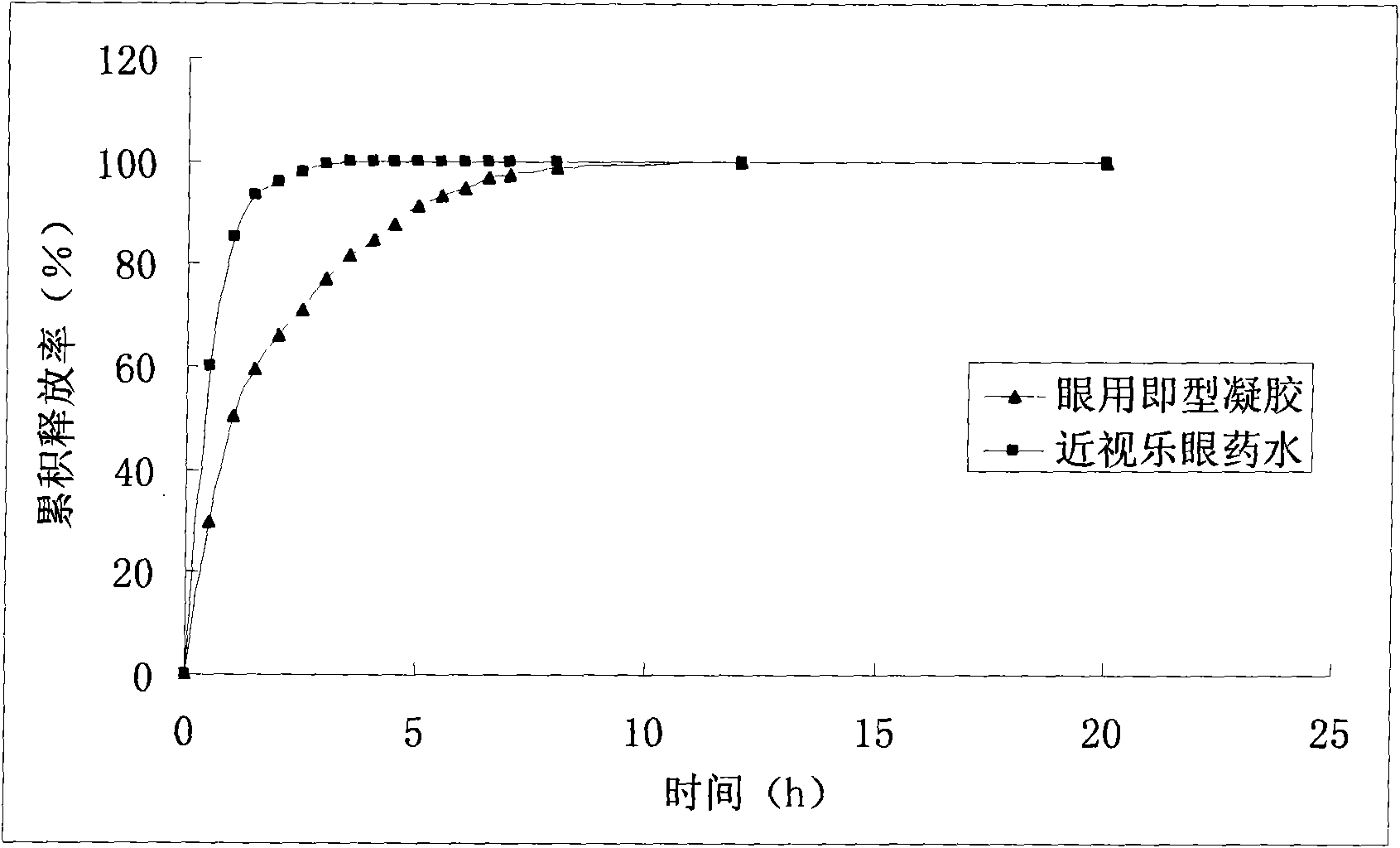
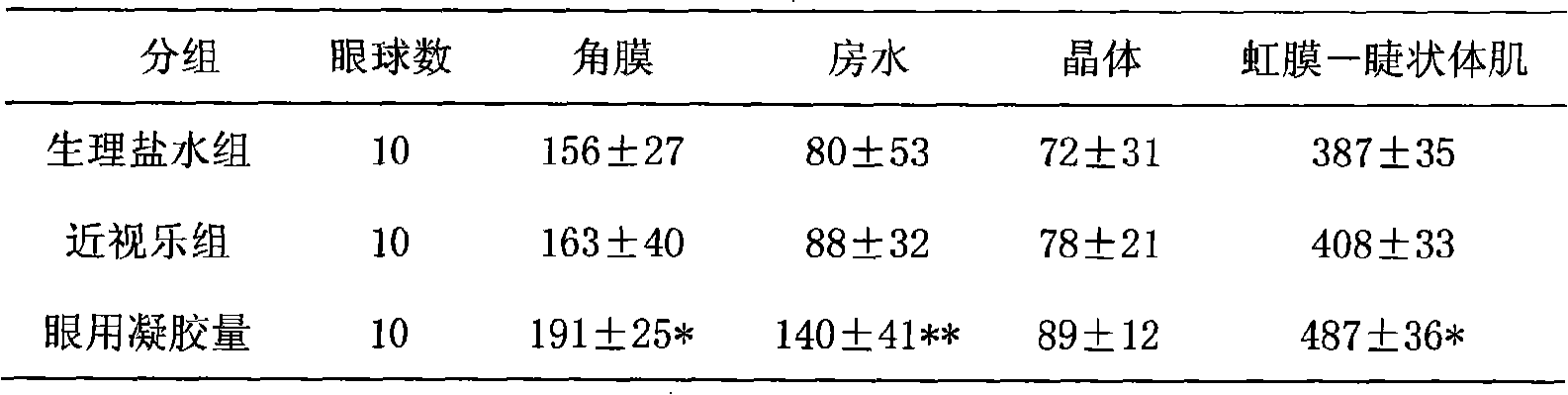
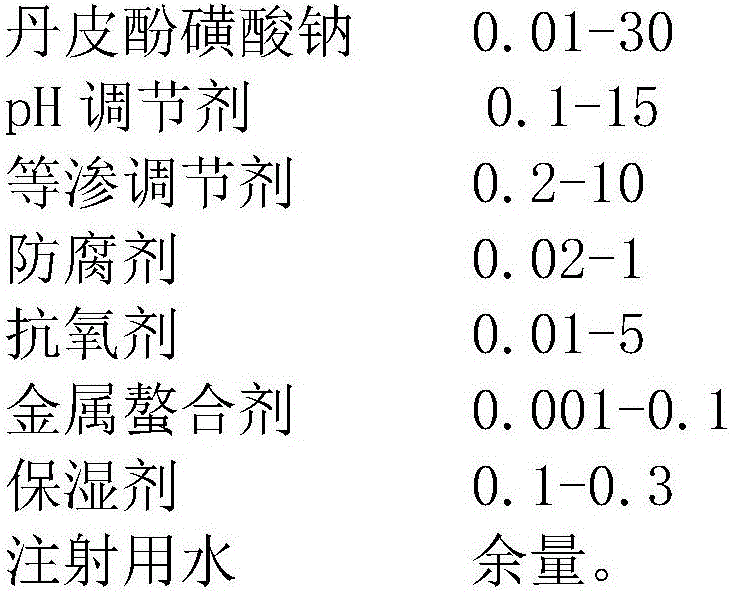
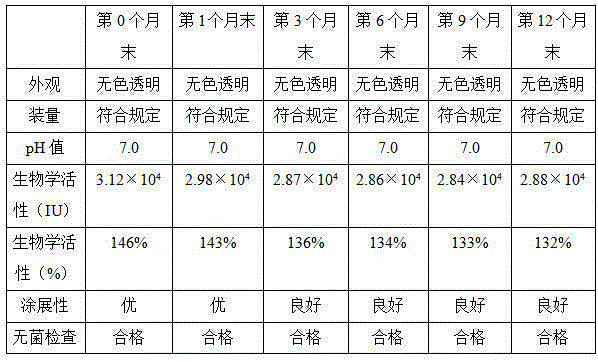
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
120 results about "Ophthalmic Gel" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
LOTEMAX (loteprednol etabonate ophthalmic gel) 0.5% contains a sterile, topical corticosteroid for ophthalmic use. Loteprednol etabonate is a white to off-white powder.
