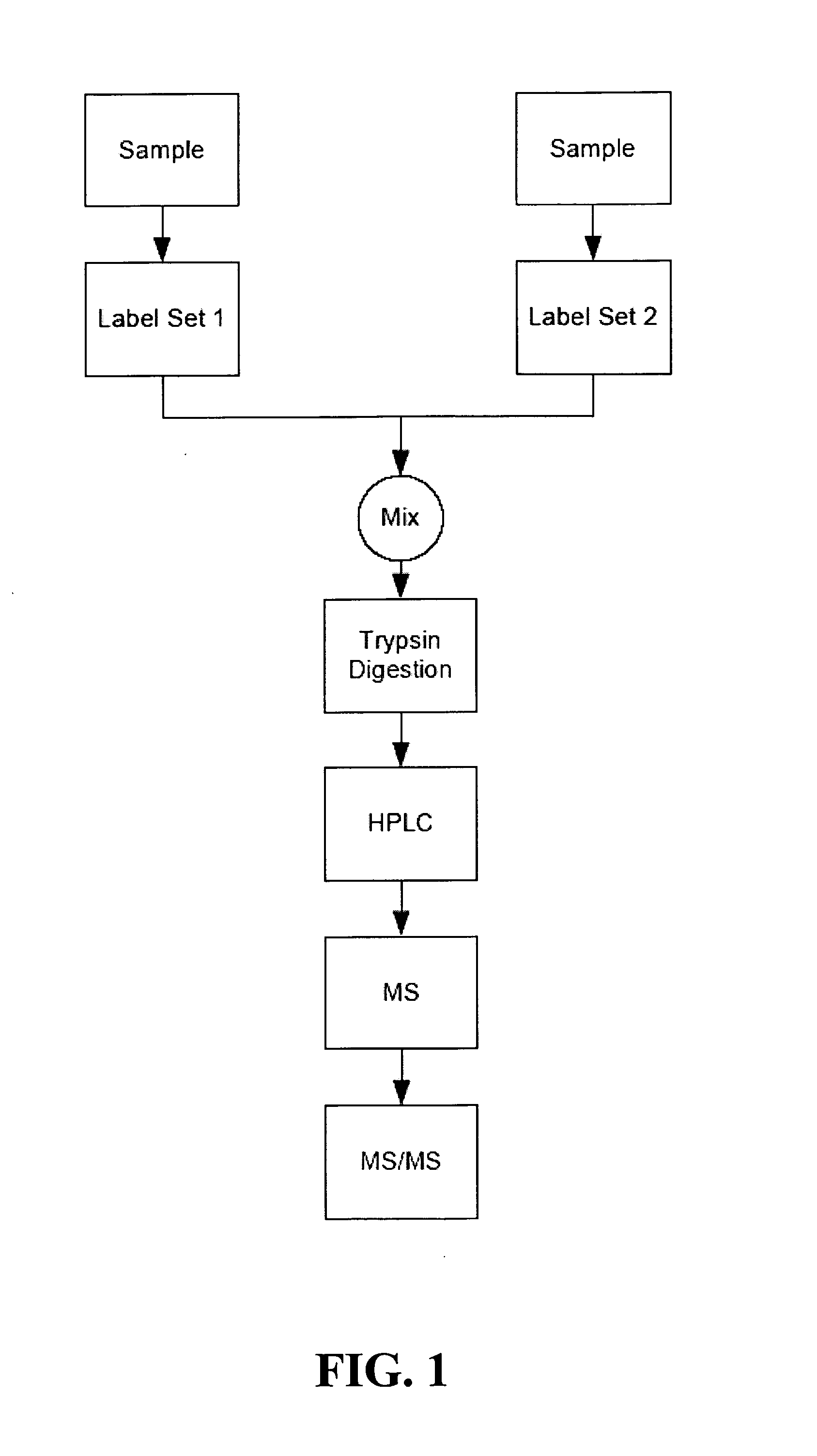
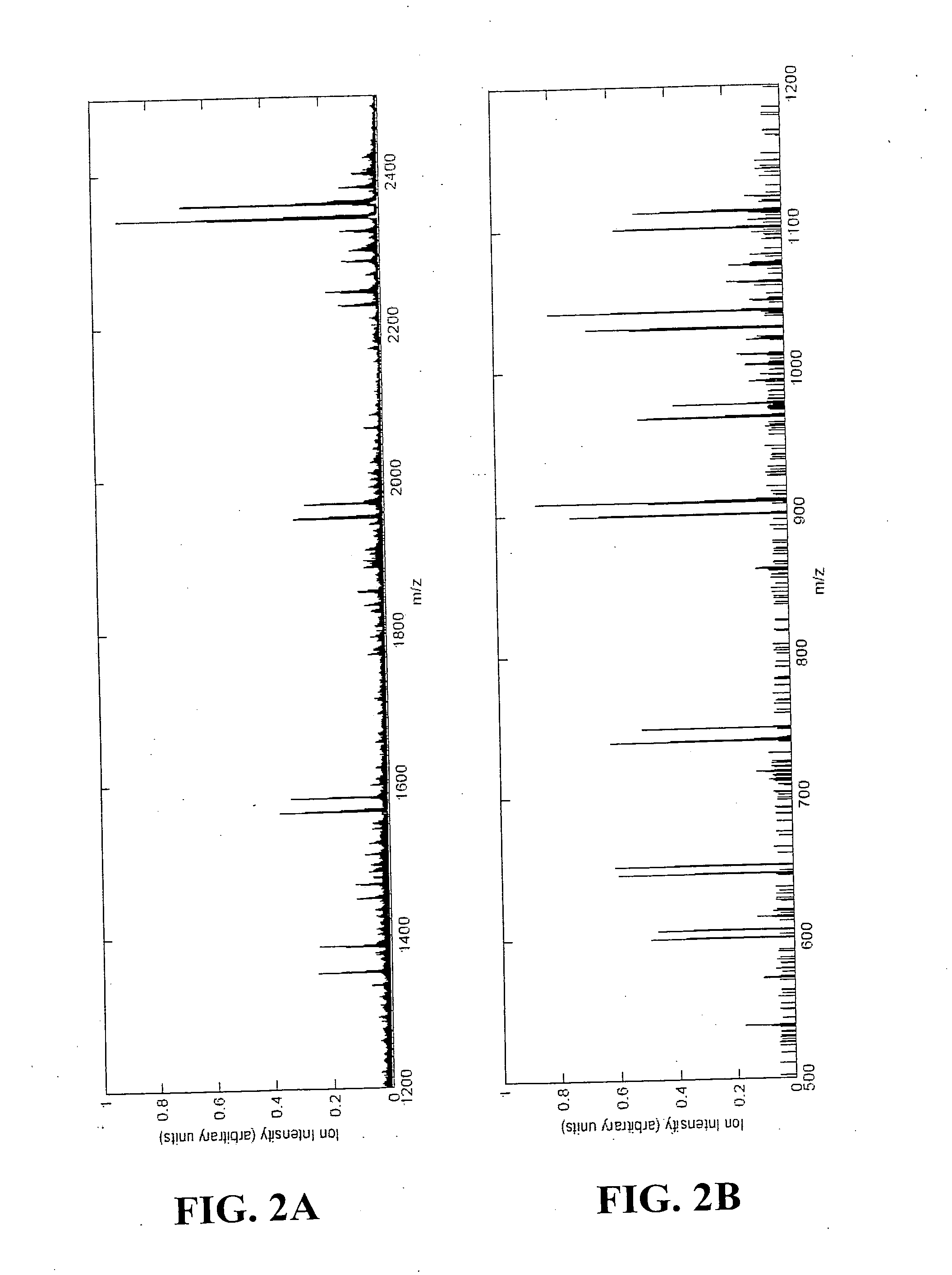
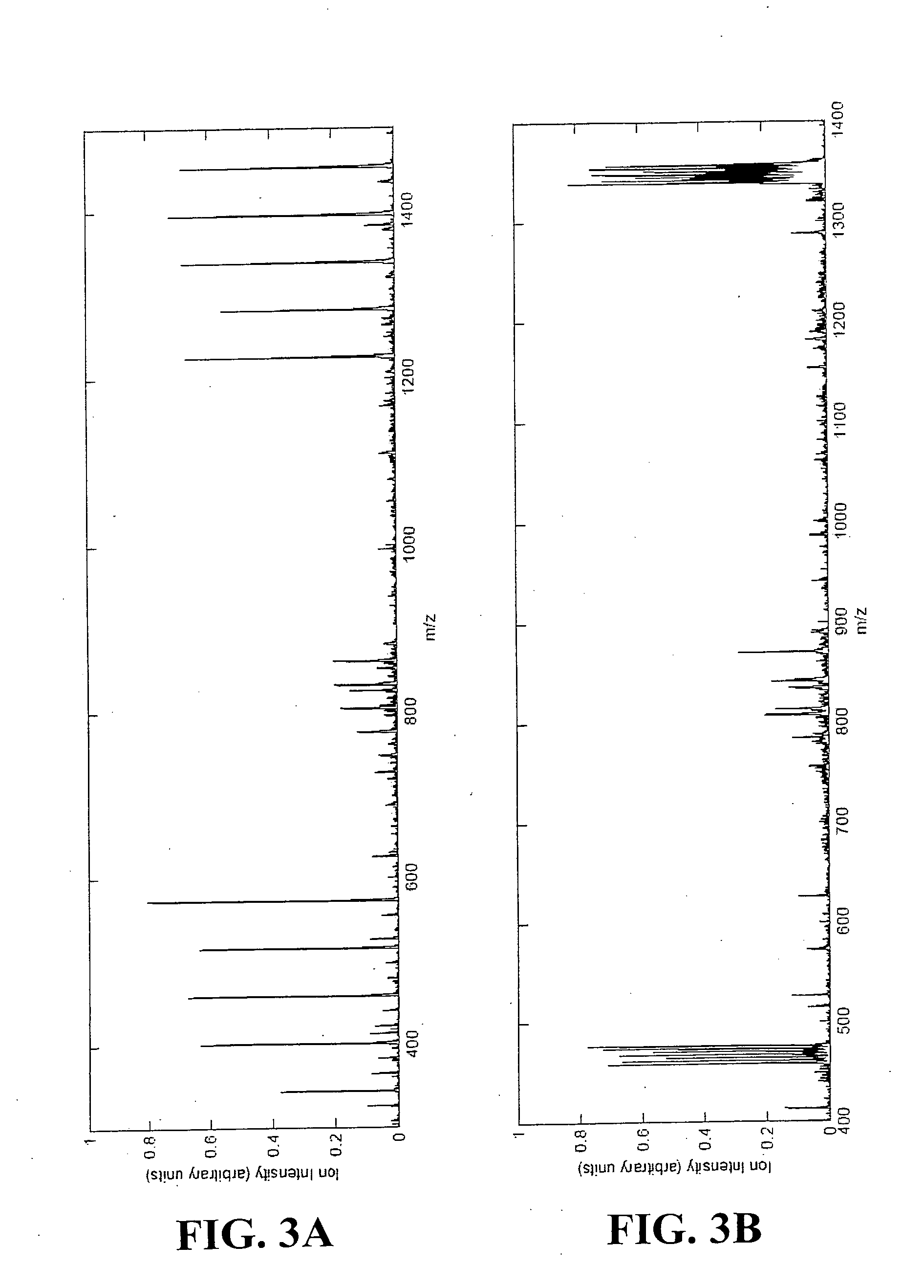
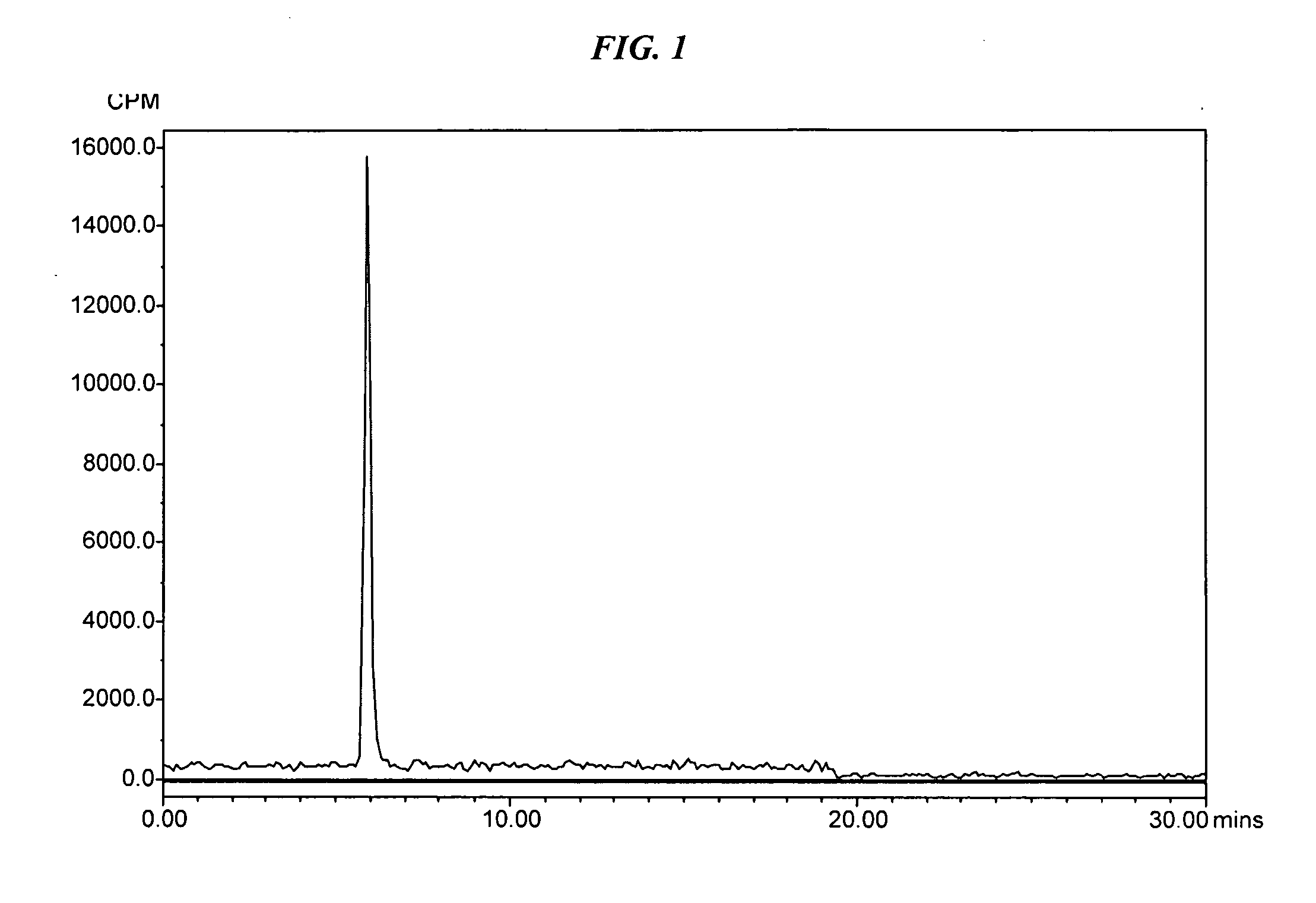
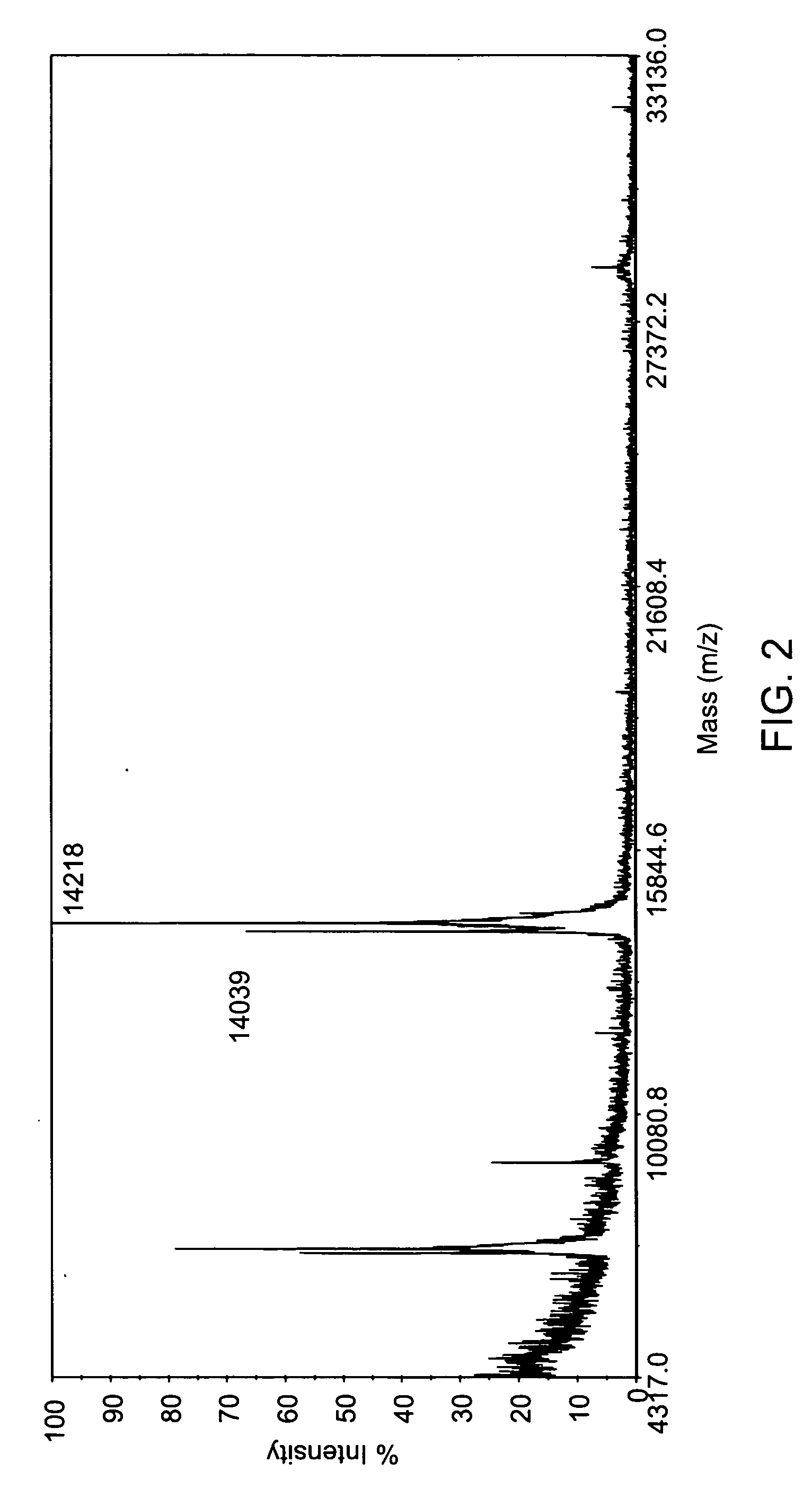
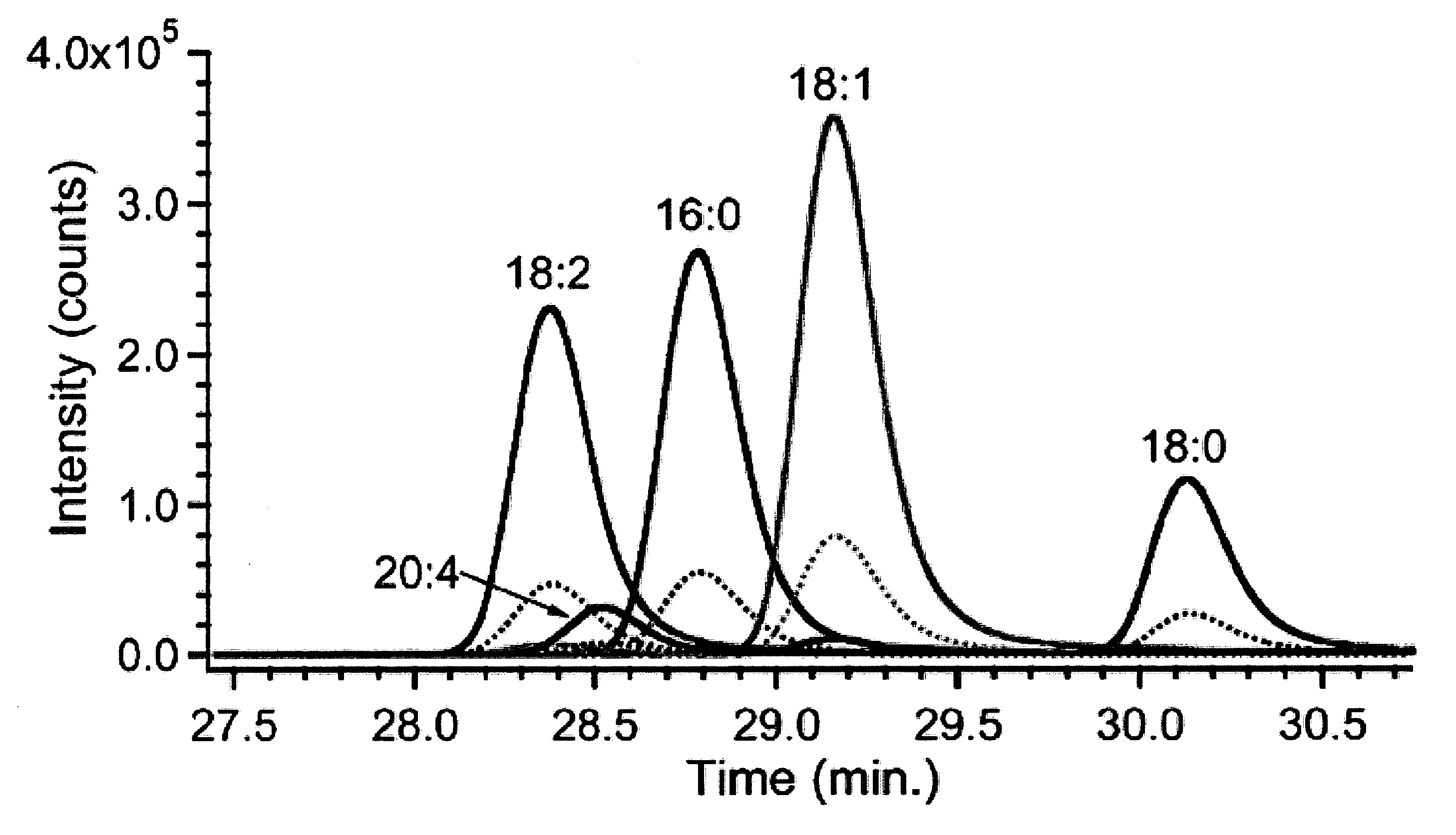
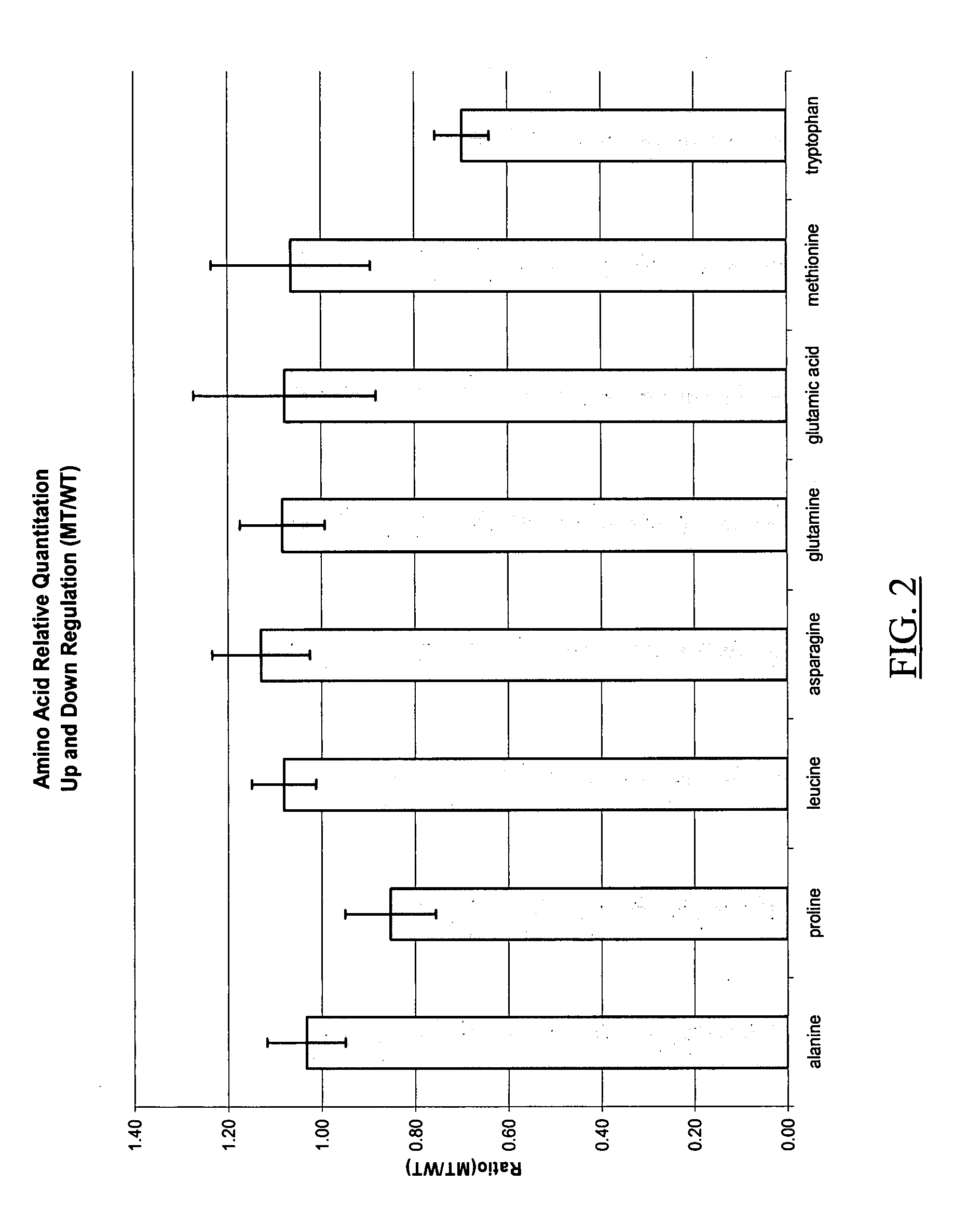
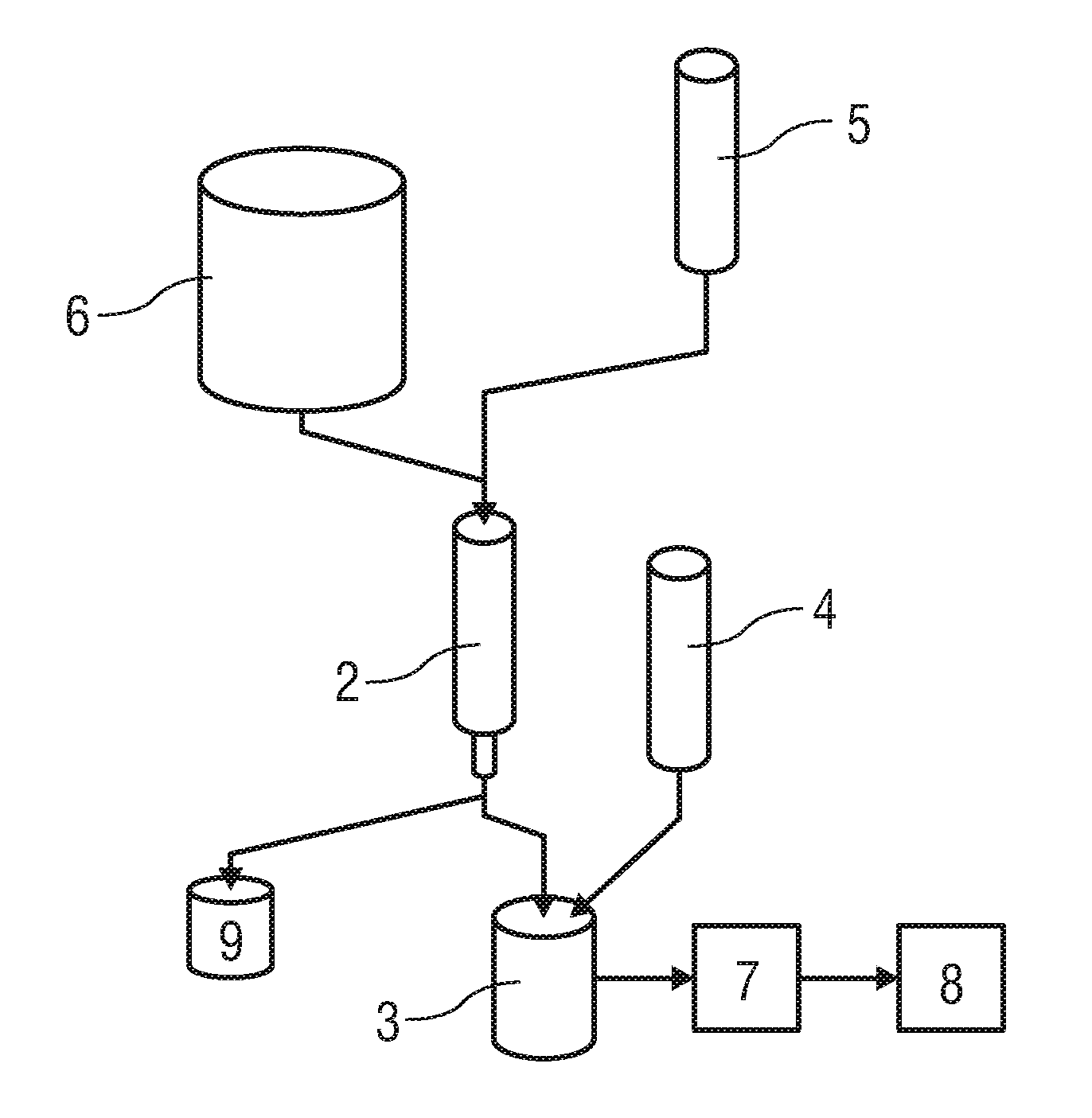
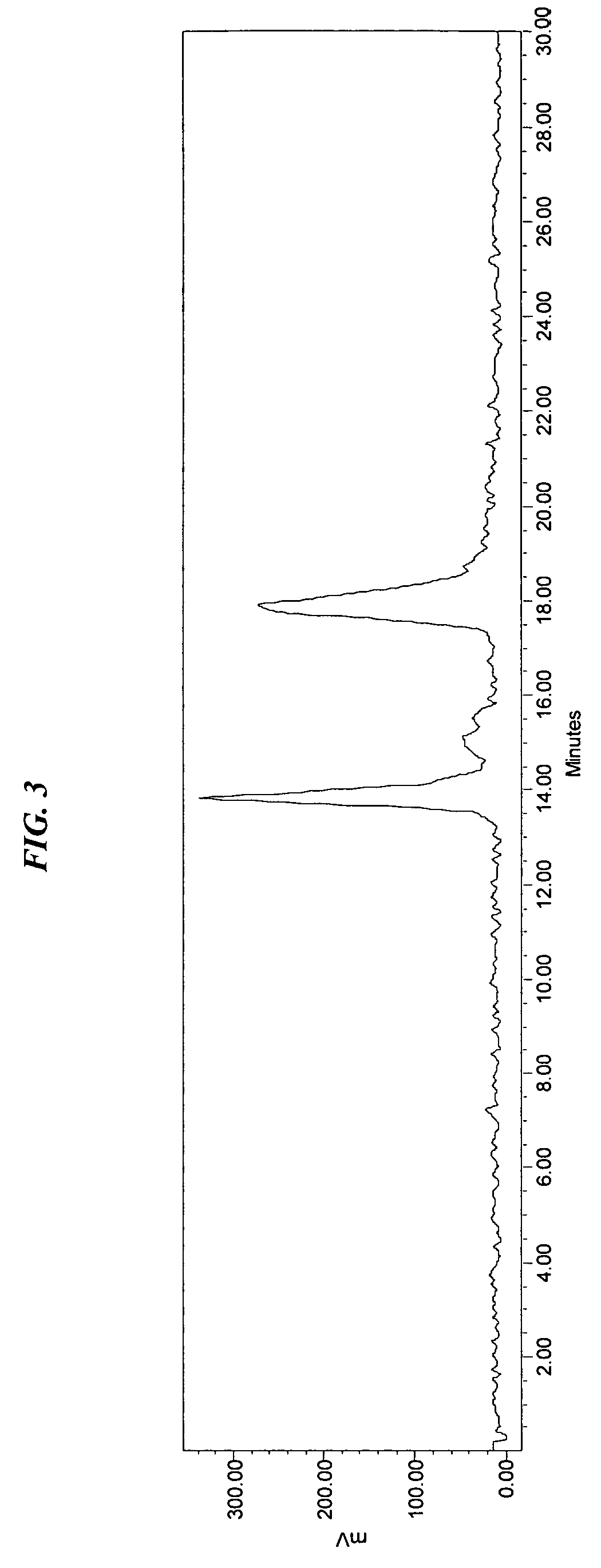
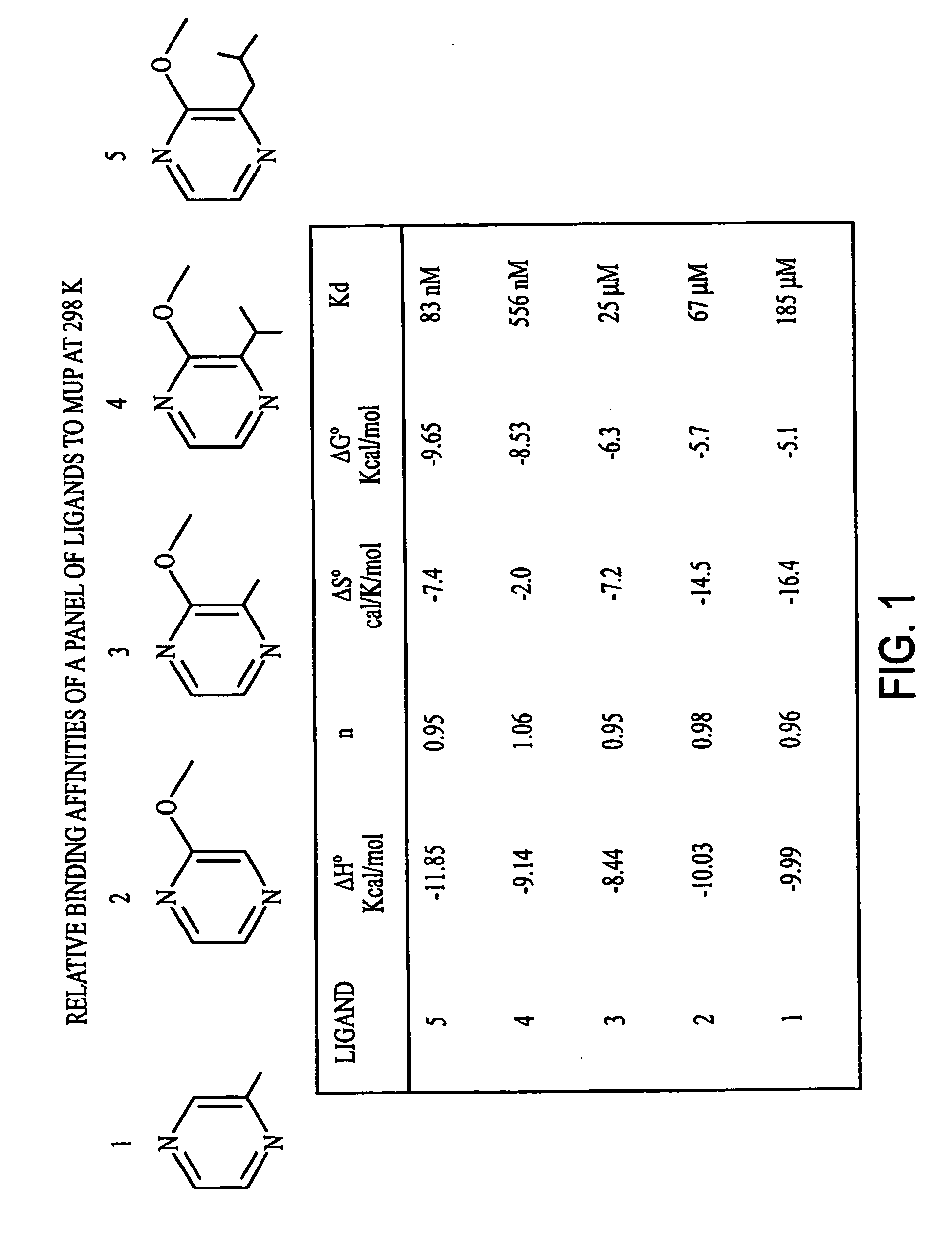
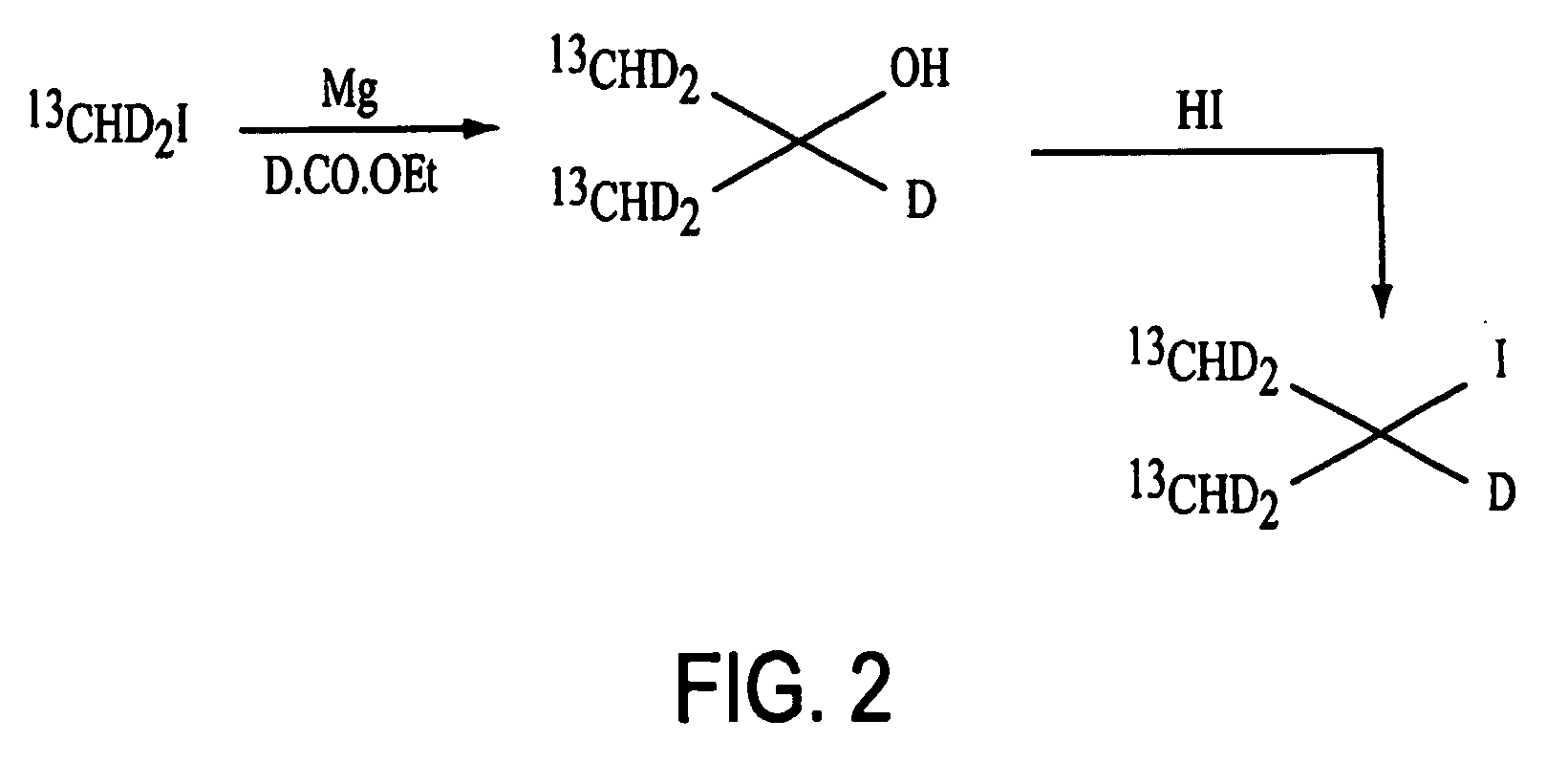
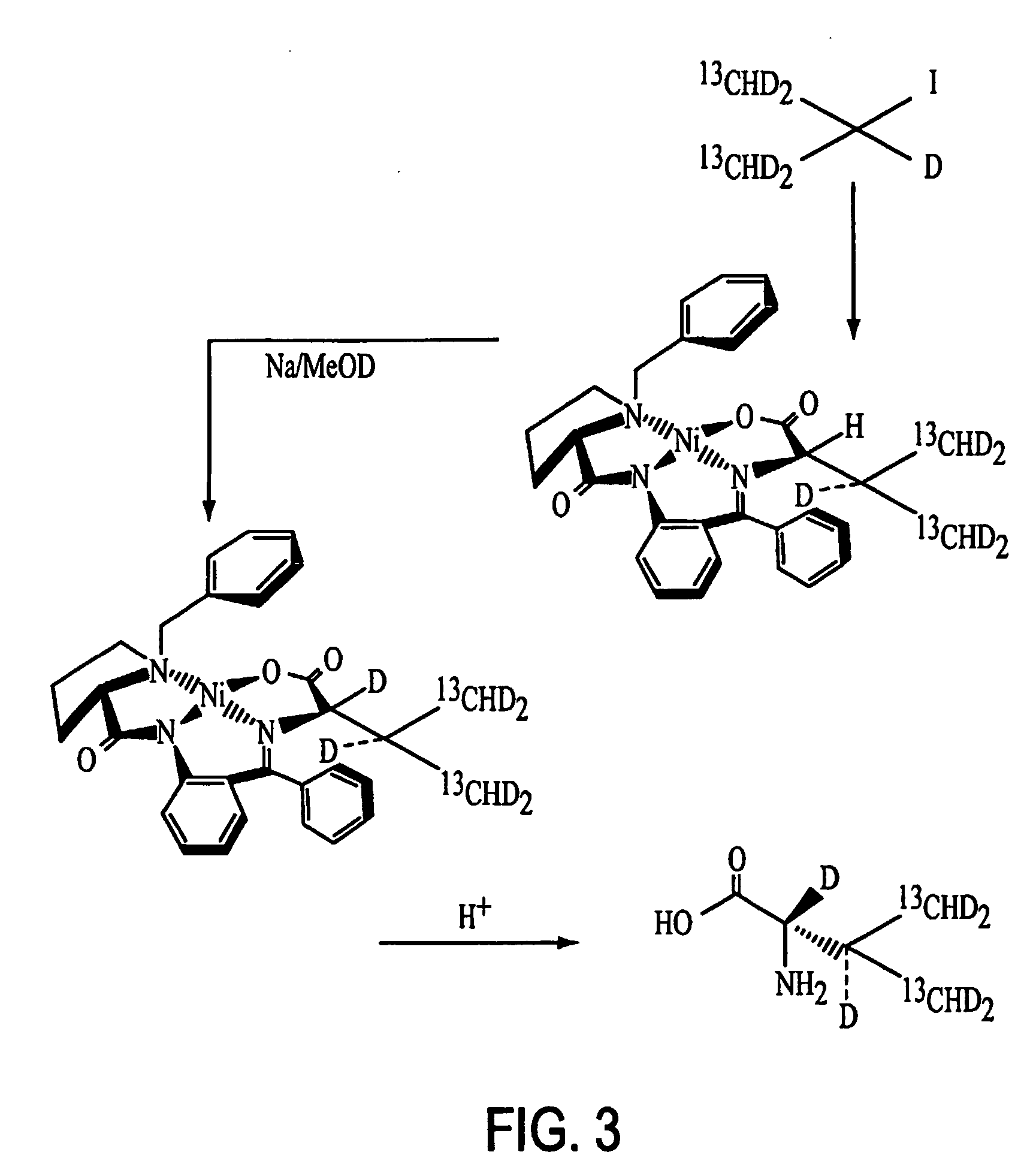
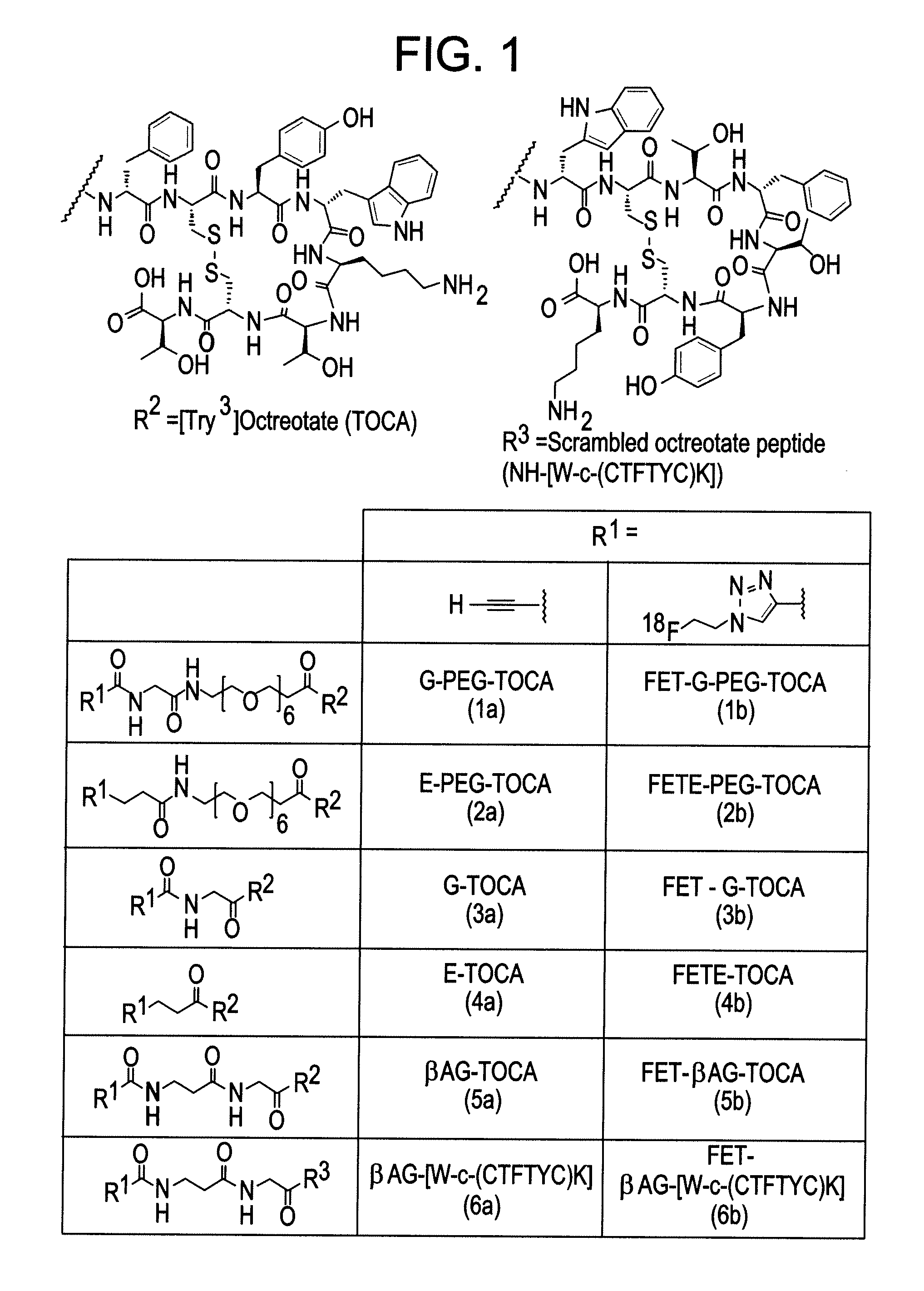
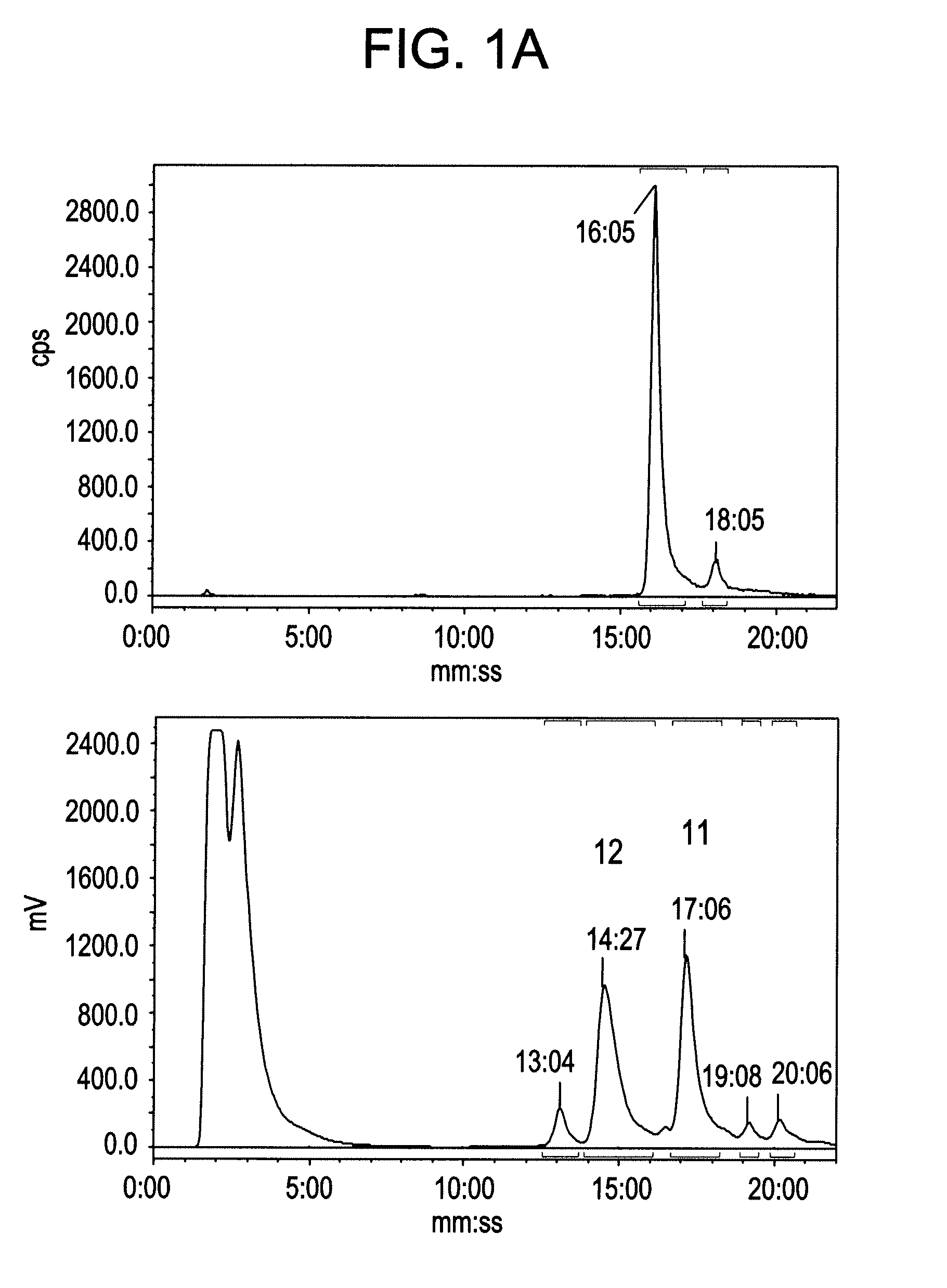
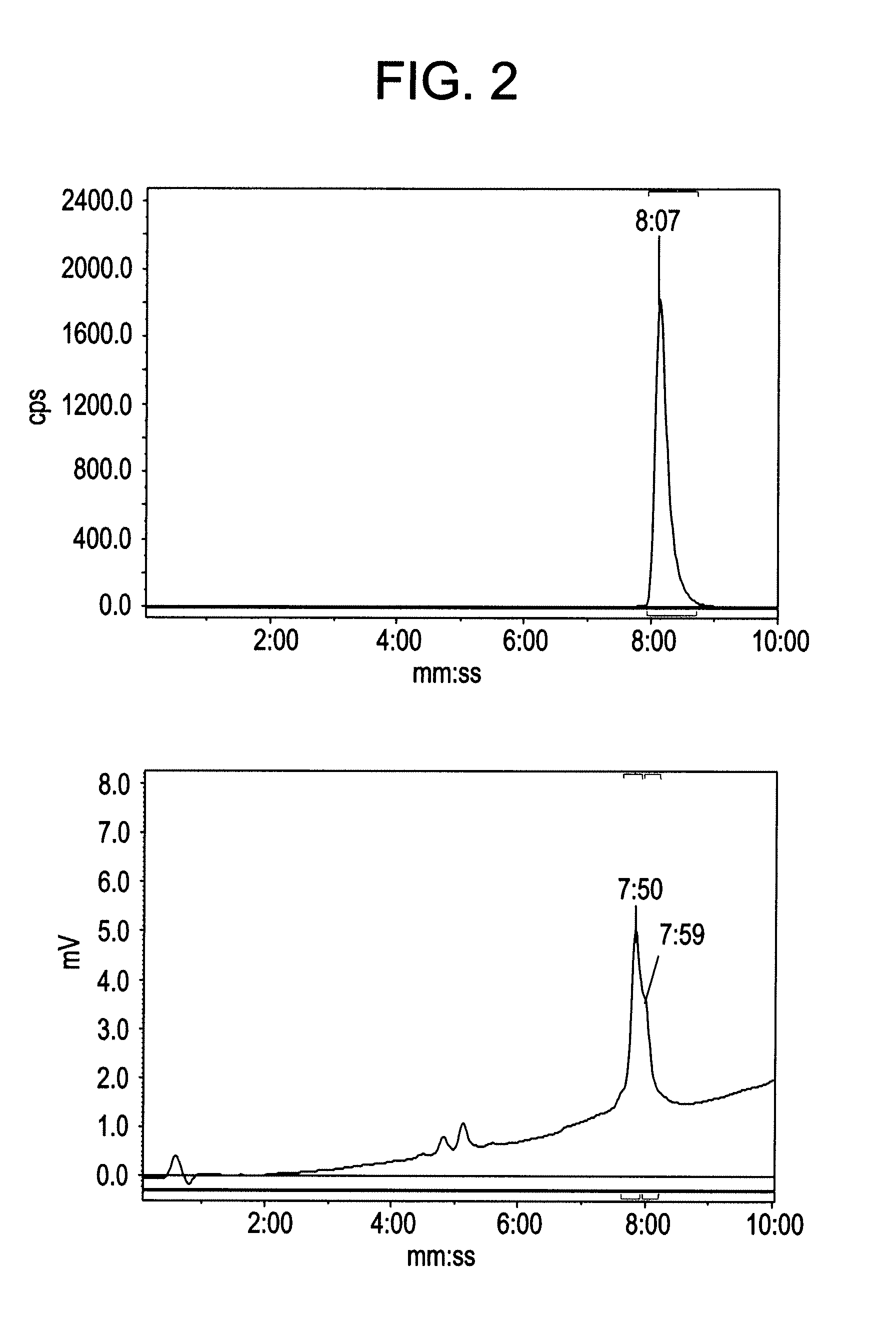
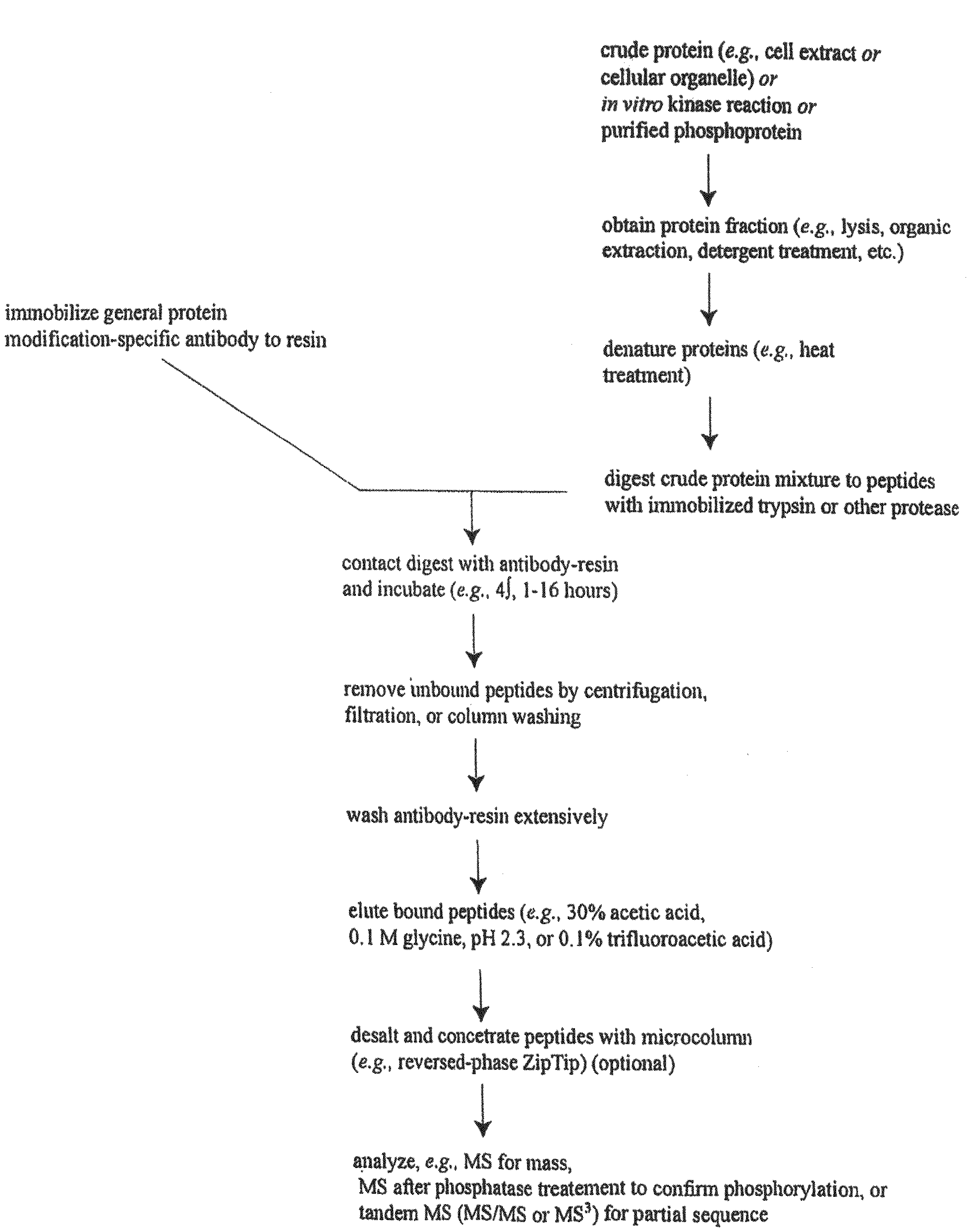
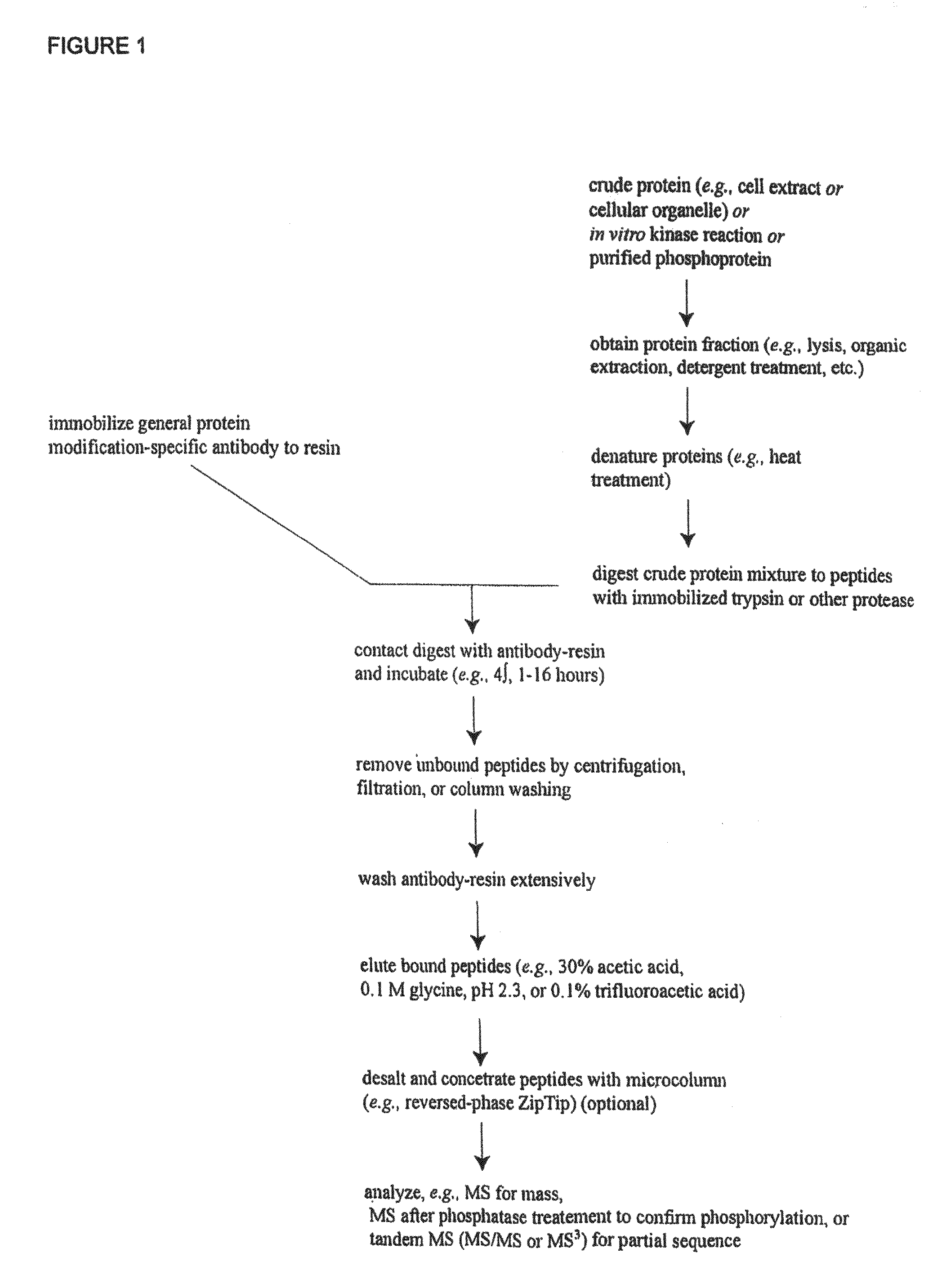
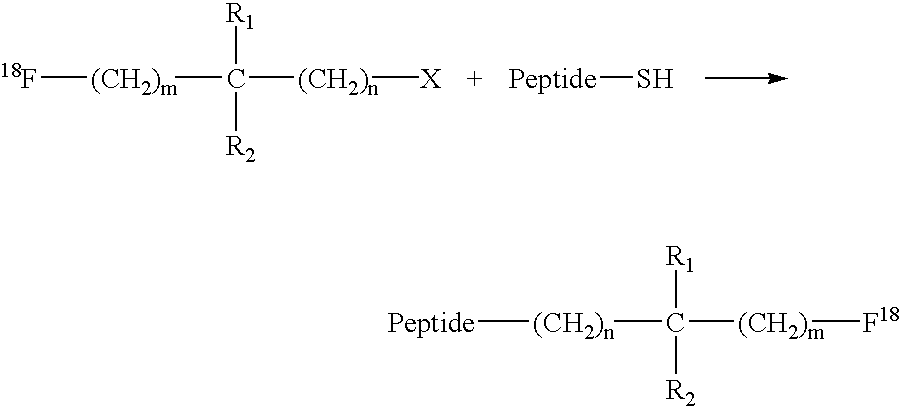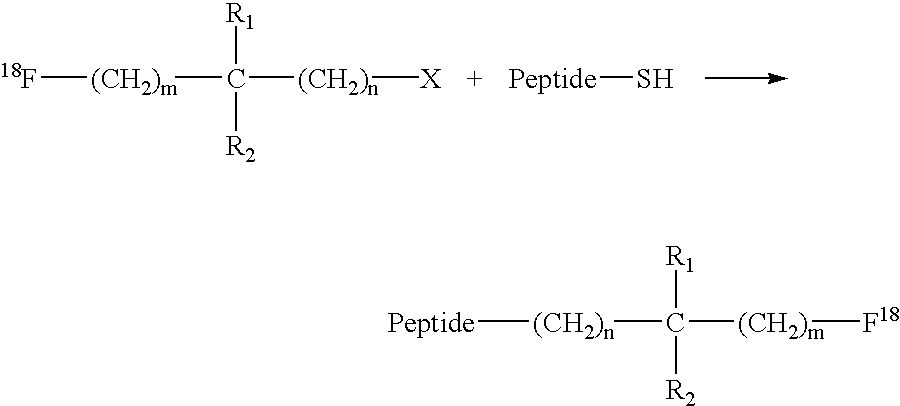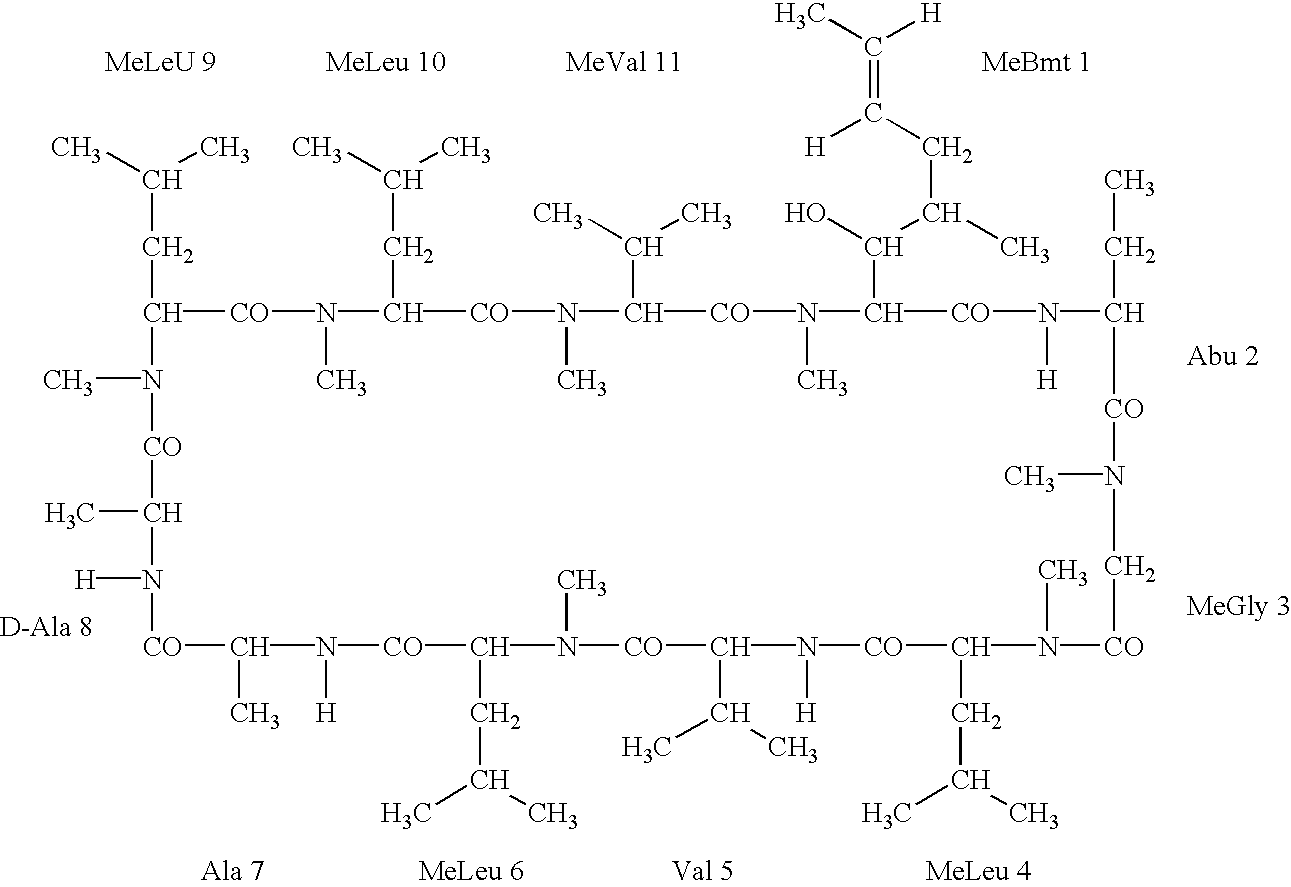Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
125results about "Isotope introduction to peptides/proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
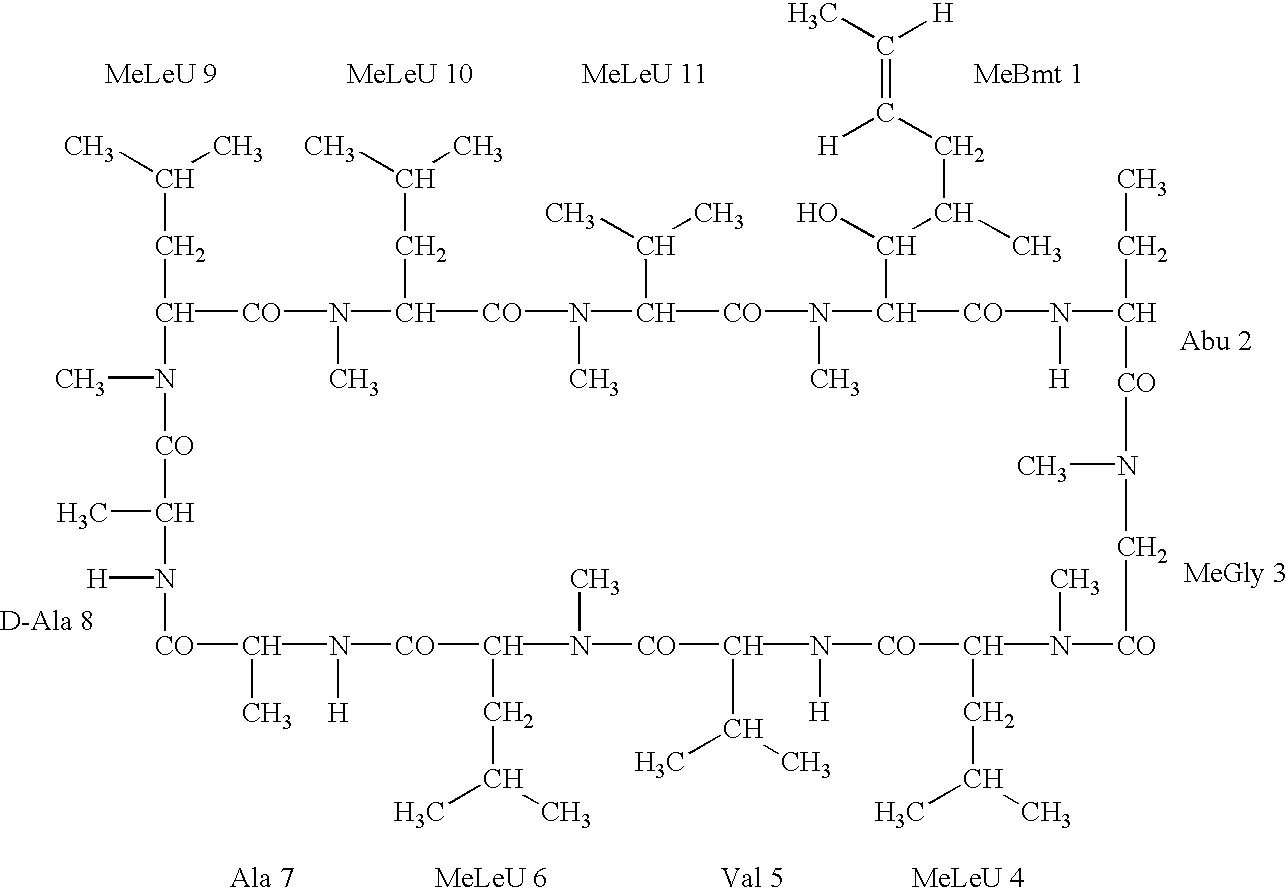
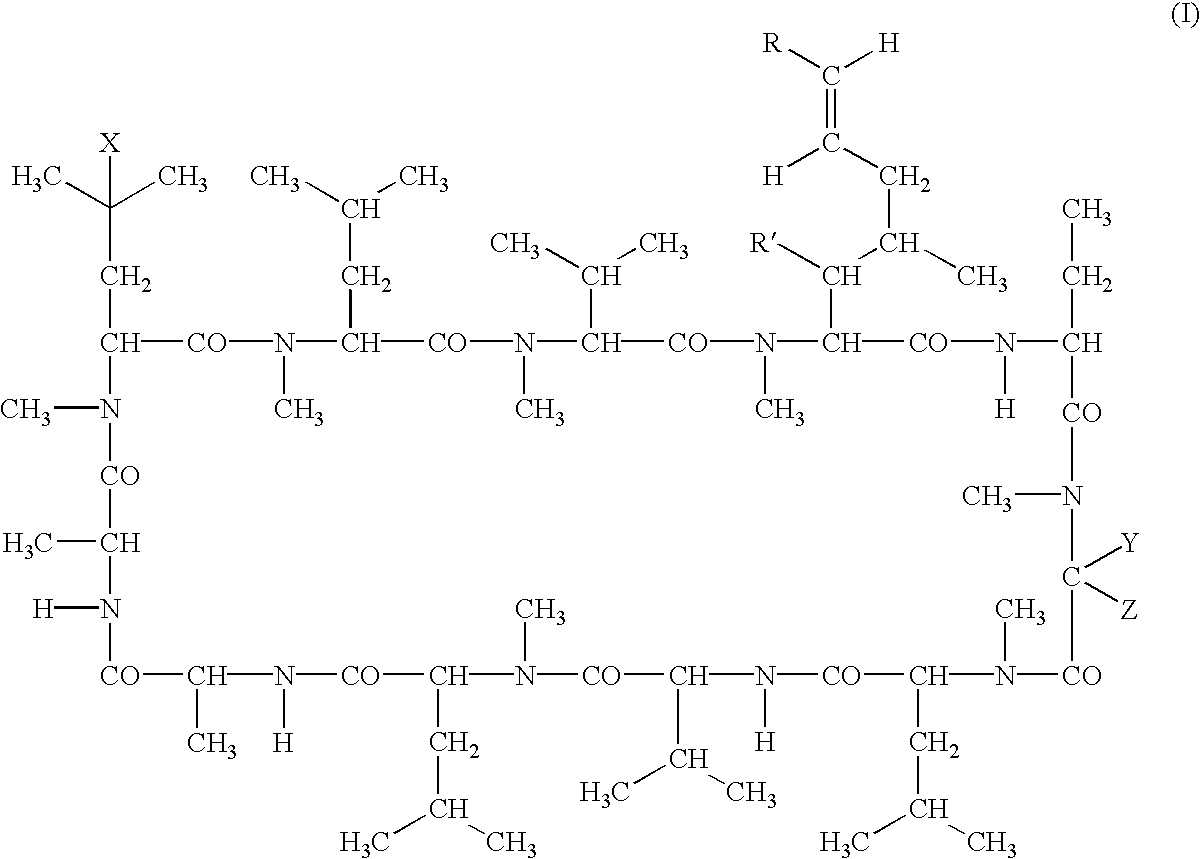
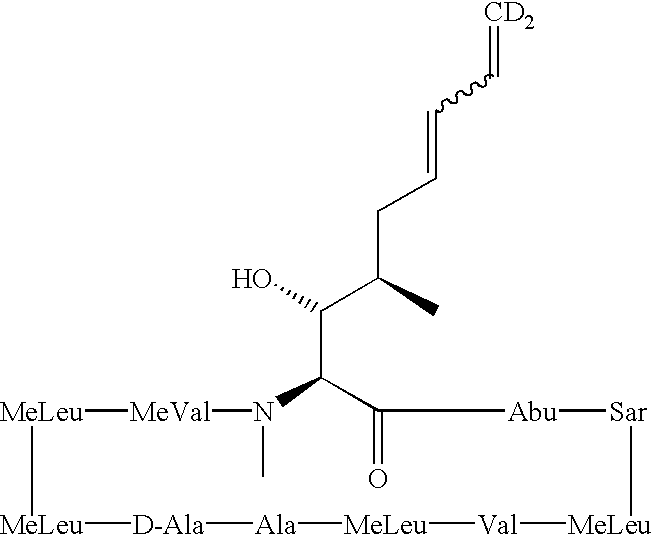
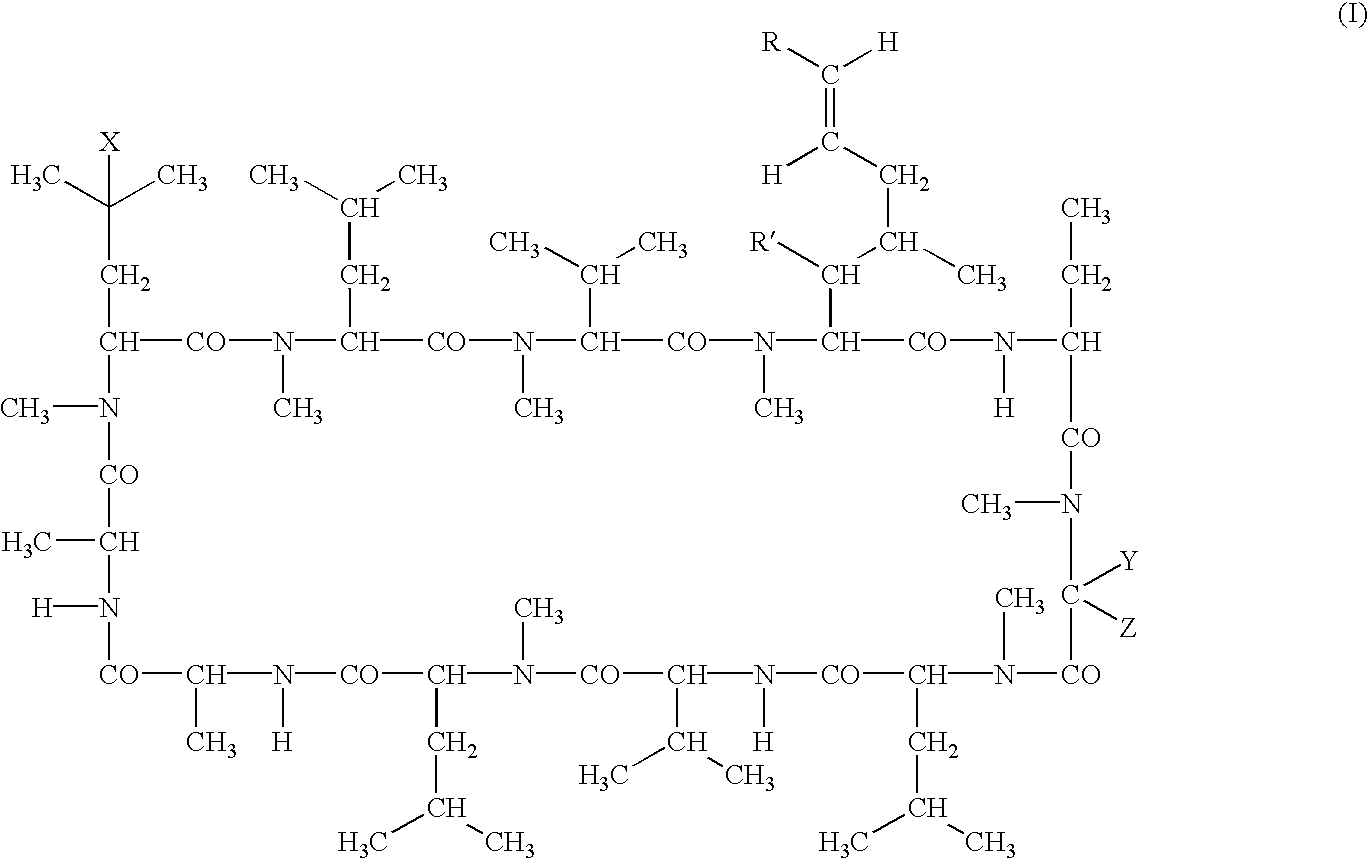
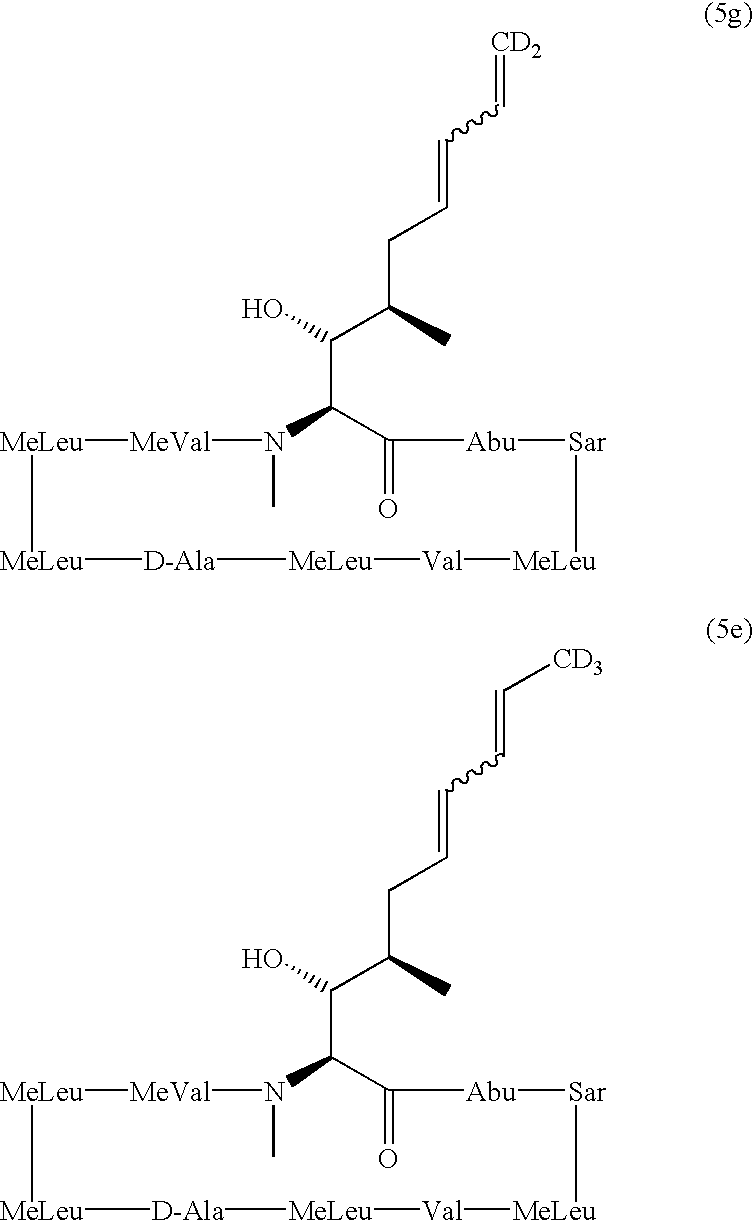
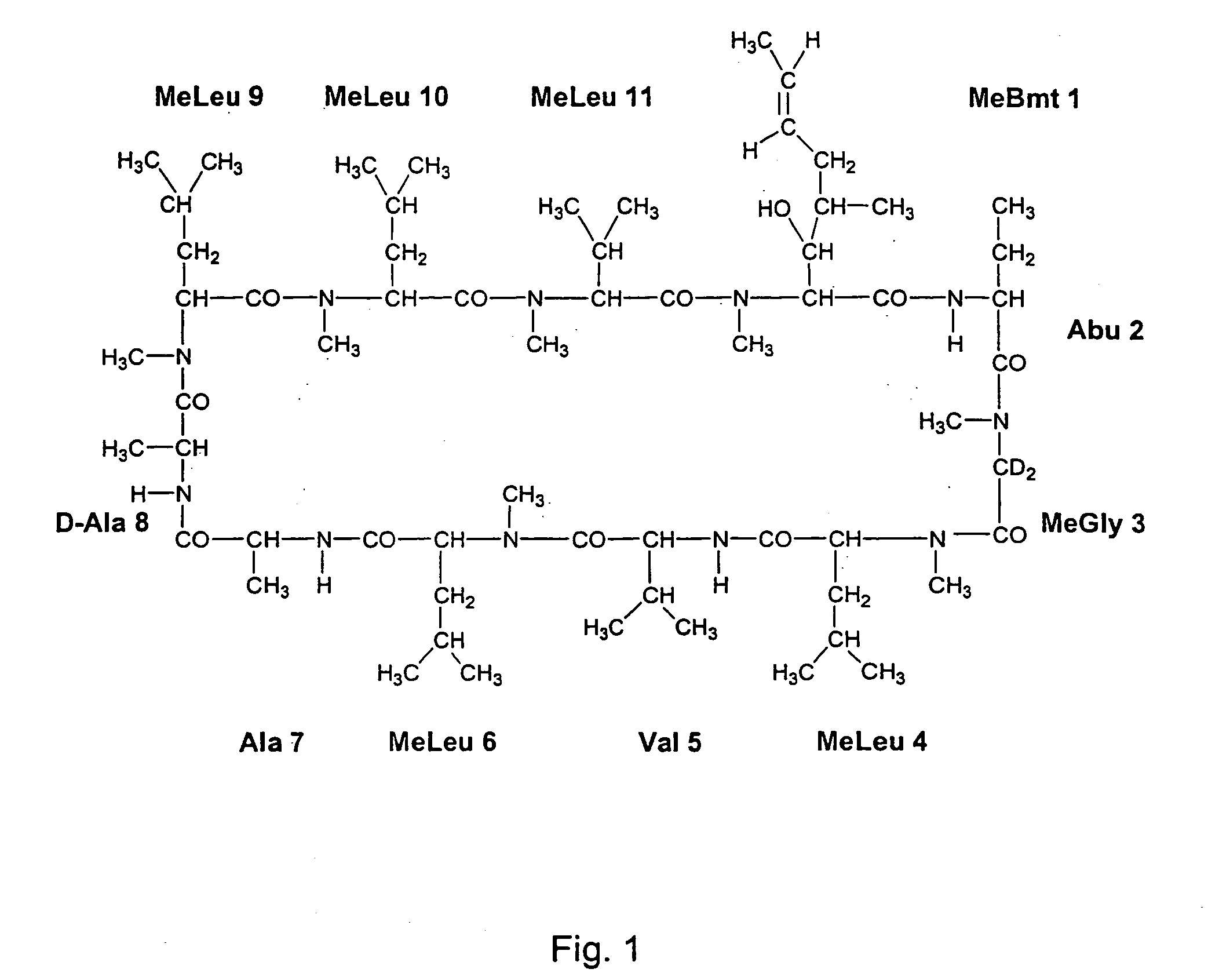
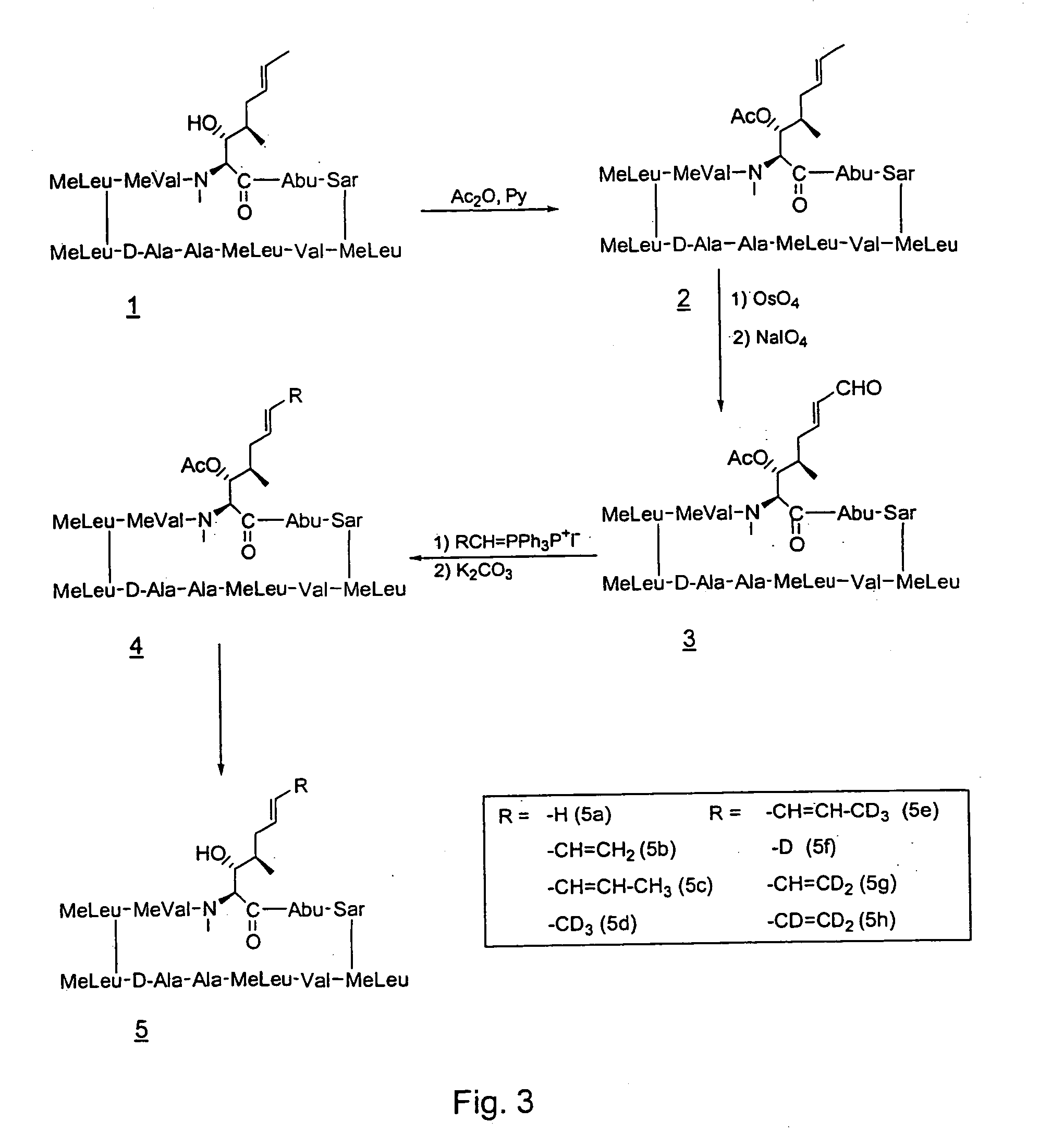
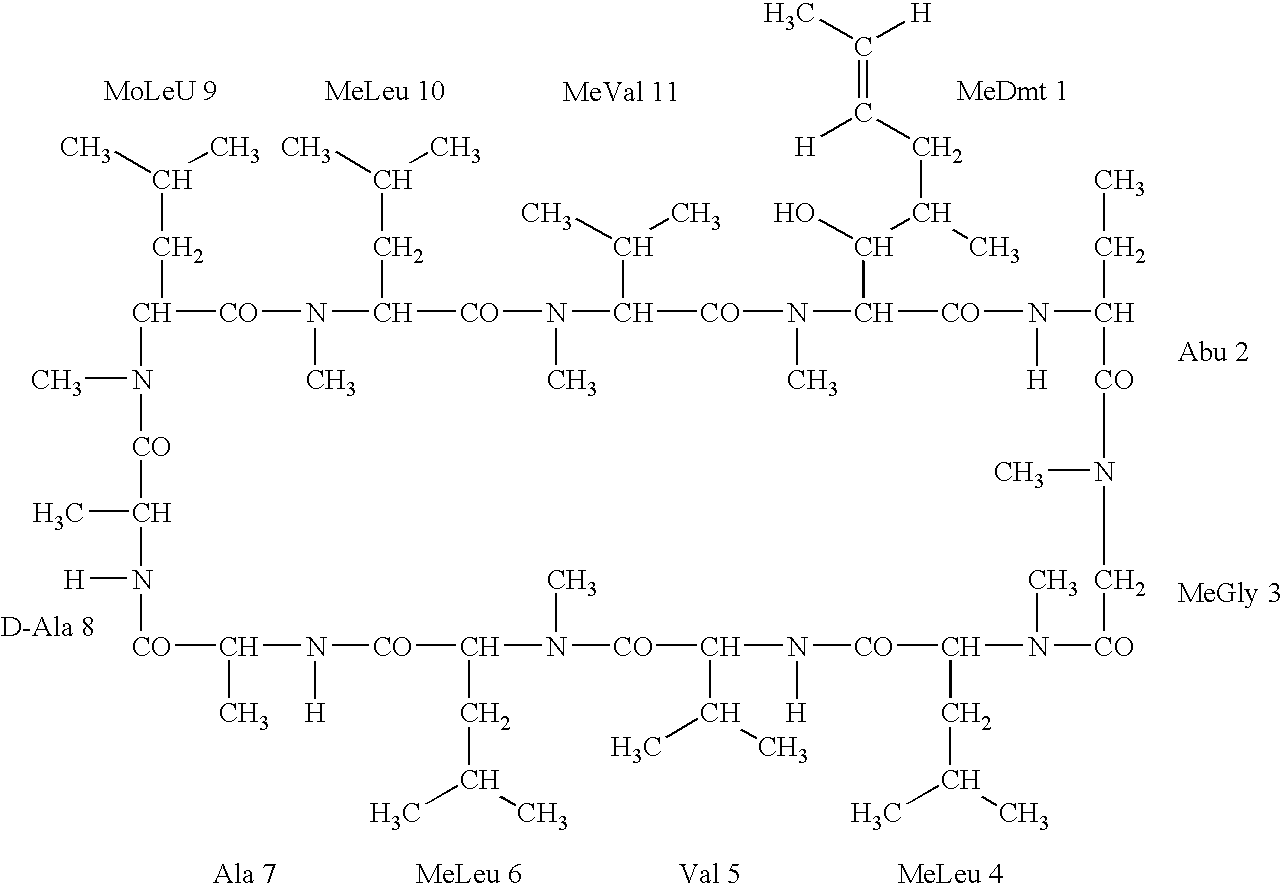
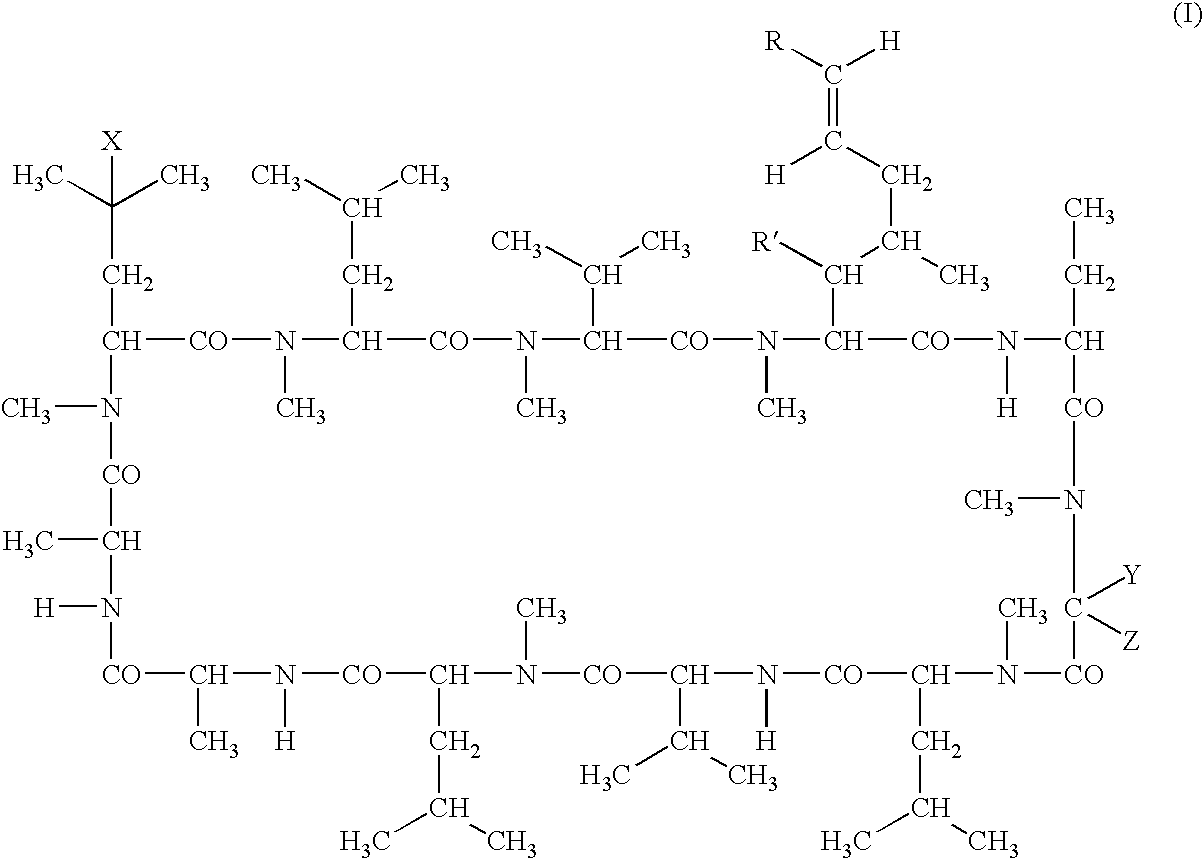
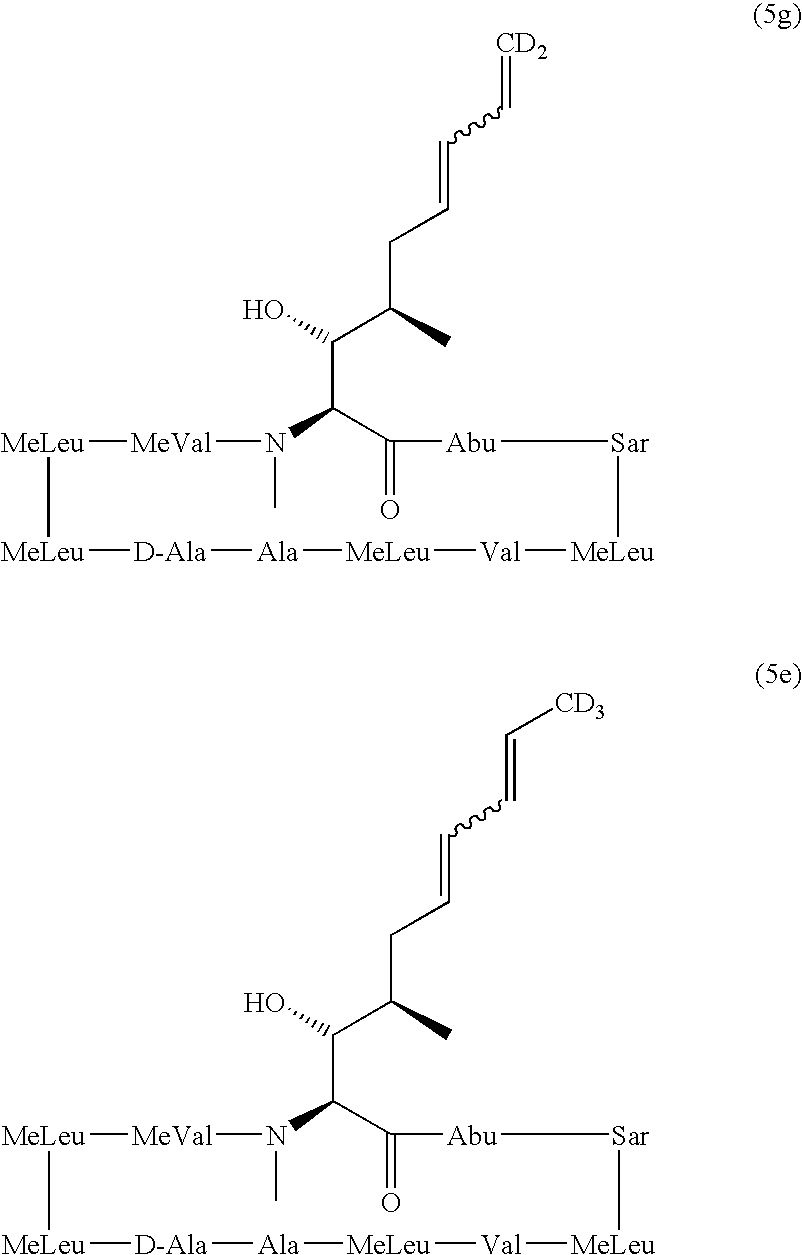
Deuterated cyclosporine analogs and their use as immunomodulating agents
InactiveUS6605593B1Improve practicalityAltered physicochemical and pharmacokinetic propertyNervous disorderAntipyreticCyclosporinsImmunomodulating Agent
Cyclosporine derivatives are disclosed which possess enhanced efficacy and reduced toxicity over naturally occurring and other presently known cyclosporins and cyclosporine derivatives. The cyclosporine derivatives of the present invention are produced by chemical and isotopic substitution of the cyclosporine A (CsA) molecule by: (1) Chemical substitution and optionally deuterium substitution of amino acid 1; and (2) deuterium substitution at key sites of metabolism of the cyclosporine A molecule such as amino acids 1, 4, 9. Also disclosed are methods of producing the cyclosporine derivatives and method of producing immunosuppression with reduced toxicity with the disclosed cyclosporine derivatives.
Owner:AURINIA PHARMA
Fluorination of proteins and peptides for F-18 positron emission tomography
Thiol-containing peptides can be radiolabeled with fluorine-18 (F-18) by reacting a peptide comprising a free thiol group with an F-18-bound labelling reagent which also has a group that is reactive with thiols. The resulting F-18-labeled peptides may be targeted to a tissue of interest using bispecific antibodies or bispecific antibody fragments having one arm specific for the F-18-labeled peptide or a low molecular weight hapten conjugated to the F-18-labeled peptide, and another arm specific to the targeted tissue. The targeted tissue is subsequently visualized by clinical positron emission tomography.
Owner:IMMUNOMEDICS INC
Deuterated cyclosporine analogs and their use as immunomodulating agents
InactiveUS20020132763A1Improve practicalityAltered physicochemical and pharmacokinetic propertyNervous disorderAntipyreticCyclosporinsImmunomodulating Agent
