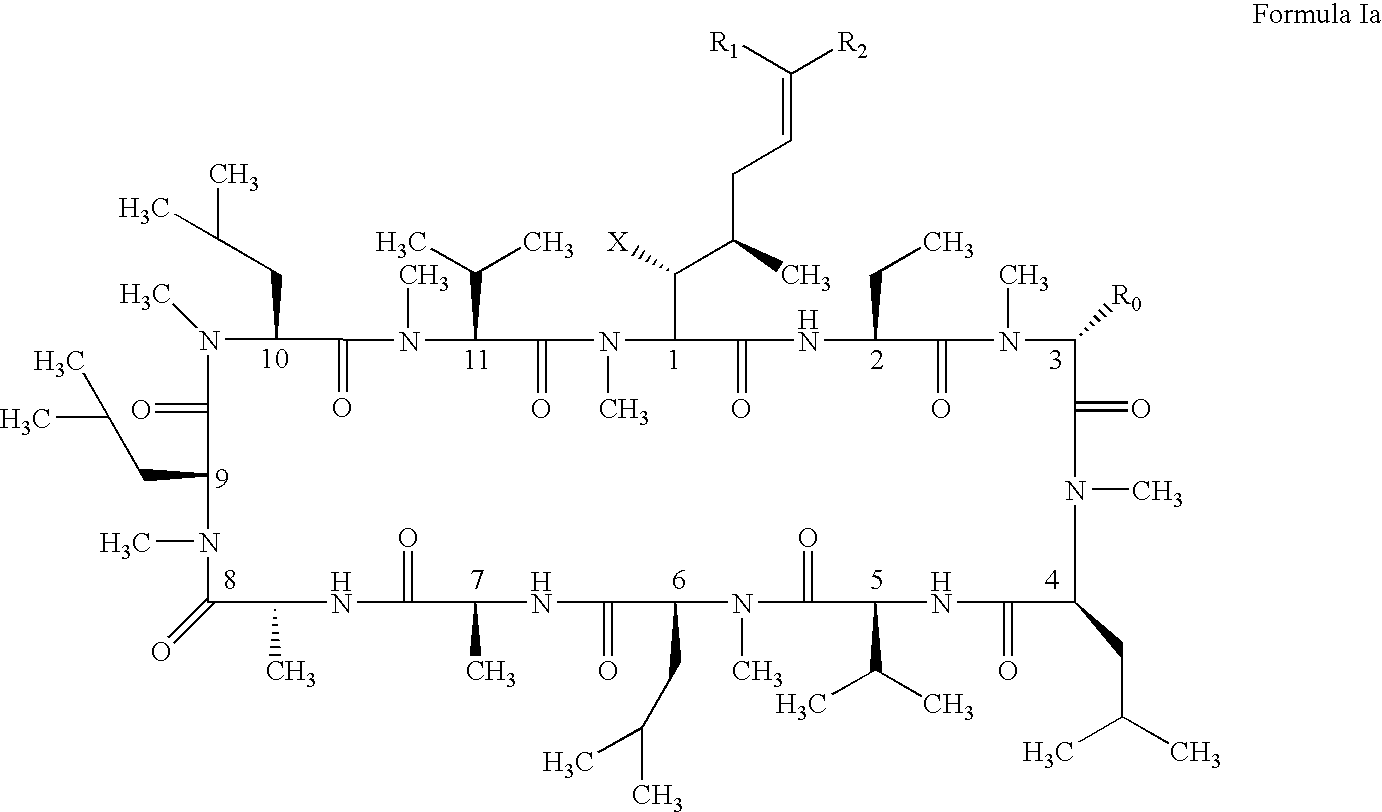
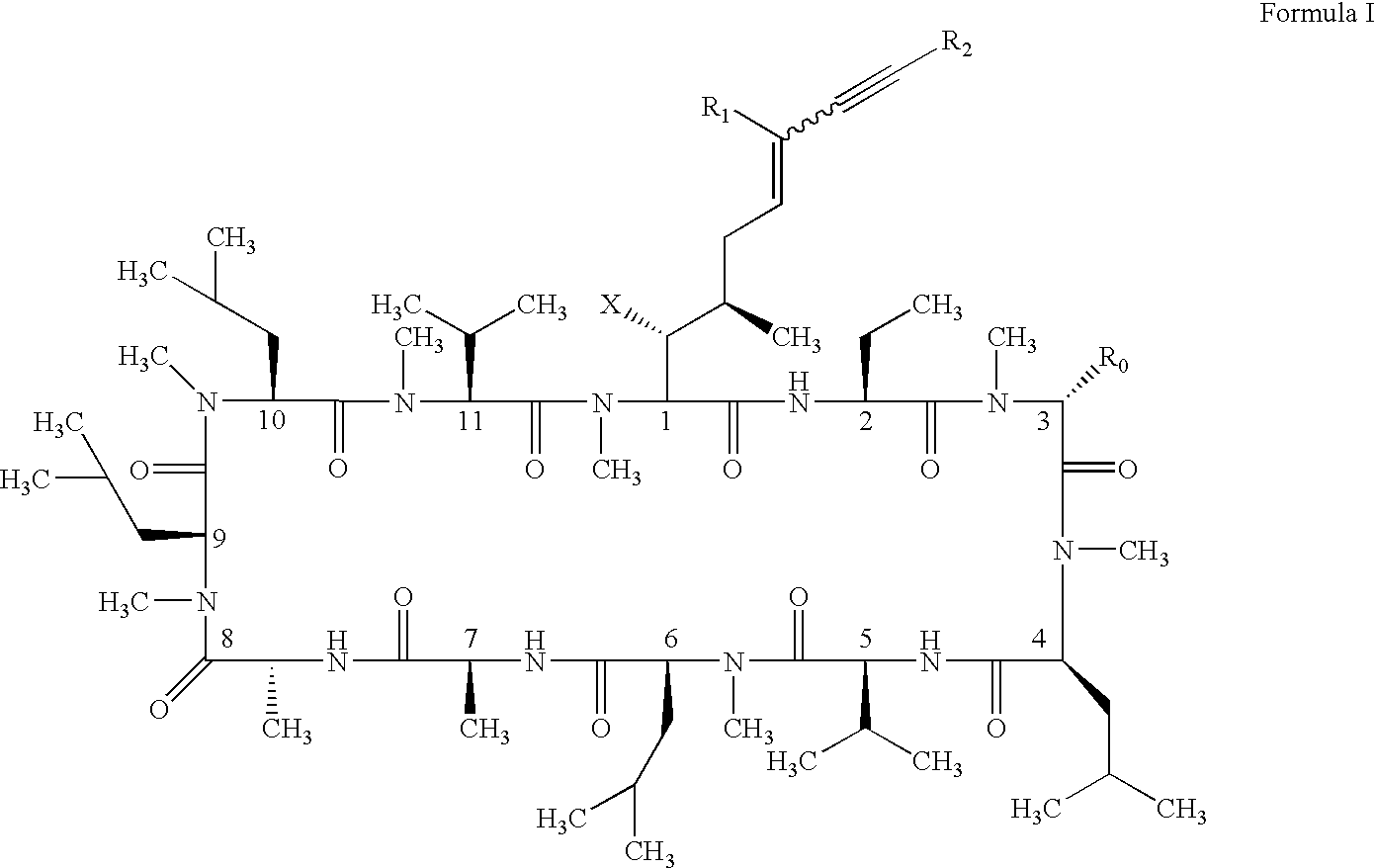
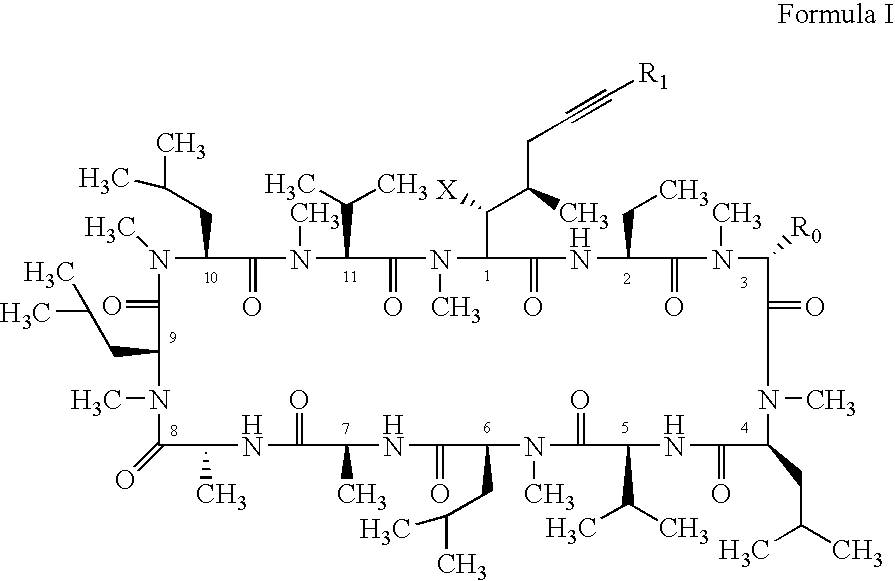
Cyclosporin alkyne analogues and their pharmaceutical uses
InactiveUS20060069016A1High activityPossess utility in the treatmentSenses disorderNervous disorderChemical structureCyclosporins
