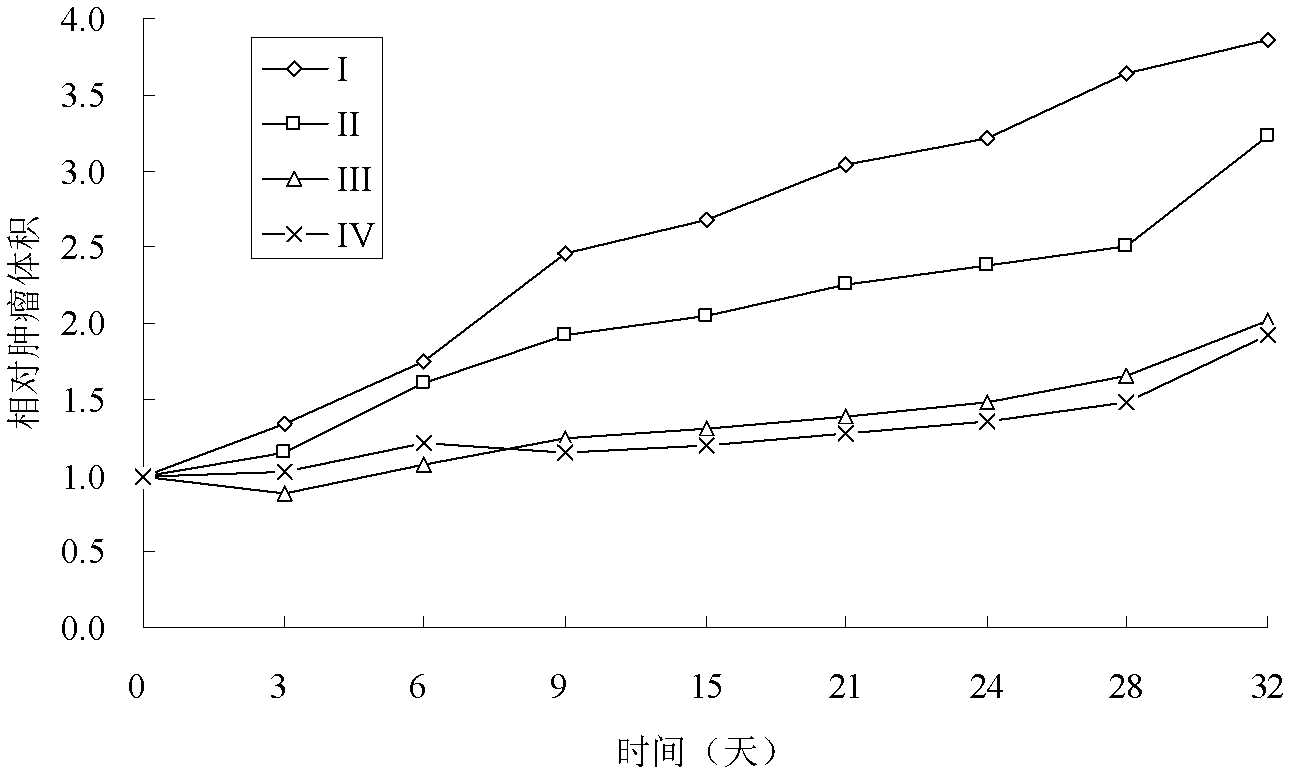
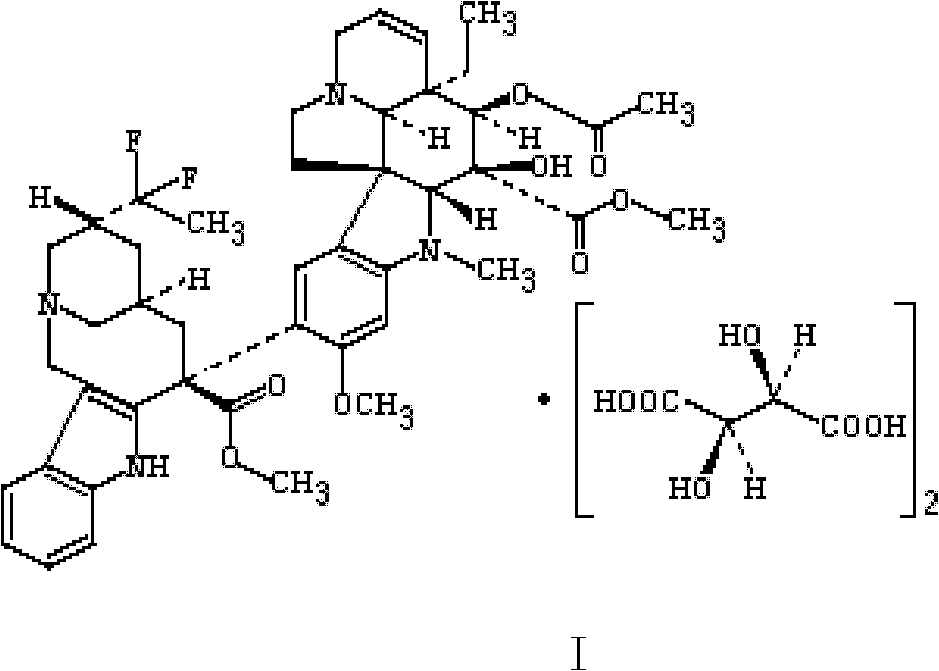
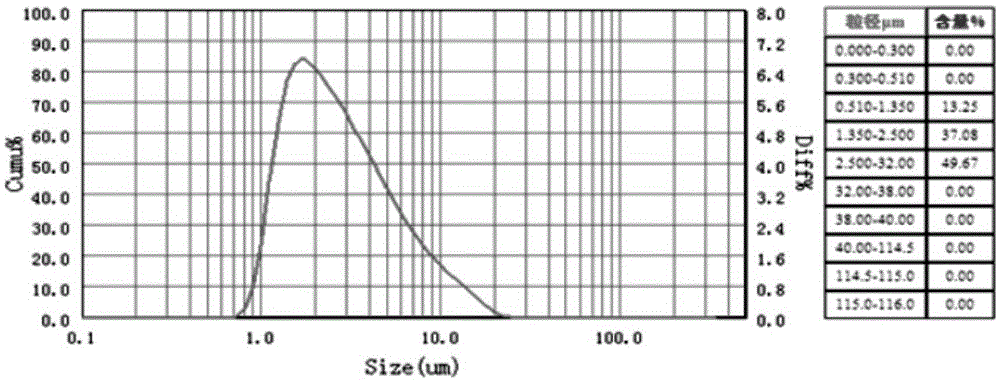
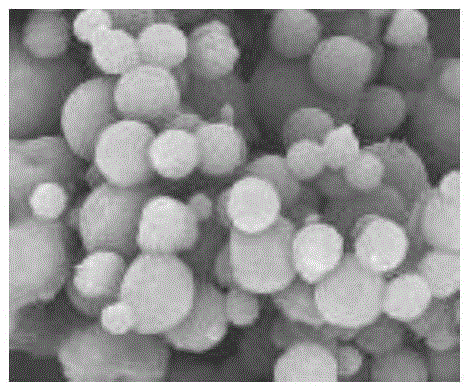
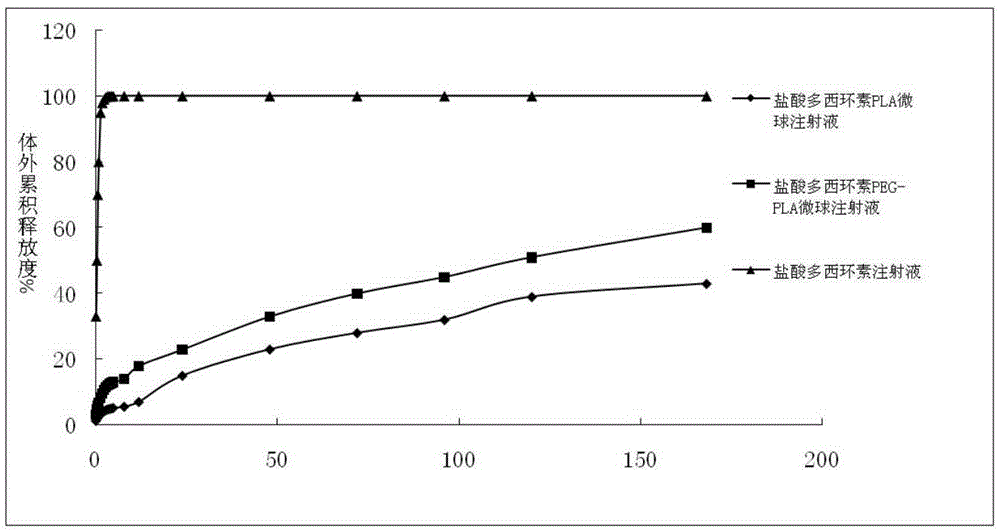
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
69results about How to "Slow drug release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
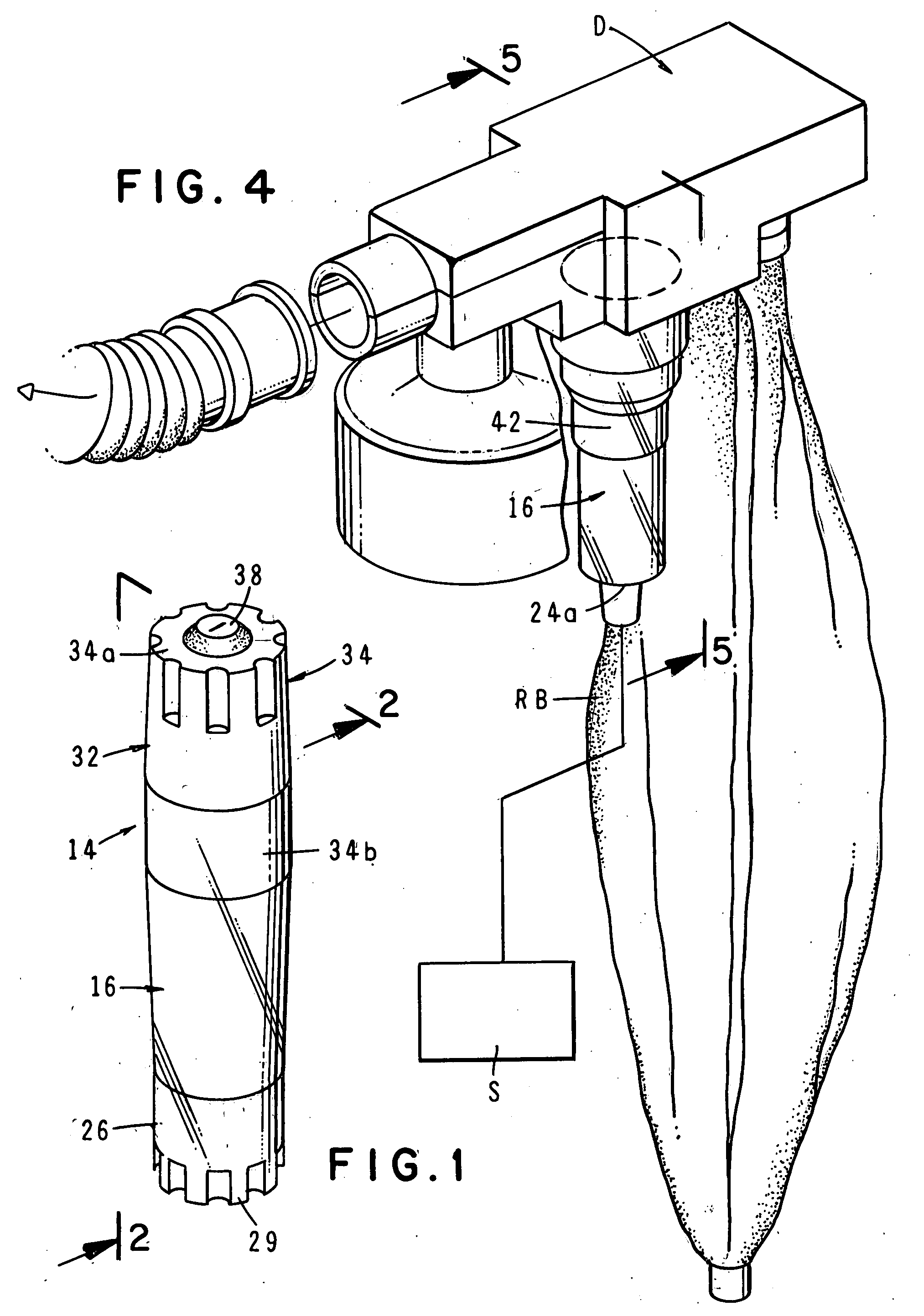
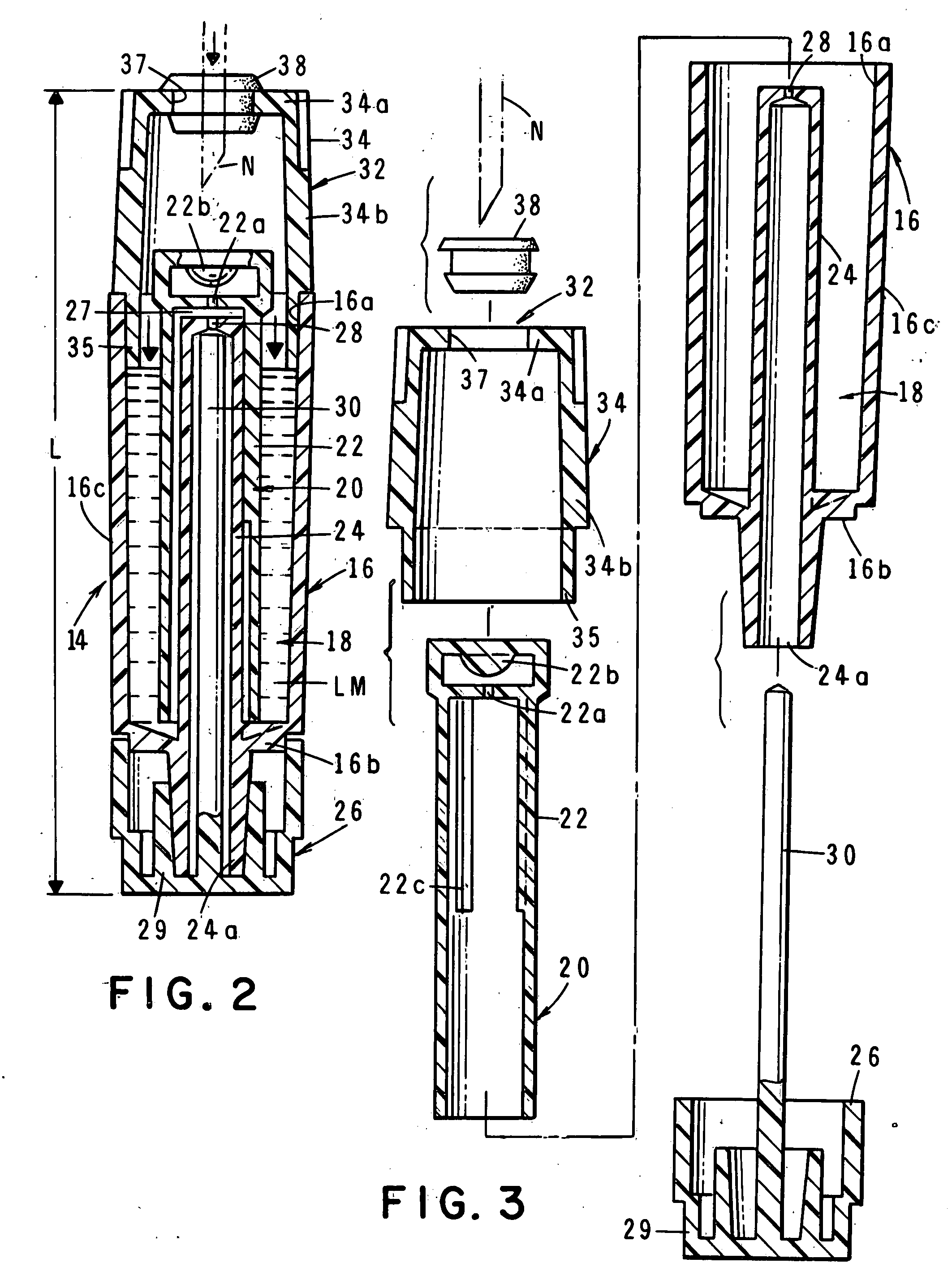
Drug delivery system for zero order, zero order-biphasic, ascending or descending drug delivery
InactiveUS7195778B2Delay swellingSlow drug releaseOrganic active ingredientsNervous disorderDrug deliveryPharmaceutical preservatives
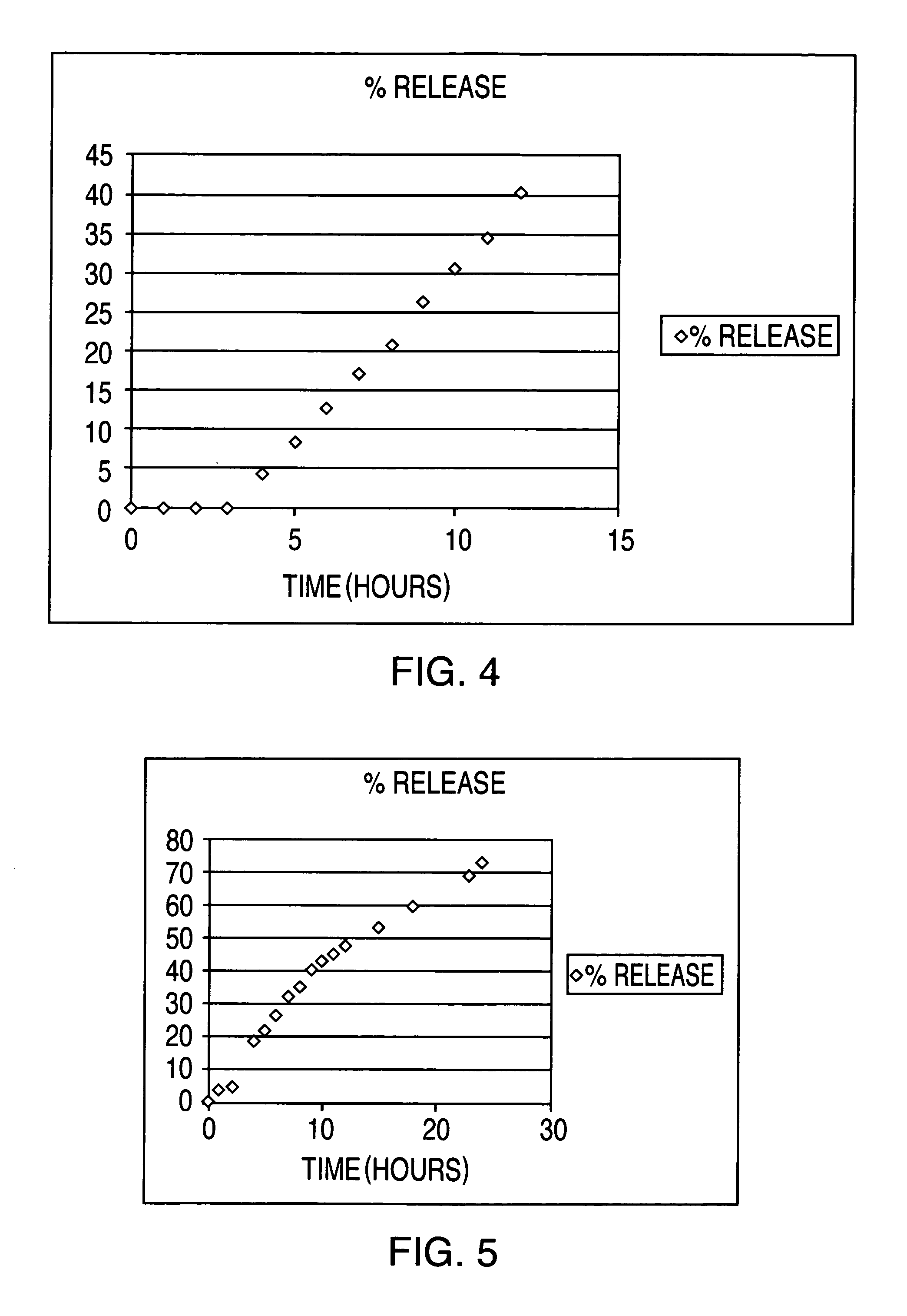
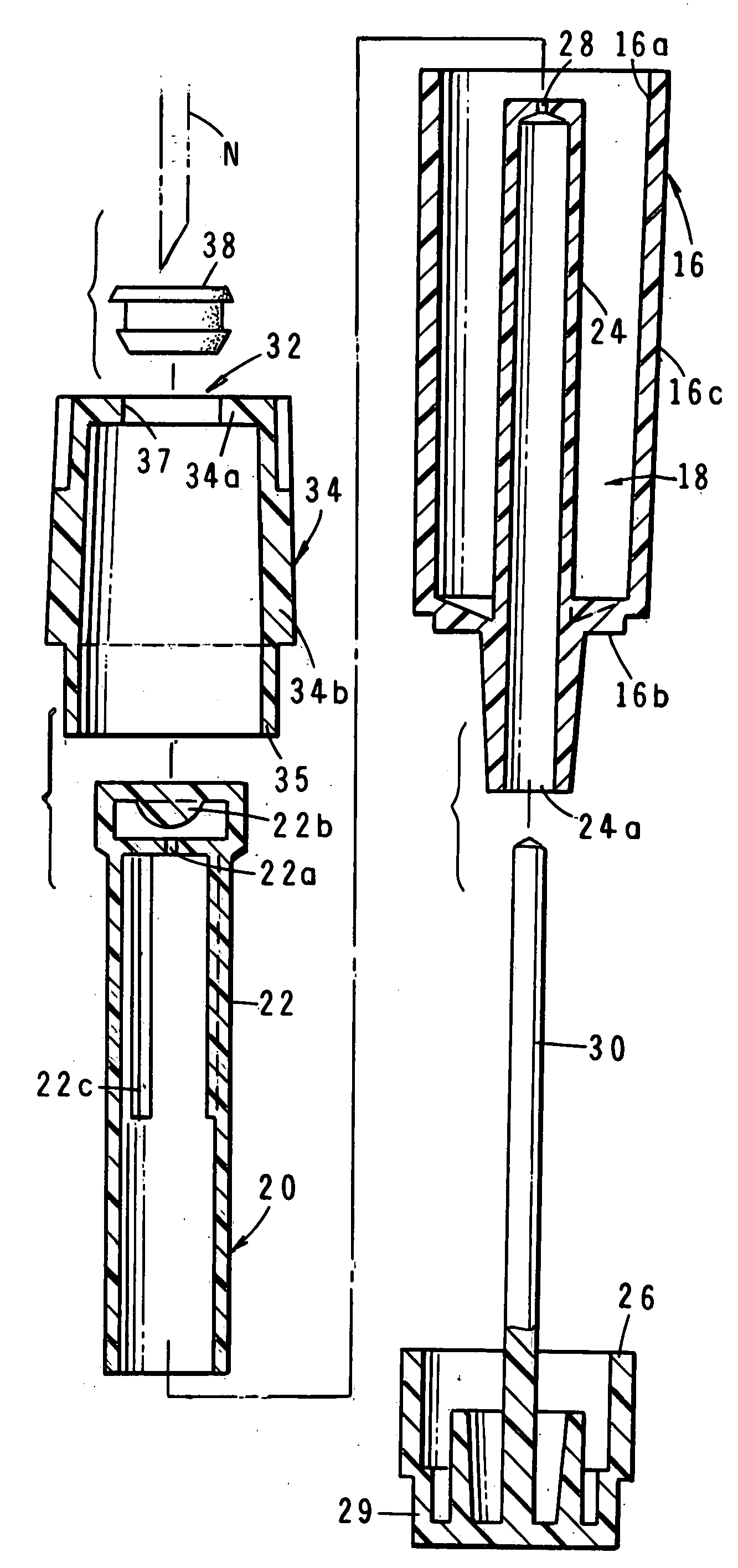
The invention is directed to a drug delivery device for controlled release of a drug, comprising a core that has a cylindrical plug embedded therein; and a coating that at least partially surrounds the core. The core is comprised of a drug and excipients. The coating surrounding the core is essentially impermeable to the drug. The cylindrical plug, which is embedded in the core, may be hollow or solid. The drug delivery device enables zero-order drug release profiles as well as more complicated release profiles to be obtained. The invention is also directed to a method of making the drug delivery device.
Owner:TEVA PHARM USA INC
Micro/sub-micro emulsion in situ gel rubber preparation of cyclosporins A for eyes and preparation thereof
InactiveCN101244256AImprove comfortObvious side effectsSenses disorderPharmaceutical delivery mechanismChemistrySide effect
The invention relates to an ophthalmic microemulsion / submicron emulsion in situ gel preparation with cyclosporine A and the preparation method. The preparation is prepared through shearing and high pressure homogenization process using cyclosporine A, oil phase, emulsifier, coemulsifier, thickener, isosmotic adjustment agent, bacteriostat, pH regulator and purified water. The invention is characterized in that safe and nonirritant nonionic surface-active material polyoxyethylene castor oil, polyoxyethylene hydrogenated castor oil, tween or phosphatides are adopted as the emulsifier; macromolecular material with ion and / or pH sensitive properties is used as the thickener. The average particle size of emulsion