Cyclosporine derivatives are disclosed which possess enhanced efficacy and reduced toxicity over naturally occurring and other presently known cyclosporins and cyclosporine derivatives. The cyclosporine derivatives of the present invention are produced by chemical and isotopic substitution of the cyclosporine A (CsA) molecule by: (1) Chemical substitution and optionally deuterium substitution of amino acid 1; and (2) deuterium substitution at key sites of metabolism of the cyclosporine A molecule such as amino acids 1, 4, 9. Also disclosed are methods of producing the cyclosporine derivatives and method of producing immunosuppression with reduced toxicity with the disclosed cyclosporine derivatives.
Owner:NAICKER SALVARAJ +2
Ultra-sensitive detection systems using multidimension signals
InactiveUS20070207555A1Target protein fragments can be alteredReduce eliminate expressionIsotope introduction to peptides/proteinsBiological material analysisAnalyteSecondary analysis
Disclosed are compositions and methods for sensitive detection of one or multiple analytes. In general, the methods involve the use of special label components, referred to as multidimension signals. In the disclosed methods, analysis of multidimension signals can result in one or more predetermined patterns that serve to indicate whether a further level of analysis can or should be performed and / or which portion(s) of the analyzed material can or should be analyzed in a further level of analysis. In some forms, isobaric and non-isobaric elements can be used together in the same assay or assay system. Isobaric and non-isobaric multidimension signals used together can generate one or more predetermined patterns during analysis. The pattern generated in this first level of analysis indicates whether the second level of analysis should be performed. The second level of analysis can involve distinguishing the isobaric multidimension signals.
Owner:PERKINELMER HEALTH SCIENCES INC
F-18 peptides for pre targeted positron emission tomography imaging
InactiveUS20070048217A1Reduce needImprove simplicityIn-vivo radioactive preparationsIsotope introduction to peptides/proteinsHydroxylamineHydrazine compound
F-18 radiolabeled peptides are prepared by reacting a peptide comprising a hydroxylamine, a thiosemicarbazide, a hydrazine or a free amine group with 4-[18F]Fluorobenzaledyde. Specific, non-limiting examples of F-18 radiolabeled peptides are described herein. The labeled peptides are useful, for example, in clinical positron emission tomography.
Owner:IMMUNOMEDICS INC
Fluorine-labeled compounds
InactiveUS20080139787A1Peptide/protein ingredientsIsotope introduction to peptides/proteinsBioconjugationAldehyde
Methods for introducing fluorine atom onto a polypeptide are provided. Also provided are linkers, bioconjugates, and bifunctional compound agents made using the methods, linkers, and bioconjugates. The methods comprise: (i) providing a linker comprising a thiol-reactive terminus and an aldehyde-reactive terminus; (ii) reacting the thiol-reactive terminus of the linker with a polypeptide comprising at least one thiol group or a reactive derivative thereof; and (iii) subsequently reacting the aldehyde-reactive terminus of the linker with a fluorine-substituted aldehyde.