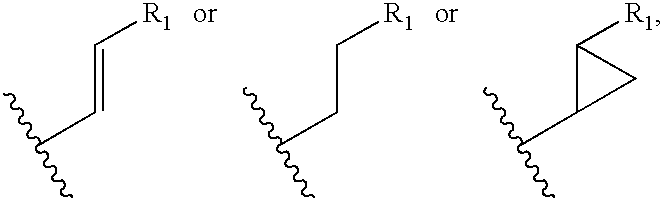
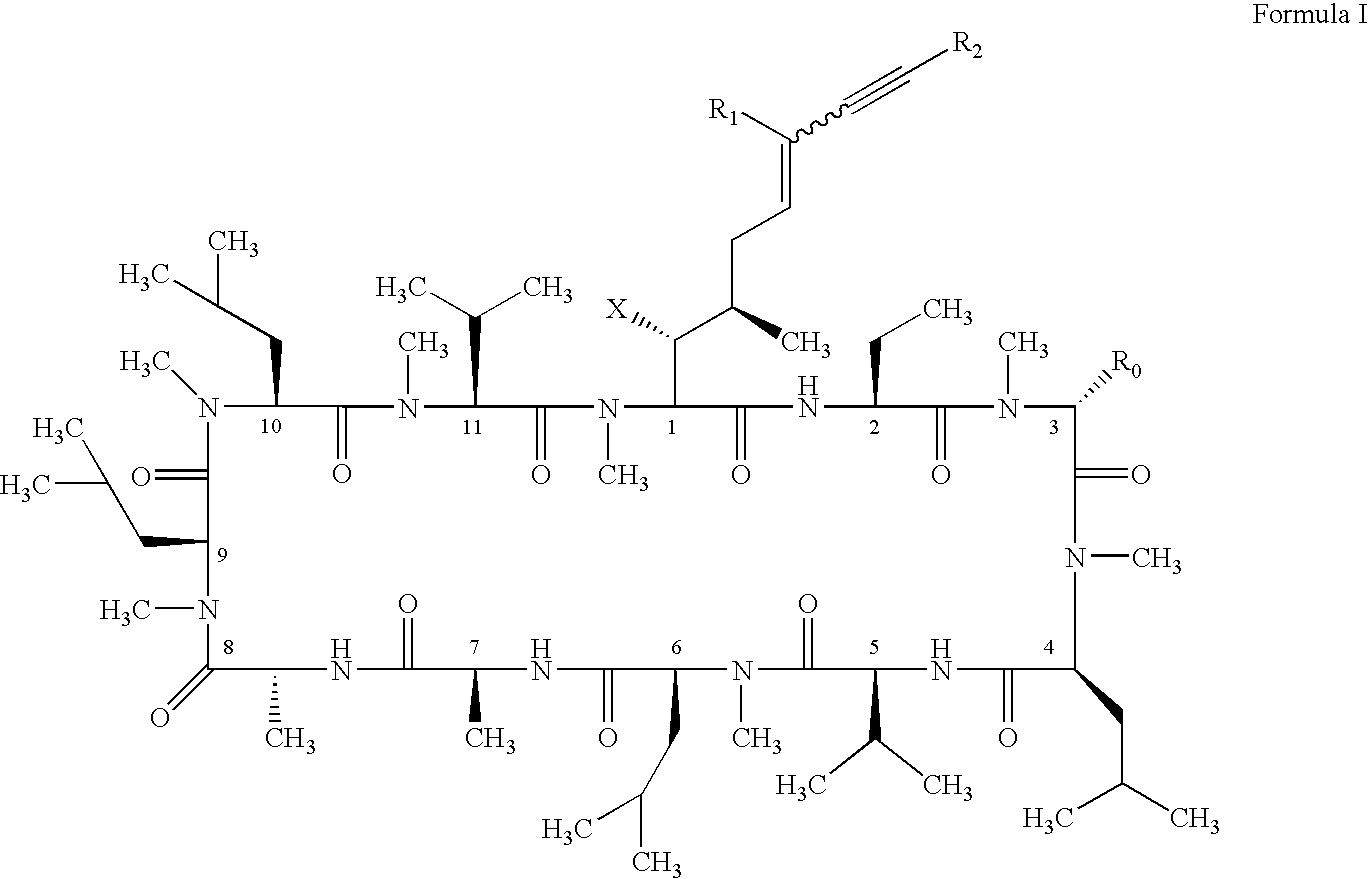
The compounds of the present invention are represented by the chemical structure found in Formula I: or a pharmaceutically acceptable salt thereof, with X, R0, R1, and R2 defined herein.