Pirenzepine ophthalmic gel
Owner:VF PHARMA
In-vivo gel preparatino able to be dropped in eyes and its preparing process
InactiveCN1397272ALow toxicityLess irritatingSenses disorderPharmaceutical delivery mechanismTreatment effectIrritation
An eyedrops able to become gel after it is dropped in eye for treating bacterial conjuctivities, keratitis, keratohelcosis, dacryocystitis, etc is prepared from antibacterial infilammatino-relieving medicine, thickening agent, antiseptic, isotonic regulator, pH regulator and water. Its advantages are long stay time in eye, high curative effect, and low poison and irritation.
Owner:SHANGHAI XINGKANG PHARMA RES & DEV
Azithromycin eye drops and preparing process thereof
InactiveCN101103992AReduce dosageEnsure clarityOrganic active ingredientsSenses disorderSide effectWhole body
The invention discloses azithromycin eye drops and the preparation. The eye drops is a stable ophthalmic preparation made by azithromycin or axithromycin salt as the active ingredient which is assisted by a stabilizer, a pH regulator, an antiseptic, an isotonic agent and a pasting agent; wherein the optimized stabilizer is propanetriol, the pH regulator is sodium phosphate buffer, the antiseptic is Ethyl p-hydroxybenzoate, the isotonic agent is sodium chloride and the pasting agent is sodium hyaluronate. Compared with oral delivery, the invention has the advantages of less dose, small side effect on a whole body, direct absorption at the ocular region, quick achieving effective antibacterial concentration and quick developing treating function; and compared with ophthalmic gel or ophthalmic ointment, the invention has the advantages of convenient application and good tolerance for a patient; thus the reasonable preparation process and stable performance of the invention guarantees the clarity and stability of eye drops.
Owner:陕西省眼科研究所
Metronidazole ophthalmic gel and preparation method and application thereof