drops of the ophthalmic microemulsion / submicron emulsion in situ gel preparation is less than 200nm; the cumulative value of 90% particle size is no more than 500nm; as low-viscosity liquid with good fluidity outside the body, the ophthalmic microemulsion / submicron emulsion in situ gel preparation can rapidly form hydrogel after being dropped into eyes, thereby increasing residence time of the medicine and bioavailability. The ophthalmic microemulsion / submicron emulsion in situ gel peparation has the advantages of no stimulation and poison and side effect to eyes and stable quality of the preparation.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Drug delivery system for zero order, zero order biphasic, ascending or descending drug delivery of methylphenidate
InactiveUS20100151020A1Slow drug releaseEasy to reachBiocideNervous disorderMethylphenidate HydrochlorideControlled release
The invention is directed to a drug delivery device for controlled release of a drug, such as methylphenidate hydrochloride. The drug deliver device has a drug core, having a plug embedded therein, and at least a first coating that at least partially surrounds the core. The plug may be hollow or solid, and swells upon absorption of water, bursting through the first coating. The drag delivery device enables zero-order drug release profiles as well as more complicated release profiles to be obtained.
Owner:TEVA PHARM USA INC
Pixantrone maleate liposome preparation and preparation process thereof
InactiveCN103479578AGood tumor targetingIncrease the amount of distributionOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolTumor targeting
The invention belongs to the field of pharmaceutical preparations, and discloses a pixantrone maleate liposome preparation and a preparation process thereof. The preparation is prepared from pixantrone maleate, phospholipid, cholesterol and a gradient establishment substance. The preparation process comprises: preparing a blank liposome, preparing a gradient liposome, loading a drug, and other steps. According to the present invention, the preparation process is simple and easy to perform, the obtained preparation has characteristics of good stability and high encapsulation efficiency, tumor targeting of drugs can be increased, and toxic-side effects of drugs can be reduced.
