Method for separating and determining raltitrexed and its impurities by high performance liquid chromatography
A technology for raltitrexed and impurities, applied in the field of separation and determination of raltitrexed and its impurities by high performance liquid chromatography, to achieve the effect of ensuring quality controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 impurity C
[0038] (1) Preparation of 5-nitro-2-thiophenoyl chloride
[0039] Put 30g of 5-nitro-2-thiophenecarboxylic acid and 50ml of thionyl chloride into a 100ml three-neck flask, raise the temperature, control the inner temperature to 75℃~80℃ and stir for 2 hours, take about 1ml of the reaction solution, add 2ml of anhydrous Methanol derivatization, using 5-nitro-2-thiophenecarboxylic acid as a control, TLC detection (ethyl acetate: n-hexane = 1:1) to the end of the reaction. The reaction solution was transferred to a 250ml single-necked bottle, concentrated to dryness, and then dried under reduced pressure by adding 30ml of toluene to obtain green needle crystals.
[0040] (2) Preparation of N-(5-nitro-2-thiophenoyl)-L-glutamic acid diethyl ester
[0041] Put 35.0g of L-diethyl glutamate, 150ml of dichloromethane and 12.5ml of diisopropylethylamine into a 250ml three-necked bottle, lower the temperature, and slowly add 50ml of di...
Embodiment 2
[0048] The preparation of embodiment 2 impurity F
[0049] (1) Preparation of N-(5-amino-2-thiophenoyl)-L-glutamic acid diethyl ester
[0050] Prepare with reference to steps (1), (2), and (3) of Example 1.
[0051] (2) Diethyl N-(5-methylamino-2-thienyl)-L-glutamate