InactiveCN102552114APromote absorptionExtended stayAntibacterial agentsOrganic active ingredientsAnaerobic infectionAnaerobic bacteria
The invention discloses metronidazole ophthalmic gel and a preparation method and an application thereof, which belong to the technical field of medicine. Metronidazole serving as a related medicinal active ingredient is prepared into ophthalmic gel for the first time. The metronidazole ophthalmic gel is applied to prevention and treatment of blepharitis ciliaris, hordeolum and conjunctivitis caused by worm acarus infection, and a dry eye syndrome caused by meibomian gland dysfunction, and is applied to prevention and treatment of infection of acanthamoeba protozoan and anaerobic bacteria in the wearing process of corneal contact lenses. The metronidazole ophthalmic gel consists of metronidazole and a pharmaceutically-acceptable vector, and the using amount of metronidazole in every 100g of the metronidazole ophthalmic gel is 0.1-3.0g by weight.
Owner:段亚东
Ophthalmic bacterial-infection resisting medicine for external use
ActiveCN101766628AGood intraocular penetrationInhibit or kill growthAntibacterial agentsSenses disorderDiseaseSide effect
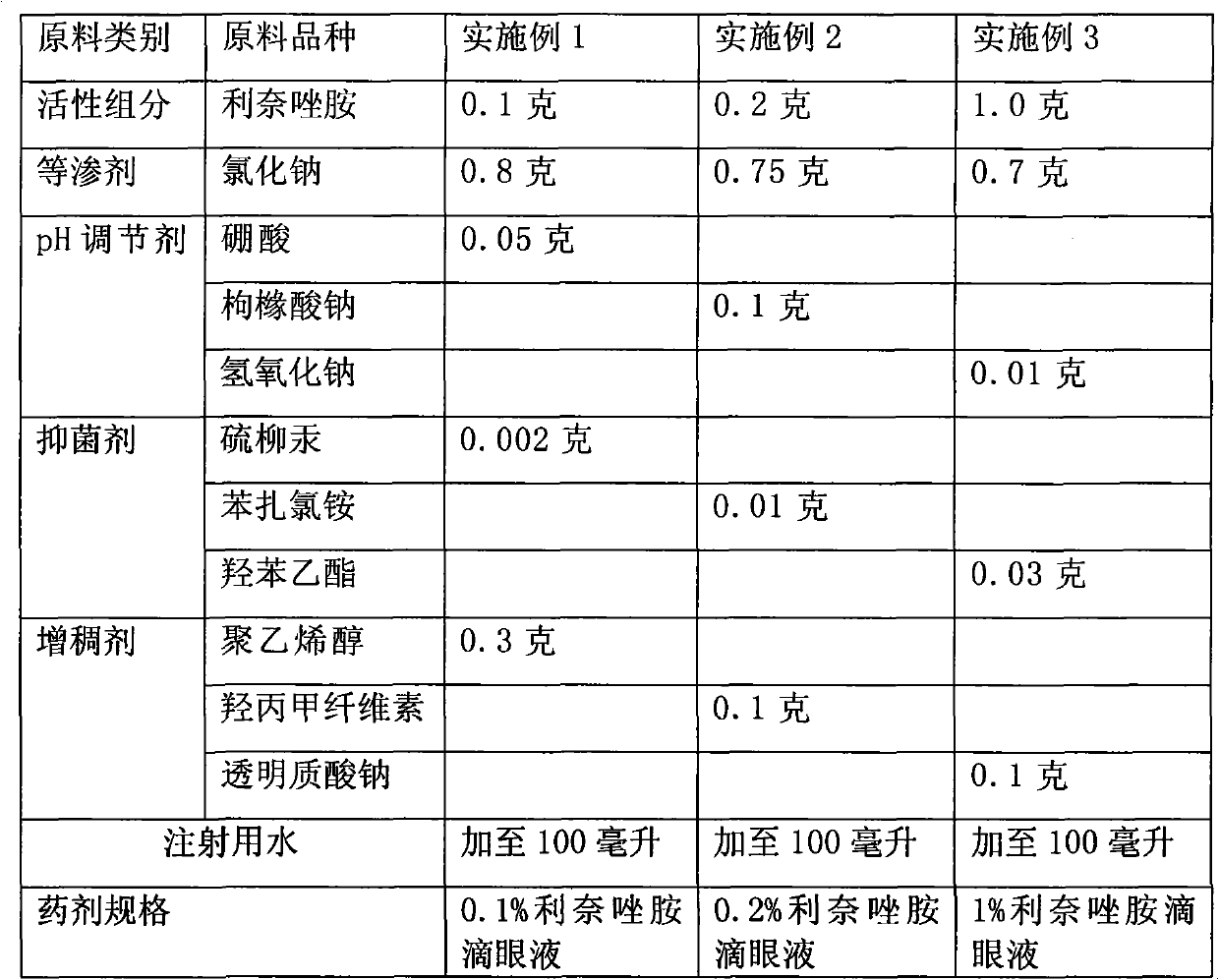
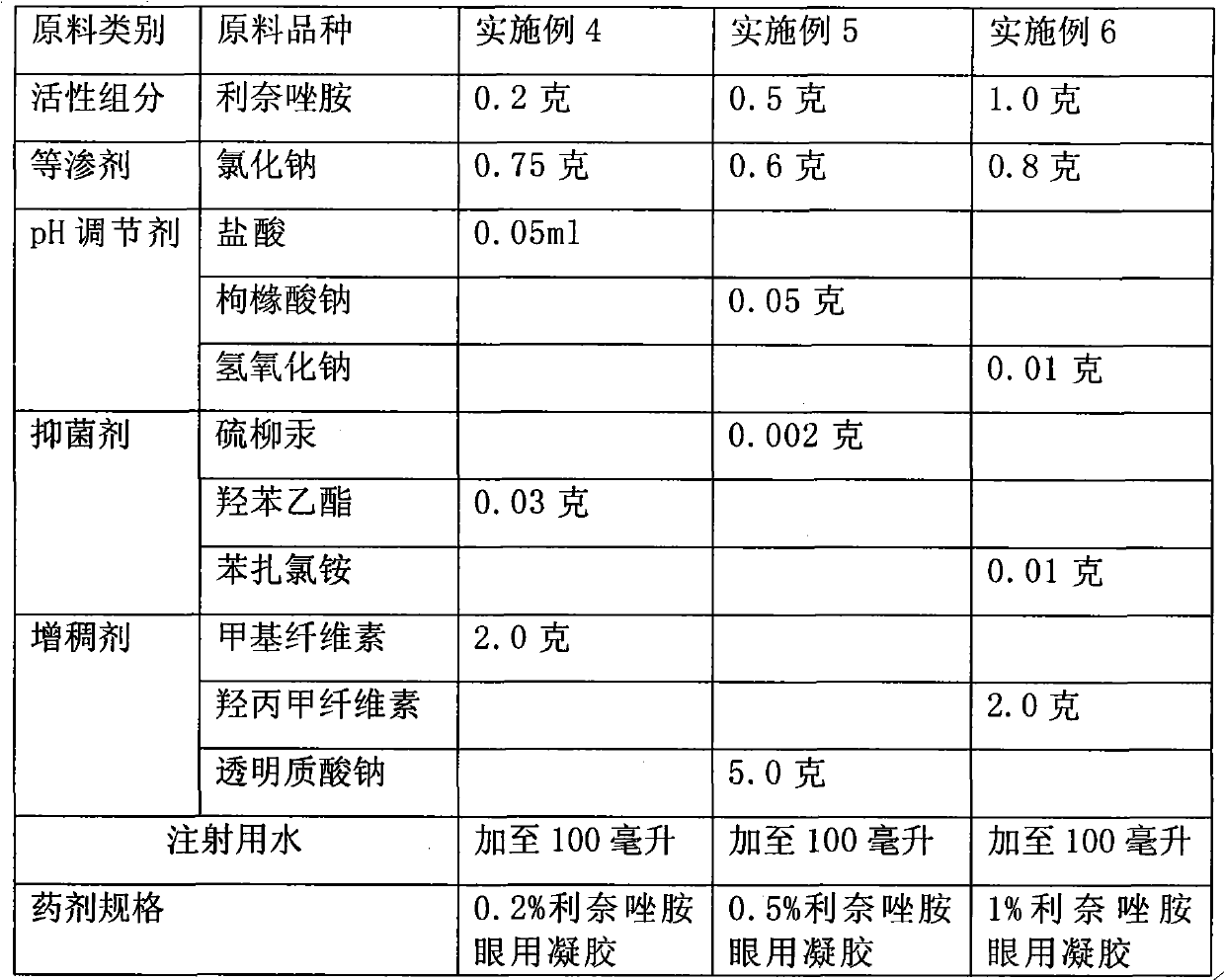
The invention provides ophthalmic bacterial-infection resisting medicine for external use. Linezolid is adopted as medicative raw materials to prepare eye drops, ophthalmic gel and eye ointments. The content of the linezolid in every 100 parts by weight of a medicament is within 0.1 to 1.0 parts by weight. The medicine is for external use at partial positions of eyes, and has good intraocular penetrability and mild toxic as well as side effects; moreover, the medicine is suitable for treating as well as preventing bacterial infection at partial positions of the eyes, including diseases of conjunctivitis, keratitis, keratohelcosis, iritis, ocular traumas, chemical injuries, ophthalmic postoperative infections and the like.