Owner:SHENYANG PHARMA UNIVERSITY
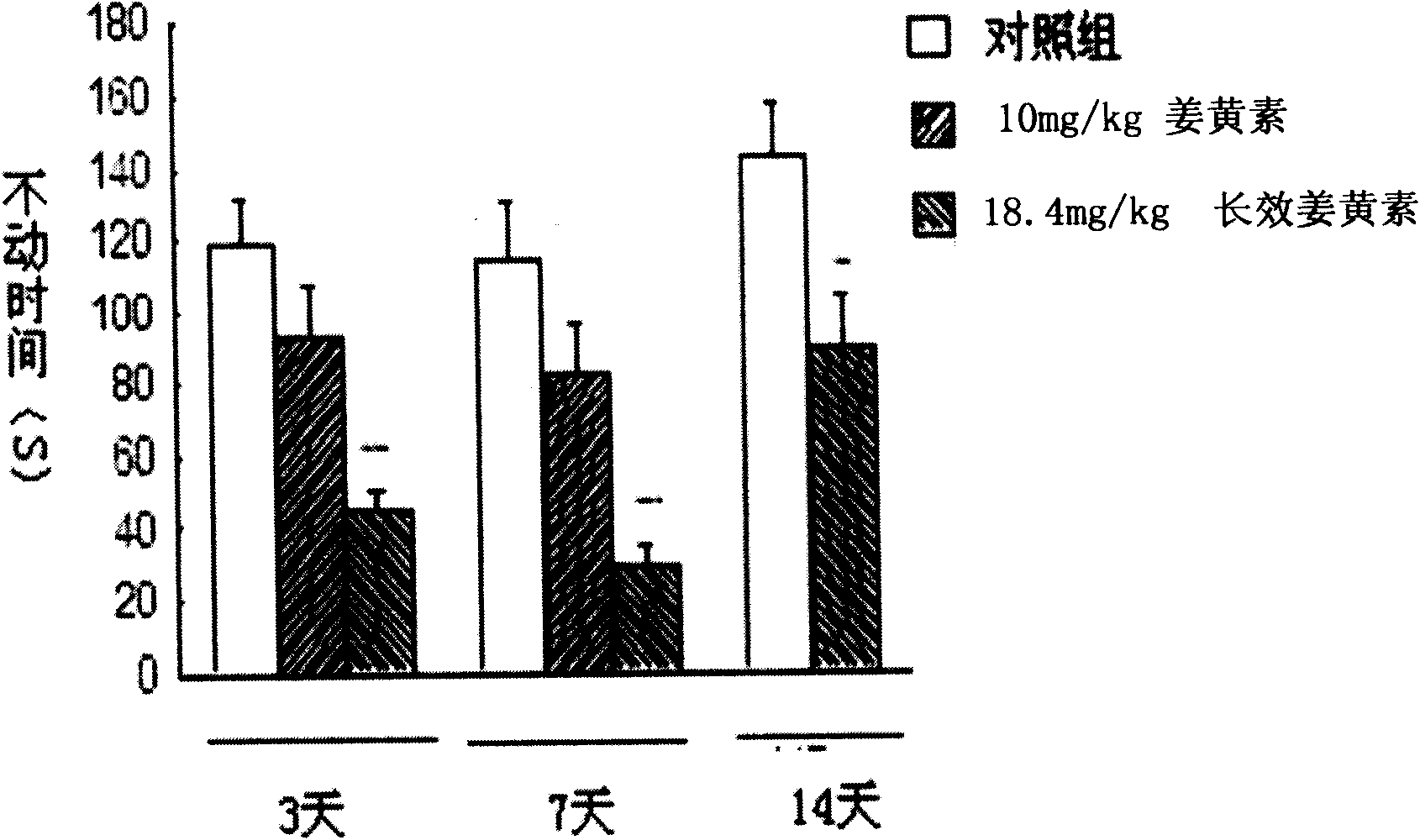
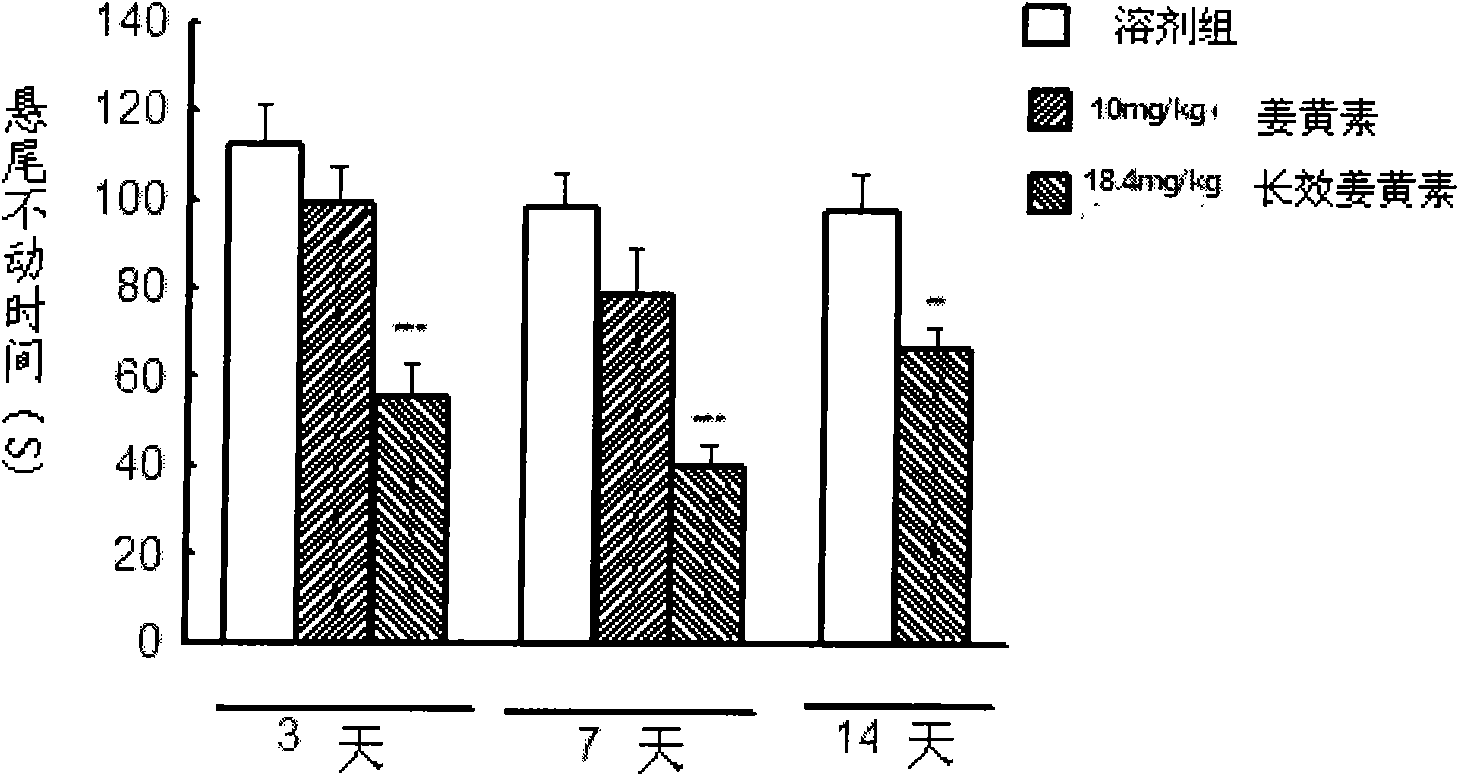
Application of long effective curcumin derivative in preparing anti-tumor disease drug
ActiveCN101669931AImprove stabilityImprove bioavailabilityKetone active ingredientsPharmaceutical non-active ingredientsDiseaseFood additive
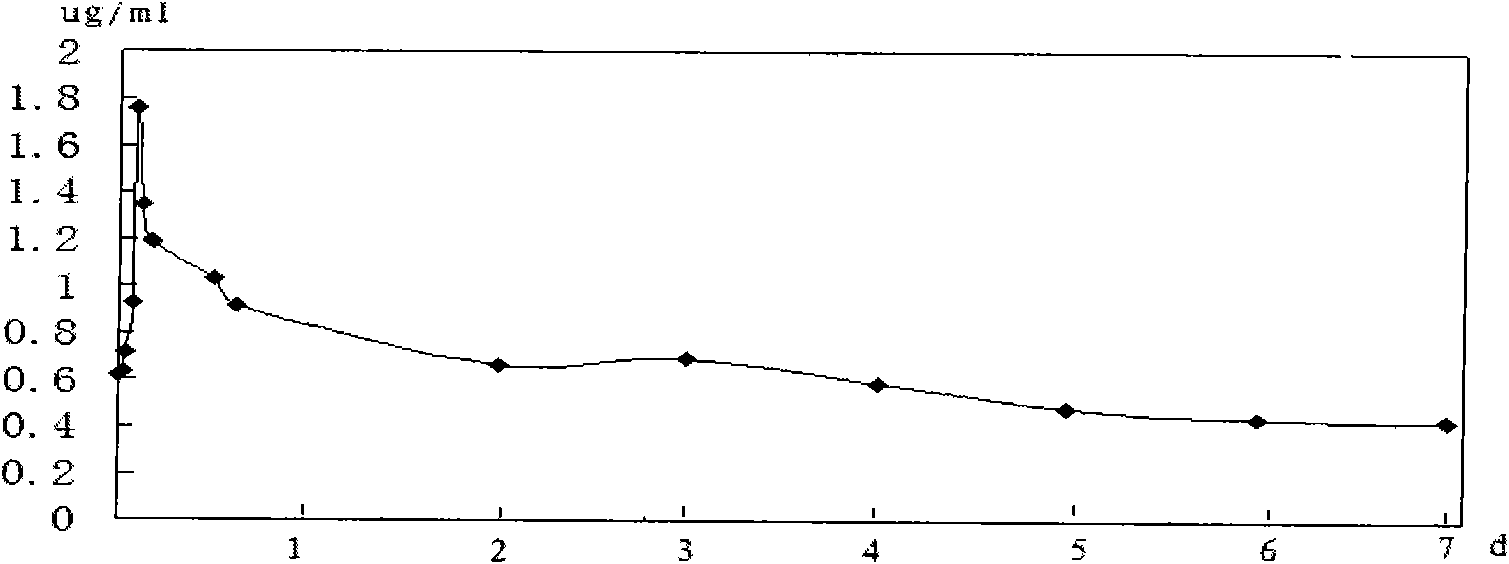
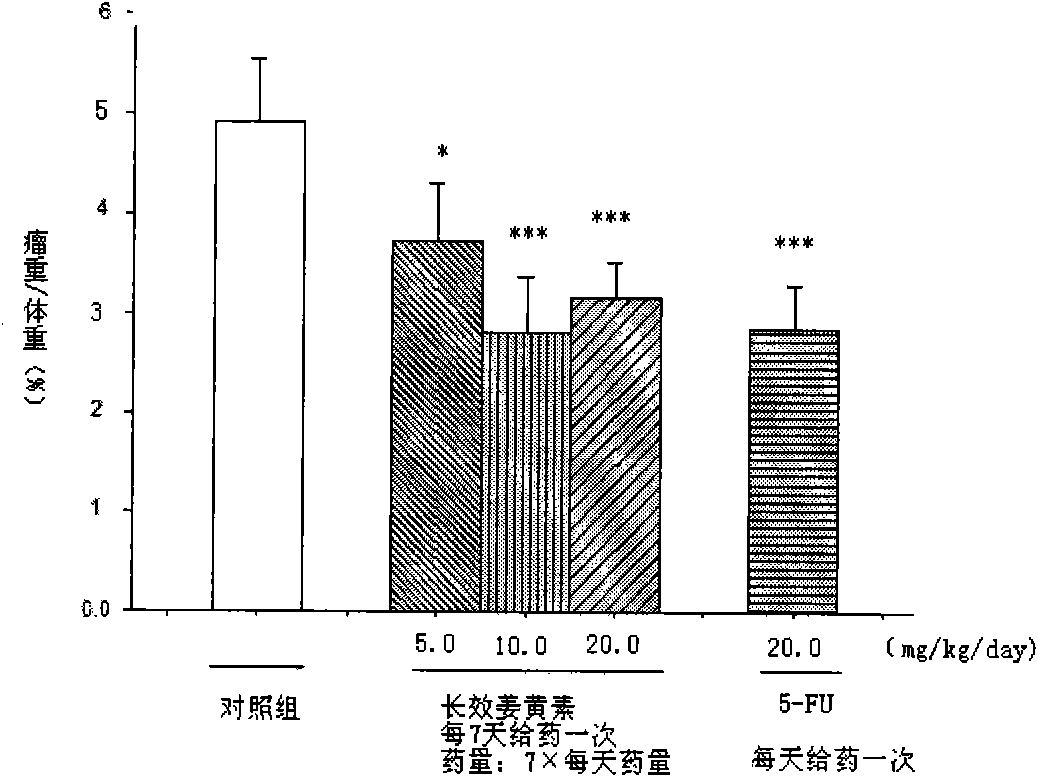
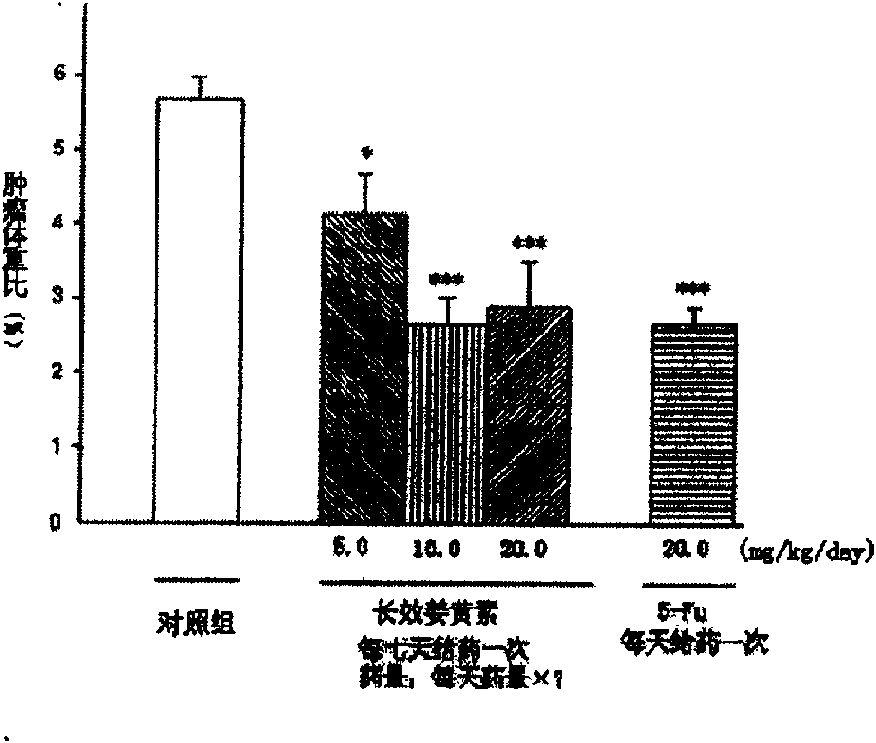
The invention relates to an application of a long effective curcumin derivative in preparing an anti-tumor disease drug. The long effective curcumin derivative is an ester which is formed in such a way that C1 to C50 saturated or unsaturated fatty acids or acyls thereof react with hydroxyl groups of curcumin substances by esterification respectively and independently. Through the research on the anti-tumor action of various animal models, the long effective curcumin derivative can reserve the inherent anti-tumor drug effect of the curcumin and also has the effects of the slow release and the prolongation of the drug effect and a favorable prospect of clinical application. The long effective curcumin derivative can be also used for preparing anti-tumor drinks, foods, food additives or health products.
