Self-microemulsifying soft capsule content combination of ciclosporin A and preparation method thereof
A technology for cyclosporine and contents, which is applied in the field of self-microemulsifying soft capsule contents combination of cyclosporine A and the field of preparation thereof, can solve the problems such as cyclosporine nephrotoxicity that cannot effectively increase the clinical efficacy of cyclosporine, and the like, Achieve the effect of improving bioavailability, simple preparation process and improving dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Weigh 10 parts of cyclosporine A, 10 parts of phosphatidylcholine (80% pure soybean lecithin), 15 parts of absolute ethanol, 6 parts of propylene glycol, 45 parts of polyoxyethylene hydrogenated castor oil CremophorRH4045 parts, 8019 parts of Span and medium chain fatty acid 25 parts of triglycerides were placed in a homogeneous emulsification pot and heated to 50°C, heated for 0.5h, and stirred to dissolve the drug to make a content solution; the content solution was pressed into soft capsules with a rotary molding machine, and processed with 95% ethanol Surface treatment, wash off the surface oil layer, ventilate and dry to get the finished product; the drying temperature is 25°C.
Embodiment 2
[0022] Weigh 12 parts of cyclosporine A, 12 parts of phosphatidylcholine (80% pure egg yolk lecithin), 10 parts of absolute ethanol, 10 parts of propylene glycol, 4060 parts of polyoxyethylene hydrogenated castor oil CremophorRH, 8010 parts of Span and medium chain fatty acid Put 20 parts of