Owner:GUANGDONG WHOLEWIN TECH
Gatifloxacin external and ophthalmic gel preparation
InactiveCN1448137ADegradableGood film formingOrganic active ingredientsSenses disorderOphthalmic Gel Dosage FormDisease
The Gatifloxacin gel preparation for external use and eye use has Gatifloxacin as main component and its supplementary material includes chitosan as gel substrate, iso-osmotic regulator, pH regulator, preservative, injection water, etc. The preparation has Gatifloxacin content of 0.1-3 wt% and chitosan content of 0.3-3 wt%. It has obvious anti-infection function and functions of speeding heal of wound, promoting epidermal growth, inhibiting formation of scar tissue, maintaining local medicine density for long term, etc. It is used in treating burns, scalds, skin infection, folliculitis, bacterial conjunctivitis, keratitis, etc.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Preparation and application of gatifloxacin temperature and pH sensitive ophthalmic gel
InactiveCN101966143AExtended stayImprove bioavailabilityOrganic active ingredientsSenses disorderPhase variationCurative effect
The invention relates to the technical field of medicaments and a new gatifloxacin ophthalmic formulation, in particular to the preparation and application of gatifloxacin temperature and pH sensitive ophthalmic gel. The invention is characterized in that: the formulation is solution in a non-physiological state and is converted into gel in a physiological state through phase variation. The temperature sensitive ophthalmic gel is prepared by performing systematic observation on an auxiliary material and using poloxamer 407 and poloxamer 188 in a proper ratio. The pH sensitive ophthalmic gel is prepared by regulating the using amount of poloxamer 980 and hydroxypropyl methyl cellulose (HPMC). Due to the adoption of the formulation, the detention time of the medicaments on eyes is greatly prolonged, the bioavailability is enhanced and the curative effect is improved; and the formulation has ideal application prospects.
Owner:胡容峰
Ganciclovir ophthalmic gel and its prepn. method
InactiveCN1868449ACrystal appearanceGood biocompatibilityOrganic active ingredientsSenses disorderIrritationOphthalmology
An eye gel of Ganciclovir for treating viral keratitis is proportionally prepared from ganciclovir and eye gel matrix through mixing Ganciclovir, eye gel matrix and water, stirring, regulating pH value, adding water, stirring and sterilizing.
Owner:湖北科益药业股份有限公司
Polymerisable yellow dye, ophthalmic lens material and ophthalmic lens
ActiveCN103483854AGood blue light blocking rateGood physical propertiesPreparation by acylationOptical elementsChemical structureYELLOW DYE
The invention relates to a polymerisable yellow dye which is used for ophthalmic lenses and is capable of reducing or preventing blue light from penetrating ophthalmic lenses. The chemical structure is represented by formula (I), wherein R1 represents hydrogen or -NHCOCH3, R2 represents hydrogen or C1-C3 alkyl, R3 and R4 represent hydrogen or -OCOR5 independently, R5 represents isopropenyl or substituted isopropenyl-R6-(R7O)n-COC(CH3)CH2, R6 represents -NH- or a structure shown in the description, R7 represents C1-C5 alkylene, and n is an integer ranging from 1 to 40. An ophthalmic lens prepared by copolymerization of the polymerisable yellow dye and an ophthalmic lens moulding material possesses excellent blue light blocking rate.
Owner:BENQ MATERIALS +1
Alficetin in situ forming eye gel
InactiveCN101352408AExtended stayAvoid discomfortOrganic active ingredientsSenses disorderWater useSide effect
The invention discloses a chloramphenicol eye ophthalmic gel and a preparation method thereof; every 1000ml of the chloramphenicol eye ophthalmic gel contains 5-50g of chloramphenicol, 0.05-1g of preservative, 1.0-7.5g of osmoregulation agent, 30-150g of gel stroma, and rest amount of acid-base buffering agent and water used for injection. Due to the optimization and technique improvement of the auxiliary materials, the medicine dosage forms of the chloramphenicol are enriched, detention time of the medicine in the eyes are greatly prolonged, and the medicine can exert the drug effect, thus not only improving healing efficacy, but also reducing the times and days for taking medicine, decreasing the part side effect of the medicine and the untoward effect caused by the whole body absorption, as well as having no pessimal stimulation response.
Owner:肖正连
Bendalysine eye gel preparation and its making method
ActiveCN1895219ADelays the development of cloudinessEffective concentration time is longOrganic active ingredientsSenses disorderGel preparationCurative effect
An eye gel for preventing and treating hyperglycemic cataract is proportionally prepared from bendalysin, gel matrix and pharmacologically acceptable carrier. Its preparing process is also disclosed.
Owner:湖北远大天天明制药有限公司
Ophthalmic gel and preparation method thereof
ActiveCN102085175ALess irritatingReduce systemic adverse reactionsOrganic active ingredientsSenses disorderWhole bodyExcipient
The invention relates to an ophthalmic gel and a preparation method thereof, in particular to the ophthalmic gel. The ophthalmic gel comprises effective quantity of latanoprost and timolol or salts thereof for treatment and / or prevention, a gel matrix, a surfactant and water. The invention also comprises the ophthalmic gel, an ophthalmic preparation of a medicinal excipient mixed with the ophthalmic gel before being used, and the preparation method of the ophthalmic gel. The ophthalmic gel has the favorable effects of treating eye diseases, and has less adverse effects on a cardiovascular system and a respiratory system of the whole body.
Owner:SHENYANG XINGQI PHARM CO LTD
Metronidazole gel free of preservative and preparation method thereof
InactiveCN105769754ALittle changeImprove securityOrganic active ingredientsAerosol deliveryPreservative freeHydrogen phosphate
The invention provides metronidazole gel free of a preservative and relates to the field of biological medicines. The metronidazole gel consists of the following components in percentage by mass: 0.01-0.1% of metronidazole, 0.1-0.5% of carbomer, 0.1-0.5% of hydroxypropyl methylcellulose, 0.1-1% of triethanolamine, 0.01-0.2% of sodium hydrogen phosphate, 0.02-0.2% of sodium dihydrogen phosphate, 1-3% of glycerinum and the balance of water. Correspondingly, the invention further provides a preparation method of the gel. Compared with the prior art, the metronidazole ophthalmic gel of the technical scheme obtained through tests and studies has relatively small influence on variation of medicinal substances (metronidazole), and is relatively good in stability; meanwhile, as no preservative is used, the metronidazole gel is relatively good in security when being used by a patient.
Owner:ZHUHAI ESSEX BIO PHARMA
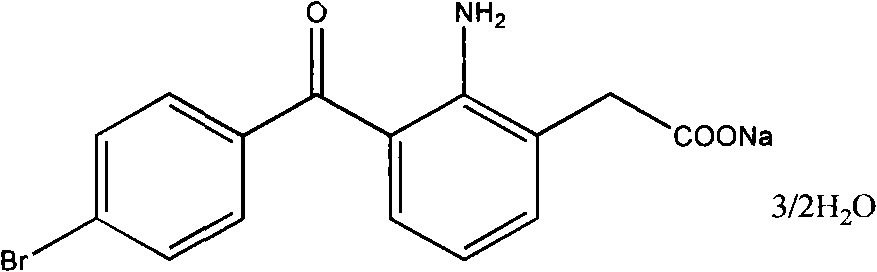
Gel for eye containing bromfenac sodium hydrate and preparation thereof
InactiveCN101322683AOrganic active ingredientsSenses disorderTherapeutic effectBULK ACTIVE INGREDIENT
The invention relates to an ophthalmic gel containing the active ingredient of bromfenac sodium hydrate and a preparation method thereof; the bromfenac sodium hydrate ophthalmic gel contains the active ingredient of bromfenac sodium hydrate, macromolecular hydrogel substrate, osmotic pressure regulator, bacteriostat, antioxidant, metal ion chelating agent, pH regulator and water for injection; the ophthalmic gel has convenient application and quick effect; particularly, the gel possesses certain viscosity and can stay in the eyes for a long time, thus overcoming the defect that eye drops can only stay in the eyes for a short time after being diluted by tears and achieving more effective therapeutic effect.