Owner:北京鼎国昌盛生物技术有限责任公司
Pre-filled, single-use, disposable small volume medication nebulizer
InactiveUS20090050141A1Easy to useEffectively mitigates against possibilityMedical devicesSpray nozzlesMechanical ventilatorsNebulizer
A miniaturized pre-filled, single-use, disposable, small volume medication nebulizer unit for medicinal use that delivers a mist of properly sized aerosol particles of medicament to the patient with a very high level of efficiency. The nebulizer can be effectively used in conjunction with conventional tee and mouthpiece patient interface devices as well as with more sophisticated interface devices such as dosimetric / reservoir systems, or mechanical ventilator systems.
Owner:KING RUSSELL WAYNE +1
Liposome nanometer carrier situ gel preparation used for eye epidermal growth factor
InactiveCN101057966AGood physical and chemical stabilityReduce exposureSenses disorderPeptide/protein ingredientsLipid formationGel preparation
The invention relates to a liposome nano carrier in-situ gelling preparation for eyes containing epidermal growth factor, which is prepared from epidermal growth factor, lipid material, emulsifying agent, thickening agent, isotonic conditioning agent, bacteria inhibitor, pH regulator and purified water, wherein non-irritating phosphatide is employed as the emulsifying agent, macromolecular material having ion and / or pH responsive characteristics is used as the thickening agent.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
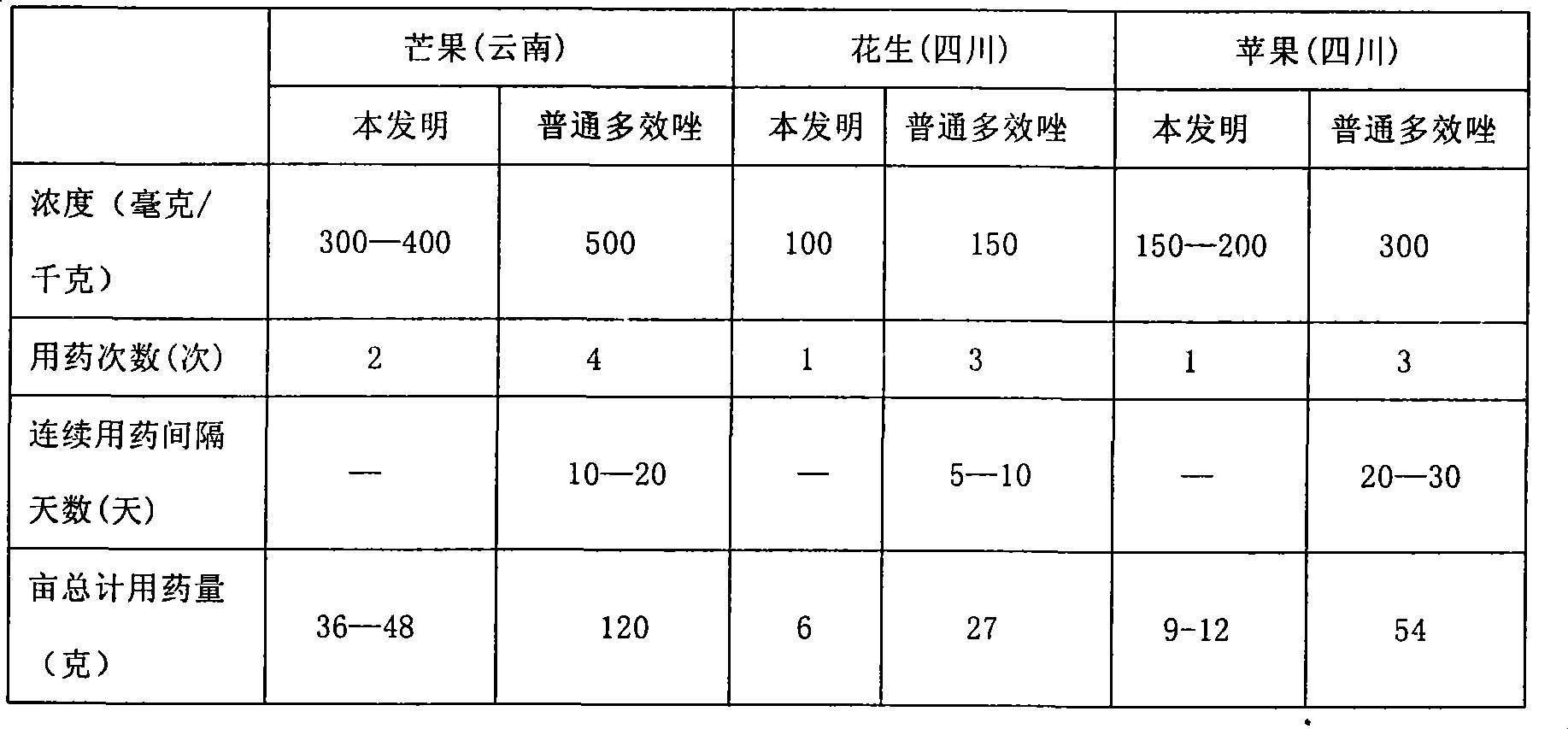
Multiple-effect azole microcapsule suspension formulation with slow-release function and preparation method thereof
InactiveCN101361476AGood slow releaseImprove stabilityBiocidePlant growth regulatorsEngineeringPaclobutrazol
The invention provides a paclobutrazol micro-capsule suspension preparation provided with sustained-release function, which includes the following components according to 100 parts by weight: 10 to 30 parts of film-forming materials, 5 to 40 parts of paclobutrazol, 5 to 10 parts of emulsifying agent and the rest parts of water. The potency of the suspension preparation is more moderate, the potency releases slower and lasts long, thus ensuring the adjustment of steady growth of plants and having the sustained-release function. The invention also provides a preparation method of the paclobutrazol micro-capsule suspension preparation.
Owner:SICHUAN LANYUE SCI & TECH
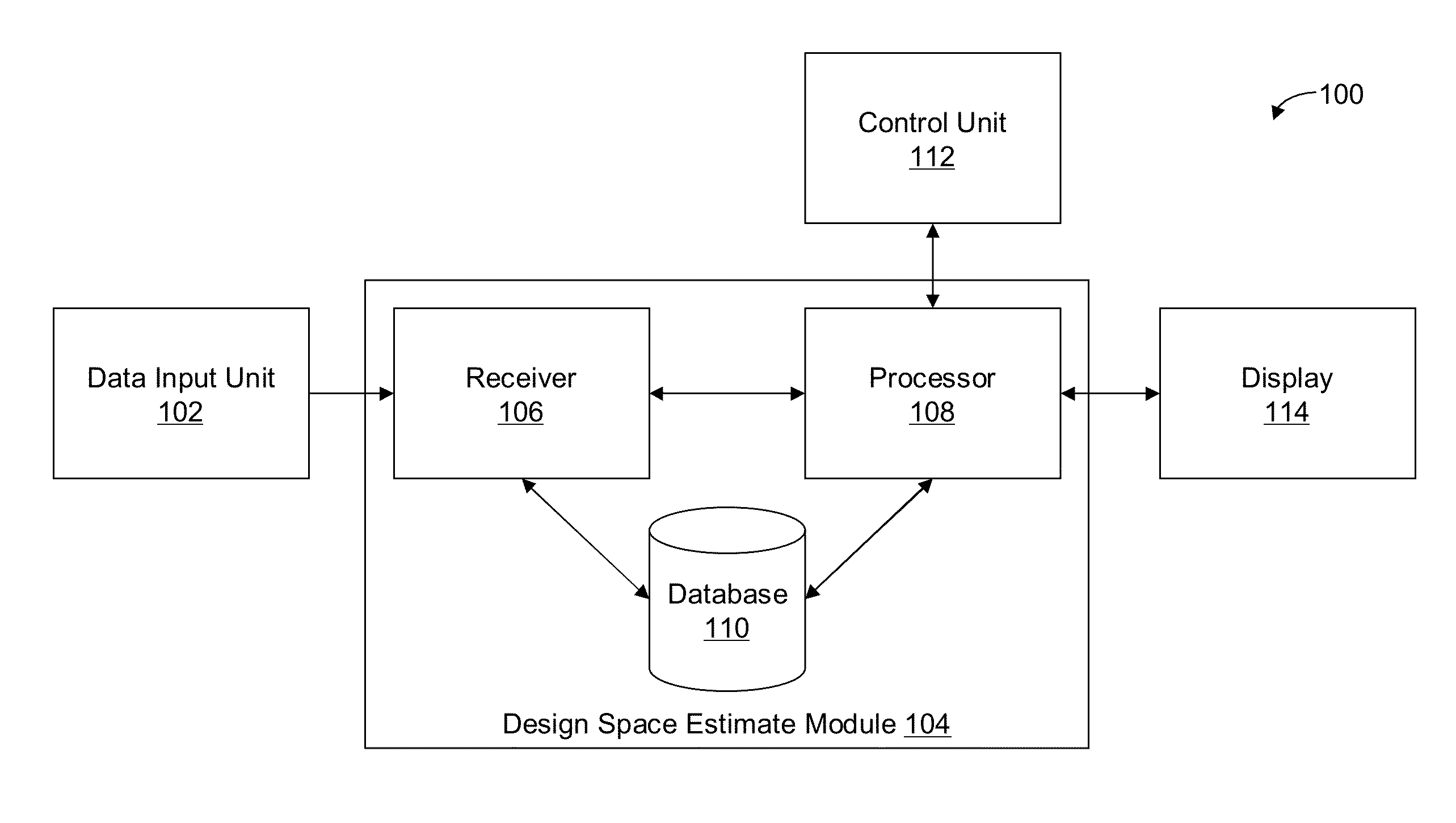
