Novel application of edaravone
A technology of pharmaceutical excipients and ophthalmic gel, applied in the field of medicine, can solve problems such as affecting the therapeutic effect, and achieve the effects of prolonging the residence time, excellent therapeutic effect and less irritation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The preparation of embodiment 1 Edaravone eye drops
[0016] Edaravone eye drops preparation method:
[0017] (1) Add 0.5g of sodium hyaluronate to 700ml of water for injection, and stir for 30 minutes to completely dissolve it;
[0018] (2) Add 0.5g of sodium chloride, 0.5g of sodium edetate, 0.1g of benzalkonium bromide and 0.3g of sodium bisulfite to the above-mentioned sodium hyaluronate solution, stir to dissolve, and use phosphate Adjust the pH value of the buffer to 6.8, and set the volume to 1000ml to obtain the blank matrix of eye drops;
[0019] (3) Add 0.5 g of edaravone to the blank matrix of the above-mentioned eye drops, stir well to dissolve, filter and sterilize through a 0.22 μm filter, and then pack it separately to obtain the product.
Embodiment 2
[0020] The preparation of embodiment 2 Edaravone ophthalmic gel
[0021] Edaravone ophthalmic gel prescription: Edaravone, 0.5g; Carbomer 940, 5.0g; Propylene glycol, 0.5g; Sodium bisulfite, 0.3g; Benzalkonium bromide, 0.2g; Hydrogen phosphate Disodium, sodium dihydrogen phosphate, appropriate amount; dilute to 1000g with water for injection.
[0022] Edaravone ophthalmic gel preparation method:
[0023] (1) Take 5.0 g of Carbomer 940, add 700 g of water for injection, stir evenly, let it stand still, make it swell, adjust the pH value to neutral with phosphate buffer solution, and obtain the matrix;
[0024] (2) Take another 0.5g of edaravone, 0.2g of benzalkonium bromide, 0.5g of propylene glycol, 0.3g of sodium bisulfite and water for injection and stir to form a mixture, slowly add it to the above matrix, stir evenly, and add water to 1000g, vacuum degassed, subpackaged, sterilized, ready to use.
[0025] application testing
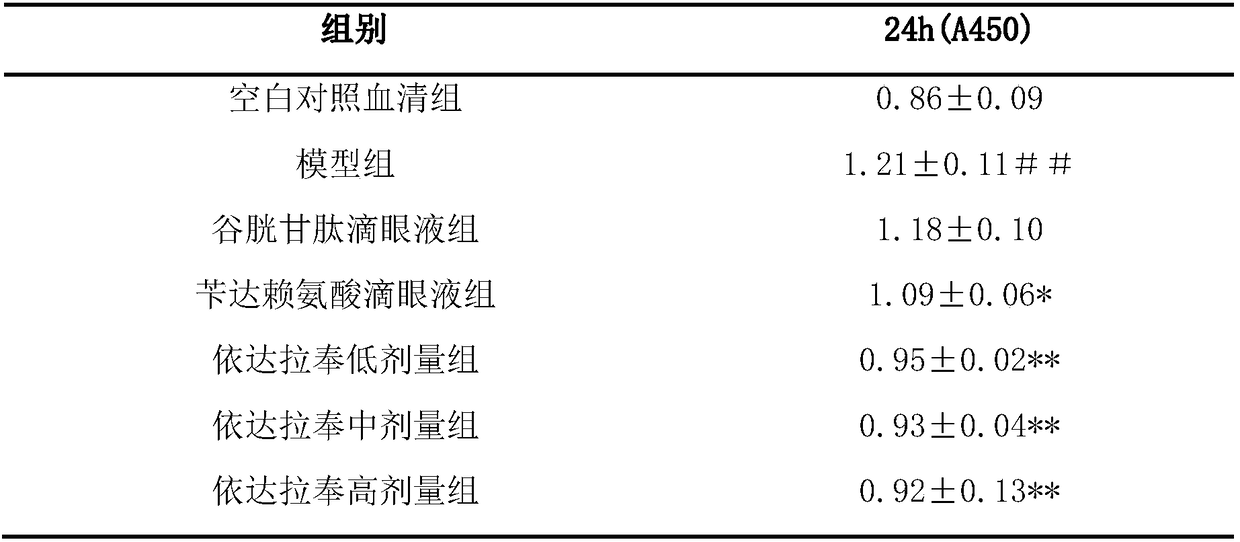
[0026] 1. Test the protective effect of ed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com