Owner:ALBANY MOLECULAR RESEARCH INC
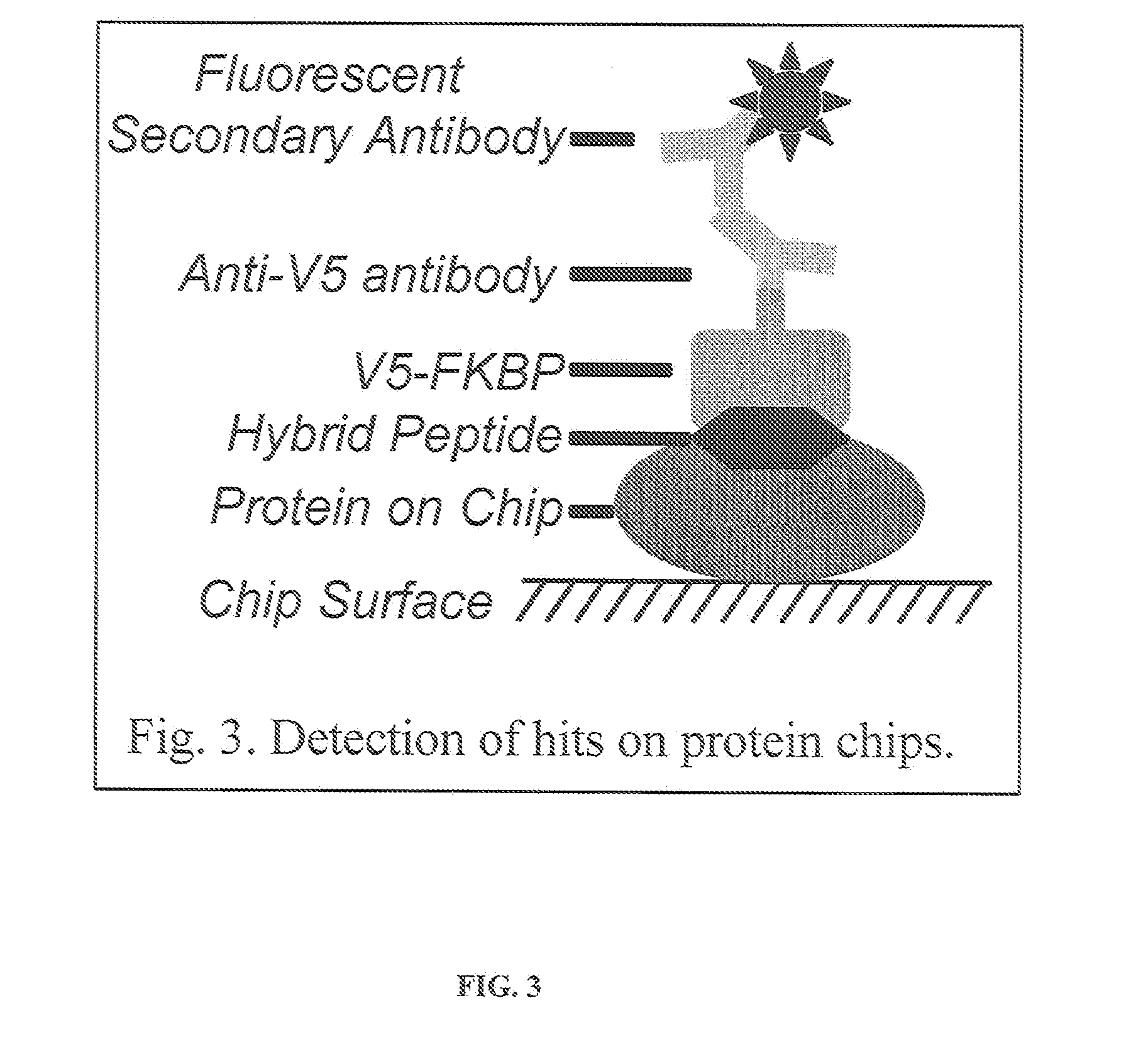
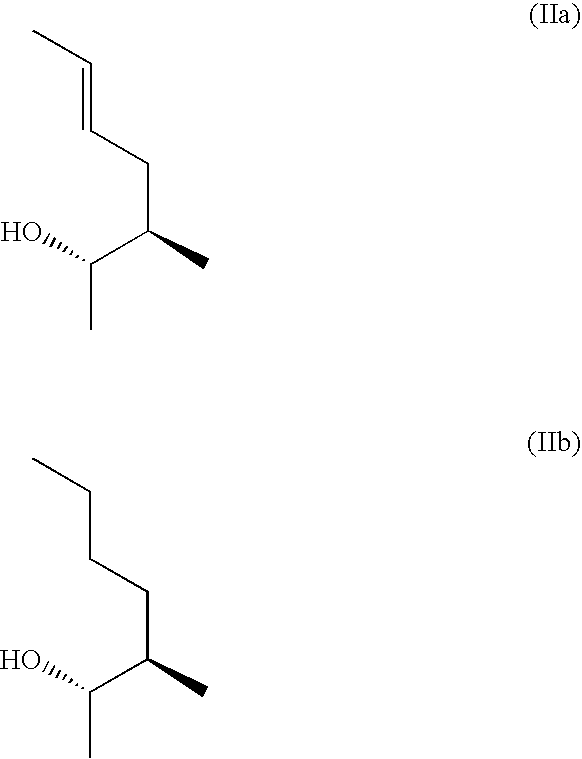
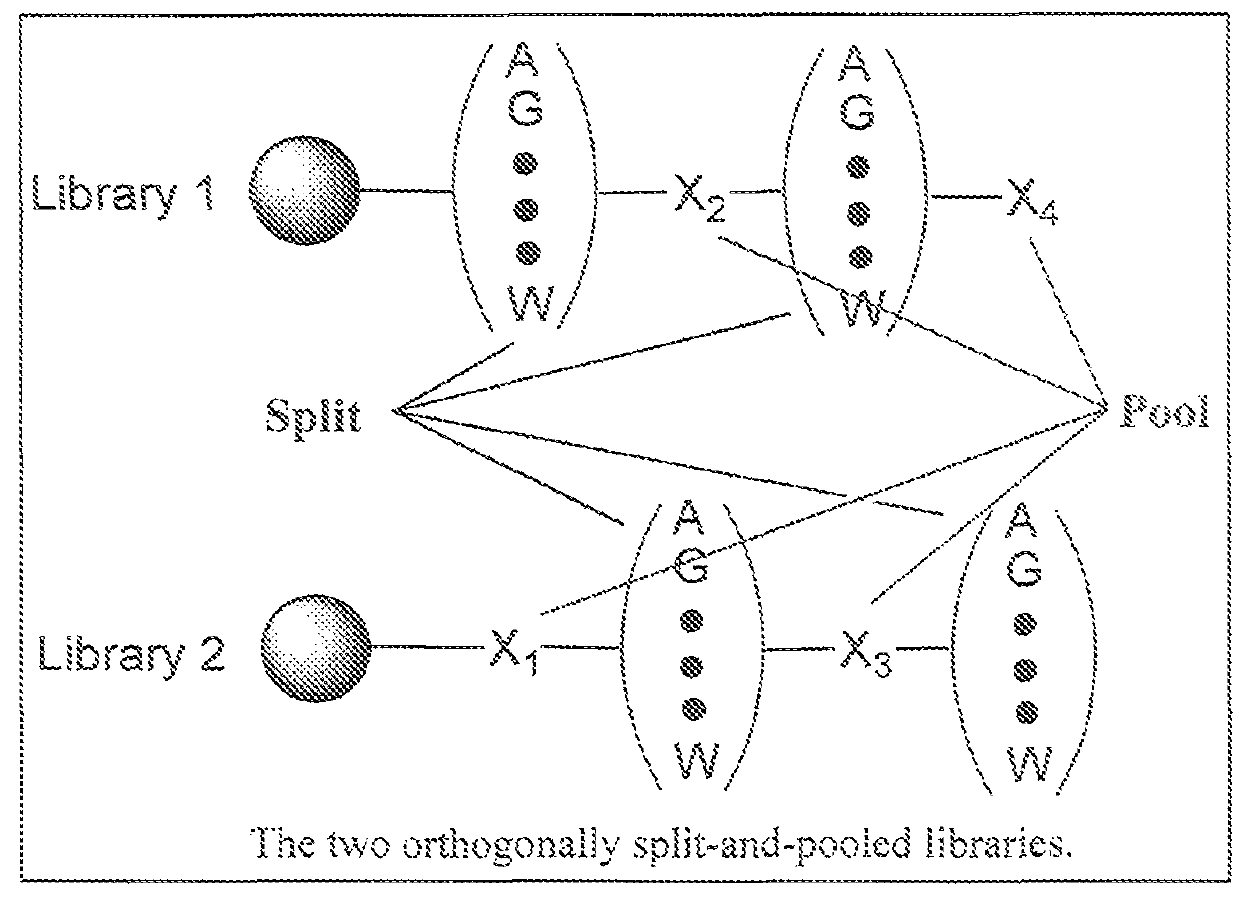
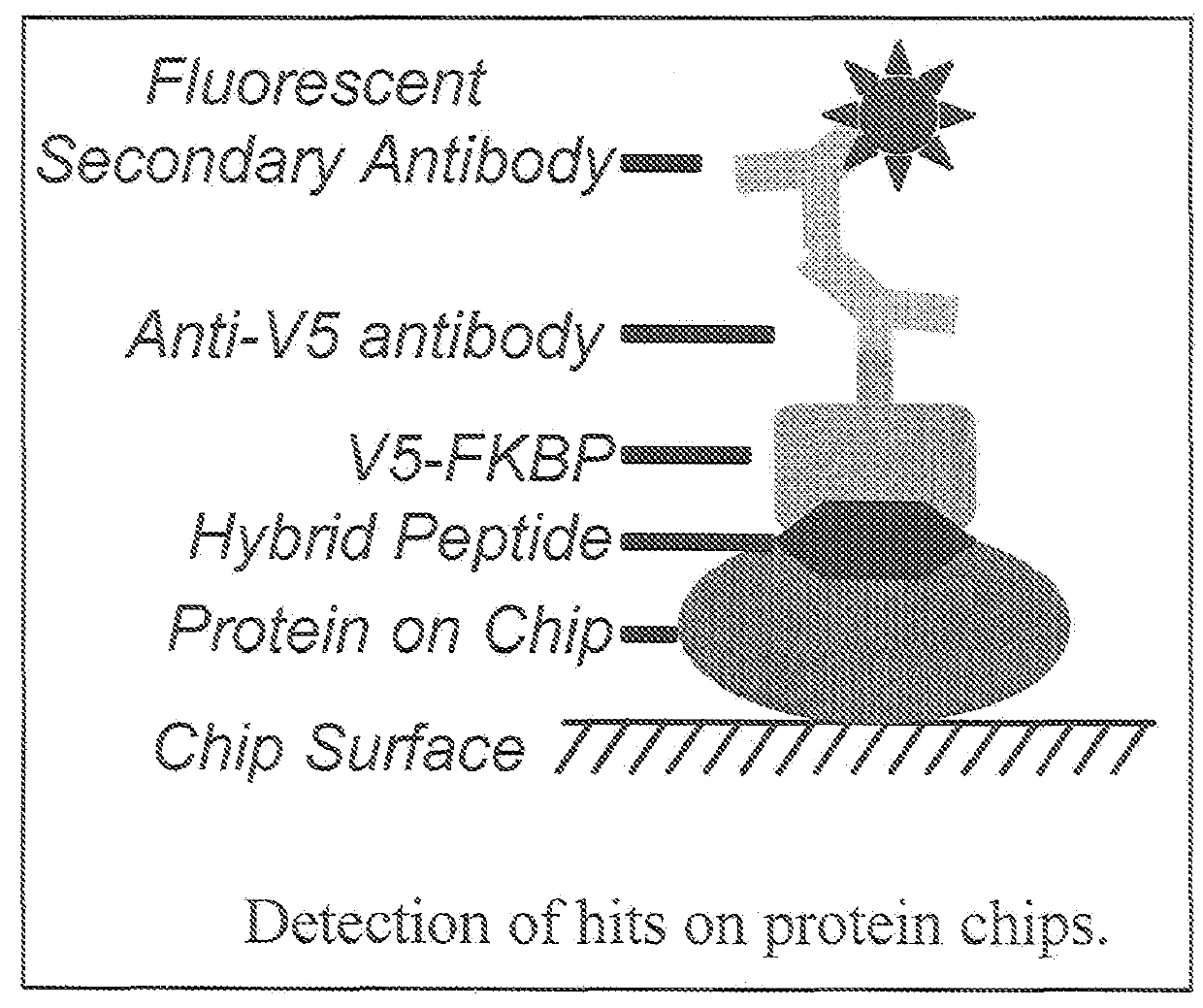
Hybrid Cyclic Libraries and Screens Thereof
Provided are novel types of hybrid cyclic libraries that contain a known protein binding domain of a natural product. Also provided are synthetic methods to make such libraries and methods for the deconvolution of hits using partially split-pooled library compounds. Such methods are applicable for use with the entire human proteome to screen such libraries that bind and for the identification of hits.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
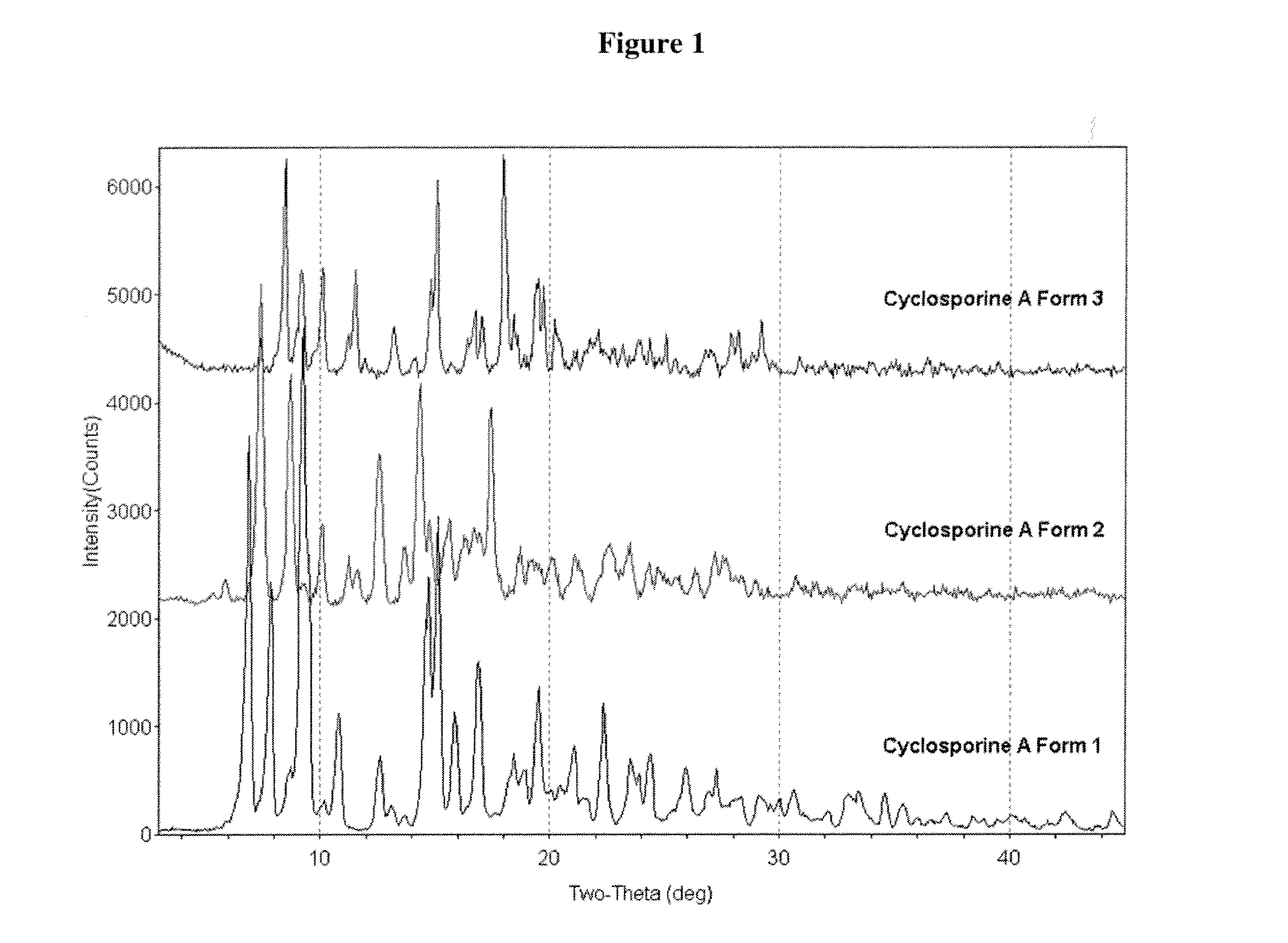
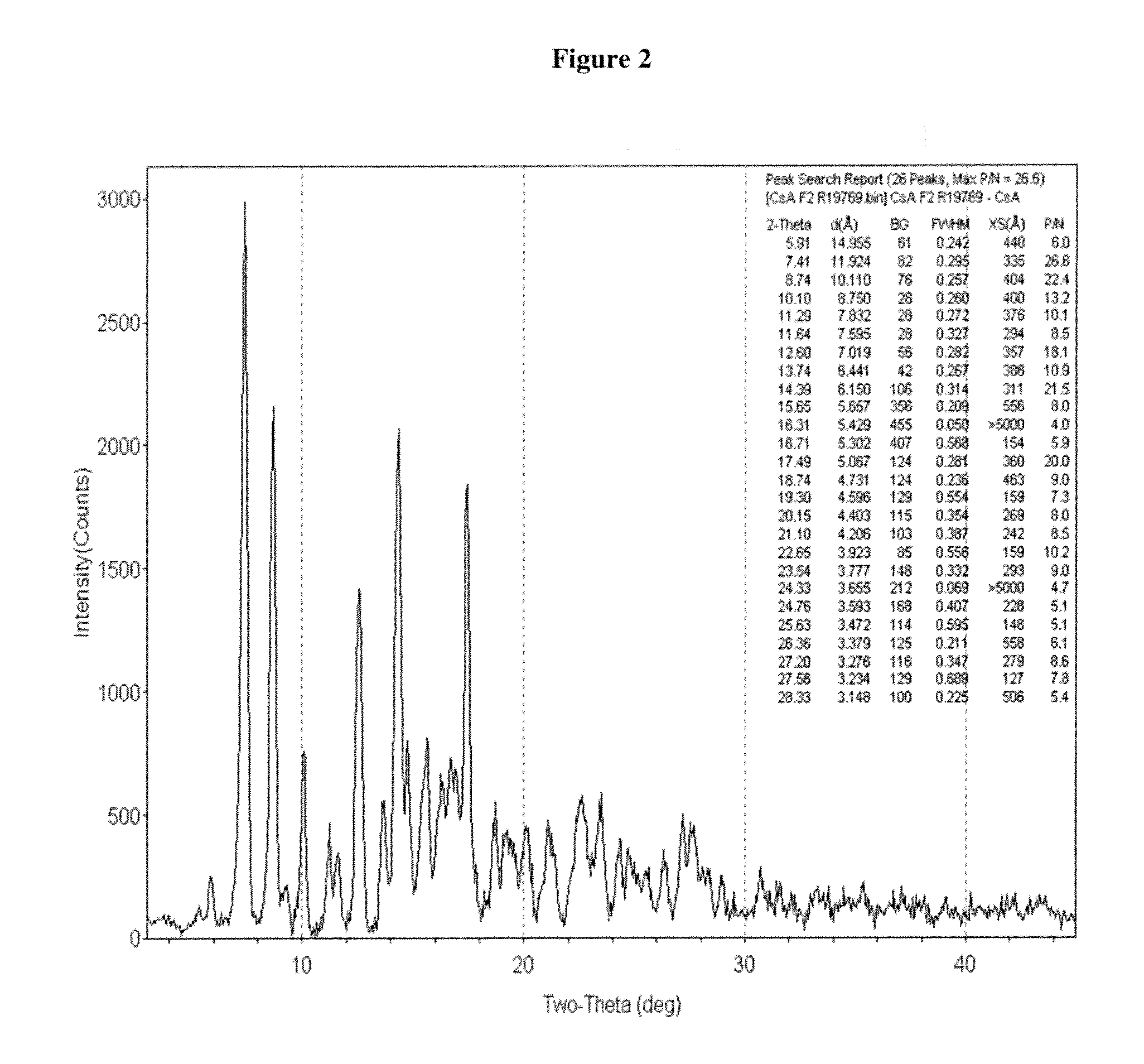
Crystalline form of cyclosporine a, methods of preparation, and methods for use thereof
Owner:ALLERGAN INC
Cyclosporin a conjugates and uses therefor