triglycerides in a homogeneous emulsification pot and heat to 60°C, heat for 0.8h, and stir to dissolve the drug to make a content solution; press the above-prepared content solution into a soft capsule with a rotary molding machine, And use 95% ethanol to carry out surface treatment, wash off the surface oil layer, ventilate and dry to obtain the finished product, and the drying temperature is 27°C.
Embodiment 3
[0024] Weigh 8 parts of cyclosporine A, 8 parts of phosphatidylcholine (45% pure soybean lecithin), 6 parts of absolute ethanol, 16 parts of propylene glycol, 65 parts of polyoxyethylene hydrogenated castor oil CremophorRH4065 parts, 808 parts of Span and medium chain fatty acid 15 parts of triglycerides were placed in a homogeneous emulsification pot and heated to 40°C, heated for 1.0 h, and stirred to dissolve the drug to make a content solution; the content solution prepared above was pressed into a soft capsule with a rotary molding machine, And use 95% ethanol for surface treatment, wash off the surface oil layer, and ventilate and dry to obtain the finished product; the drying temperature is 28°C.
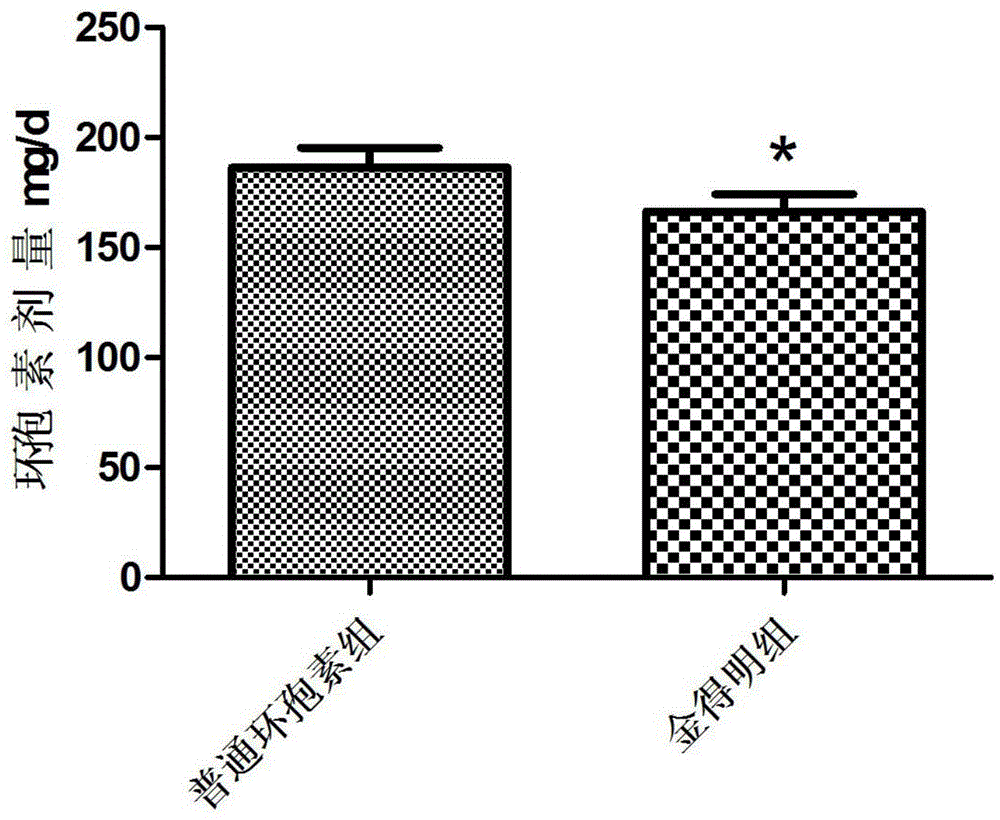
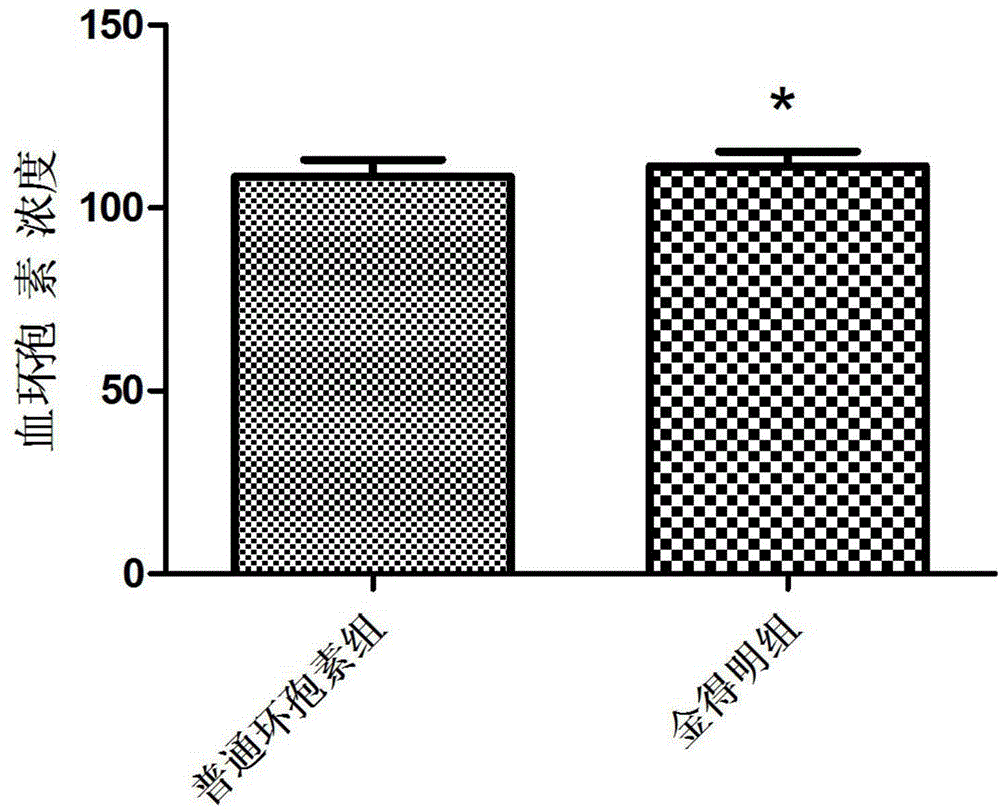
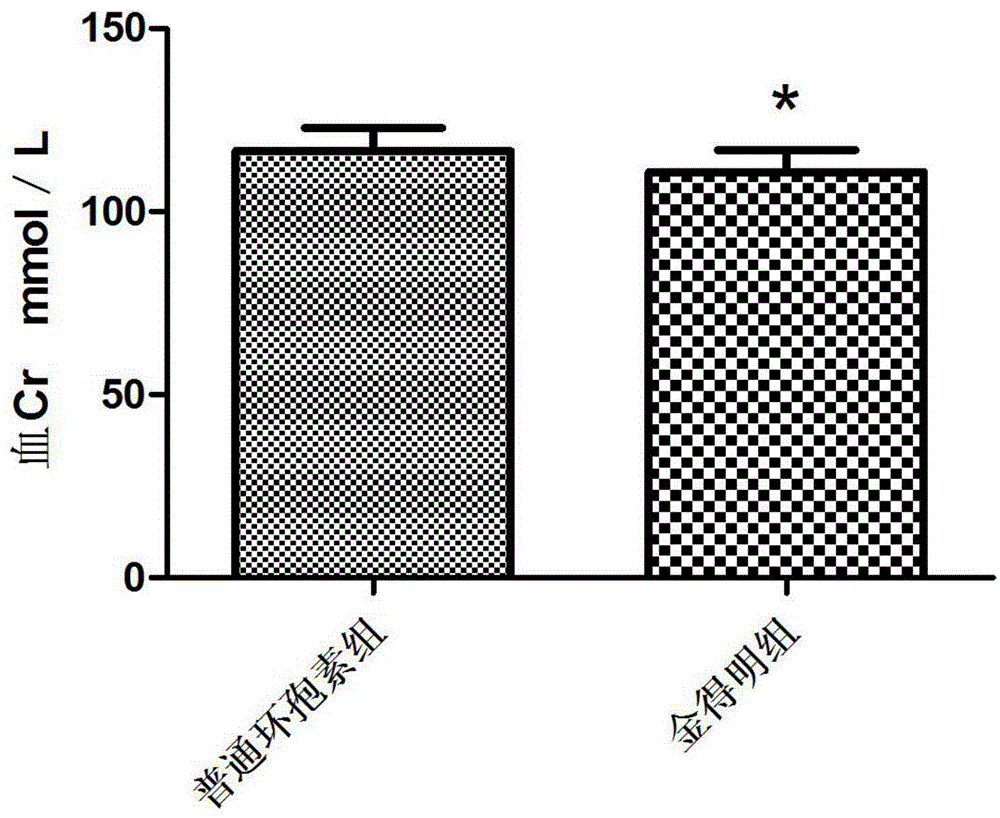
[0025] Cyclosporine A self-microemulsifying soft capsules prepared by using the above three components are suitable for preventing rejection of allogeneic kidney, liver, heart, bone marrow and other organ or tissue transplants, and are also suitable for preventing and treating...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com