Owner:ZHEJIANG SHENGBOKANG PHARMA CO LTD
Brimonidine tartrate in situ forming eye gel
InactiveCN101352441AVarious dosage formsExtended stayOrganic active ingredientsSenses disorderWater usePreservative
The invention discloses a brimonidine tartrate eye drops ophthalmic gel and a preparation method thereof; every 1000ml of brimonidine tartrate eye drops ophthalmic gel contains 1-4g of brimonidine tartrate eye drops, 0.05g of preservative, 1.0-7.5g of osmoregulation agent, 30-150g of gel stroma, and rest amount of acid-base buffering agent and water used for injection. Due to the optimization and technique improvement of the auxiliary materials, the medicine dosage forms of the brimonidine tartrate eye drops are enriched, detention time of the medicine in the eyes are greatly prolonged, the healing effect is improved, and no pessimal stimulation response exists.
Owner:肖正连
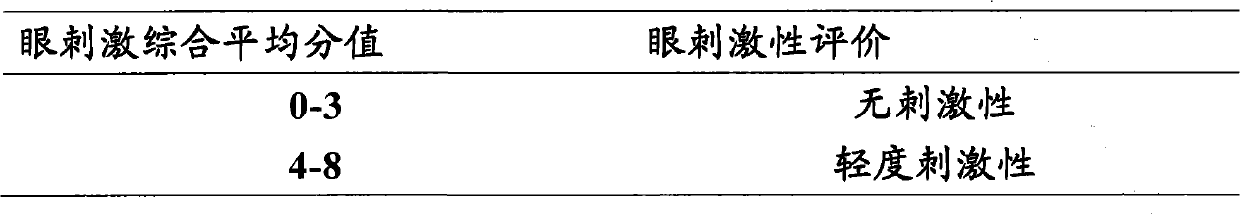
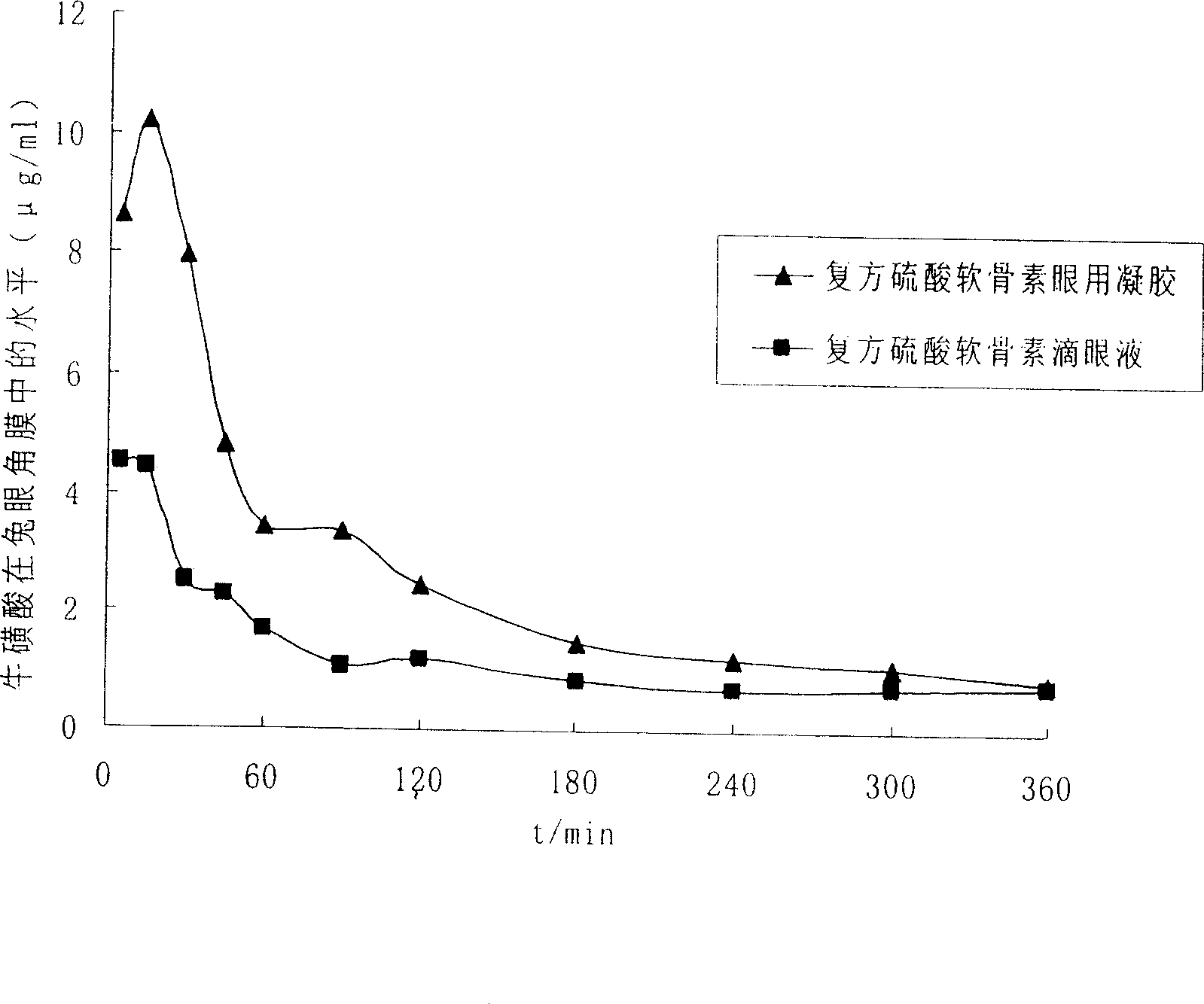
Ophthalmic gel containing chondroitin sulfate and method for preparing same
ActiveCN101543509AGood lookingSmooth appearanceSenses disorderPharmaceutical delivery mechanismIrritationBiocompatibility Testing
The invention relates to an ophthalmic gel containing chondroitin sulfate and a method for preparing the same. The ophthalmic gel comprises chondroitin sulfate and taurine in effective dose and an aqueous gel substrate in proper amount. Compared with eyedrop, the ophthalmic gel has the advantages of good biocompatibility, small stimulation, simple preparation process and the like.
Owner:SHENYANG XINGQI PHARM CO LTD
Ophthalmic gel composition
ActiveCN102188695APromote absorptionGood biocompatibilityOrganic active ingredientsSenses disorderMedicineGram
The invention discloses an ophthalmic gel composition. Each gram of the composition comprises necessary components of: 1000 to 100000 IU (international unit) of epidermal growth factors (EGF), 0.5 to 3.5 mg of hyaluronate sodium, and rest components of pharmaceutical acceptable carriers. The invention also discloses a preparation method and a purpose of the composition.