InactiveUS6316405B1Highly effective to treat or prevent neurological disordersAct synergisticallyNervous disorderMetabolism disorderAmyotrophic lateral sclerosisAmyloid
Disclosed are conjugates of Abeta-binding peptides and CsA analogs and conjugates of Abeta-binding peptides and FK506 Binding Peptide inhibitors. These conjugates chemically induce dimerization of either cyclophilin or FK506 Binding Peptide with Abeta peptide, a major component of amyloid plaques found in neurological disorders such as Alzheimer's disease, multiple sclerosis, and amyotrophic lateral sclerosis. The conjugates are useful in the treatment of neurological diseases involving the formation of amyloid plaques because they inhibit and / or prevent the aggregation and deposition of Abeta peptide into plaques.
Owner:WISCONSIN ALUMNI RES FOUND
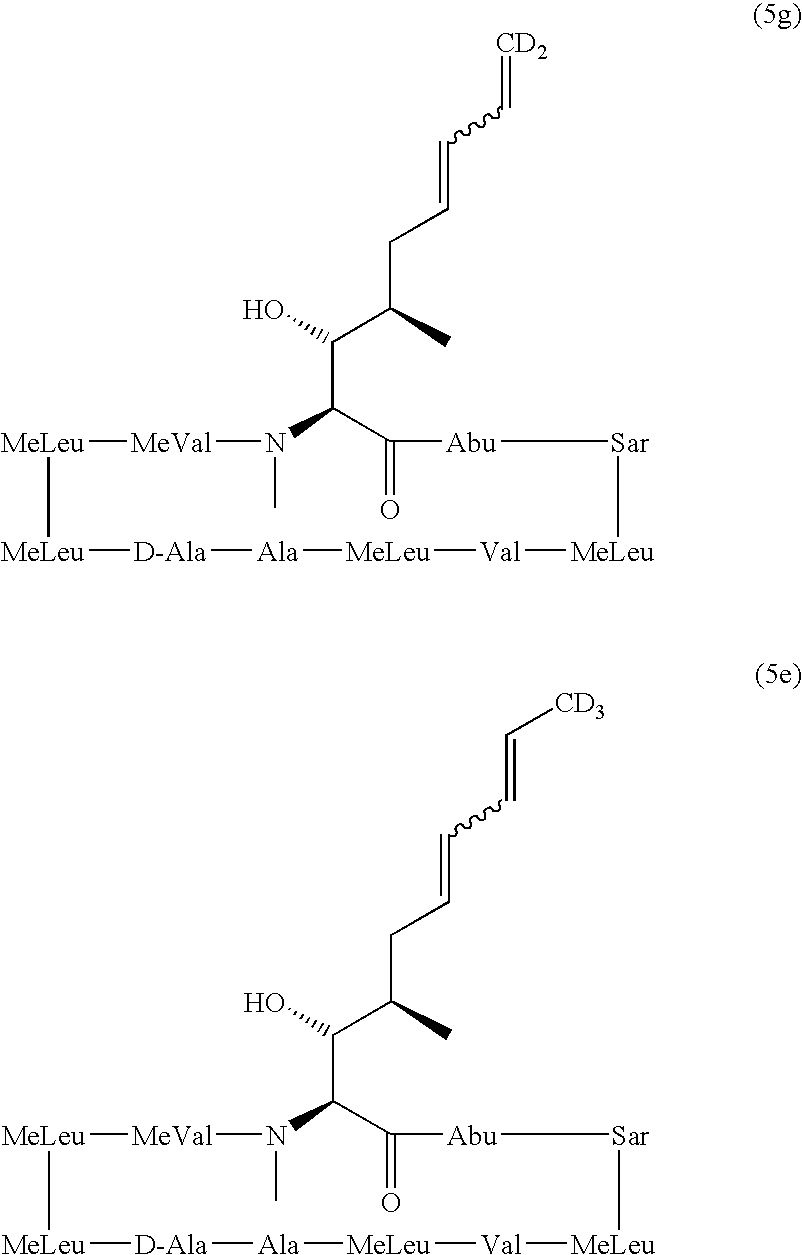
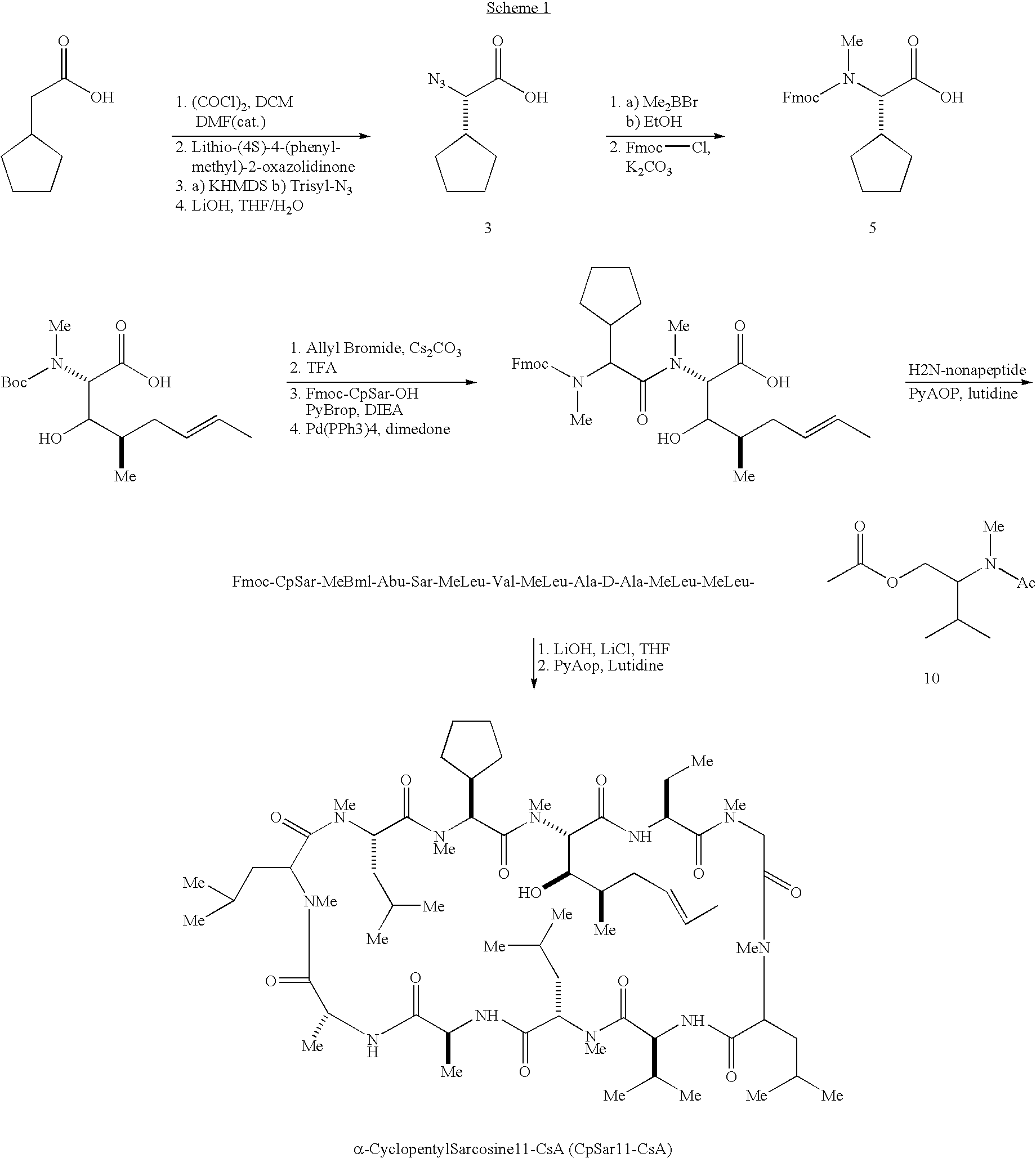
Cyclosporin analogues
ActiveUS20100209390A1Inhibition of replicationAntibacterial agentsBiocideHepatitis c viralCyclosporins