Owner:GENERAL ELECTRIC CO
Ionizable isotopic labeling reagents for relative quantification by mass spectrometry
ActiveUS20080050833A1Improve ETD analysisConvenient charging statusOrganic compound preparationComponent separationMetaboliteIsotopic labeling
Relative quantification of metabolites by Electrospray Ionization Mass Spectrometry (ESI-MS) requiring a mechanism for simultaneous analysis of multiple analytes in two or more samples. Labeling reagents that are reactive to particular compound classes and differ only in their isotopic kit facilitating relative quantification and providing tangible evidence for the existence of specific functional groups. Heavy and light isotopic forms of methylacetimidate were synthesized and used as labeling reagents for quantification of amine-containing molecules, such as biological samples. Heavy and light isotopic forms of formaldehyde and cholamine were also synthesized and used independently as labeling reagents for quantification of amine-containing and carboxylic acid-containing molecules, such as found in biological samples. Advantageously, the labeled end-products are positively charged under normal acidic conditions involving conventional Liquid Chromatography Mass Spectrometry (LC / MS) applications. Labeled primary and secondary amine and carboxylic acid end-products also generated higher signals concerning mass-spectra than pre-cursor molecules and improved sensitivity. Improved accuracy concerning relative quantification was achieved by mixing heavy and light labeled Arabidopsis extracts in different ratios. Labeling strategy was further employed to ascertain differences in the amounts of amine-containing metabolites for two strains of Arabidopsis seeds.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
Method and Kit for Preparing a Radiopharmaceutical
ActiveUS20130310537A1Reduce processingHigh purityAnalysis using chemical indicatorsPeptide/protein ingredientsElutionCombinatorial chemistry
The invention relates to a method and a kit for preparing a radiopharmaceutical, the method comprising the steps:elution of a 68Ge / 68Ga-Generator using hydrochloric acid as an eluent for obtaining a generator eluate comprising 68Gallium,feeding the generator eluate through a cation exchange cartridge, which collects the 68Gallium,separating the used eluent,eluting the 68Gallium from the cation exchange cartridge using a solution comprising sodium chloride and hydrochloric acid and feeding the resulting eluate into an aqueous precursor mixture comprising at least a labelling precursor thereby forming a reaction solution.
Owner:ZENTRALKLINIK BAD BERKA
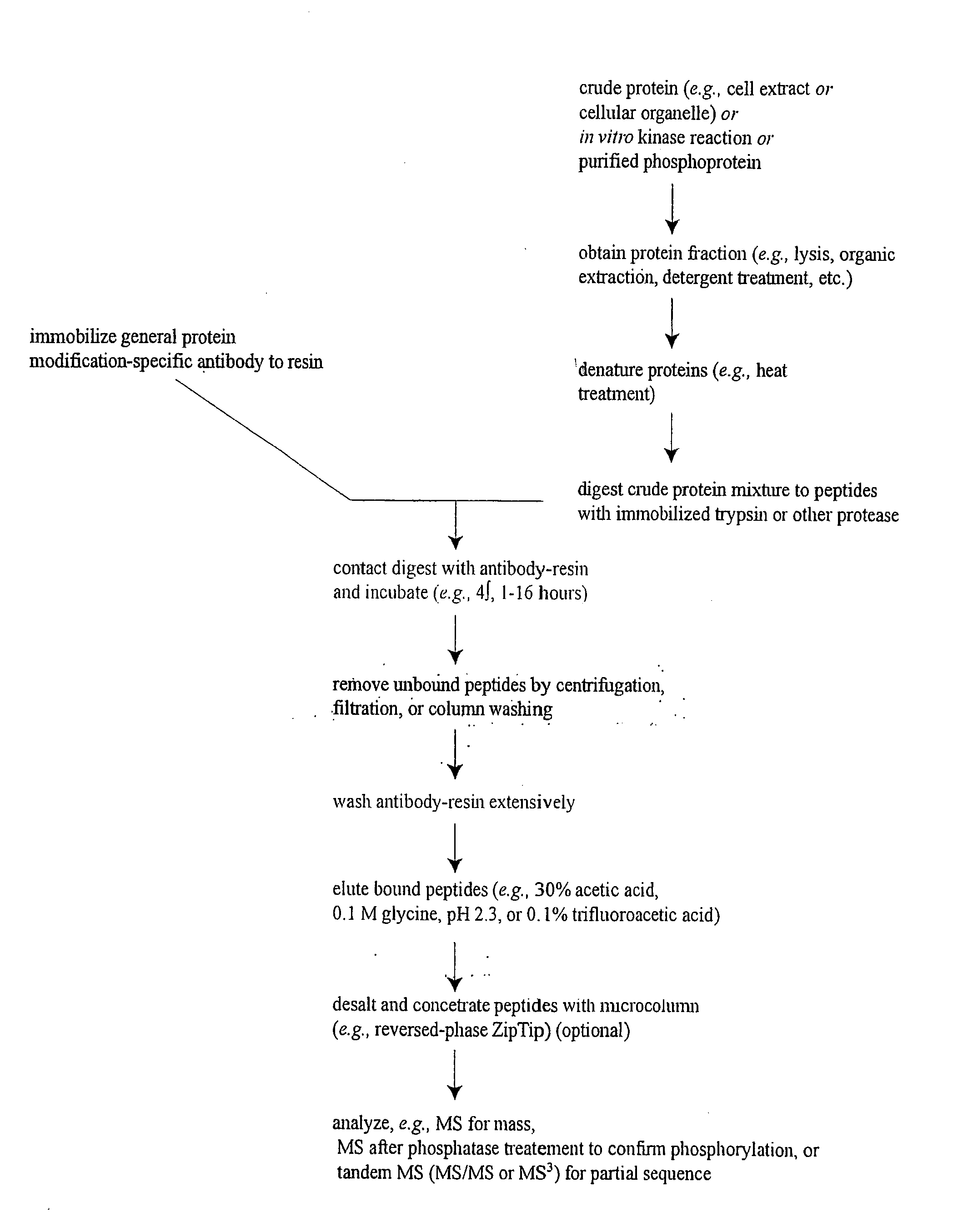
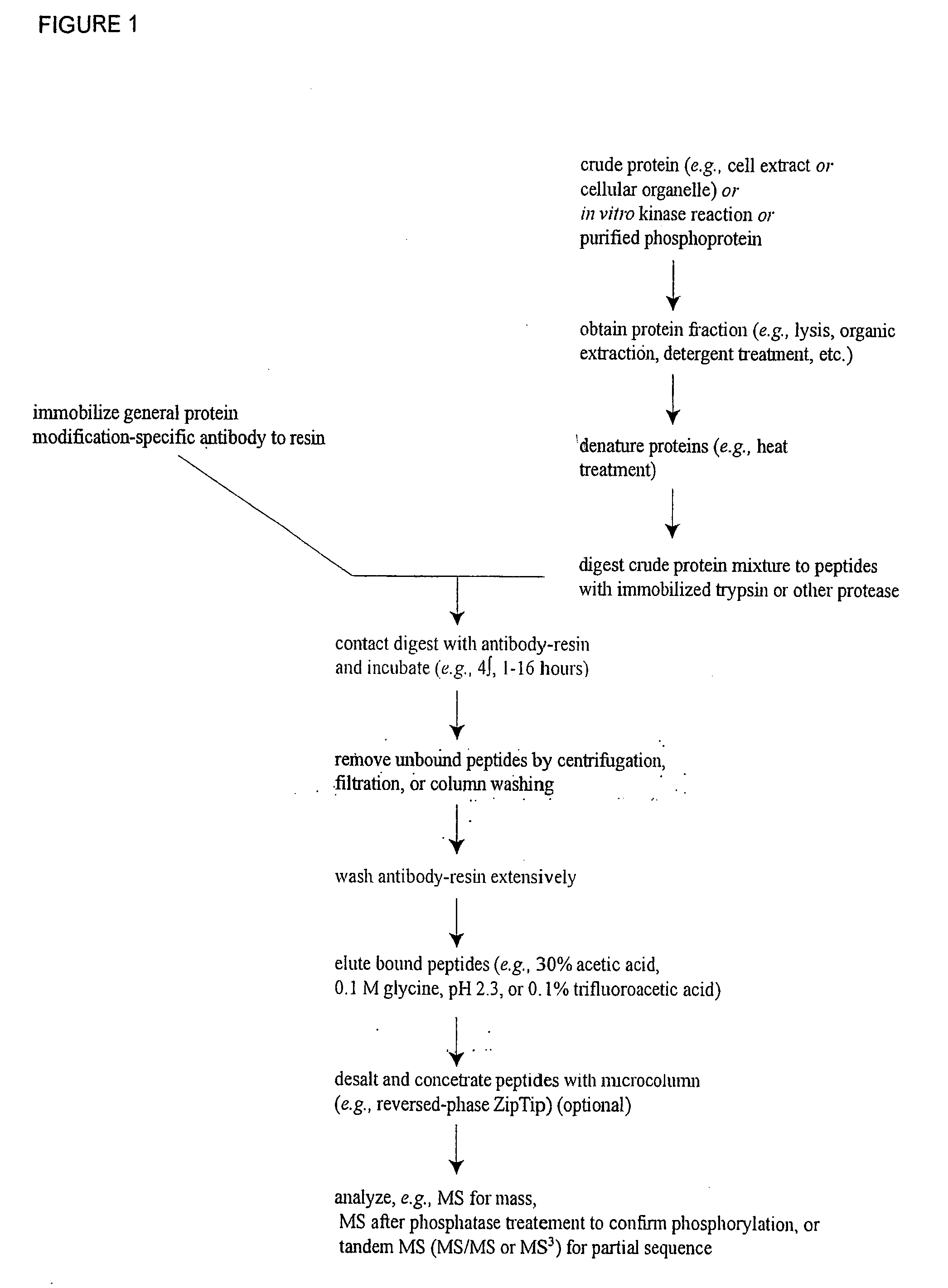
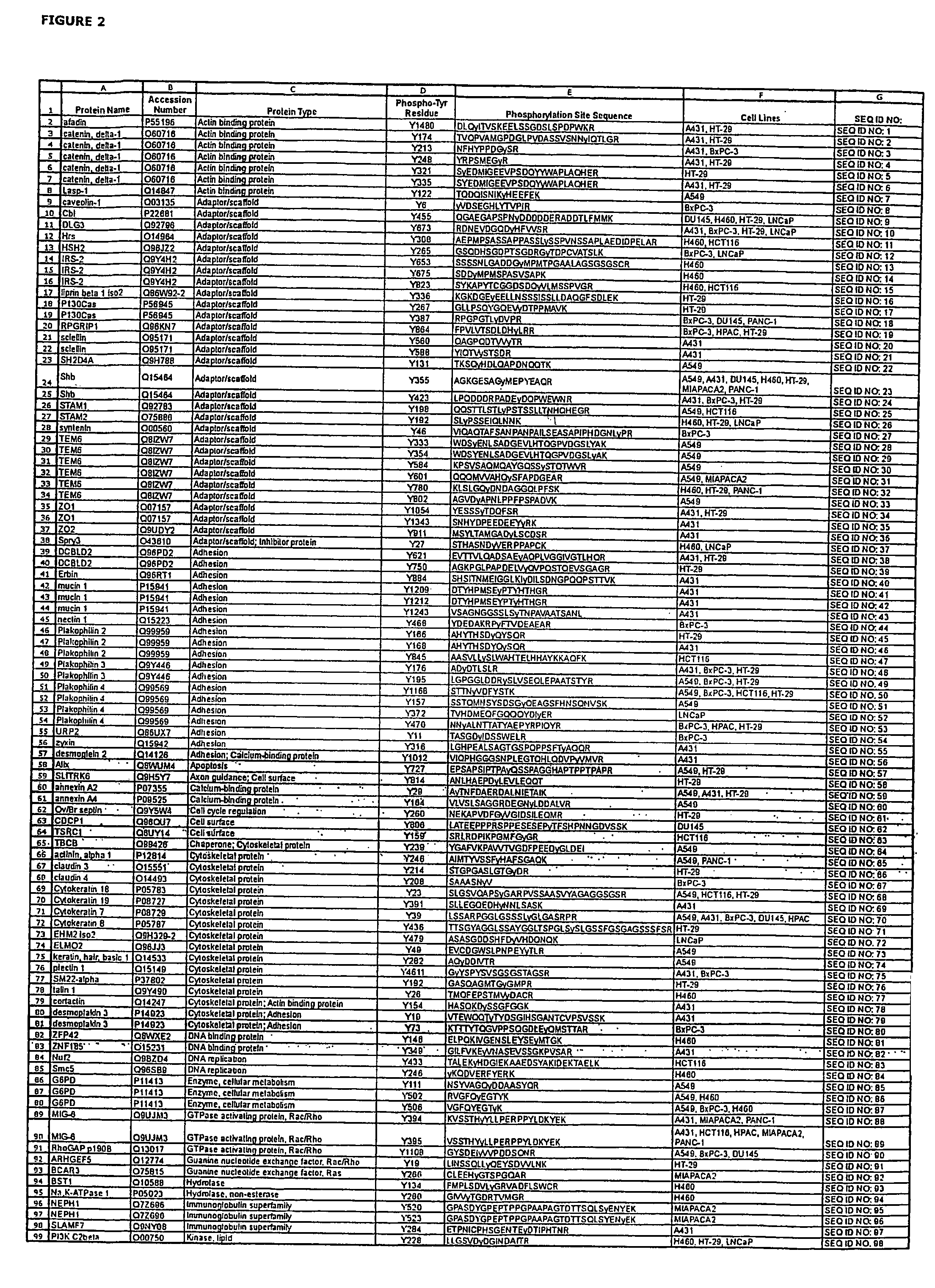
Reagents for the detection of protein phosphorylation in EGFR-signaling pathways
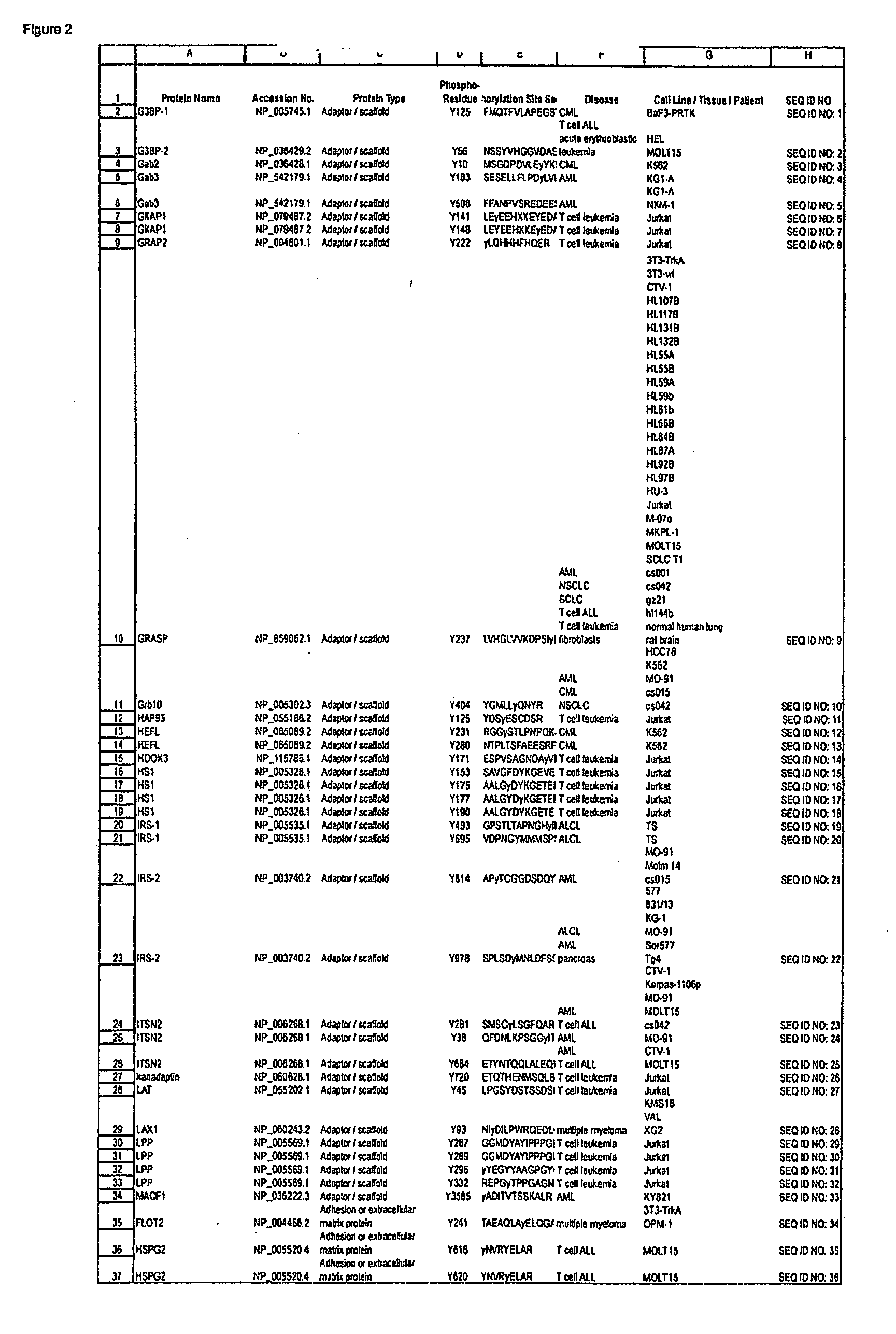
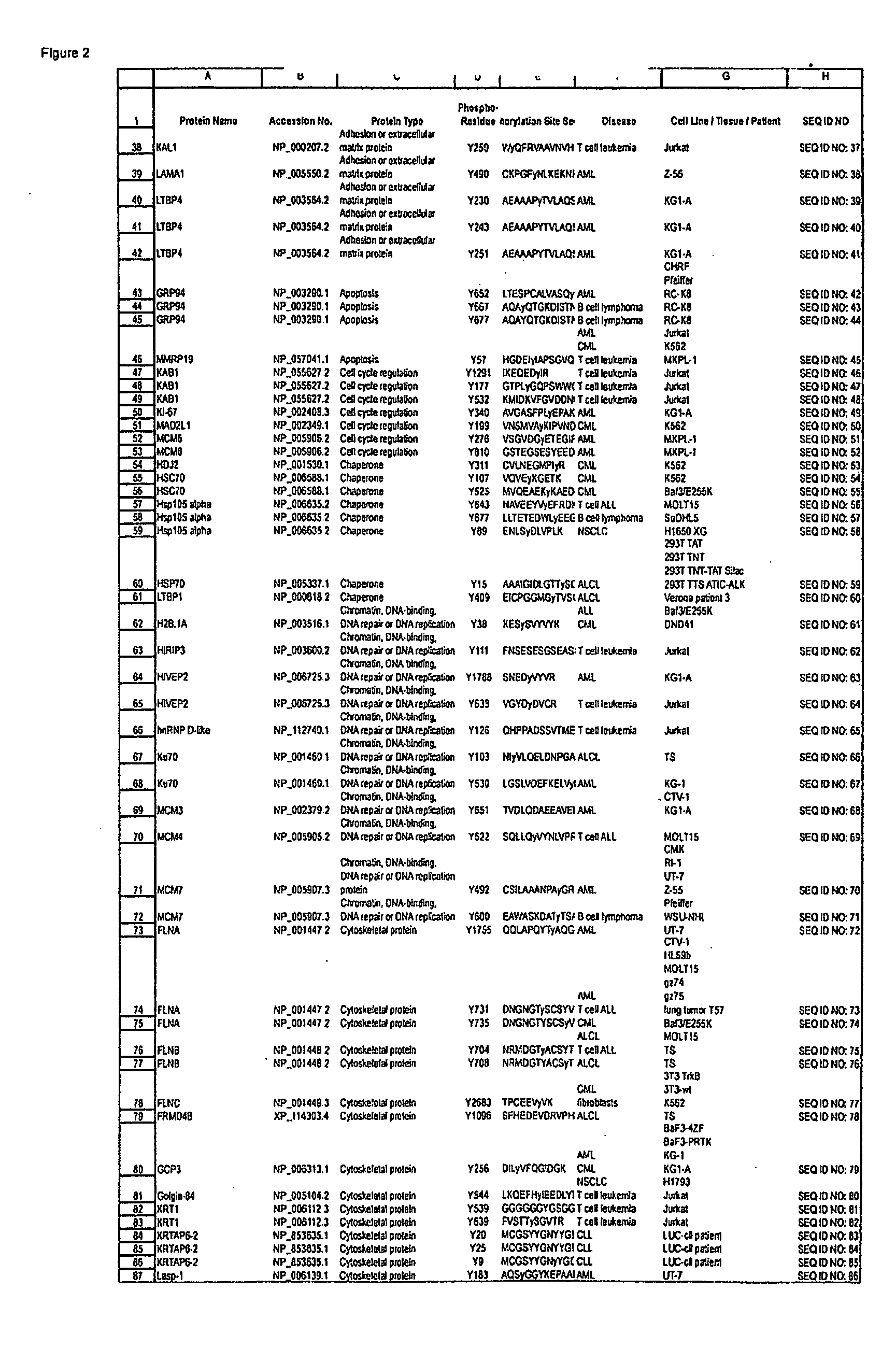
ActiveUS20080108795A1Isotope introduction to peptides/proteinsImmunoglobulins against animals/humansActin-binding proteinScaffold protein