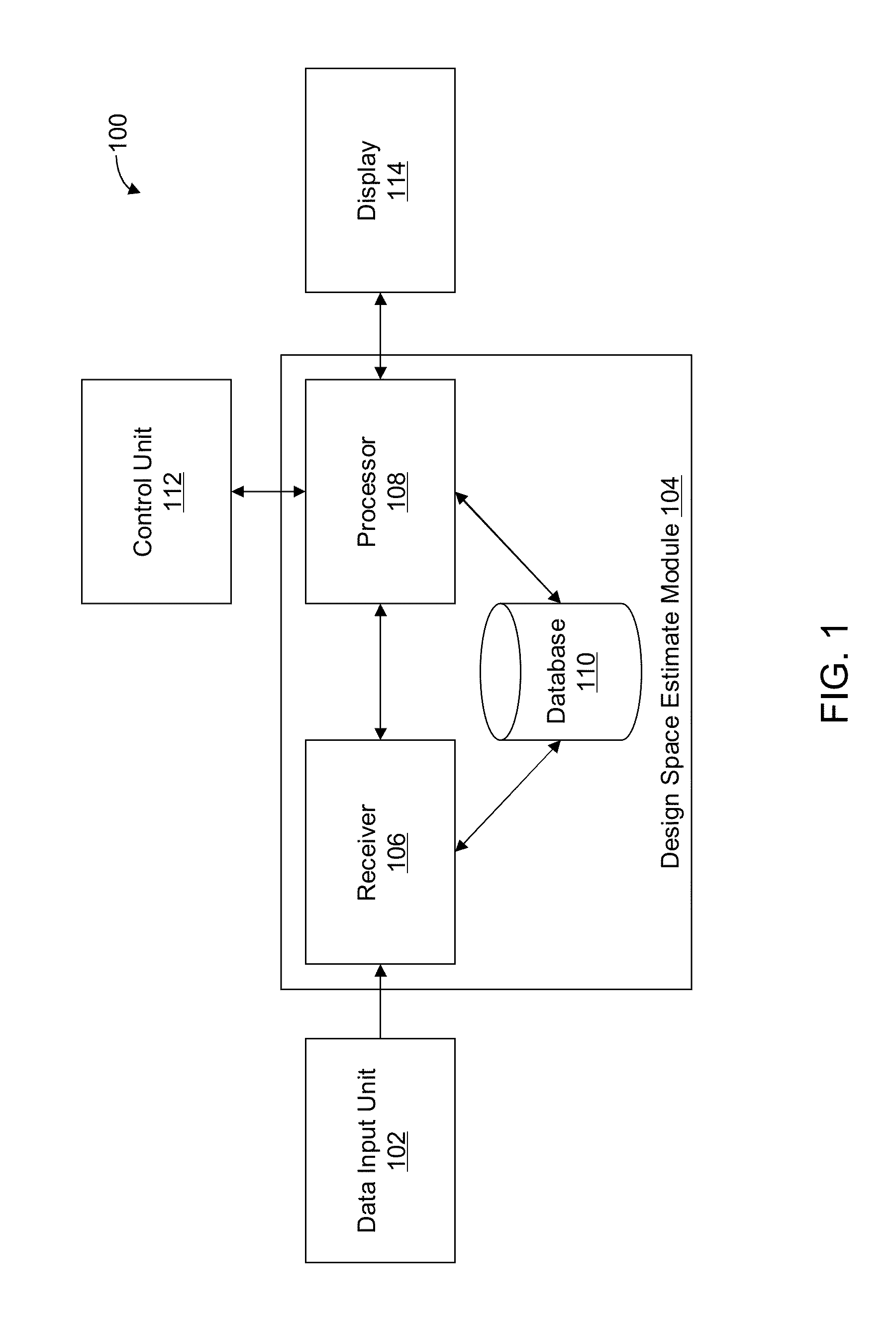
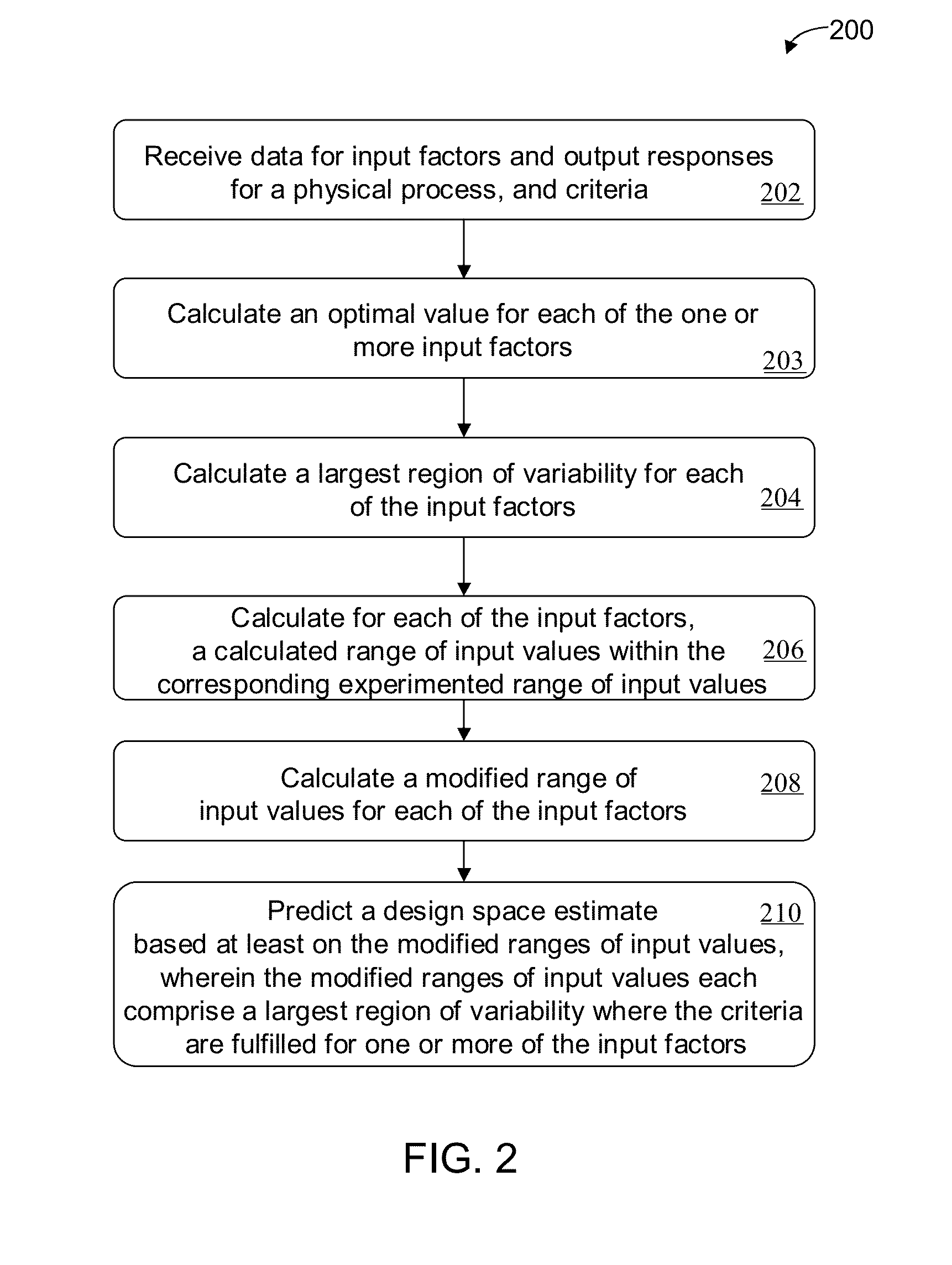
Methods and apparatus for automated predictive design space estimation
ActiveUS20130218529A1Facilitate continuousFacilitate stable drug intakeComputation using non-denominational number representationComputer aided designComputer science
Described are computer-implemented methods and apparatuses, including computer program products, for estimating an optimal value for each input factor of a design space. The design space is defined by the input factors and output responses for a physical process. The optimal values for the input factors represent a starting point for estimating the design space. Data is received for the input factors, the output responses and criteria. An initial design space is estimated based on the received data. The optimal values for the input factors are determined from the initial design space.
Owner:SARTORIUS STEDIM DATA ANALYTICS AB
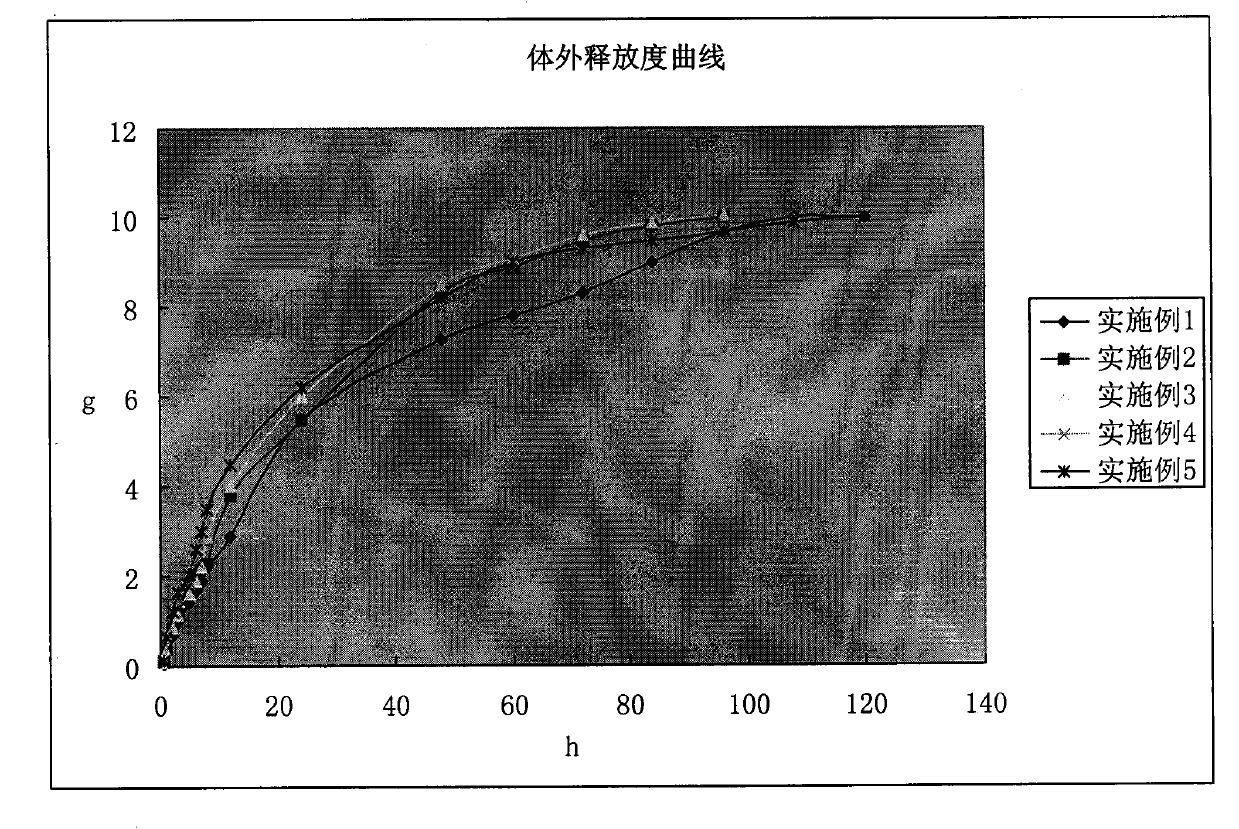
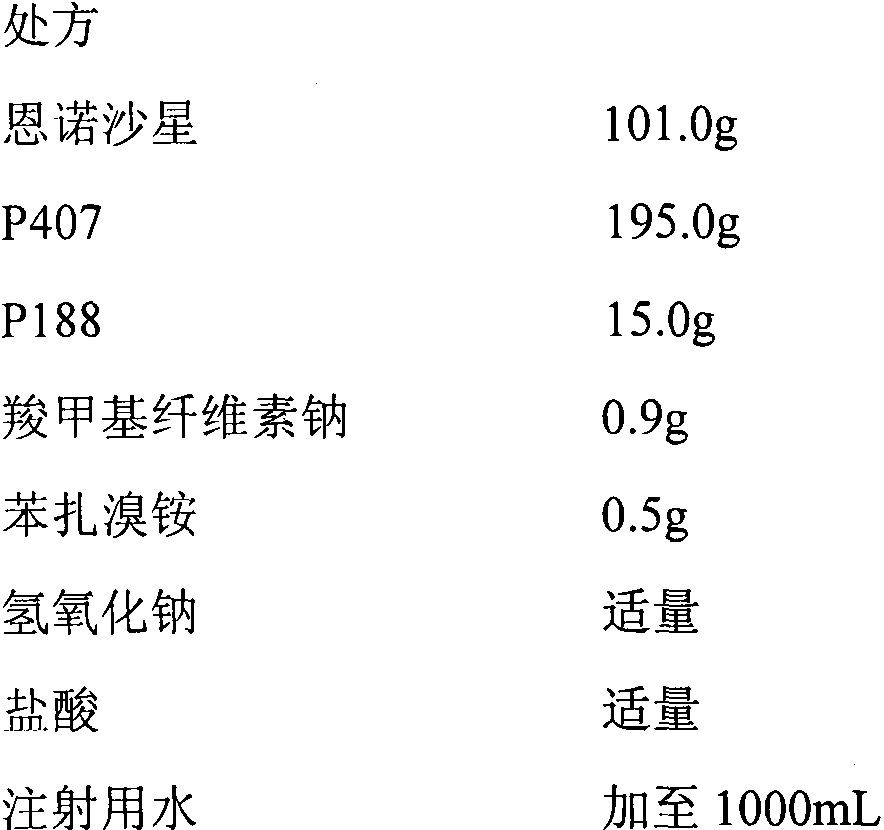
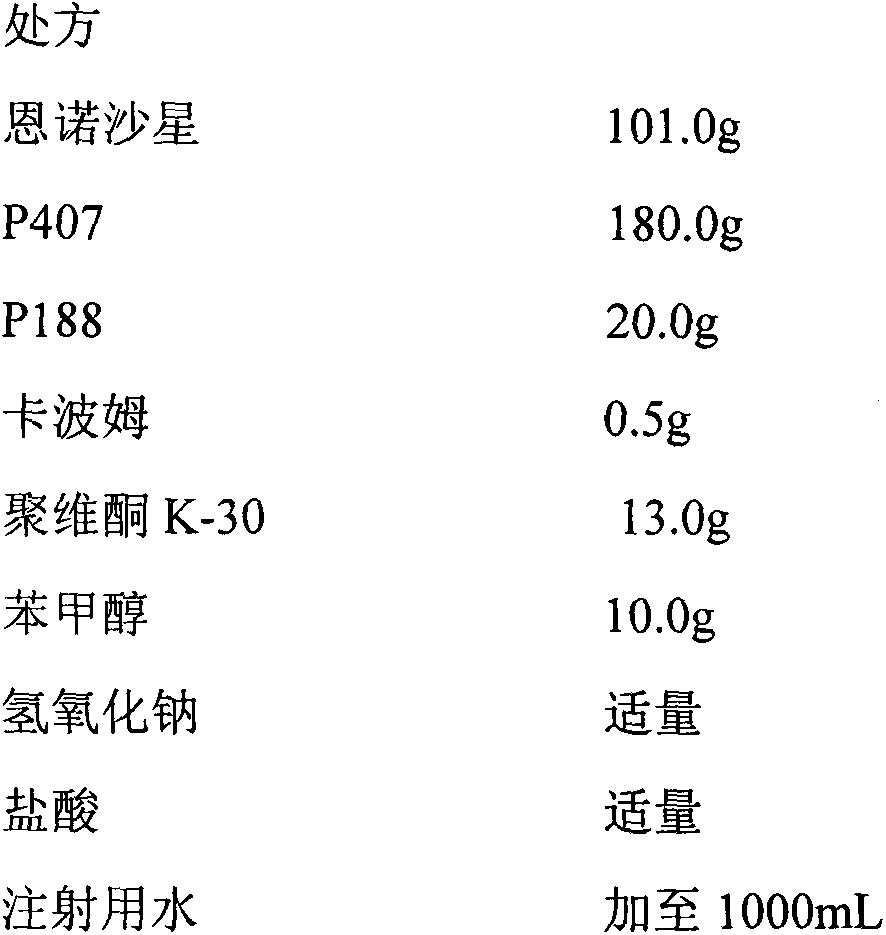
In-situ gel for enrofloxacin injection and preparation method thereof
InactiveCN103385850AImprove complianceSlow drug releaseAntibacterial agentsOrganic active ingredientsDiseaseGel preparation
The invention belongs to the field of special pharmaceutical preparations for animals, and relates to an in-situ gel preparation for enrofloxacin injection and a preparation method thereof. The preparation comprises the following components in parts by weight: 5.0-20.0 parts of enrofloxacin, 14.5-32.5 parts of P407, 0.5-10.5 parts of P188, 25.0-90.0 parts of water, 0.01-10.0 parts of a high molecular retardant, 0.01-3.0 parts of a preservative and appropriate amount of a pH adjustor. The preparation provided by the invention is a freely flowing liquid at room temperature, and forms a semi-solid gel through dosage by intramuscular injection or subcutaneous injection, so that the preparation is just delivered once in a course of treatment. The preparation has the advantages of stable property, long lasting time for delivery, exact curative effect, strong animal adaptability, no toxic and side effects and adverse reaction and the like, and is simple to prepare and convenient to delivery. The preparation provided by the invention has broad-spectrum antibacterial effect, and can be used as a medicine for preventing and treating diseases of animals such as pigs, sheep, dogs, cats and cattle.