Owner:SHANGHAI HAOHAI BIOLOGICAL TECH
Ready-to-use puerarin ophthalmic gel
InactiveCN101579305AStrong ability to formHigh transparencyOrganic active ingredientsSenses disorderReduced doseRetention time
The invention discloses a ready-to-use puerarin ophthalmic gel preparation belonging to the field of traditional Chinese medicines. The active ingredient of the preparation is puerarin, and the gel base material of the preparation mainly comprises gellan gum accounting for 0.4-0.7 percent of the total weight. The technical scheme overcomes the deficiencies that the prior puerarin ophthalmic preparation has low bioavailability, inconvenient use, short retention time, and the like and has the effects of reducing dose, prolonging the retention time in eyes, having convenient use and accurate dose, and the like.
Owner:BEIJING HERUN INNOVATION PHARMA TECH DEV
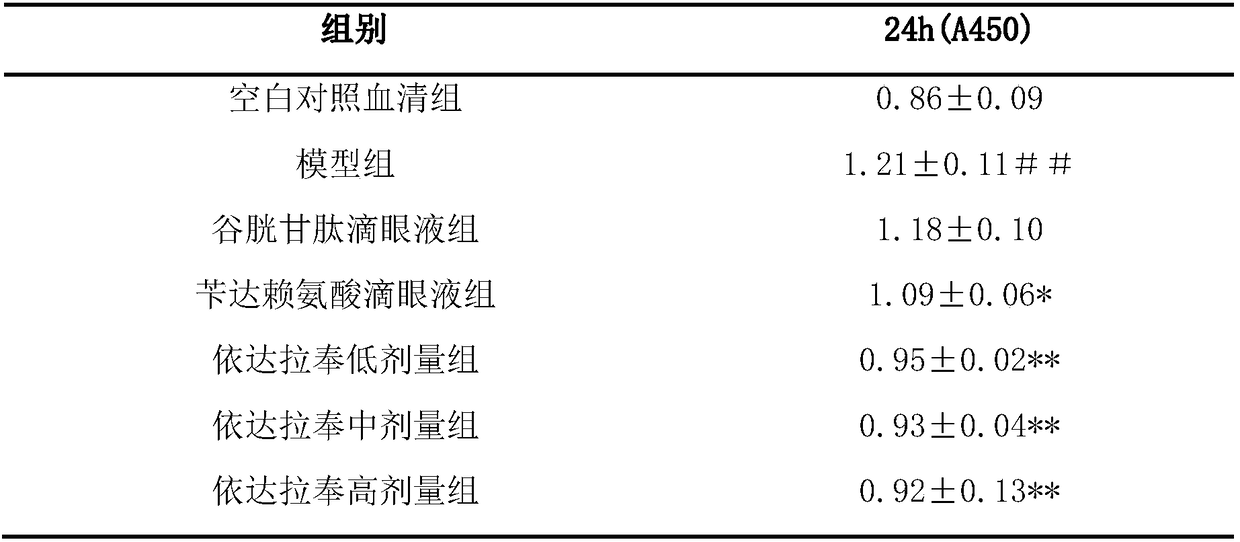
Novel application of edaravone
InactiveCN108403691AExtended stayImprove stabilityOrganic active ingredientsSenses disorderIrritationCurative effect
The invention relates to application of edaravone in the preparation of medicine for treating cataract and belongs to the technical field of medicine. The medicine comprises the edaravone and pharmaceutical auxiliary materials, wherein the concentration of the edaravone is 0.03-0.5%. The edaravone and the pharmaceutical auxiliary materials are prepared into eye drops and gel for the eyes, and theeye drops and gel for the eyes are good in stability, low in irritation to the eyes, capable of prolonging the staying time of the edaravone in eye tissue and capable of bringing the curative effect of the edaravone in the eye tissue into full play and have an evident better curative effect on the cataract as compared with that of current preparations for the eyes on the market.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Ophthalmic gel and its preparation method
ActiveCN101006974AHigh viscosityExtended staySenses disorderPharmaceutical delivery mechanismRetention periodMass ratio
The invention relates to an eye gel which comprises base material, reactive Chinese medicinal components, auxiliary material and water by a mass ratio of 0.05-10.0:0.1- 40.0:1-14:50-98. The gel is capable of increasing viscosity of the active Chinese medicinal constituents, raising the retention period of the medicament in eyes, and reducing medicinal loss.
Owner:TEYI PHARMACEUTICAL GROUP CO LTD
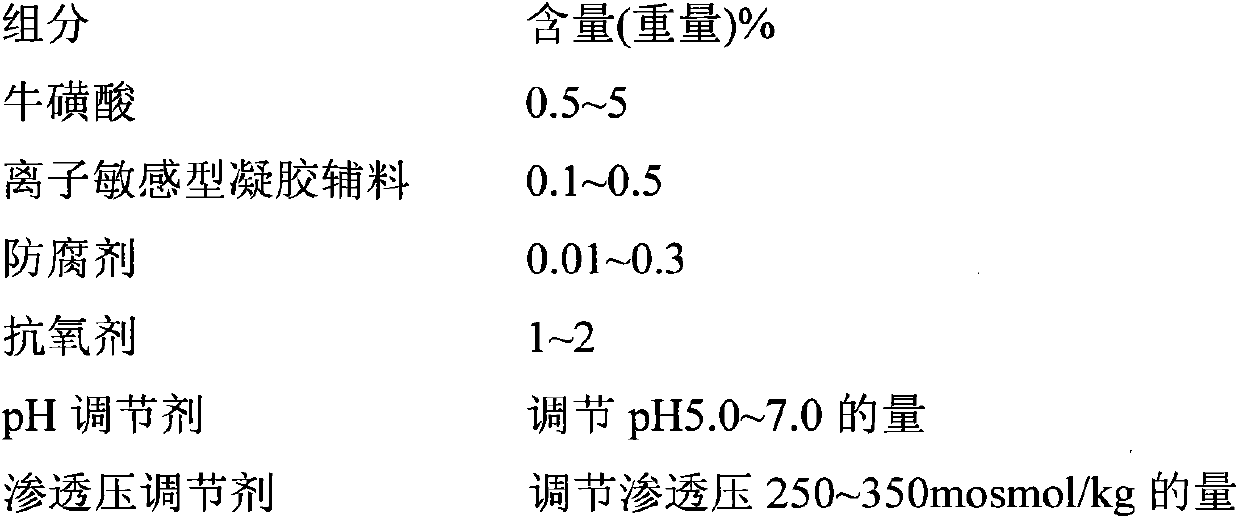
Taurine-containing ophthalmic in-vivo gel preparation and preparation method thereof
InactiveCN103720641AEffectively maintain effective concentrationLow toxicitySenses disorderAerosol deliveryDiseaseAdjuvant