The invention discloses 168 novel phosphorylation sites identified in signal transduction proteins and pathways downstream of, and including, EGFR kinase, and provides phosphorylation-site specific antibodies and heavy-isotope labeled peptides (AQUA peptides) for the selective detection and quantification of these phosphorylated sites / proteins, as well as methods of using the reagents for such purpose. Among the phosphorylation sites identified are sites occurring in the following protein types: Actin Binding proteins, Adaptor / Scaffold proteins, Calcium-Binding Proteins, Cell Cycle Regulation proteins, Cytoskeletal proteins, DNA Binding and Replication Proteins, GTPase Activating proteins, Guanine Nucleotide Exchange Factor proteins, Lipid Kinases, Receptor Tyrosine Kinases, Receptor Tyrosine Kinase ligands, Protein Kinases, Receptor and Protein Phosphatases, Transcription Factor proteins, Tumor Suppressor proteins, and Vesicle proteins.
Owner:CELL SIGNALING TECHNOLOGY
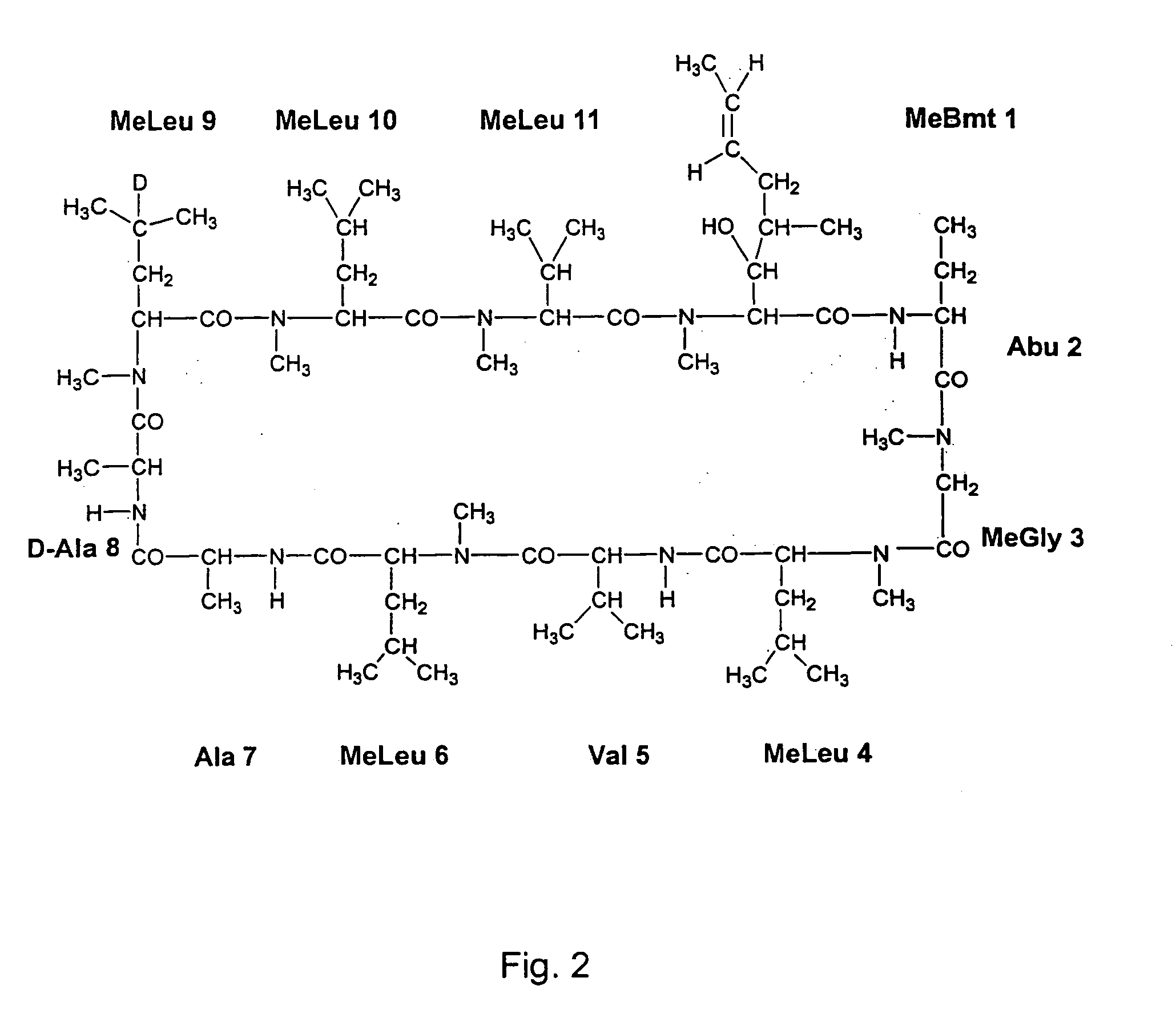
Deuterated cyclosporin analogs and their use as immunomodulating agents
InactiveUS20050176628A1Good curative effectAltered pharmacokinetic and pharmacodynamic parametersIsotope introduction to peptides/proteinsPeptide preparation methodsCyclosporinsImmunomodulating Agent
Cyclosporine derivatives are disclosed which possess enhanced efficacy and reduced toxicity over naturally occurring and other presently known cyclosporins and cyclosporine derivatives. The cyclosporine derivatives of the present invention are produced by chemical and isotopic substitution of the cyclosporine A (CsA) molecule by: (1) Chemical substitution and optionally deuterium substitution of amino acid 1; and (2) deuterium substitution at key sites of metabolism of the cyclosporine A molecule such as amino acids 1, 4, 9. Also disclosed are methods of producing the cyclosporine derivatives and method of producing immunosuppression with reduced toxicity with the disclosed cyclosporine derivatives.
Owner:AURINIA PHARMA
Fluorine-labeled compounds
InactiveUS7902332B2Peptide/protein ingredientsIsotope introduction to peptides/proteinsBioconjugationThiol group
Methods for introducing fluorine atom onto a polypeptide are provided. Also provided are linkers, bioconjugates, and bifunctional compound agents made using the methods, linkers, and bioconjugates. The methods comprise: (i) providing a linker comprising a thiol-reactive terminus and an aldehyde-reactive terminus; (ii) reacting the thiol-reactive terminus of the linker with a polypeptide comprising at least one thiol group or a reactive derivative thereof; and (iii) subsequently reacting the aldehyde-reactive terminus of the linker with a fluorine-substituted aldehyde.
Owner:GENERAL ELECTRIC CO
F-18 peptides for pre targeted positron emission tomography imaging
InactiveUS7842279B2Improve efficiencyReduce needIn-vivo radioactive preparationsIsotope introduction to peptides/proteinsHydroxylamineHydrazine compound
F-18 radiolabeled peptides are prepared by reacting a peptide comprising a hydroxylamine, a thiosemicarbazide, a hydrazine or a free amine group with 4-[18F]Fluorobenzaldehyde. Specific, non-limiting examples of F-18 radiolabeled peptides are described herein. The labeled peptides are useful, for example, in clinical positron emission tomography.
Owner:IMMUNOMEDICS INC