Owner:NANJING AGRICULTURAL UNIVERSITY
Enhanced targeted drug delivery system via chitosan hydrogel and chlorotoxin
InactiveUS9522114B1Improve stabilityEasy to storeOrganic active ingredientsPeptide/protein ingredientsScorpion VenomsMucoadhesion
Specific drug delivery to tumor cells without affecting normal cells is a major challenge in cancer treatment. The present invention incorporates embedding nonionic surfactant vesicles (niosomes) containing an anti-cancer therapeutic agent with chlorotoxin into a thermosensitive chitosan hydrogel network. Chitosan has mucoadhesive property which can be used in the targeting of the tumor cells with the mucin over expression. Chlorotoxin is a 36 amino acid peptide obtained from Leiurus quinquestriatus scorpion venom which binds preferentially to tumor cells of neuroectodermal origin but not to normal cells. The incorporation of chlorotoxin along with niosomes in the chitosan hydrogel is used as the second targeting strategy to further improve the specific delivery of drugs to tumor cells such as glioma.
Owner:UNIV OF SOUTH FLORIDA
Albumin conjugated temperature and pH-sensitive multi-block copolymer, a method of preparation thereof and drug delivery system using the same
ActiveUS20160022823A1Reducing initial burst release of drugSlow drug releaseBiocidePeptide/protein ingredientsAmino estersSustained release drug
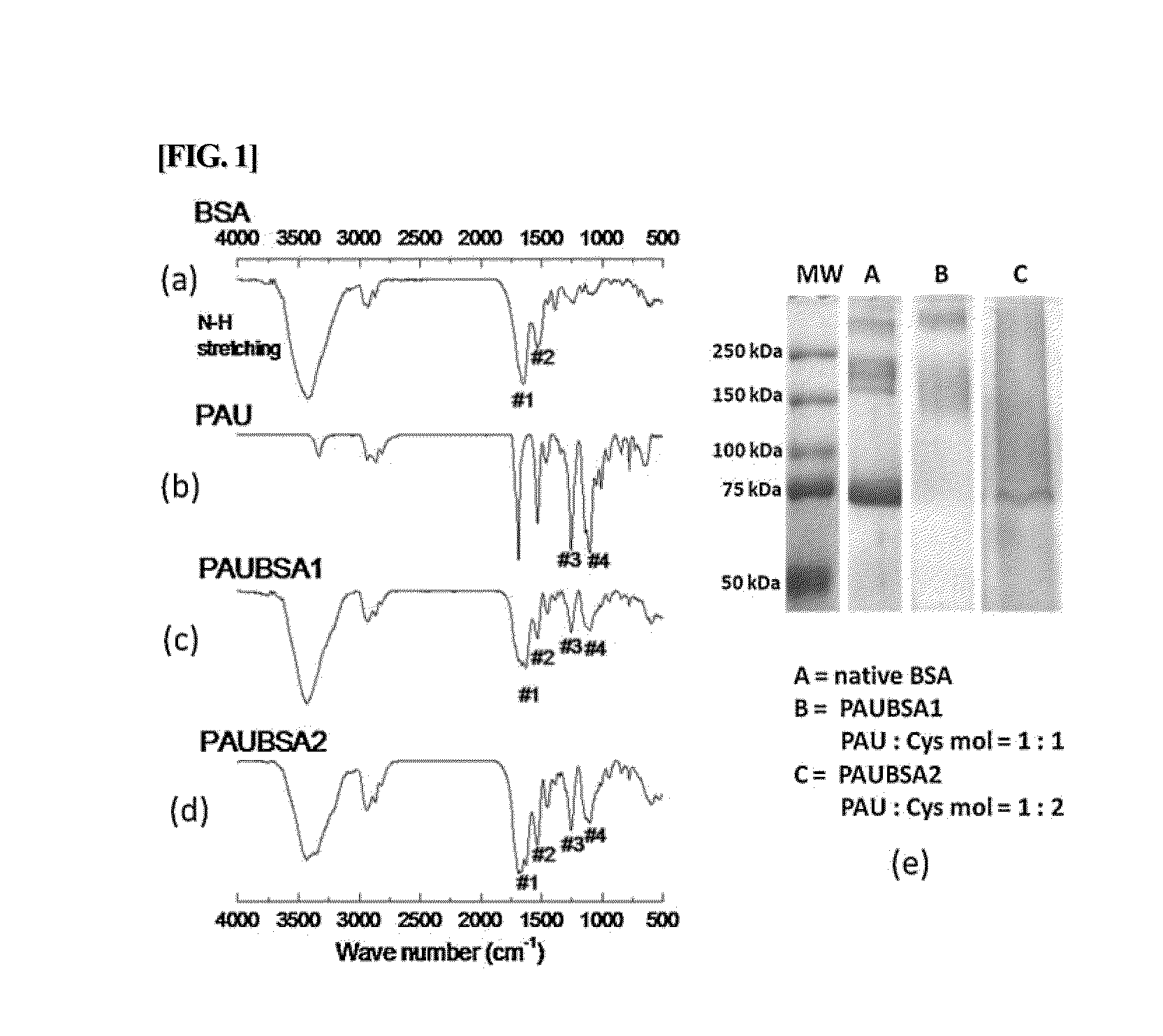
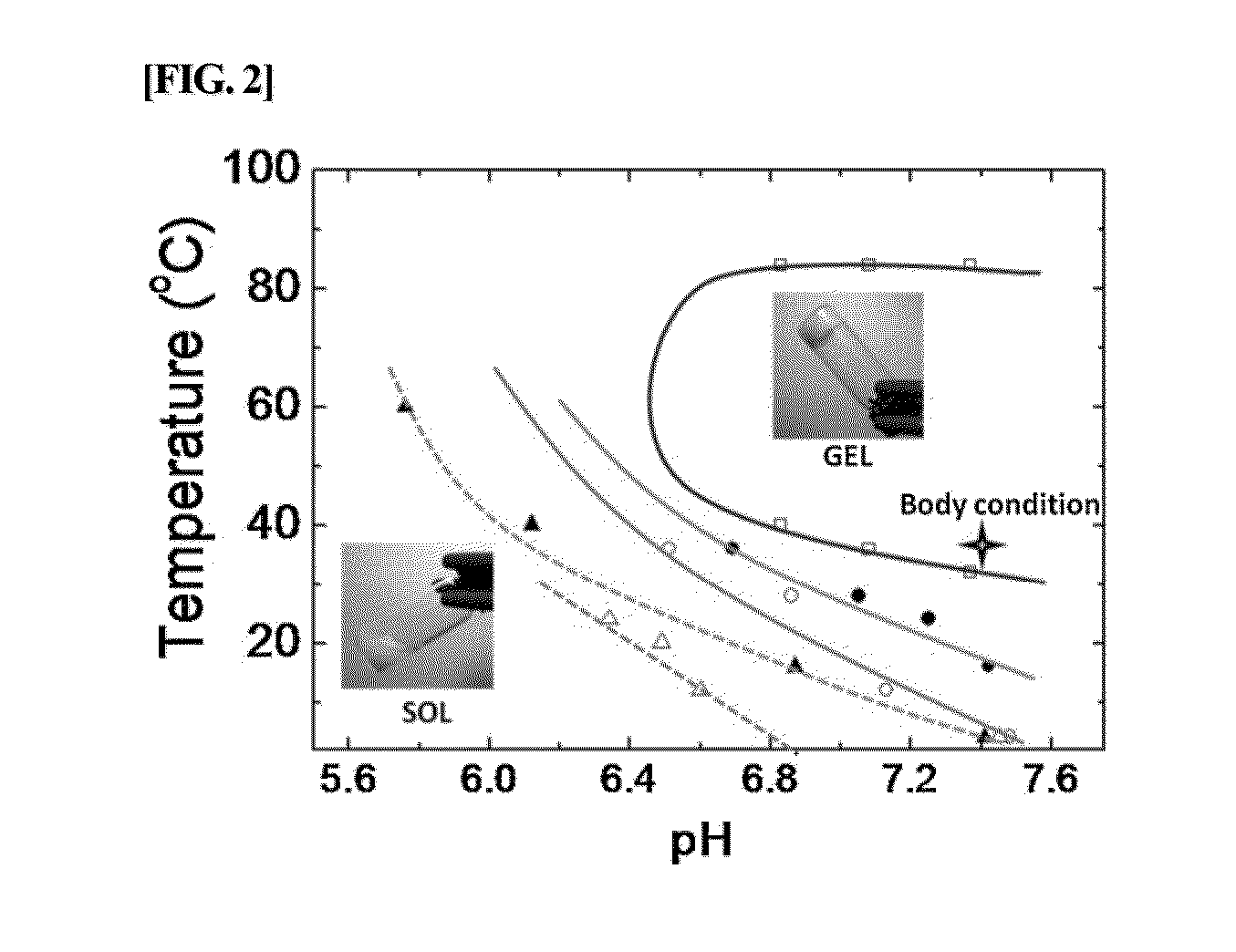
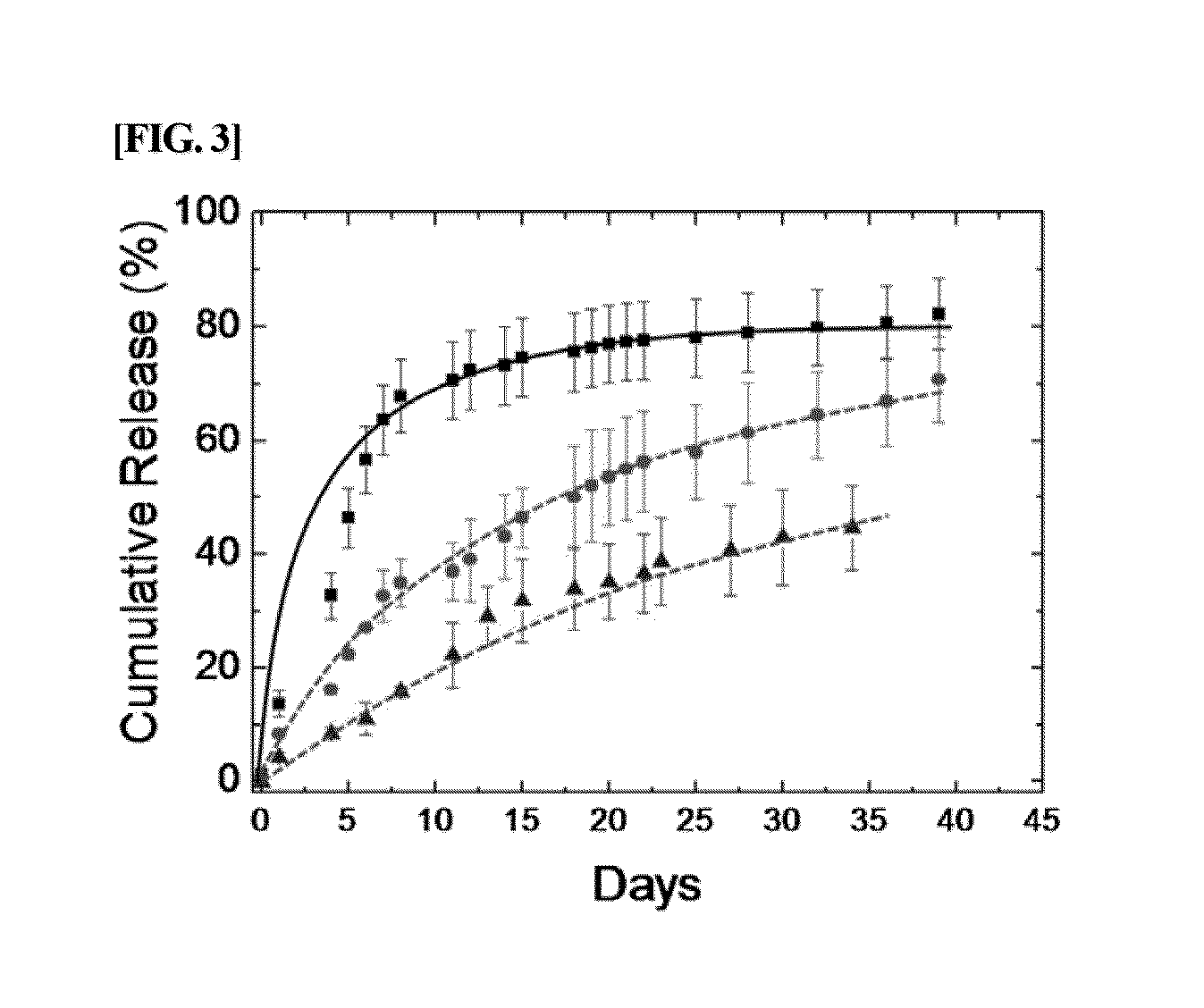
The present invention relates to a conjugate of albumin and a temperature- and pH-sensitive multi-block copolymer, a method of preparation thereof, and a sustained-release drug carrier comprising the same, and more specifically, to a conjugate in which polyethylene glycol-poly(amino urethane) (PEG-PAU) or polyethylene glycol-poly(amino ester urethane) (PEG-PAEU) multi-block copolymer is conjugated to albumin, a method of preparing the same, and a long-term sustained-release drug carrier comprising the same, capable of reducing an initial burst release of drugs and improving an affinity to drugs.