The present invention provides cyclosporin analogues of formula I,and compositions comprising these compounds, as well as processes for their preparation, intermediates in their synthesis, and methods of use thereof for prevention of organ transplantation rejection, the treatment of immune disorders and inflammation, and treatment of viral (particularly hepatitis C viral) infection.
Owner:ENANTA PHARM INC
3-Ether and 3-Thioether Substituted Cyclosporin Derivatives For the Treatment and Prevention of Hepatitis C Infection
InactiveUS20100167996A1Increased safety marginImproved hepatotoxicityDigestive systemAntiviralsMedicineCyclosporins
Owner:FLIRI HANS GEORG +1
Hybrid cyclic libraries and screens thereof
Provided are novel types of hybrid cyclic libraries that contain a known protein binding domain of a natural product. Also provided are synthetic methods to make such libraries and methods for the deconvolution of hits using partially split-pooled library compounds. Such methods are applicable for use with the entire human proteome to screen such libraries that bind and for the identification of hits.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Cyclosporin analogues for preventing or treating hepatitis c infection
Owner:ENANTA PHARM INC
3-Ether and 3-thioether substituted cyclosporin derivatives for the treatment and prevention of hepatitis C infection
InactiveUS20060160727A1Increased safety marginImproved hepatotoxicityBiocideDigestive systemCyclosporinsPharmaceutical drug
Owner:SCYNEXIS INC
Novel cyclosporin alkynes and their utility as pharmaceutical agents
InactiveUS20060074015A1Good immunosuppressive activityHigh activitySenses disorderNervous disorderChemical structureCyclosporins
The compounds of the present invention are represented by the chemical structure found in Formula I: or a pharmaceutically acceptable salt thereof, with X, R0, and R1 defined herein.