Deuterated cyclosporine analogs and their use as immunodulating agents
InactiveUS20030220234A1Good curative effectAltered pharmacokineticIsotope introduction to peptides/proteinsPeptide preparation methodsCyclosporinsIsotope
Cyclosporine derivatives are disclosed which possess enhanced efficacy and reduced toxicity over naturally occurring and other presently known cyclosporins and cyclosporine derivatives. The cyclosporine derivatives of the present invention are produced by chemical and isotopic substitution of the cyclosporine A (CsA) molecule by: (1) Chemical substitution and optionally deuterium substitution of amino acid 1; and (2) deuterium substitution at key sites of metabolism of the cyclosporine A molecule such as amino acids 1, 4, 9. Also disclosed are methods of producing the cyclosporine derivatives and method of producing immunosuppression with reduced toxicity with the disclosed cyclosporine derivatives.
Owner:NAICKER SELVARAJ +2
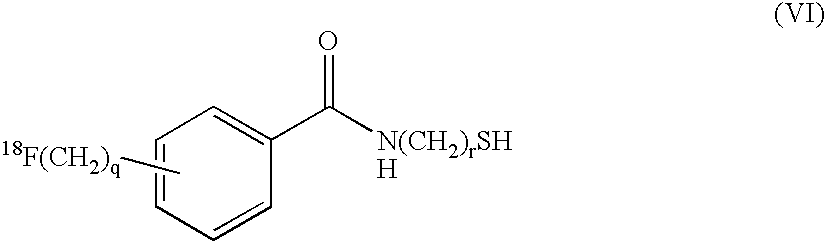
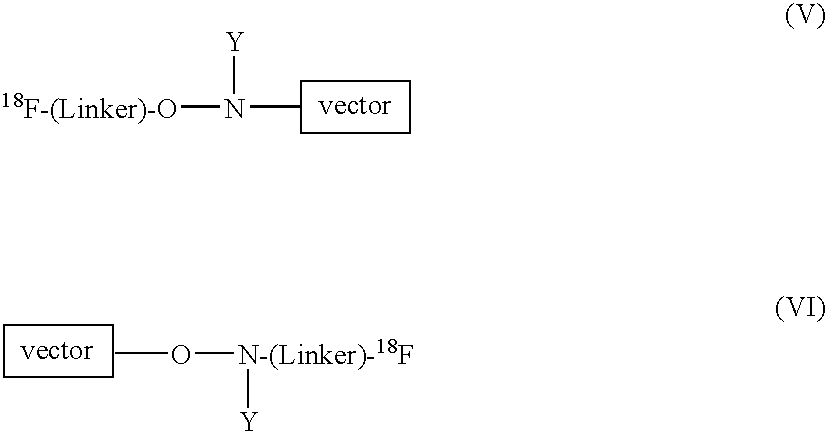
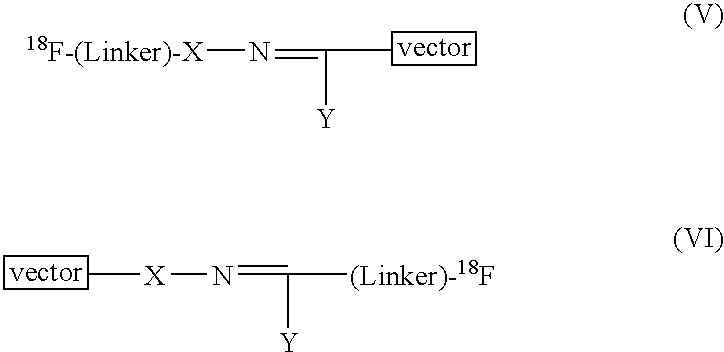
Radiofluorination methods
InactiveUS7368474B2BiocideIsotope introduction to peptides/proteinsCombinatorial chemistryPositron emission tomography
The present invention relates to methods and reagents for [18F]-fluorination, particularly of peptides. The resultant 18F-labelled compounds are useful as radiopharmaceuticals, specifically for use in Positron Emission Tomography (PET). Thus, a compound of formula 18F-(Linker)-SH, such as a compound of formula (IV), (V), or (VI):18F—(CH2CH2O)n—(CH2)m—SH (IV)18F—(CH2)p—SH (V)may be reacted with an activated peptide as a means for 18F-labelling.
Owner:GE HEALTHCARE LTD
Ionizable isotopic labeling reagents for relative quantification by mass spectrometry
ActiveUS7982070B2Improve ETD analysisConvenient charging statusComponent separationOrganic compound preparationMetaboliteIsotopic labeling
Relative quantification of metabolites by Electrospray Ionization Mass Spectrometry (ESI-MS) requiring a mechanism for simultaneous analysis of multiple analytes in two or more samples. Labeling reagents that are reactive to particular compound classes and differ only in their isotopic kit facilitating relative quantification and providing tangible evidence for the existence of specific functional groups. Heavy and light isotopic forms of methylacetimidate were synthesized and used as labeling reagents for quantification of amine-containing molecules, such as biological samples. Heavy and light isotopic forms of formaldehyde and cholamine were also synthesized and used independently as labeling reagents for quantification of amine-containing and carboxylic acid-containing molecules, such as found in biological samples. Advantageously, the labeled end-products are positively charged under normal acidic conditions involving conventional Liquid Chromatography Mass Spectrometry (LC / MS) applications. Labeled primary and secondary amine and carboxylic acid end-products also generated higher signals concerning mass-spectra than pre-cursor molecules and improved sensitivity. Improved accuracy concerning relative quantification was achieved by mixing heavy and light labeled Arabidopsis extracts in different ratios. Labeling strategy was further employed to ascertain differences in the amounts of amine-containing metabolites for two strains of Arabidopsis seeds.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