Owner:RES & BUSINESS FOUND SUNGKYUNKWAN UNIV
Vinflunine liposome preparation and preparation method of vinflunine liposome preparation
InactiveCN102805729AProlong half-life in vivoAvoid phagocytosisOrganic active ingredientsPharmaceutical non-active ingredientsVinflunineInjection solution
The invention relates to a vinflunine liposome preparation and the preparation method thereof. The vinflunine liposome preparation mainly comprises vinflunine or salt, phospholipid, cholesterin and polyethylene glycol-distearoyl phosphatidyl ethanolamine of the vinflunine, wherein salt, phospholipid, cholesterin and polyethylene glycol-distearoyl phosphatidyl ethanolamine are pharmaceutically acceptable, and the vinflunine liposome preparation can be prepared into an injection or a lyophilized preparation. The preparation method is simple in technology, easy to operate and suitable for industrial production, and long-circulating vinflunine liposomes prepared by using the method are high in encapsulation rate and good in stability.
Owner:QILU PHARMA CO LTD
Termite-resistant adhesive used for polyethylene pipeline, adhesive tape and preparation of adhesive tape
ActiveCN104559839AEasy to produceLow priceMineral oil hydrocarbon copolymer adhesivesNon-macromolecular adhesive additivesEnvironmental effectAdhesive
The invention provides a termite-resistant adhesive used for a polyethylene pipeline, an adhesive tape and a preparation of the adhesive tape. The termite-resistant adhesive comprises the following components by mass percent: 5-10% of thermoplastic elastomer, 15-25% of amorphous state alpha-olefin copolymer, 15-25% of middle-molecular polyisobutene, 10-15% of tackifying resin, 30-45% of inorganic enhanced powder, 1-2% of an antioxygen, 1-2% of black color master batch and 1-2% of a termite-resistant agent. The adhesive tape containing the termite-resistant adhesive is excellent in termite-resistant effect, slow in pesticide effect release, not easy to degrade, small in impact on environment, durable in pesticide effect and environmentally friendly, also has good adhesive sealing properties, can realize long-acting sealing protection performance in the process of protecting the pipeline to prevent insects, can achieve good termite-resistant effect so long as the adhesive tape is wound and coated on the surface of a protected object when being used and can be widely applied to termite resistance of a PE gas pipeline and various electric wires.
Owner:CYG CHANGTONG NEW MATERIAL +1
Chinese and western combine compound uterus perfusate for treating cow endometritis
InactiveCN101310734AReduce dosageStrong bioadhesionAntibacterial agentsTetracycline active ingredientsDiseasePregnancy
The invention discloses compound intrauterine infusion liquid of western medicine and Chinese traditional medicine for treating cow endometritis and a preparation method thereof, the infusion liquid takes oxytetracycline, motherwort herb, peach seed, Chinese angelica, Szechuan lovage rhizome, membranous milkvetch root, liquoric root and a plurality of excipients as raw materials and is prepared by decoction, filtration, concentration, pumping filtration, concentration, spray drying, micro-powderization of oxytetracycline, homogenization and other processes; the infusion liquid has the advantages that firstly, the raw materials constitute the safe, effective and comprehensive infusion liquid which can not only realize the rapid anti-inflammation, but can also restore the functions of the body with the diseases after the inflammation, promote the pregnancy and treat the infertility; secondly, the infusion liquid adopts the formulation of microparticle suspension, the unit solvent drug-loaded amount is great, the drug delivery volume is small, the chemical properties of the drug microparticles are stable, the bioadhesion is stronger, the drug release is sustained, the infusion liquid can be adhered on the cow uterine endometrium for a long time, thus directly stimulating a uterine receptor, ensuring the drug to be fully absorbed as well as better and rapidly playing the roles.
Owner:史义林 +1
Chinese medicinal gel formulation and its preparing process
Disclosed is a Chinese medicinal gelling agent which comprises, 7.5-12.5 wt% of mentha-camphor, 5.5-8.5 wt% of curcuma longa pieces, 5.5-8.5 wt% of dahurian angelica root pieces, 2.5-4.4 wt% of pollen pieces, 2.5-4.4 wt% of red peony root pieces, 2-4 wt% of nitrogen ketone, 1.5-3 wt% of Carbomer, 0.3-0.5 wt% of polysorbate, 0.1-0.3 wt% of triethanolamine and balancing water. The invention also discloses the preparing process.
Owner:WUHAN UNIV
Traditional Chinese medicine microecological preparation and preparation method thereof
The invention discloses a traditional Chinese medicine microecological preparation and a preparation method thereof. The traditional Chinese medicine microecological preparation is prepared by adopting traditional Chinese medicine ultra-fine powder as the main ingredient of fermentation culture media, adopting bacillus coagulans as the producing strain and adopting the fermentation technology. The traditional Chinese medicine ultra-fine powder comprises the composition of 20-60 parts of milkvetch roots, 20-40 parts of glossy privet fruits and 20-40 parts of epimedium herb with the grain size of 1-60 micrometers. The preparation method comprises the steps of strain separation and purification, inclined face activation, seed solution preparation, fermentation and culture, ingredient extraction, granulation forming and the like. The traditional Chinese medicine microecological preparation and the preparation method thereof have the advantages that the medicine effect is good, the extraction rate is high, and the medicinal material utilization rate is high.
Owner:GUANGDONG RONGDA BIOENG CO LTD
Blank high-molecular microspheres and preparation method thereof
InactiveCN106474071AGood compatibilityAvoid rapid degradationGranular deliveryMacromolecular non-active ingredientsControlled releaseMicrosphere
The invention provides blank high-molecular microspheres prepared by a spray drying method. With use of the instant drying ability of spray drying and the excellent dissolving ability of dichloromethane and the like, a high-molecular material is made into the microspheres. Through the characterization observation and particle size determination of the microspheres, the diameter size and surface appearance of a slow-release carrier support material are known, and the diameter of the blank high-molecular microspheres is in line with the size for injection. Compared with other microsphere preparation methods, the spray drying method is simple, the repetition rate is high, and the structure of the microspheres is clear. Animal in-vivo pharmacokinetics and in-vitro release degree evaluation show that the microsphere carrier is used as a good sustained-release carrier for different treatment drugs in the field of drug sustained or controlled release.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Liraglutide multivesicular liposome and preparation method and application thereof
ActiveCN110339166AAvoid problems prone to degradationHigh encapsulation efficiencyPeptide/protein ingredientsMetabolism disorderMedication costSide effect
The invention relates to the field of medicines, and particularly relates to a liraglutide multivesicular liposome and a preparation method and an application thereof. The liraglutide multivesicular liposome provided by the invention comprises liraglutide, a membrane material, an osmotic pressure regulator and a stabilizer. The liraglutide multivesicular liposome prepared by the invention has goodstability, high drug encapsulation rate, large drug loading capacity, slow and steady drug release rate and no burst release phenomenon, significantly improves the bioavailability of the drug, thereby improving the curative effect, reduces dose-related side effects of the drug and medication cost and has a higher application value. Experiments shows that the liposome provided by the present invention can continuously release the drug in vitro for about 432 hours, and provide a stable blood concentration in vivo, significantly prolongs in-vivo retention time compared to other injections, showsobvious pharmacokinetic characteristics of a sustained release preparation, can provide a normal steady blood glucose level, plays hypoglycemic effect for 312 hours, and has the relative bioavailability of 661% to injections.
Owner:SHENYANG PHARMA UNIVERSITY
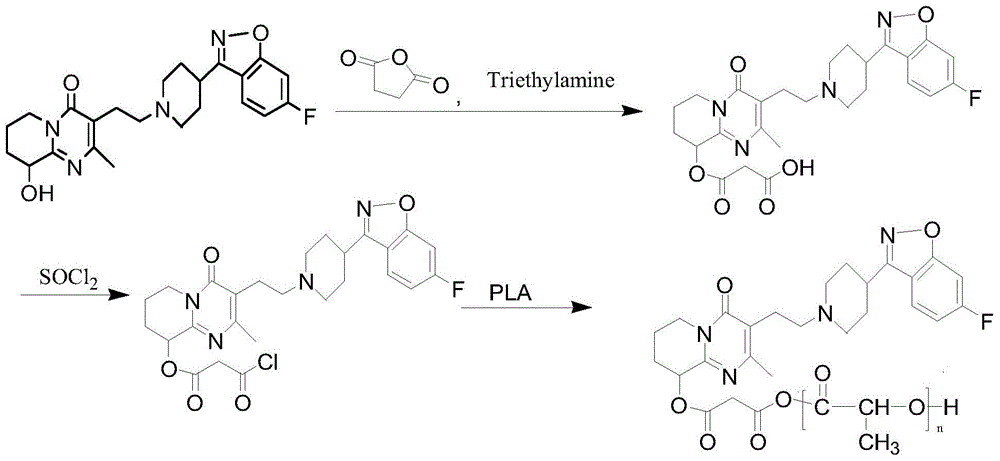
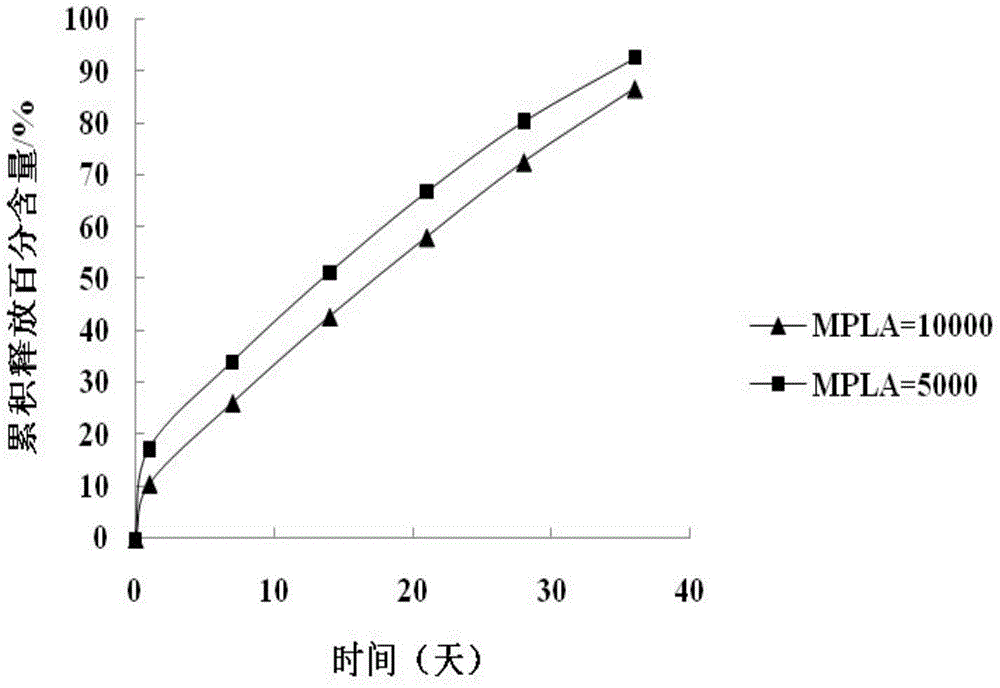
Preparation method of paliperidone sustained release microsphere injection
InactiveCN104940150AImprove toleranceStable blood concentrationPowder deliveryOrganic active ingredientsMicrosphereDrug release
The invention discloses a preparation method of a paliperidone sustained release microsphere injection. The preparation technology comprises the following steps: (1) paliperidone and an acid anhydrides reagent are respectively dissolved in a solvent to be subjected to esterification reaction under the effect of an alkaline catalyst; (2) the esterification product obtained in the step (1) is dissolved in anhydrous dichloromethane to be subjected to acylation reaction with the added acylation reagent with DMF (N, N- dimethylformamide) being the catalyst; (3) the acylated paliperidone is subjected to esterification reaction with polylactic acid (PLA) through chemical bond bonding; (4) paliperidone sustained release microspheres are prepared by adopting solvent evaporation method, and the paliperidone sustained release microspheres are prepared into the microsphere injection after being cooled and dried. The preparation method is safe to operate, mild in reaction condition and simple and clear in steps; the total yield reaches 50% or above; the particle sizes of the obtained paliperidone microspheres are 30-50 micron; the paliperidone sustained release microsphere injection provided by the invention is high in drug loading capacity, stable in drug release, long in drug release time and good in sustained release effect.