The invention relates to a taurine-containing ophthalmic in-vivo gel preparation and a preparation method thereof. The taurine-containing ophthalmic gel is an ophthalmic preparation which is in a liquid state in vitro and forms a gel state after dropped into eyes. The taurine-containing ophthalmic gel is prepared by taking taurine as an active substance and supplementing a gel adjuvant, a thickening agent, a preservative, an osmotic-pressure conditioning agent, a pH conditioning agent and water for injection. The taurine-containing ophthalmic gel is suitable for treatment of diseases such as retinopathy and the like; and the ophthalmic gel is in the liquid state in vitro, a dosage is easy and accurate to control, the use is convenient, the ophthalmic gel can be uniformly spread after dropped into the eyes, gelates, is retained in the eyes for longer time and is not prone to loss, effective drug concentration can be effectively maintained, the treatment effect is enhanced, further, the toxicity is low, the irritation is small, the biocompatibility is good, and the taurine-containing ophthalmic gel is effective, facilitates clinical application, and has a wide development prospect.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
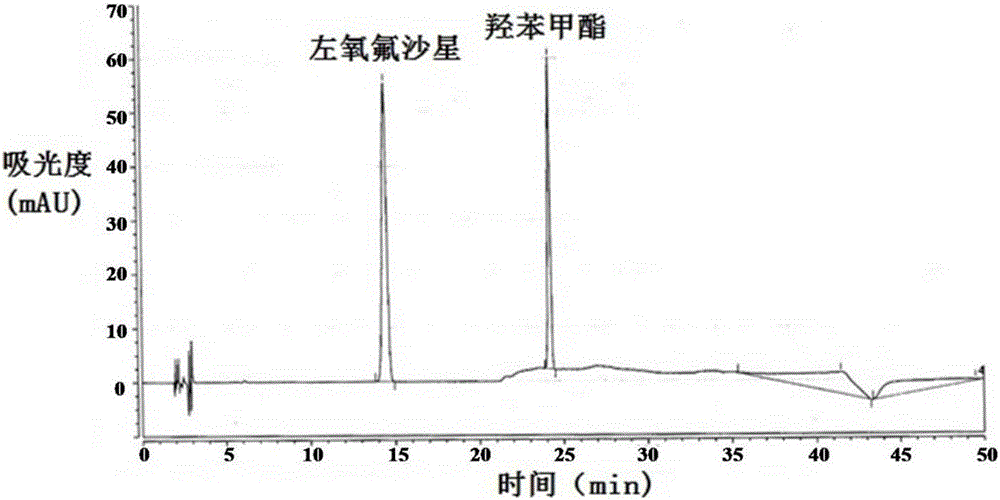
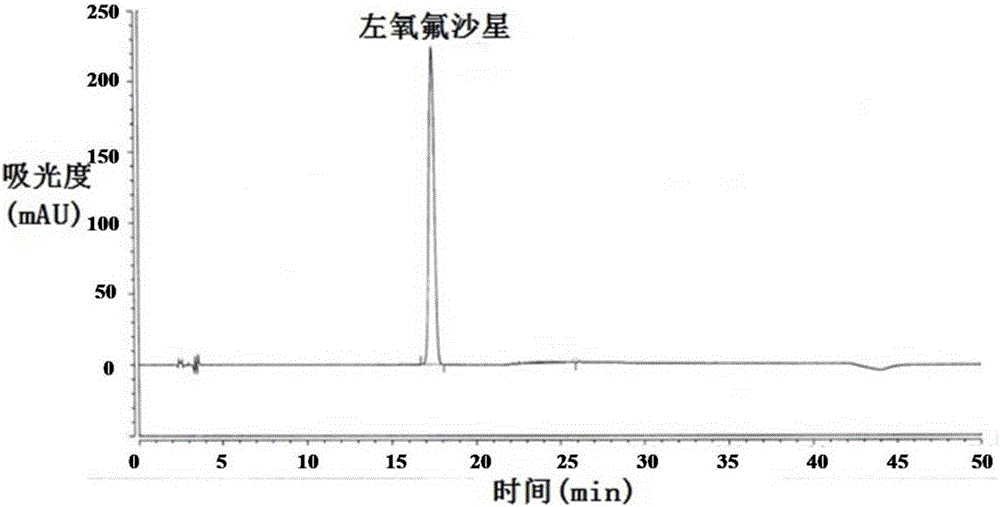
Method for separating and/or detecting levofloxacin and methylparaben in levofloxacin hydrochloride ophthalmic gel
ActiveCN106769963AEnsure quality controllabilityGuaranteed effective contentColor/spectral properties measurementsChromatographic separationSilanes
The application discloses a method for separating and / or detecting levofloxacin and methylparaben in levofloxacin hydrochloride ophthalmic gel. The method is a high performance liquid chromatography, and concretely comprises the following steps: preparing a test solution and a reference solution; using octadecyl silane bonded silica gel as a chromatographic column, wherein chromatographic separation test conditions are as follows: the column temperature is 30-40 DEG C, the flow velocity of a mobile phase is 0.9-1.3mL / min, and the ultraviolet detection wavelengths of the levofloxacin and the methylparaben are respectively 240-260nm and 280-300nm; detecting: respectively and precisely absorbing 10mu L of the test solution and 10mu L of the reference solution, injecting into a high performance liquid chromatograph, and reading the chromatographic peak areas of the levofloxacin and the methylparaben in the test solution. The method can accurately and rapidly measure the contents of the levofloxacin and the methylparaben in the levofloxacin hydrochloride ophthalmic gel at the same time, thus providing guarantee for the quality controllability of the levofloxacin hydrochloride ophthalmic gel.
Owner:湖北远大天天明制药有限公司
Ophthalmic gel of gatifloxacin and preparation method thereof
ActiveUS20130040960A1Reduced bioavailabilityPoor patient complianceAntibacterial agentsOrganic active ingredientsSodium hyaluronateGatifloxacin
An ophthalmic gel of gatifloxacin and the preparation method thereof are disclosed. The gel comprises gatifloxacin or its pharmaceutical salts, matrix and water. Said matrix is one or more selected from carbomer, mixture of carbomer and HPMC, and sodium hyaluronate.