In vivo copper-free click chemistry for delivery of therapeutic and/or diagnostic agents
InactiveCN103221072AAntibacterial agentsIsotope introduction to peptides/proteinsChemical reactionDiagnostic agent
The present application discloses compositions and methods of synthesis and use involving click chemistry reactions for in vivo or in vitro formation of therapeutic and / or diagnostic complexes. Preferably, the diagnostic complex is of use for 18F imaging, while the therapeutic complex is of use for targeted delivery of chemotherapeutic drugs or toxins. More preferably, a chelating moiety or targetable construct may be conjugated to a targeting molecule, such as an antibody or antibody fragment, using a click chemistry reaction involving cyclooctyne, nitrone or azide reactive moieties. In most preferred embodiments, the click chemistry reaction occurs in vivo. In vivo click chemistry is not limited to 18F labeling but can be used for delivering a variety of therapeutic and / or diagnostic agents.
Owner:IMMUNOMEDICS INC
Labeled ligands for lectin-like oxidized low-density lipoprotein receptor (LOX-1)
InactiveUS20050089471A1Ultrasonic/sonic/infrasonic diagnosticsGeneral/multifunctional contrast agentsCoronary artery diseaseImaging agent
Embodiments of the present invention relates to compounds labeled with imaging agents that also are capable of binding lectin-like oxidized low-density lipoprotein (LOX-1). The labeled compounds are useful for the diagnosis and monitoring of diseases in which inflammation plays a role, such as various cardiovascular diseases including but not limited to atherosclerosis, vulnerable plaque, and coronary artery disease, as well as rheumatoid arthritis.
Owner:GENERAL ELECTRIC CO
Reagents for the detection of protein phosphorylation in signaling pathways
InactiveUS20100009463A1Animal cellsIsotope introduction to peptides/proteinsCell Surface ProteinsPhosphorylation
The invention discloses novel phosphorylation sites identified in signal transduction proteins and pathways, and provides phosphorylation-site specific antibodies and heavy-isotope labeled peptides (AQUA peptides) for the selective detection and quantification of these phosphorylated sites / proteins, as well as methods of using the reagents for such purpose. Among the phosphorylation sites identified are sites occurring in the following protein types: adaptor / scaffold proteins, adhesion / extracellular matrix protein, apoptosis proteins, calcium binding proteins, cell cycle regulation proteins, chaperone proteins, chromatin, DNA binding / repair / replication proteins, cytoskeletal proteins, endoplasmic reticulum or golgi proteins, enzyme proteins, G / regulator proteins, inhibitor proteins, motor / contractile proteins, phosphatase, protease, Ser / Thr protein kinases, protein kinase (Tyr)s, receptor / channel / cell surface proteins, RNA binding proteins, transcriptional regulators, tumor suppressor proteins, ubiquitan conjugating system proteins and proteins of unknown function.