Owner:ALBANY MOLECULAR RESEARCH INC
Cyclosporin derivatives for the treatment of immune disorders
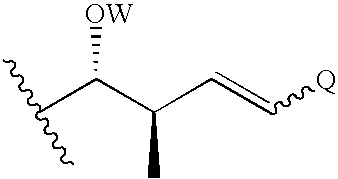
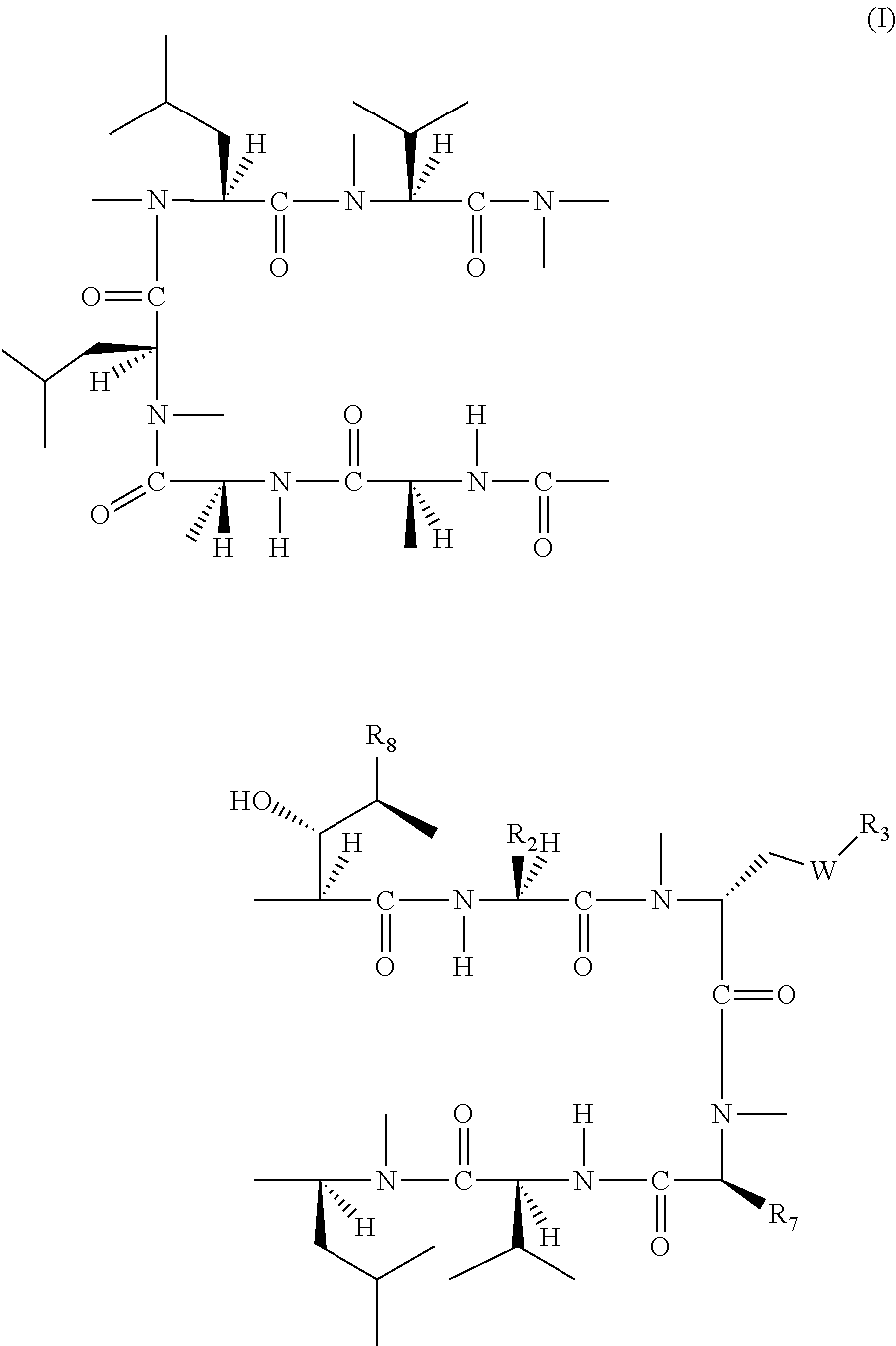
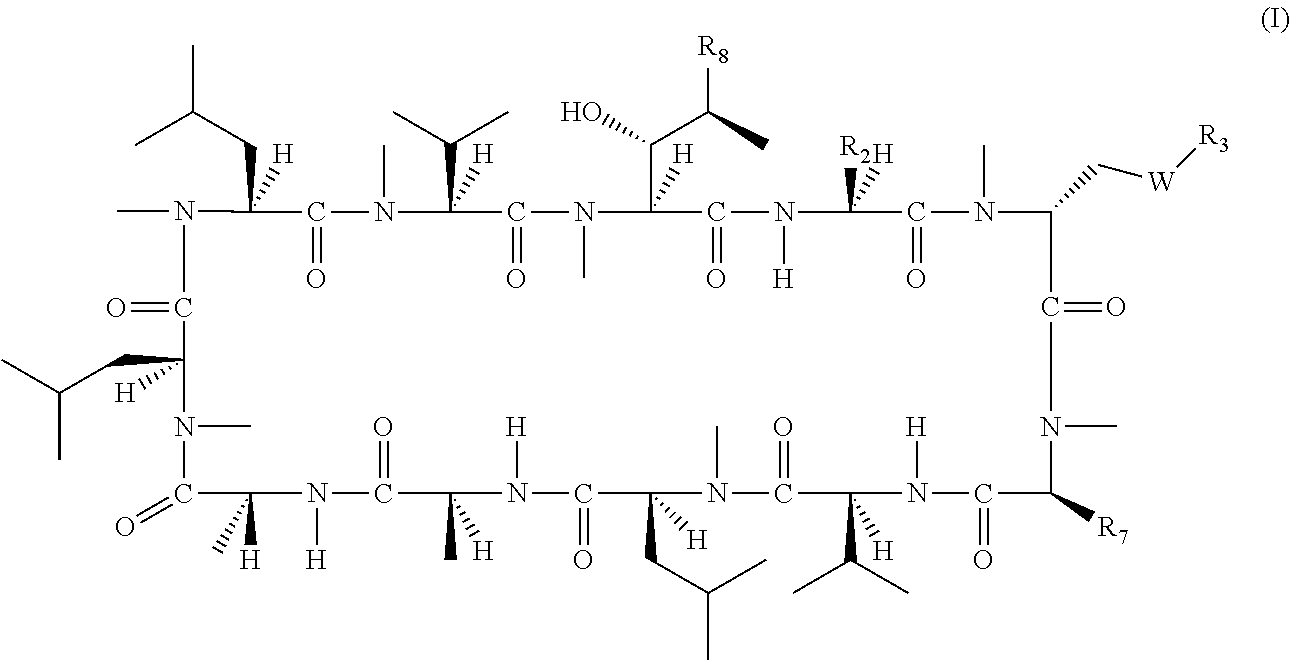
The present invention relates to a cyclosporin analog of the following formula (I) or a pro-drug or pharmaceutically acceptable salt thereof:whereinA is of the formula:where Q, W, X, Y, and Z are defined herein. In a second embodiment, the present invention relates to pharmaceutical compositions comprising pro-drugs or pharmaceutically acceptable salts of the compounds of the present invention and the use thereof for treating autoimmune diseases or for the prevention of organ transplantation rejection in a subject. In a third embodiment, the present invention relates to processes for the production of novel cyclosporin analogs of the present invention.
Owner:ENANTA PHARM INC
Deuterated cyclosporin analogs and their use as immunomodulating agents
InactiveUS20050176628A1Good curative effectAltered pharmacokinetic and pharmacodynamic parametersIsotope introduction to peptides/proteinsPeptide preparation methodsCyclosporinsImmunomodulating Agent
Cyclosporine derivatives are disclosed which possess enhanced efficacy and reduced toxicity over naturally occurring and other presently known cyclosporins and cyclosporine derivatives. The cyclosporine derivatives of the present invention are produced by chemical and isotopic substitution of the cyclosporine A (CsA) molecule by: (1) Chemical substitution and optionally deuterium substitution of amino acid 1; and (2) deuterium substitution at key sites of metabolism of the cyclosporine A molecule such as amino acids 1, 4, 9. Also disclosed are methods of producing the cyclosporine derivatives and method of producing immunosuppression with reduced toxicity with the disclosed cyclosporine derivatives.
