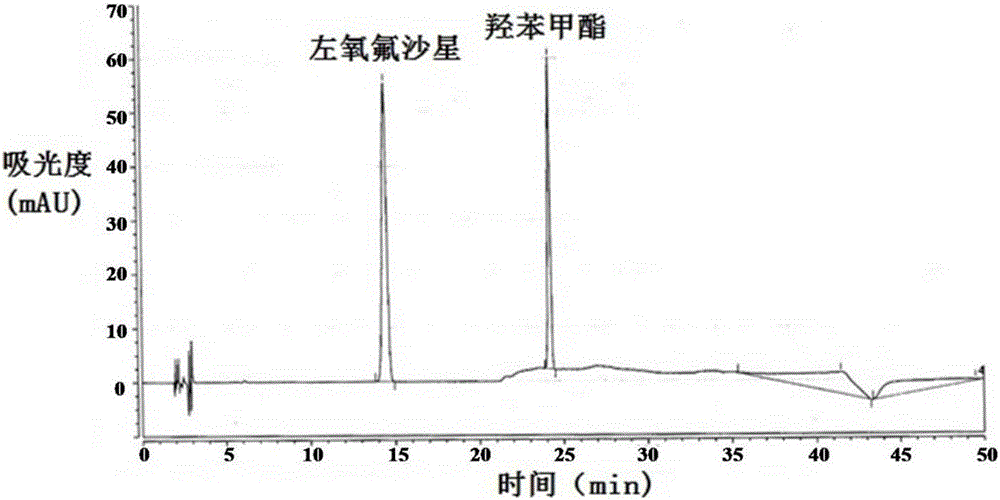
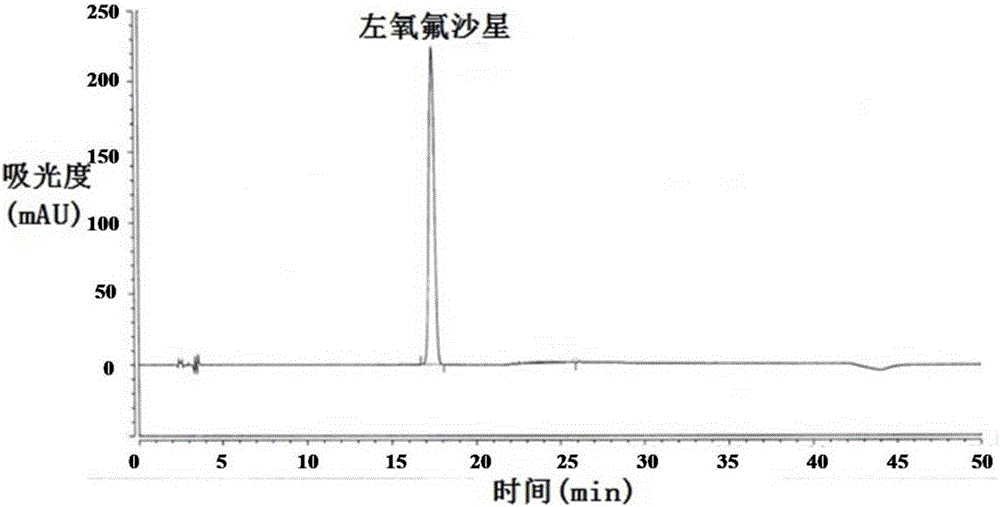
Method for separating and/or detecting levofloxacin and methylparaben in levofloxacin hydrochloride ophthalmic gel
A technology of levofloxacin hydrochloride and levofloxacin, which is applied in the field of drug analysis, can solve the problems of inability to guarantee the quality attributes of levofloxacin hydrochloride ophthalmic gel, harmful effects on patients, etc., and achieves the quality controllability, easy operation, and short detection time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Instrument: Diana U3000 HPLC chromatograph
[0053] Chromatographic column: Dionex Acclaim120 C18 chromatographic column (250mm×4.6mm, 5μm, )
[0054] Mobile phase: mobile phase A: mixed solution of acetonitrile-3.32mg / mL ammonium acetate and 5.26mg / mL sodium perchlorate (adjust pH=2.2 with phosphoric acid)=15:85; mobile phase B: acetonitrile
[0055] The volume content of mobile phase A in the elution time and its corresponding mobile phase is as follows: at 0~18min, the volume content of mobile phase A is 100%; at 18~25min, the volume content of mobile phase A decreases from 100% to 70%; at 25-39min, the volume content of mobile phase A is 70%; at 39-40min, the volume content of mobile phase A increases from 70% to 100%; at 40-50min, the volume content of mobile phase A is 100%.
[0056] Column temperature: 40°C
[0057] Flow rate: 1.0mL / min
[0058] Detection wavelength of levofloxacin and methylparaben: 294nm; 252nm.
[0059] Reference substance solution prep...
Embodiment 2
[0068] Instrument: Agilent 1260 HPLC chromatograph
[0069] Chromatographic column: Agilent ZORBAX Extend-C18 chromatographic column (250mm×4.6mm, 5μm, )
[0070] Mobile phase: acetonitrile-3.00mg / mL ammonium acetate and 5.43mg / mL sodium perchlorate mixed solution (adjust pH to 2.5 with phosphoric acid)=14:86; mobile phase B: acetonitrile
[0071] The volume content of mobile phase A in the elution time and its corresponding mobile phase is as follows: at 0~18min, the volume content of mobile phase A is 100%; at 18~25min, the volume content of mobile phase A decreases from 100% to 70%; at 25-39min, the volume content of mobile phase A is 70%; at 39-40min, the volume content of mobile phase A increases from 70% to 100%; at 40-50min, the volume content of mobile phase A is 100%.
[0072] Column temperature: 38°C
[0073] Flow rate: 1.1mL / min
[0074] The detection wavelengths of levofloxacin and methylparaben are 296nm and 254nm, respectively.
[0075] Relevant solution p...
Embodiment 3
[0081] Instrument: Waters Alliance2695 HPLC chromatograph
[0082] Chromatographic column: Thermo Hypersil-C18 chromatographic column (250mm×4.6mm, 5μm, )
[0083] Mobile phase: acetonitrile-3.85mg / mL ammonium acetate and 6.00mg / mL sodium perchlorate mixed solution (adjust pH=2.4 with phosphoric acid)=11:89; mobile phase B: acetonitrile
[0084] The volume content of mobile phase A in the elution time and its corresponding mobile phase is as follows: at 0~18min, the volume content of mobile phase A is 100%; at 18~25min, the volume content of mobile phase A decreases from 100% to 70%; at 25-39min, the volume content of mobile phase A is 70%; at 39-40min, the volume content of mobile phase A increases from 70% to 100%; at 40-50min, the volume content of mobile phase A is 100%.
[0085] Column temperature: 35°C
[0086] Flow rate: 1.0mL / min
[0087] The detection wavelengths of levofloxacin and methylparaben are 291nm and 247nm respectively
[0088] Relevant solution prepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com