Owner:CELL SIGNALING TECHNOLOGY
Composition and method for the diagnosis and treatment of diseases associated with neurite degeneration
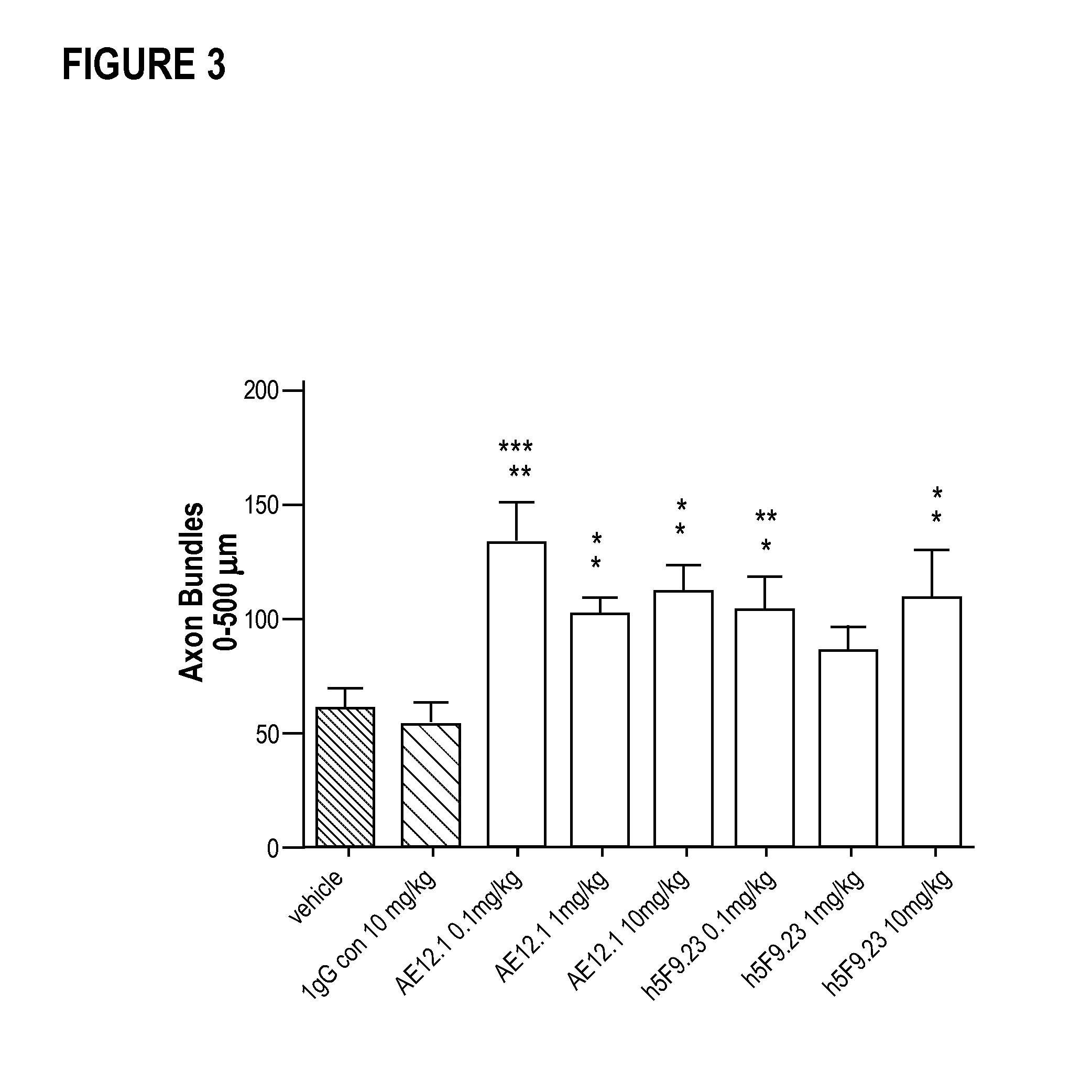
Provided herein are antibodies and methods of using the antibodies to treat and diagnose neurite degenerative diseases and disorders.
Owner:ABBVIE INC +1
Nucleic acid sequencing processes using non-radioactive detectable modified or labeled nucleotides or nucleotide analogs, and other processes for nucleic acid detection and chromosomal characterization using such non-radioactive detectable modified or labeled nucleotides or nucleotide analogs
ActiveUS8097405B1Sugar derivativesIsotope introduction to peptides/proteinsNucleic acid detectionNucleotide
A process for determining the sequence of nucleic acids of interest employs nucleotides or nucleotide analogs that have been made detectable by non-radioactive modifying or labeling. Such nucleotides or nucleotide analogs are modified on the sugar moieties, the phosphate moieties or the base moieties, including base analogs. Modified nucleotide analogs can be attached to or coupled to or incorporated into DNA or RNA. The modified or labeled nucleotides or nucleotide analogs are also useful in processes for detecting the presence of nucleic acids of interest and for characterizing chromosomal sequences. Detection processes using the modified or labeled nucleotides or nucleotide analogs extend to the use of a gel for separating or resolving hybrids formed between non-radioactively labeled oligonucleotides or polynucleotides and such nucleic acids of interest.
Owner:ENZO LIFE SCI INC
Method for obtaining dynamic and structural data pertaining to proteins and protein/ligand complexes
InactiveUS20060154323A1High sensitivityImprove resolutionMagnetic measurementsIsotope introduction to peptides/proteinsDiffusionImage resolution
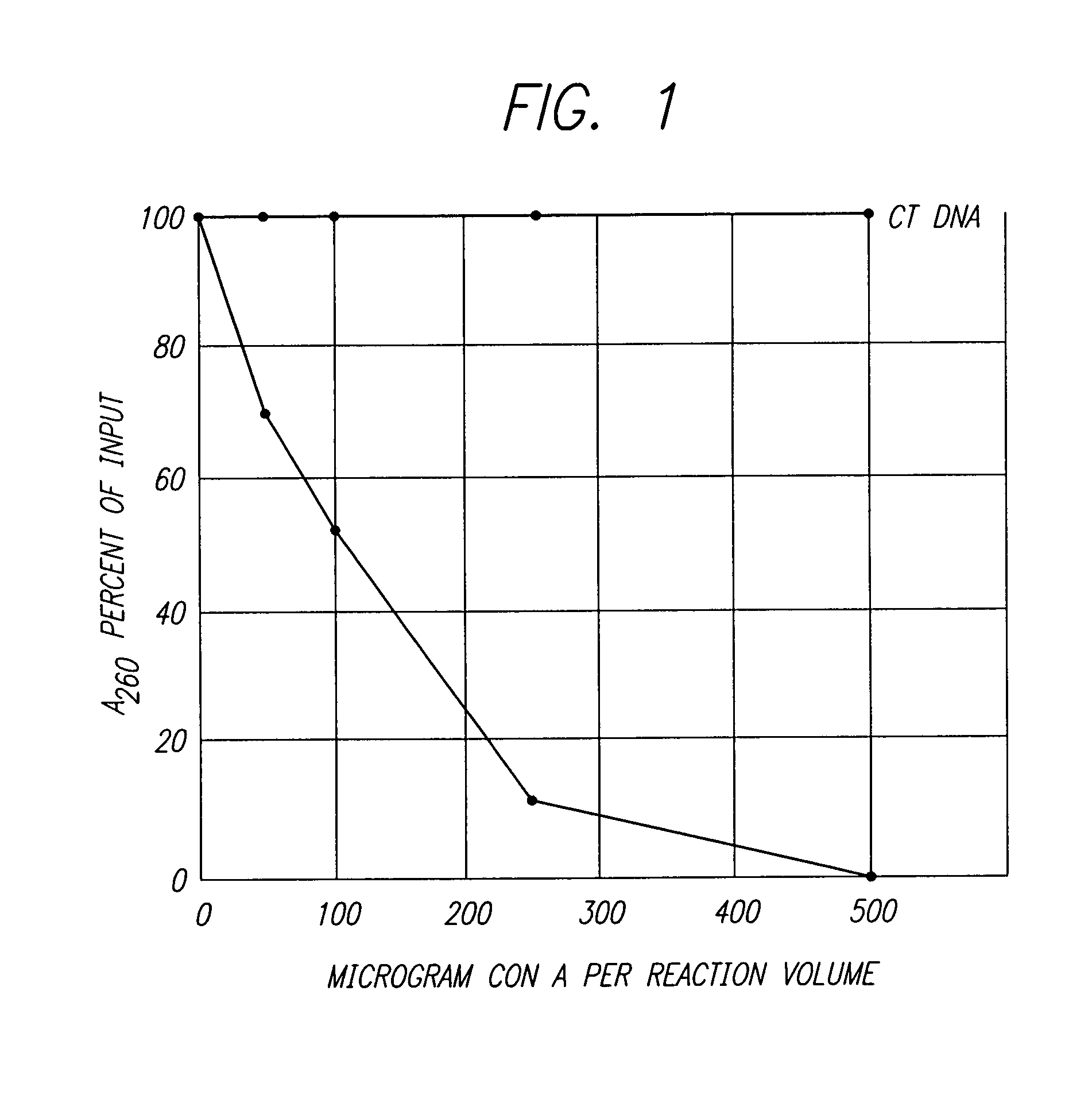
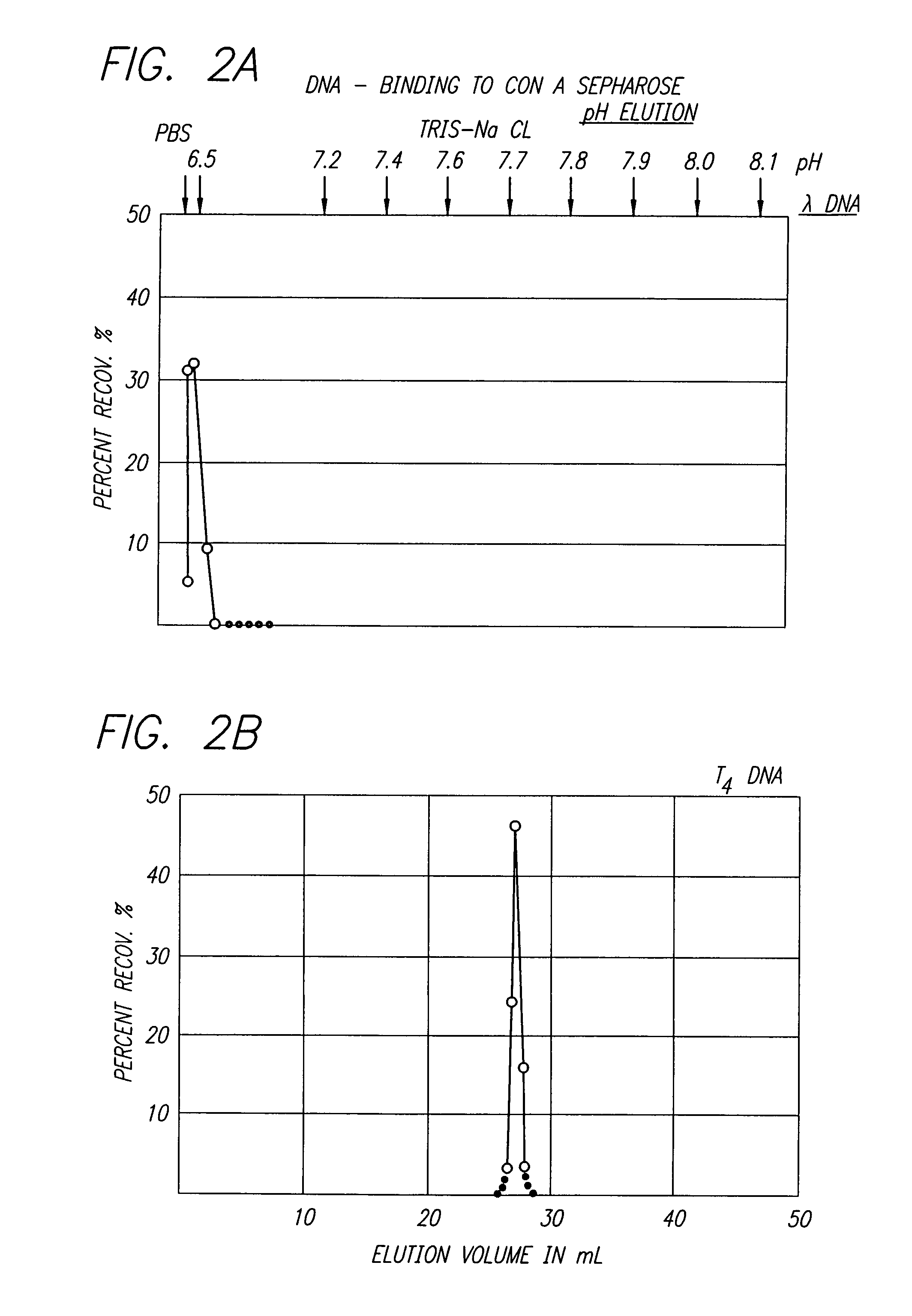
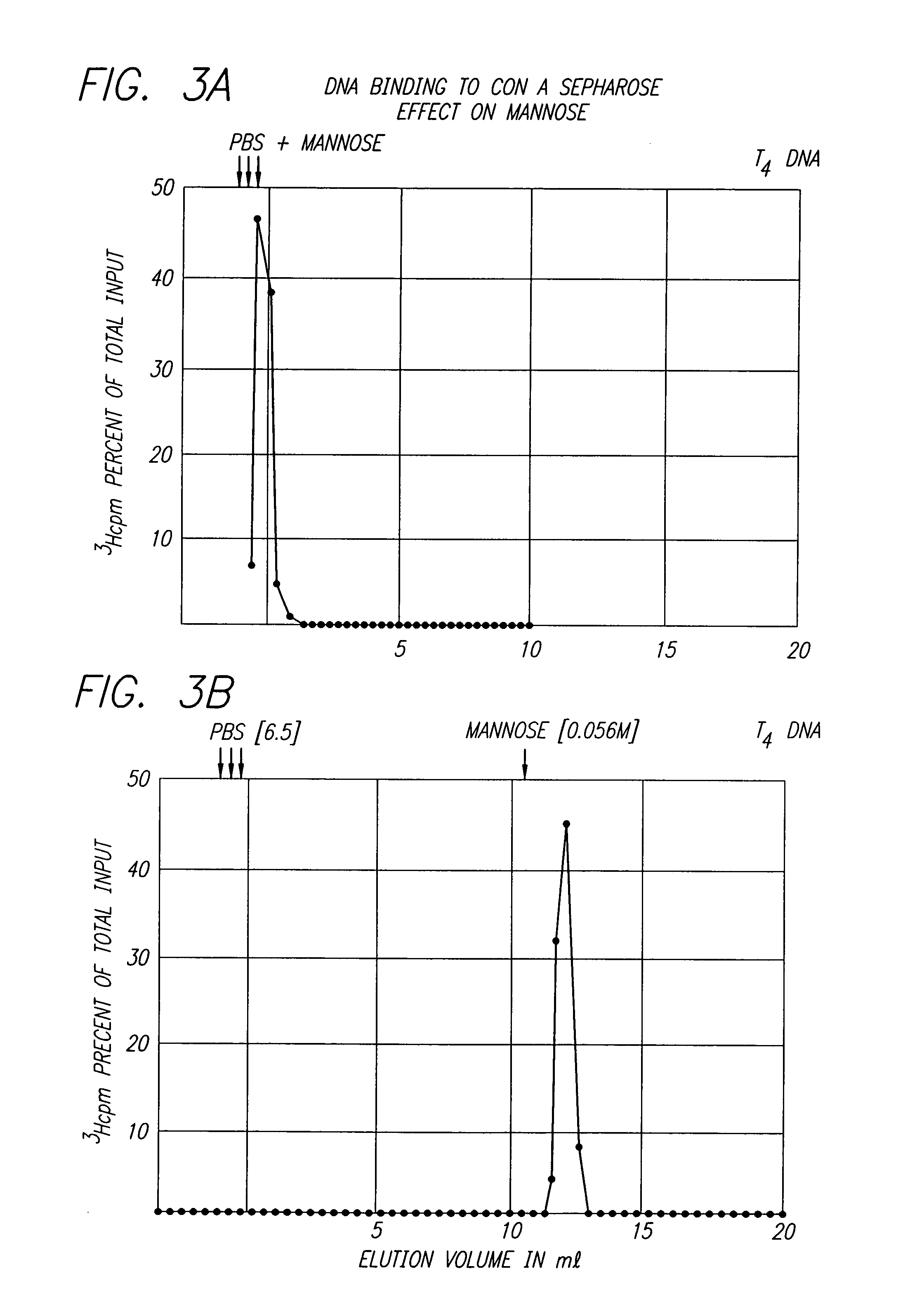
This invention provides an NMR method for obtaining both entropic and enthalpic data on proteins and protein / ligand complexes which can be used to obtain accurate structural and dynamic data of proteins and protein complexes having a wide range of molecular weights. An embodiment of the invention provides proteins which contain at least one bond vector whose dynamics are to be measured and which is surrounded by NMR inactive nuclei, and amino acids for synthesis of the proteins via chemical means or biological expression. The NMR methods using specifically labeled proteins for analysis result in maximization of the sensitivity and resolution of the NMR experiments, and minimization of the loss of signal due to diffusion.
Owner:PROSPECT PHARMA
Radiolabelled octreotate analogues as pet tracers
ActiveUS20130343990A1Rapid target localizationRapid pharmacokineticsIsotope introduction to heterocyclic compoundsPeptide/protein ingredientsRadioactive tracerGastroenteropancreatic neuroendocrine tumor
Novel radiotracer(s) for Positron Emission Tomography (PET) imaging are described. Novel radiotracer(s) for Positron Emission Tomography (PET) imaging of neuorendocrine tumors are described. Specifically the present invention describes novel [18F]Fluoroethyltriazol-[Tyr3]Octreotate analogs; in particular those that target somatostatin receptors found on the cell surface of gastroenteropancreatic neuorendocrine tumors. The present invention also describes intermediate(s), precursor(s), pharmaceutical composition(s), methods of making, and methods of use of the novel radiotracer(s).
Owner:IMPERIAL INNOVATIONS LTD
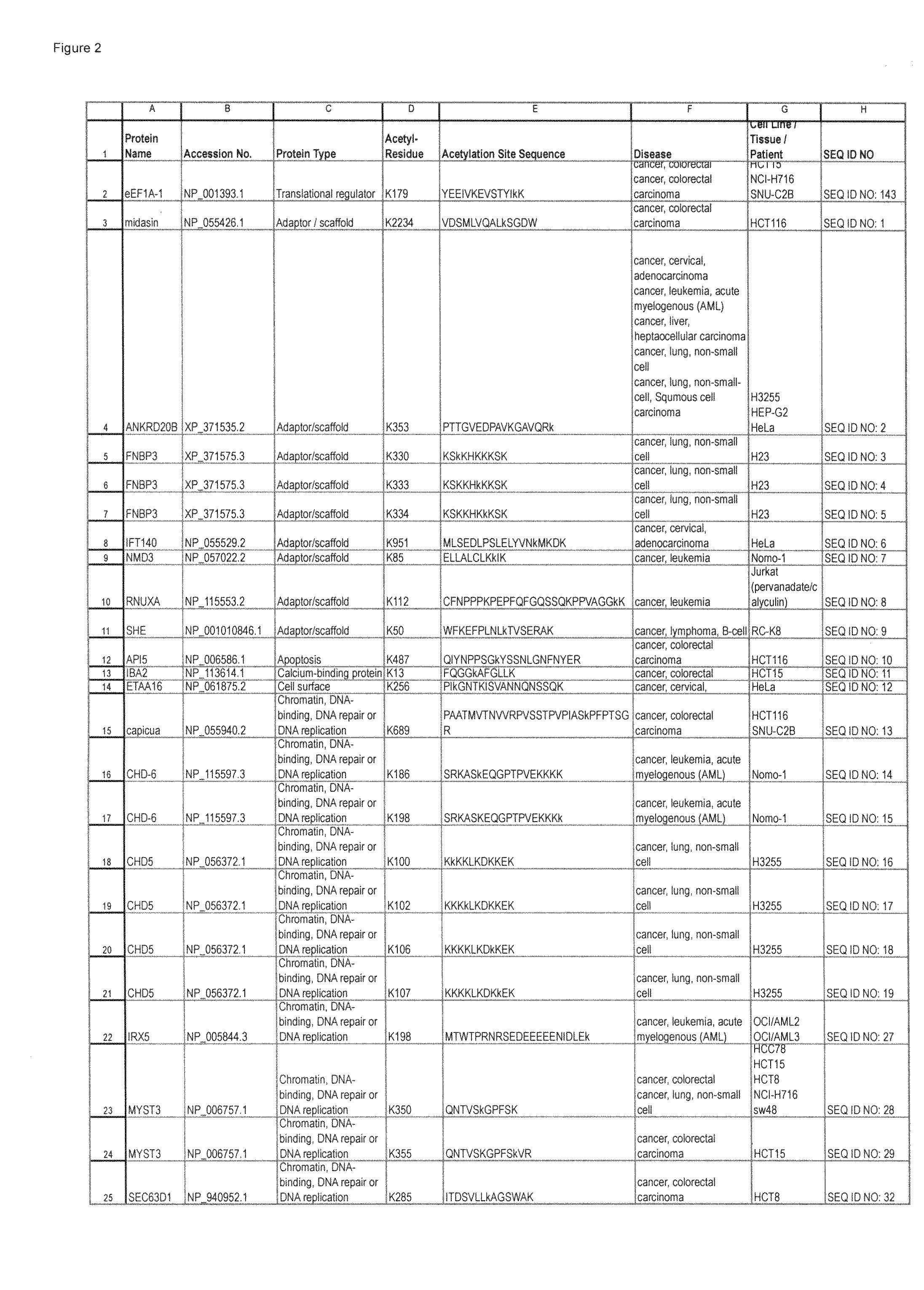
Lysine acetylation sites
InactiveUS20100092992A1Isotope introduction to peptides/proteinsImmunoglobulinsAntiendomysial antibodiesAcetylation
The invention discloses 322 novel acetylation sites identified in various cancers, peptides (including AQUA peptides) comprising a acetylation site of the invention, antibodies specifically bind to a novel acetylation site of the invention, and diagnostic and therapeutic uses of the above.