Owner:AURINIA PHARMA
Novel cyclic peptides
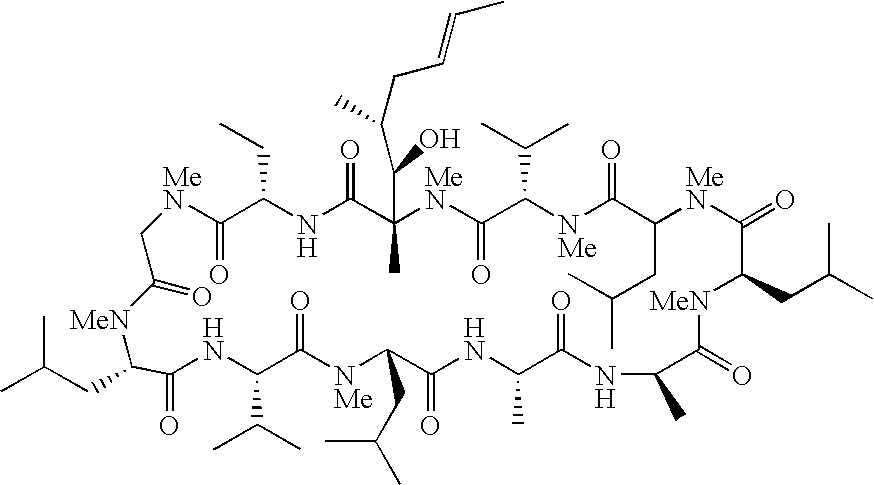
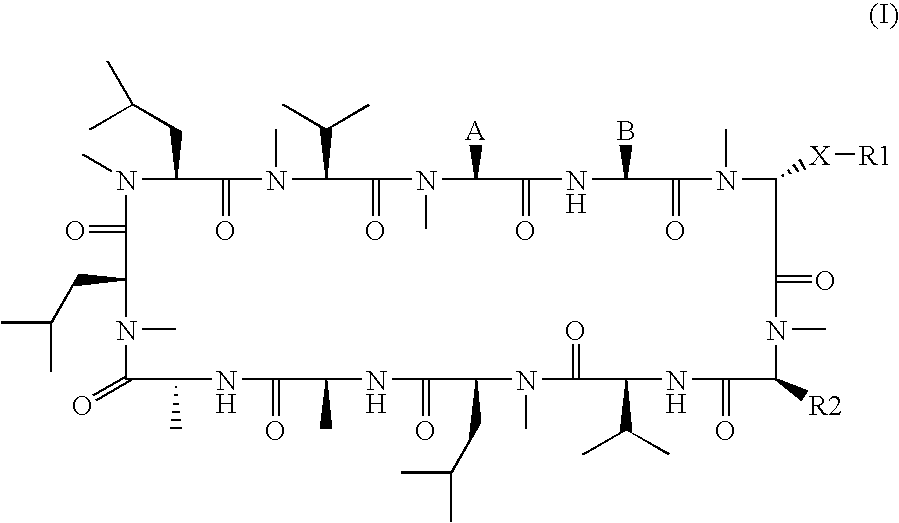
The invention relates to novel cyclic peptide derivatives of general formula (I):wherein A, B, R1 and R2 are as defined in the specification, pharmaceutically acceptable salts thereof, and their use as pharmaceuticals, in particular for the treatment of hepatitis C virus.
Owner:SCYNEXIS INC
3-ether and 3-thioether substituted cyclosporin derivatives for the treatment and prevention of hepatitis C infection
InactiveUS20060089301A1Increased safety marginImproved hepatotoxicityBiocideDigestive systemMedicineCyclosporins
Owner:SCYNEXIS INC
Novel cyclosporin derivatives and uses thereof
The present invention relates to a compound of the Formula (I)):or pharmaceutically acceptable salt thereof, wherein the symbols are as defined in the specification; a pharmaceutical composition comprising the same, a method for treating or preventing viral infections, inflammation, dry eye, central nervous disorders, cardiovascular diseases, cancer, obesity, diabetes, muscular dystrophy, and hair loss.
Owner:S&T GLOBAL
Cyclic peptides
Owner:SCYNEXIS INC
Cyclosporin alkyne analogues and their pharmaceutical uses
InactiveUS7378391B2High activityPossess utility in the treatmentSenses disorderNervous disorderChemical structureCyclosporins
The compounds of the present invention are represented by the chemical structure found in Formula I:or a pharmaceutically acceptable salt thereof, with X, R0, R1, and R2 defined herein.
Owner:ALBANY MOLECULAR RESEARCH INC
Deuterated cyclosporine analogs and their use as immunodulating agents
InactiveUS20030220234A1Good curative effectAltered pharmacokineticIsotope introduction to peptides/proteinsPeptide preparation methodsCyclosporinsIsotope
Cyclosporine derivatives are disclosed which possess enhanced efficacy and reduced toxicity over naturally occurring and other presently known cyclosporins and cyclosporine derivatives. The cyclosporine derivatives of the present invention are produced by chemical and isotopic substitution of the cyclosporine A (CsA) molecule by: (1) Chemical substitution and optionally deuterium substitution of amino acid 1; and (2) deuterium substitution at key sites of metabolism of the cyclosporine A molecule such as amino acids 1, 4, 9. Also disclosed are methods of producing the cyclosporine derivatives and method of producing immunosuppression with reduced toxicity with the disclosed cyclosporine derivatives.
Owner:NAICKER SELVARAJ +2
Cyclosporin analogs for the treatment of immunoregulatory disorders and respiratory diseases
InactiveUS20060035821A1Diminished plasma stabilityPowerfulImmunoglobulinsCyclic peptide ingredientsDiseaseCyclosporins
Owner:ARRAY BIOPHARMA
Gene therapy by cell specific targeting
InactiveUS6982082B1Selectively inhibiting proliferationPrevent proliferationBiocideCyclosporinsCell specificDisease
This invention is directed to a modified cyclosporin A and to a modified, genetically engineered version of its receptor, cyclophilin. This invention is further directed to a method for treating host versus graft disease following blood marrow transplantation by transfecting stem cells so that after introduction into a patient the stem cells will express the modified cyclophilin, and, as necessary, administer the modified cyclosporin A to the patient.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +2
Novel proline substituted cyclosporin analogues
The present invention provides novel proline substituted cyclosporinanalogue compounds, pharmaceutical compositions comprising these compounds and methods of using these compounds for the treatment of disorders and diseases, including immune disorders, inflammatory disorders and viral infections.
Owner:ENANTA PHARM INC
Cyclosporine analogue mixtures and their use as immunomodulating agents
ActiveUS20050192214A1Improve effectivenessLow toxicitySenses disorderDispersion deliveryCyclosporinsImmunomodulating Agent
The invention is directed to isomeric mixtures of cyclosporine analogues that are structurally similar to cyclosporine A. The mixtures possess enhanced efficacy and reduced toxicity over the individual isomers and over naturally occurring and other presently known cyclosporines and cyclosporine derivatives. Embodiments of the present invention are directed toward cis and trans-isomers of cyclosporin A analogs referred to as ISATX247, and derivatives thereof. Mixtures of ISATX247 isomers exhibit a combination of enhanced potency and reduced toxicity over the naturally occurring and presently known cyclosporins. ISATX247 isomers and alkylated, arylated, and deuterated derivatives are synthesized by stereoselective pathways where the particular conditions of a reaction determine the degree of siereoselectivity. The ratio of isomers in a mixture may range from about 10 to 90 percent by weight of the (E)-isomer to about 90 to 10 percent by weight of the (Z)-isomer, based on the total weight of the mixture.
Owner:AURINIA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com