[0052] Add 100ml of DMF, 2.0g of 2,6-lutidine, 10g of diethyl N-(5-amino-2-thiophenoyl)-L-glutamate, and 4.5g of methyl iodide into a 250ml three-necked flask, Stir evenly, heat up, and control the feed liquid temperature to 50-60°C for 6 hours. The completion of the reaction was monitored by HPLC, the temperature was lowered to 10-30° C., quenched by adding 80 ml of 15% saline, extracted with 200 ml of ethyl acetate in 3 times on average, and allowed to stand for liquid separation. Combine the organic phases, add anhydrous magnesium sulfate, stir and dehydrate for 2 hours, and filter with suction until no liquid flows out. The filtrate was transferred to a rotary evaporator and concentrated until no liquid ...
Embodiment 3
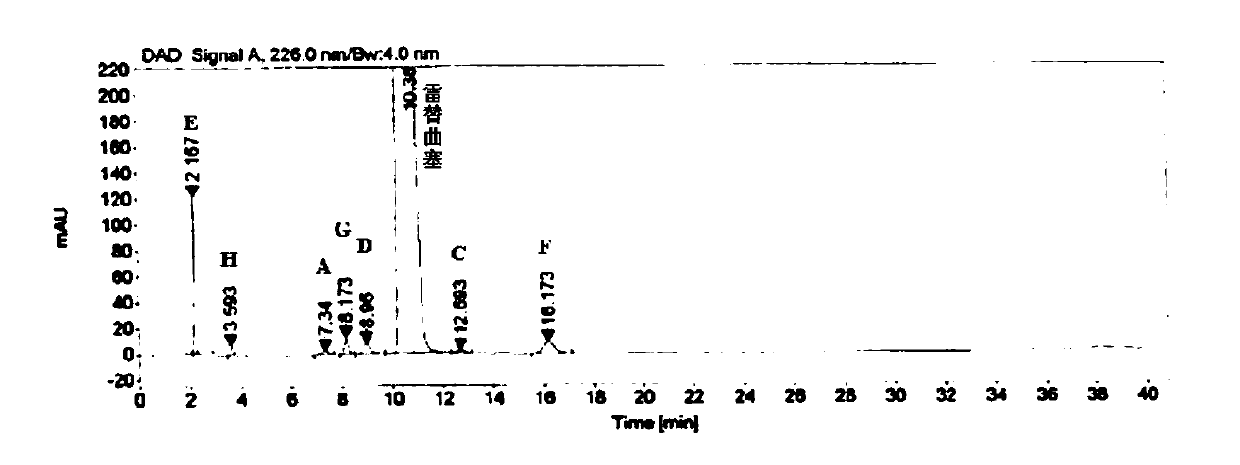
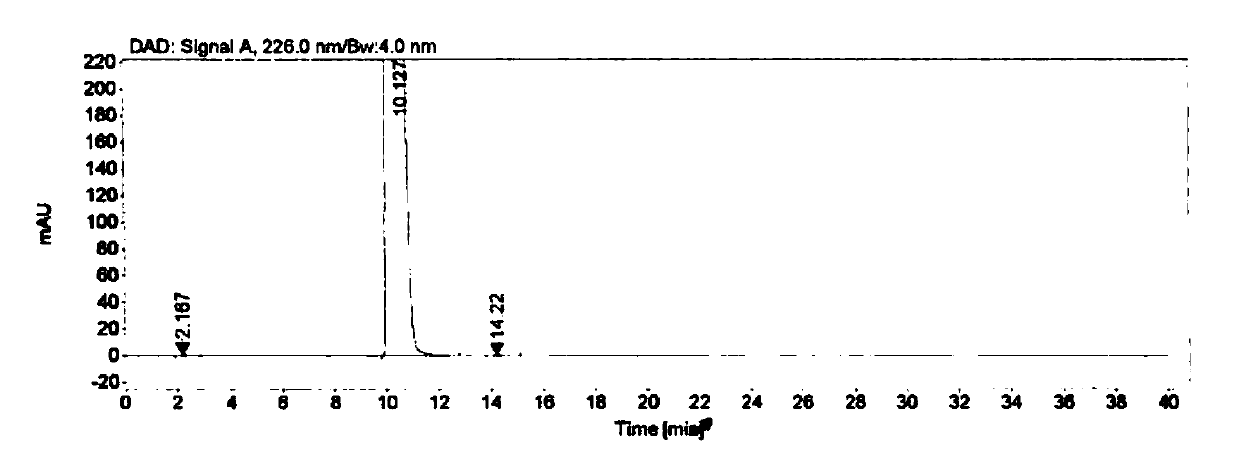
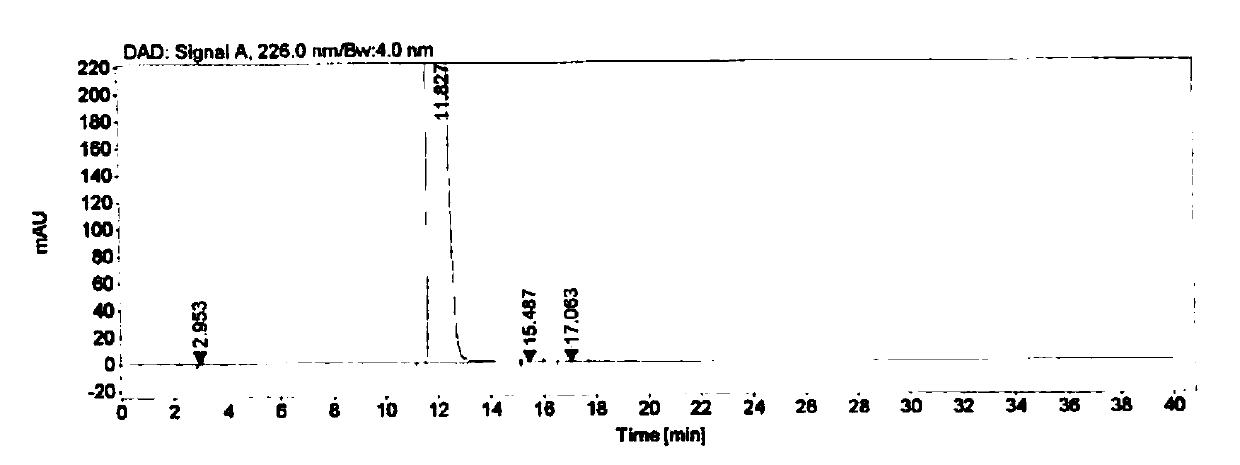
[0058] Embodiment 3 specificity test
[0059] Instrument: Agilent 1260 high performance liquid chromatograph
[0060] Detector: DAD
[0061] Workstation: Agilent OpenLAB CDS (EZChrom Edition)
[0062] Chromatographic column: Agilent Extend-C18 (4.6mm×250mm, 5μm)
[0063] Detection wavelength: 226nm
[0064] Flow rate: 1.0mL / min
[0065] Column temperature: 30°C
[0066] Injection volume: 10μL
[0067] Mobile phase: buffer-methanol (70:30), where the buffer is 0.005mol / L tetrabutylammonium bromide aqueous solution (adjust the pH to 8.5 with phosphoric acid)
[0068] Blank solution preparation: Precisely measure 0.4mL of 0.1mol / L sodium hydroxide aqueous solution, put it in a 10mL measuring bottle, add mobile phase to dilute to the mark, and shake well.
[0069] Positioning solution preparation: Accurately weigh about 10.0 mg of each impurity, put them in a 20 mL volumetric bottle, add 0.4 mL of 0.1 mol / L sodium hydroxide aqueous solution and ultrasonically dissolve them,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com