Owner:HORNBECK PETER +5
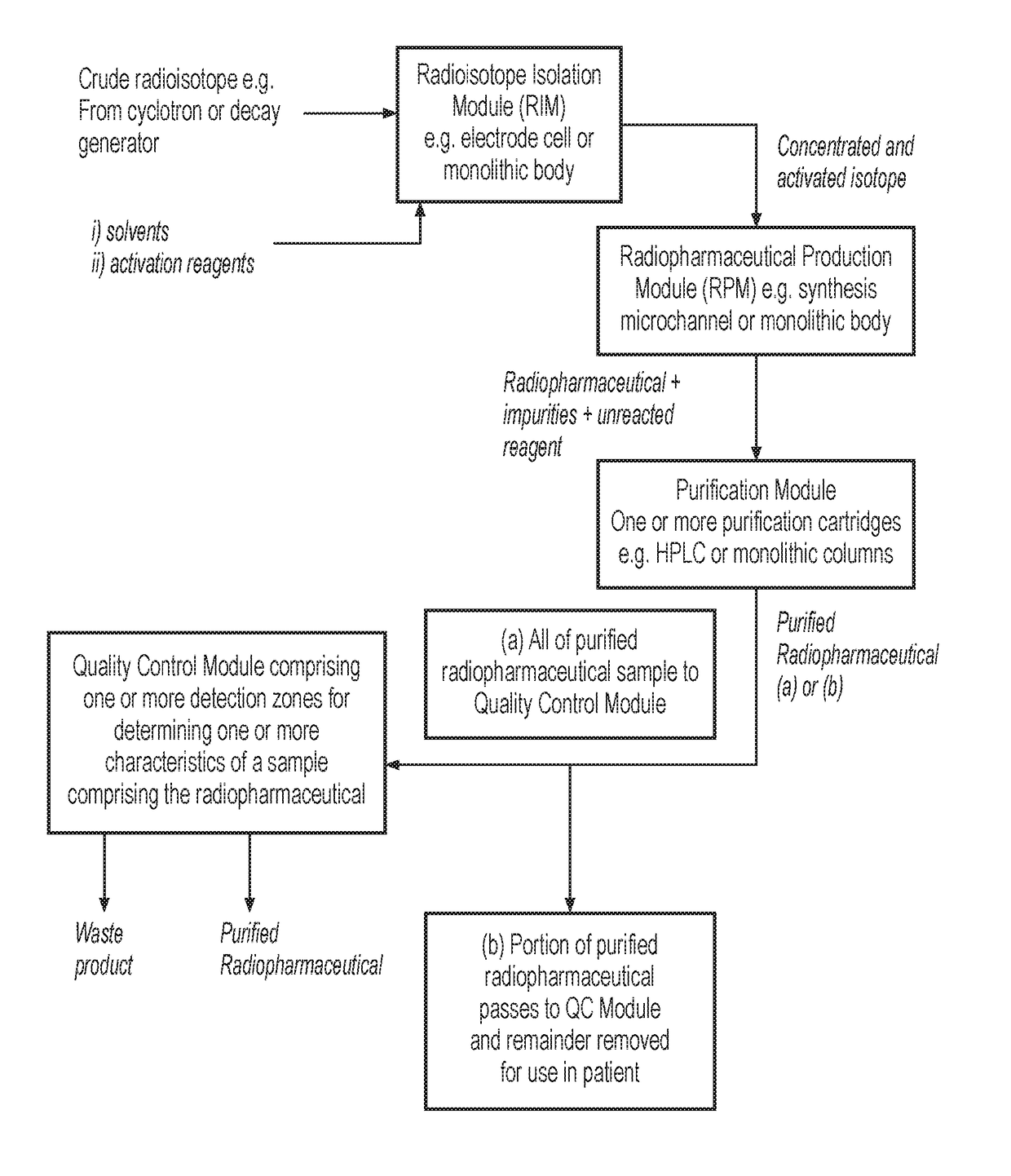
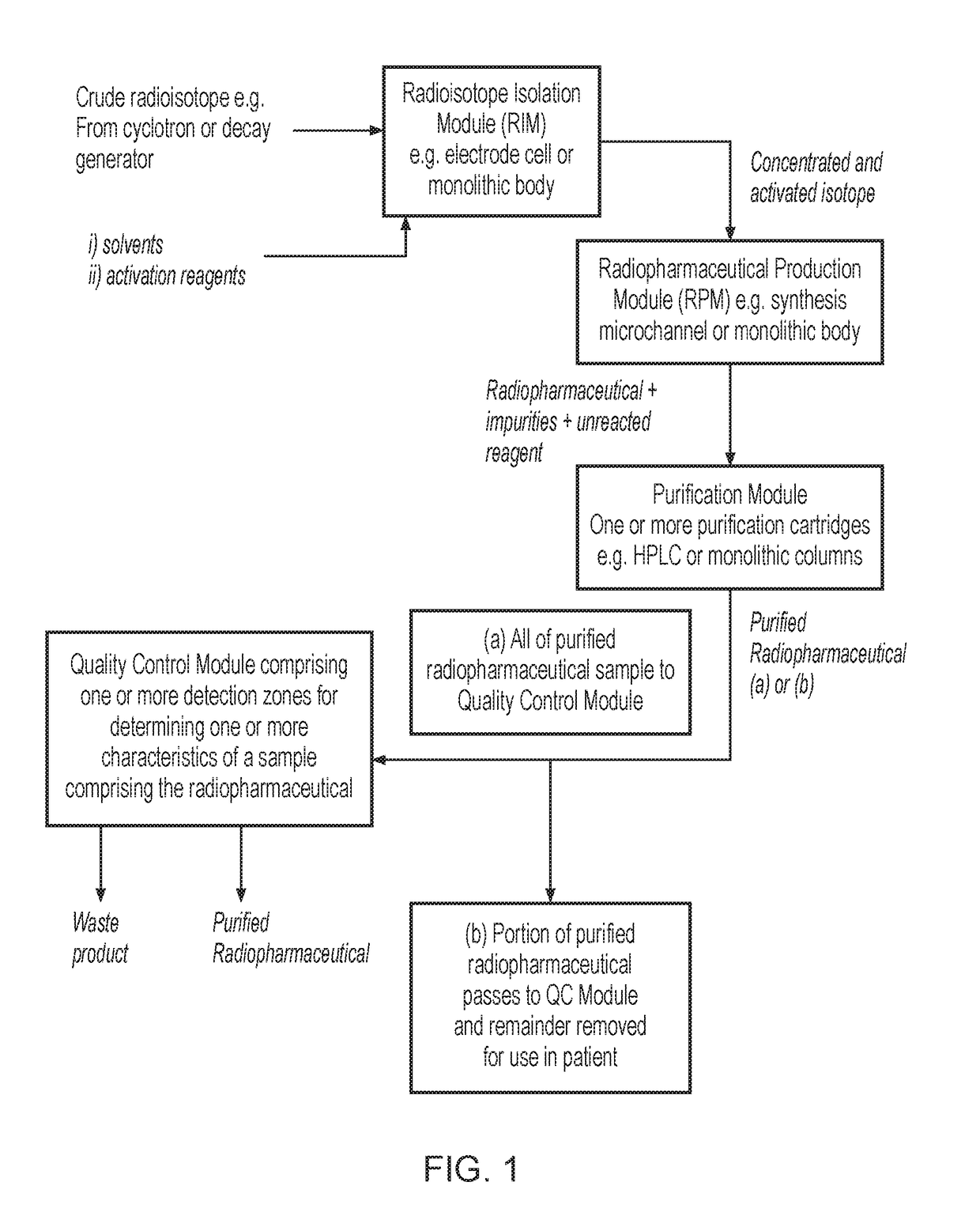
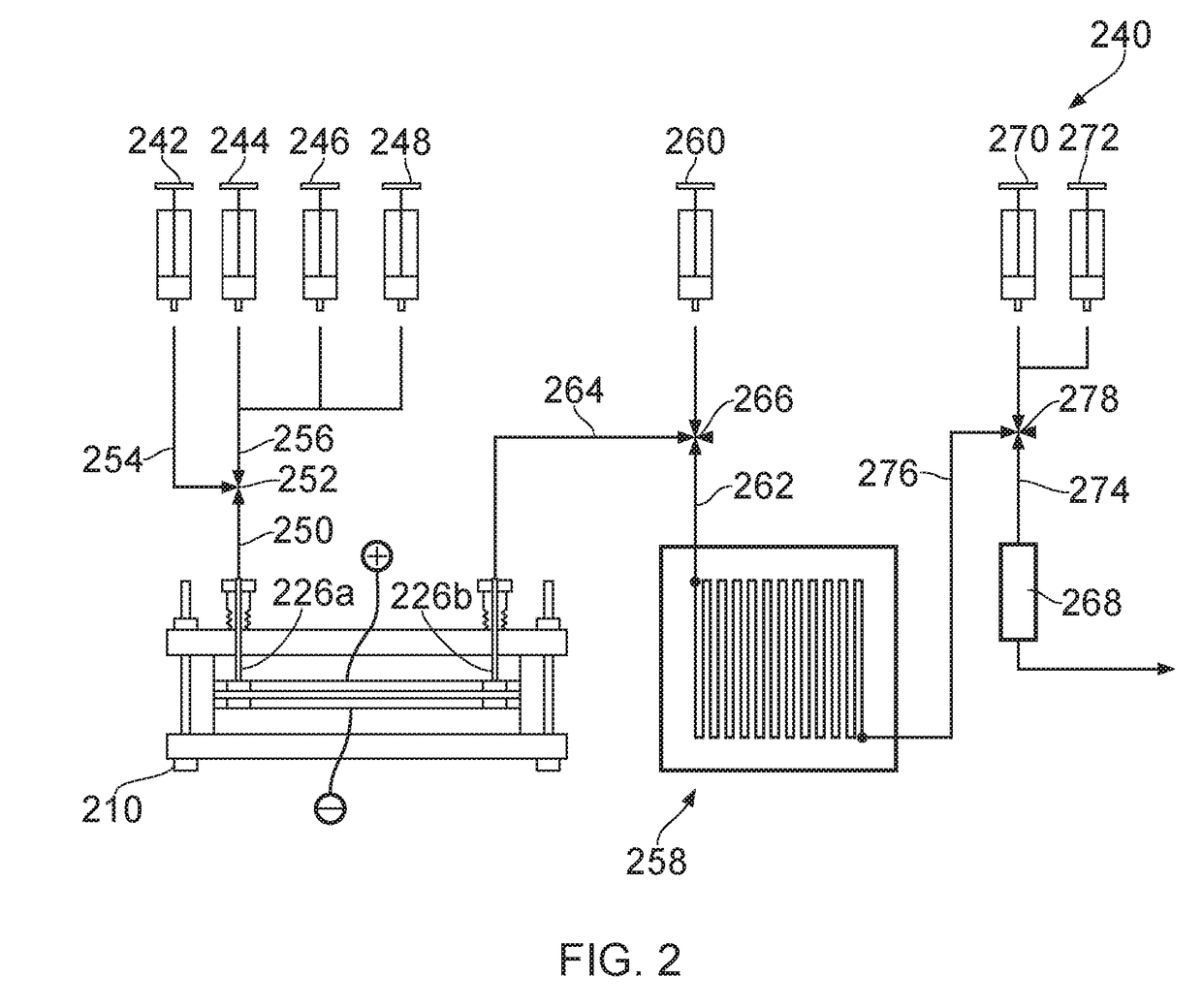
System for radiopharmaceutical production
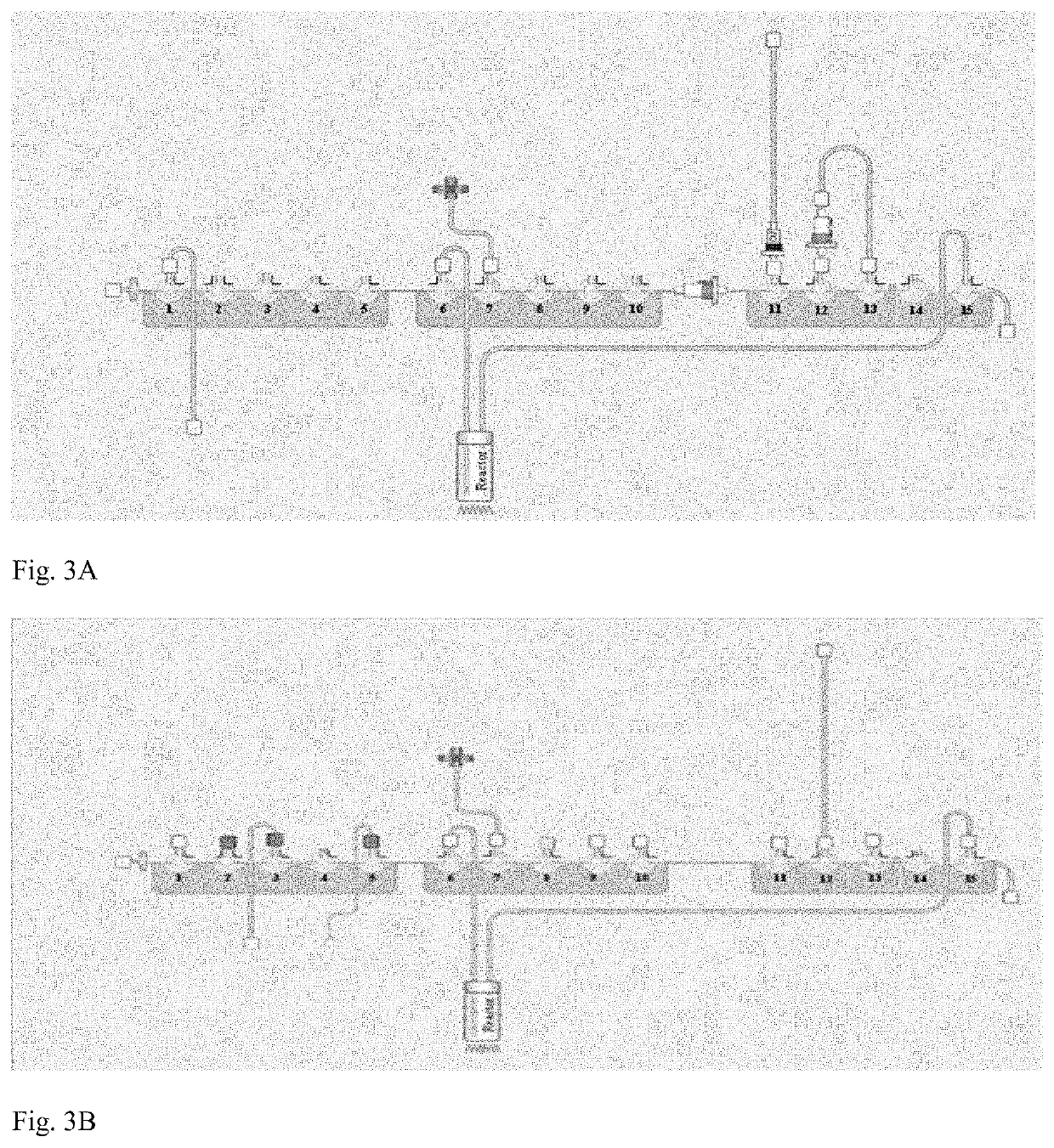
ActiveUS20180033510A1Chromatographic cation exchangersIsotope delivery systemsQuality controlEngineering
Certain embodiments of the present invention relate to a system and a method for producing a radiopharmaceutical, wherein the system is formed from and / or provides a microfluidic flow system. In certain embodiments, the system comprises a radioisotope isolation module, a radiopharmaceutical production module, a purification module and a quality control module.
Owner:UNIVERSITY OF HULL
Complex of bifunctional connecting agent realizing core coordination with carbonyl metal and preparation method thereof
ActiveCN109438517AGood biological propertiesGood effectIsotope introduction to peptides/proteinsRadioactive preparation carriersEthylenediamineFunctional connectivity
The invention relates to a complex of a bifunctional connecting agent realizing core coordination with carbonyl metal and a preparation method thereof, and belongs to the technical field of radiopharmaceutical chemistry. The bifunctional connecting agent is N,N'-bi[2-hydroxyl-5-( propyloic) benzyl] ethylenediamine-N,N'-oxalic acid, and has a structure shown in the description, wherein at least onein R1 and R2 is a targeted small molecule or polypeptide or protein big molecule; the metal is Tc or Re. The carbonyl Tc / Re core marked HBED-CC complex is successfully obtained for the first time, different nuclide (68Ga, 99mTc / Re) marks of the same marking precursors can be realized; by aiming at the mutual supplementation of two development modes (PET / SPECT) in the same target point, the 188 / 186Re complex can be used for radioactive therapy medicine study. A novel idea is provided for the development of a Tc-99m single photon radioactive tracer agents and Re-188 / 186 radioactive treatment medicine; meanwhile, the application range of the HBED-CC derivatives as radiopharmaceutical marker precursors is also expanded.
Owner:北京久杰净化工程技术有限公司
Process for the preparation of complexes of 68ga
ActiveUS20140171637A1Isotope introduction to peptides/proteinsRadioactive preparation carriersSkin complexionFormic acid
A process for the preparation of complexes containing 68Ga wherein a buffer formic acid / formate in the presence of compounds capable to sequester metal cations is used in the complexion reaction.
Owner:ADVANCED ACCELERATOR APPL INT SA
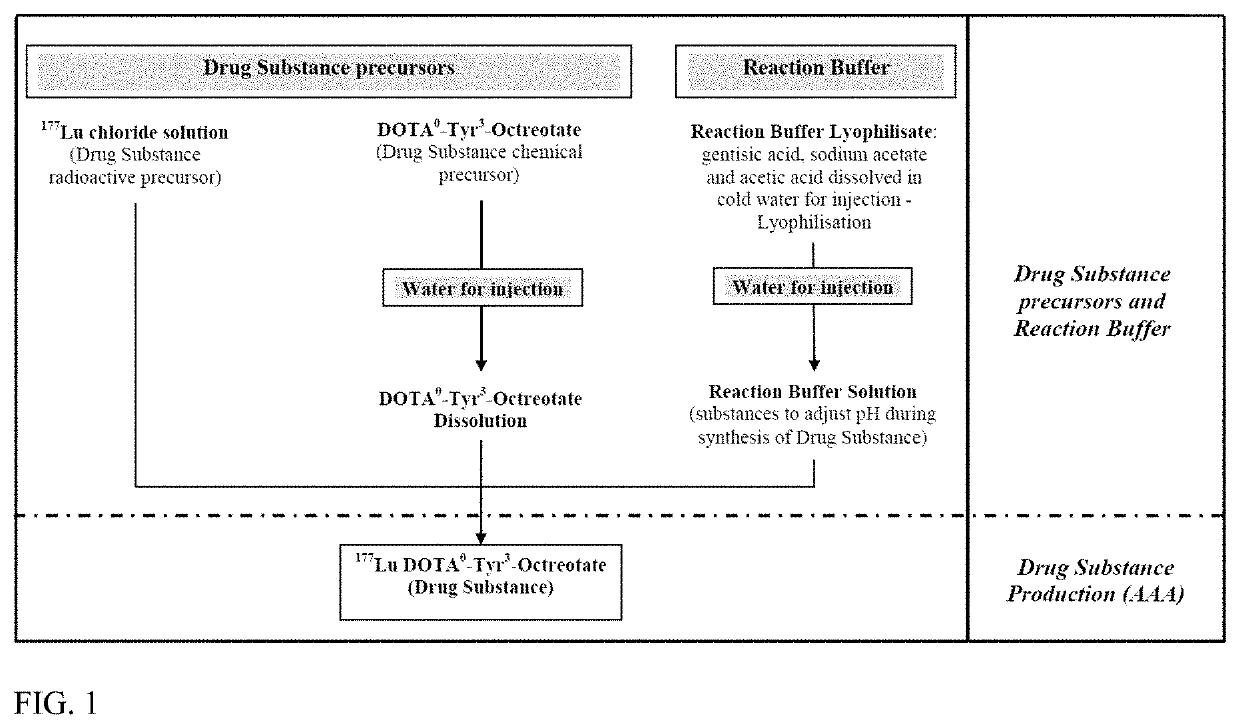
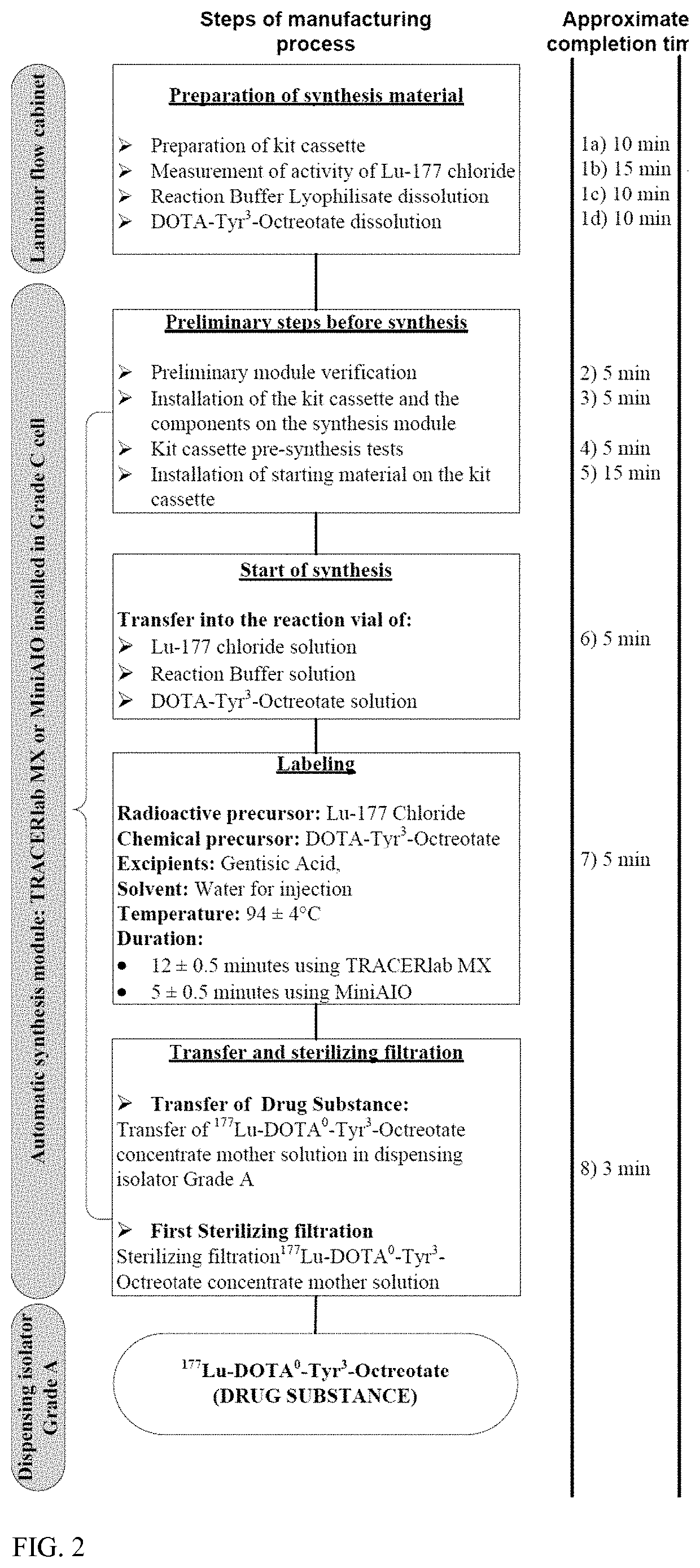
Methods for Synthesis of Radionuclide Complex
InactiveUS20200131224A1Isotope introduction to peptides/proteinsRadioactive preparation carriersRadioactive drugSomatostatin receptor binding
The present disclosure relates to the synthesis of radionuclide complex solutions, in particular for their use in the commercial production of radioactive drug substances, for diagnostic and / or therapeutic purposes. In particular, the synthesis method comprises the following steps in the following order:a. providing a radionuclide precursor solution into a first vial,b. transferring the radionuclide precursor solution into a reactor,c. providing a reaction buffer solution into said first vial containing residual radionuclide precursor solution,d. transferring the buffer reaction solution and residual radionuclide precursor solution from said first vial into the reactor,e. transferring a peptide solution comprising the somatostatin receptor binding peptide linked to a chelating agent, into the reactor,f. reacting the somatostatin receptor binding peptide linked to a chelating agent with said radionuclide in the reactor to obtain the radionuclide complex,g. recovering said radionuclide complex.
Owner:ADVANCED ACCELERATOR APPL (ITAL) SRL
Radiofluorination methods
Owner:GE HEALTHCARE LTD
Heat shock protein as a targeting agent for endothelium-specific in vivo transduction
InactiveUS7575738B2Sufficient specificityIn-vivo radioactive preparationsIsotope introduction to peptides/proteinsHeat shockImaging agent
The present invention relates to the use of Heat Shock Proteins and fragments thereof as targeting ligand. The Heat Shock Protein may be labeled with imaging agents that are capable of binding lectin-like oxidized low-density lipoprotein (LOX-1) or may be attached to a therapeutic agent. The sequences are useful for the diagnosis and monitoring of diseases as well as means for internalizing signaling moieties and therapeutics.
Owner:GENERAL ELECTRIC CO
Methods of radiofluorination of biologically active vectors
ActiveUS8197793B2Hydrazine preparationOrganic compound preparationMedicinal chemistryBiological activity
Owner:GE HEALTHCARE AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com