Owner:SHENYANG XINGQI PHARM CO LTD
Gel for lysozyme eye and preparation method thereof
InactiveCN102961324AEliminate fastExtended stayAntibacterial agentsSenses disorderDiseaseEye infection
The invention relates to a gel for lysozyme eye and a preparation method thereof. The gel for the lysozyme eye is applicable of treating various normal eye infection diseases, such as a pinkeye, an ophthalmia, a ceratitis, a keratohelcosis, a conjunctivitis and the like, which is caused by bacteria, Chlamydia, virus and drug-resistance bacteria. The gel for the lysozyme eye disclosed by the invention uses a solution containing 0.1-10.0% of lysozyme, 0.01-20% of water soluble macromolecule matrix, 0.1-10% of a pH modifier, 0.01-10% of a bacteriostat and 0.1-10% of a viscosity modifier and takes a solution with a pH value of 5.0-6.5 as a buffer solution. With the adoption of the receipt of the gel for the lysozyme eye provided by the invention, the time that drug lysozyme remains on an eye can be prolonged, and the bacteriostat efficiency of the lysozyme on the eye can be improved.
Owner:SHENYANG PHARMA UNIVERSITY
Extraction method of sheep sclera collagen, ophthalmic gel and preparation method thereof
InactiveCN105617350ASmall molecular weightHigh puritySenses disorderHydrolysed protein ingredientsPolyvinyl alcoholTissue Compatibility
The invention particularly relates to an extraction method of sheep sclera collagen and a preparation method of ophthalmic gel. An enzyme hydrolysis-salt extraction method is adopted to extract collagen from sheep eye sclera. The invention further provides the ophthalmic gel with the sheep sclera collagen. The ophthalmic gel comprises, by mass, 80-90% of the sheep sclera collagen, 0.01-2% of preservative,0.5-2% of boric acid and borax, 0.5-2% of polyvinyl alcohol, 0.3-2% of Tween, 0.5-2% of sodium chloride and the balance of water for injection. The sheep sclera collagen is similar to human eye sclera collagen in structure and has the advantages of high purity, high tissue compatibility, low molecular weight and low repellency.
Owner:王媛
Gel composition for eyes and preparation method thereof
ActiveCN101455628AImprove liquidityGood dispersionSenses disorderPharmaceutical delivery mechanismOphthalmic Gel Dosage FormCurative effect
The present invention discloses an ophthalmic gel composition and a preparing method thereof, wherein the ophthalmic gel composition according to the invention is composed of chondroitin sulfate, taurine and medical supplementary material. The invention successfully develops the ophthalmic gel composition which is currently hard through combining the prior clinical ophthalmic medicine. The ophthalmic gel composition has excellent fluidity and dispersibility and has beneficial contribution for increasing curative effect. Furthermore the prior animal experiment shows that the ophthalmic gel composition of the invention has excellent safety and provides a powerful guarantee for the further product commercialization.
Owner:杨文龙
Palmatine ophthalmic gel and preparation method thereof
InactiveCN104490765ASlow drug releaseReduce the frequency of eye dropsAntibacterial agentsOrganic active ingredientsPharmacologic therapyPharmaceutical Substances
The invention relates to palmatine ophthalmic gel and a preparation method thereof. The method comprises the following steps: adding water for injection into a matrix and an antioxidant in a formula dosage, mixing, swelling or dissolving; adding a pH regulator for uniformly stirring, thereby obtaining a matrix liquid; weighing palmatine according to the formula dosage, adding the water for injection for dissolving, adding a preservative and an isoosmotic adjusting agent, uniformly mixing, adding the mixture into the matrix liquid, adding the water for injection to 1L, uniformly shaking, filtering by virtue of a micro-aperture filter membrane, sterilizing, and split charging, thereby obtaining the product. The gel comprises the following components: 0.5-30g of palmatine, 0.1-30g of a matrix, 0.1-20g of a pH regulator, 1-100g of an isoosmotic adjusting agent, 0.01-8g of a preservative and 0.02-5g of an antioxidant. Compared with palmatine liquid type eye drops, the product disclosed by the invention has certain advantages in the dosage form, the administration frequency can be reduced, loss of the medicine in the eyes is avoided, and the effect of the medicine for treating conjunctivitis and keratitis can be obviously improved.
Owner:昆明振华制药厂有限公司
Ready-to-use Dactylicapnos scandens ophthalmic gel
InactiveCN101579403AStrong ability to formHigh transparencySenses disorderPharmaceutical delivery mechanismReduced doseRetention time
The invention discloses a ready-to-use Dactylicapnos scandens ophthalmic gel preparation, belonging to the field of traditional Chinese medicines. The active ingredient of the preparation is from Dactylicapnos scandens which is Chinese medical herbs and the gel base material of the preparation mainly comprises gellan gum accounting for 0.4-0.7 percent of the total weight. The technical scheme overcomes the deficiencies that the prior Dactylicapnos scandens ophthalmic preparation has low bioavailability, inconvenient use, short retention time, and the like and has the effects of reducing dose, prolonging the retention time in eyes, having convenient use and accurate dose, and the like.
Owner:BEIJING HERUN INNOVATION PHARMA TECH DEV
Sodium paeonolsilate ophthalmic preparation
InactiveCN106727454AEffective absorptionImprove stabilitySenses disorderPharmaceutical delivery mechanismSide effectRetention time
The invention discloses a sodium paeonolsilate ophthalmic preparation, which is prepared by adding ophthalmic preparation pharmaceutic adjuvants into sodium paeonolsilate, wherein the weight percentage of the contained sodium paeonolsilate is 0.01 to 30 percent; the balance is auxiliary materials for the ophthalmic preparation; the sodium paeonolsilate ophthalmic preparation contains eye drops, ophthalmic lipidosome, inclusion compound eye drops, eye ointment, ophthalmic gel, in-situ gel, ophthalmic liposome gel, ophthalmic injection, ophthalmic lipidosome injection and the like. The sodium paeonolsilate is used as the active effective ingredients; the ophthalmic preparation pharmaceutic adjuvants are added for preparation according to a conventional preparation method. The sodium paeonolsilate is prepared into ophthalmic medicine; the effective absorption of the medicine is enhanced; the medicine stability is improved; the retention time in the eyes can be prolonged; the toxic and side effects of the medicine are reduced; the eye bioavailability of the medicine is improved. The preparation method provided by the invention has the advantages that the process is feasible; the quality is controllable; wide industrialization prospects are very wide.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Recombinant calf alkaline fibroblast growth factor ophthalmic gel
ActiveCN104546692AImprove securityNon-irritatingSenses disorderPeptide/protein ingredientsDrug effectAlbumin
The invention relates to the field of biological products and in particular relates to recombinant calf alkaline fibroblast growth factor ophthalmic gel and a preparation method thereof. The ophthalmic gel comprises the following components: 1500-1800IU of recombinant calf alkaline fibroblast growth factors, 0.05-0.20mg of human serum albumin, 0.6-1.0mg of Carbomer, a proper amount of a pH regulator and water for injection added to 0.4ml. The ophthalmic gel disclosed by the invention is smooth, uniform, comfortable to use, lasting in drug effect, safe to eyes, non-irritating and high in stability. A bacteriostatic agent is not added into the formula, hazards brought by long-term use of the bacteriostatic agent are avoided, and the safety of the product is improved.
Owner:ZHUHAI ESSEX BIO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com