Owner:HEILONGJIANG UNIV
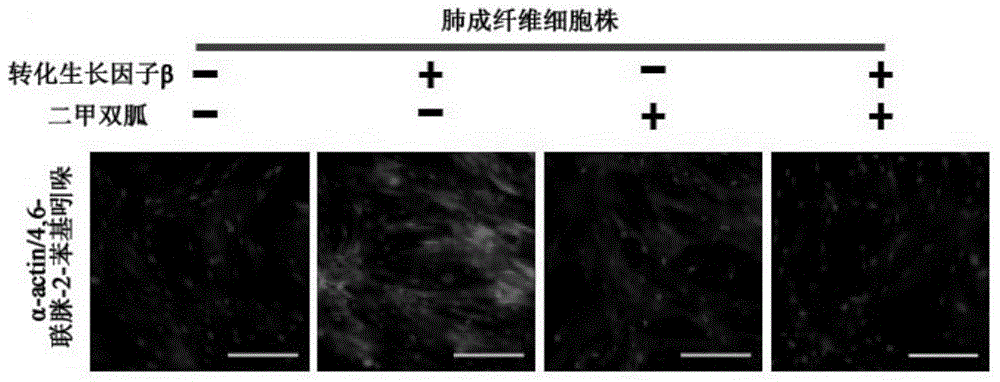
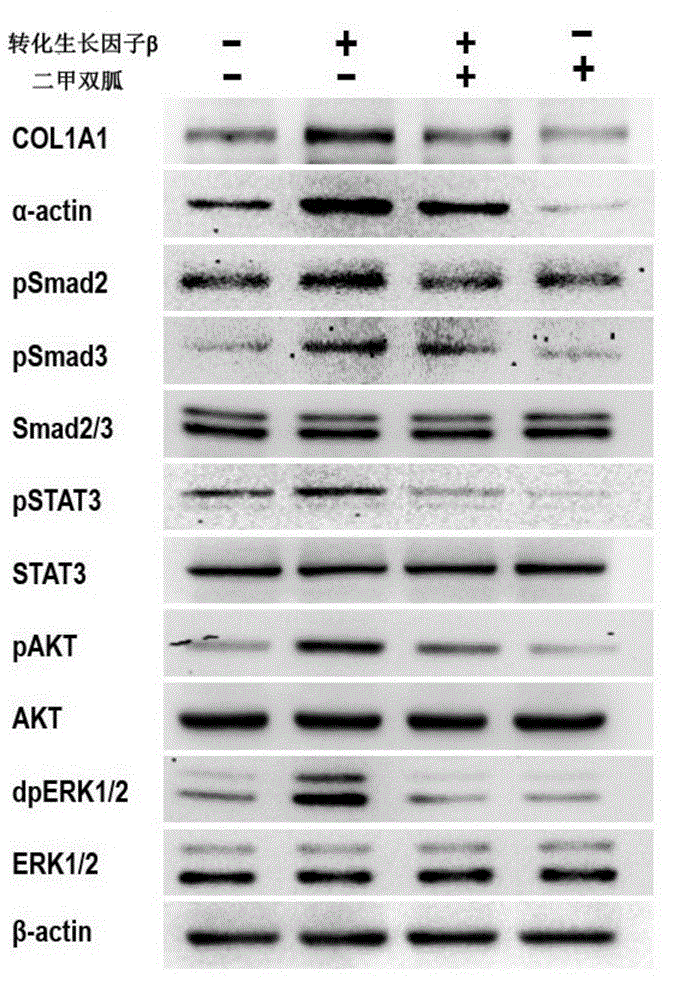
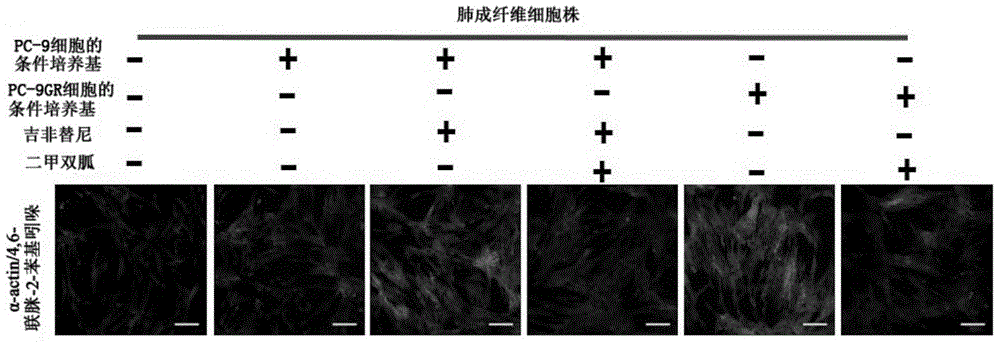
Application of biguanide drugs in preparation of drugs for preventing or weakening pulmonary fibrosis
InactiveCN106138020APrevent or slow downPrevent or slow the developmentOrganic active ingredientsRespiratory disorderTherapeutic effectNon small cell
The invention relates to new application of biguanide drugs, in particular to application of biguanide drugs in preparation of drugs for preventing or weakening pulmonary fibrosis. The pulmonary fibrosis is complications produced by EGFR-TKI or ALK-TKI in treatment of non-small cell lung cancers. The biguanide drugs can avoid the complications produced by the EGFR-TKI or ALK-TKI in treatment of non-small cell lung cancers, effectively improve an EGFR-TKI or ALK-TKI treatment effect and reduce occurrence and development of patients' complications.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Application of long effective curcumin derivative in preparing anti-depression drug
ActiveCN101669932AImprove stabilityImprove bioavailabilityNervous disorderKetone active ingredientsProlongationDrug effect
The invention relates to an application of a long effective curcumin derivative in preparing an anti-depression drug. The long effective curcumin derivative is an ester which is formed in such a way that C1 to C50 saturated or unsaturated fatty acids or acyls thereof react with hydroxyl groups of curcumin substances by esterification respectively and independently. Through the research on the anti-depression action of various animal models, the long effective curcumin derivative can reserve the inherent anti-depression drug effect of the curcumin and also has the effects of the slow release and the prolongation of the drug effect and a favorable prospect of clinical application. The long effective curcumin derivative can be also used for preparing anti-depression drinks, foods, food addiitives or health products.
Owner:北京鼎国昌盛生物技术有限责任公司
Medical povidone-iodine dressing and preparation method thereof
ActiveCN104474570ASlow drug releaseImprove the bactericidal effectAbsorbent padsBandagesWound healingPlasticizer
The invention provides a medical povidone-iodine dressing and a preparation method thereof. The dressing comprises the following components: a styrene-isoprene segmented copolymer, povidone-iodine, ethylcellulose, chitosan, a tackifier, a plasticizer, an anti-aging agent and water. The preparation method comprises the following steps: weighing the components; melting the styrene-isoprene segmented copolymer and ethylcellulose in a reaction kettle, and adding chitosan, the tackifier, the plasticizer and the anti-aging agent after the styrene-isoprene segmented copolymer and ethylcellulose are fully melted, and insulating; dissolving povidone-iodine in water, slowly adding the mixture into the povidone-iodine aqueous solution, uniformly stirring and vacuumizing and defoaming; and pouring the obtained product in a mould, freezing and standing at room temperature to obtain the dressing. The medical povidone-iodine dressing provided by the invention not only can be used for maintaining a moist environment required by wound healing, but also can be used for alleviating the efficacy release of povidone-iodine, sterilizes for a long time and has a broad application value.
Owner:GUANGZHOU CHUANGSAI BIOLOGICAL MEDICAL MATERIALS CO LTD
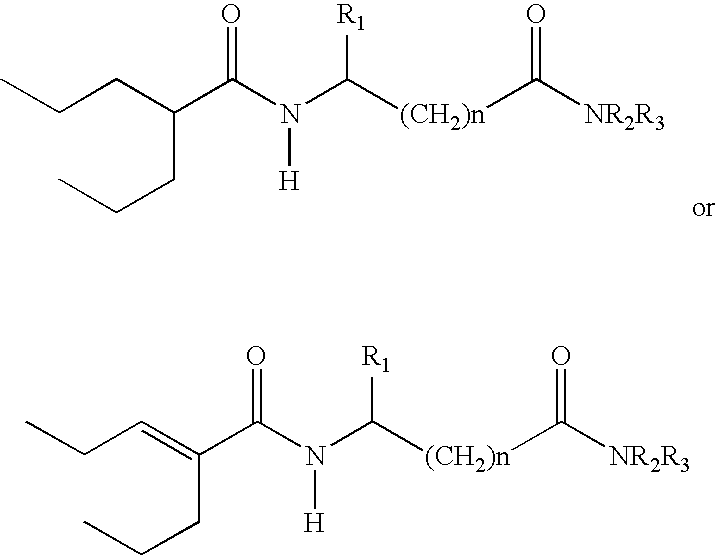
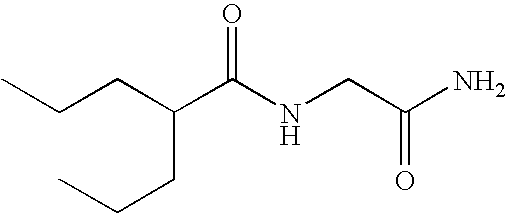
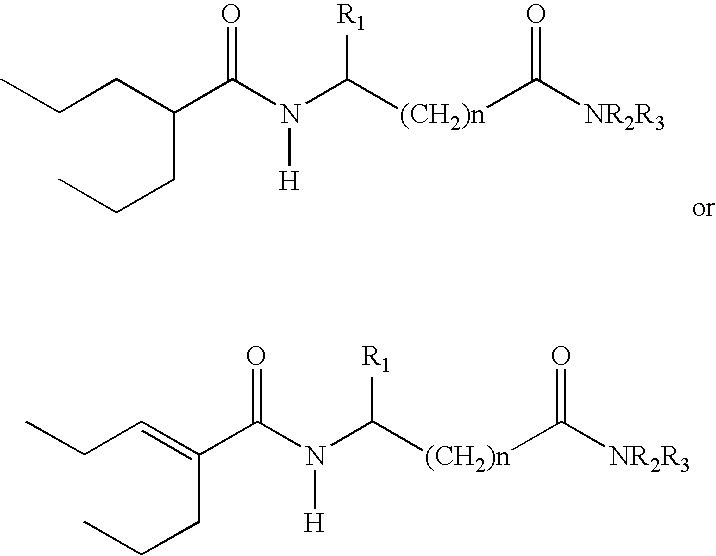
Sustained release formulation of N- (2-propylpentanoyl) glycinamide and related compounds
InactiveUS20040175423A1Maximum sustained actionHigh viscosityBiocidePill deliveryBULK ACTIVE INGREDIENTSustained Release Formulations
The subject provides a sustained release tablet comprising the following components: a) a uniform admixture of an active ingredient selected from the group consisting of valproic sodium acid, a pharmaceutically acceptable salt or ester of valproic acid, divalproex sodium, valpromide and a compound having the structure: wherein R1, R2, and R3 are independently the same or different and are hydrogen, a C1-C6 alkyl group, an aralkyl group, or an aryl group, and n is an integer which is greater than or equal to 0 and less than or equal to 3; and a binder, and b) a hydroxypropylmethyl cellulose, a process for manufacturing the tablet and a method of treating neuropathic pain, epilepsy, mania in bipolar disorder, a headache disorder, pain or of effecting pain prophylaxis in a subject.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Compound botanical fungiticide and preparation method thereof
InactiveCN110150330AHigh bactericidal activitySlow drug releaseBiocideFungicidesNicotiana tabacumAntioxidant
The invention discloses a compound botanical fungiticide, which comprises the following raw materials by mass percentage: 5%-30% of natural capsaicin, 5%-25% of ageratina adenophora extract, 1%-15% ofphytolacca acinosa extract, 1%-10% of gynostemma pentaphyllum extract, 2.5%-15% of an emulsifier, 3%-10%of a surfactant, 1%-5% of a synergist, 0.5%-5% of an antioxidant, and the balance deionized water. The invention has the beneficial effects that: the compound botanical fungiticide provided by the invention can be applied to control of common diseases in fruits, vegetables, flowers and the like, and the specific diseases to be controlled include: anthracnose, brown patch, black spot and the like in mangos, pears and apples; anthracnose, black spot, botrytis cinereal and the like in Chinesecabbage, tomatoes, cucumber and the like; root rot, late blight, anthracnose and the like in panax notoginseng, chili and tobacco.
Owner:云南宏绿辣素有限公司
Disintegrant-free delayed release doxylamine and pyridoxine formulation and process of manufacturing
InactiveUS20140335176A1Inhibition of dissolutionLess expensiveBiocideAnimal repellantsVomitingPharmaceutical preservatives
The present invention relates to a delayed release pharmaceutical composition containing doxylamine succinate and pyridoxine HCl for treatment of nausea and vomiting during pregnancy. More specifically, the present invention concerns a disintegrant-free delayed release pharmaceutical composition for oral administration comprising a core and an enteric coating, wherein said core comprising: a) at least one pharmaceutically active ingredient, and b) at least one pharmaceutically acceptable excipient, wherein said composition provides an in vitro drug release profile of about 80% of active ingredient dissolved within 20 minutes as measured by USP Type II apparatus and also a manufacturing process of said pharmaceutical composition.
Owner:PHARMASCIENCE INC
Liposome nanometer carrier situ gel preparation used for eye epidermal growth factor
InactiveCN101057966BGood physical and chemical stabilityReduce exposureSenses disorderPeptide/protein ingredientsGel preparationPhospholipid
The invention relates to a liposome nano carrier in-situ gelling preparation for eyes containing epidermal growth factor, which is prepared from epidermal growth factor, lipid material, emulsifying agent, thickening agent, isotonic conditioning agent, bacteria inhibitor, pH regulator and purified water, wherein non-irritating phosphatide is employed as the emulsifying agent, macromolecular material having ion and / or pH responsive characteristics is used as the thickening agent.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Baicalin liposome ointment and preparation method thereof
ActiveCN110227062APromote transdermal absorptionOvercome the disadvantage of poor solubilityOrganic active ingredientsNervous disorderMedicineCholesterol
The invention discloses a baicalin liposome ointment and a preparation method thereof. The ointment is prepared from baicalin liposome, azone and a matrix through grinding; and the components of the ointment include soya bean lecithin, cholesterol, baicalin, vitamin E, the azone and carboxymethylcellulose sodium. The ointment can promote the transdermal absorption of the baicalin; the disadvantages of poor dissolvability of the baicalin can be overcome; the bioavailability of the baicalin can be enhanced; and the ointment is slow in drug release speed and long in drug effect and has a certainslow release effect.
Owner:QUANZHOU NORMAL UNIV
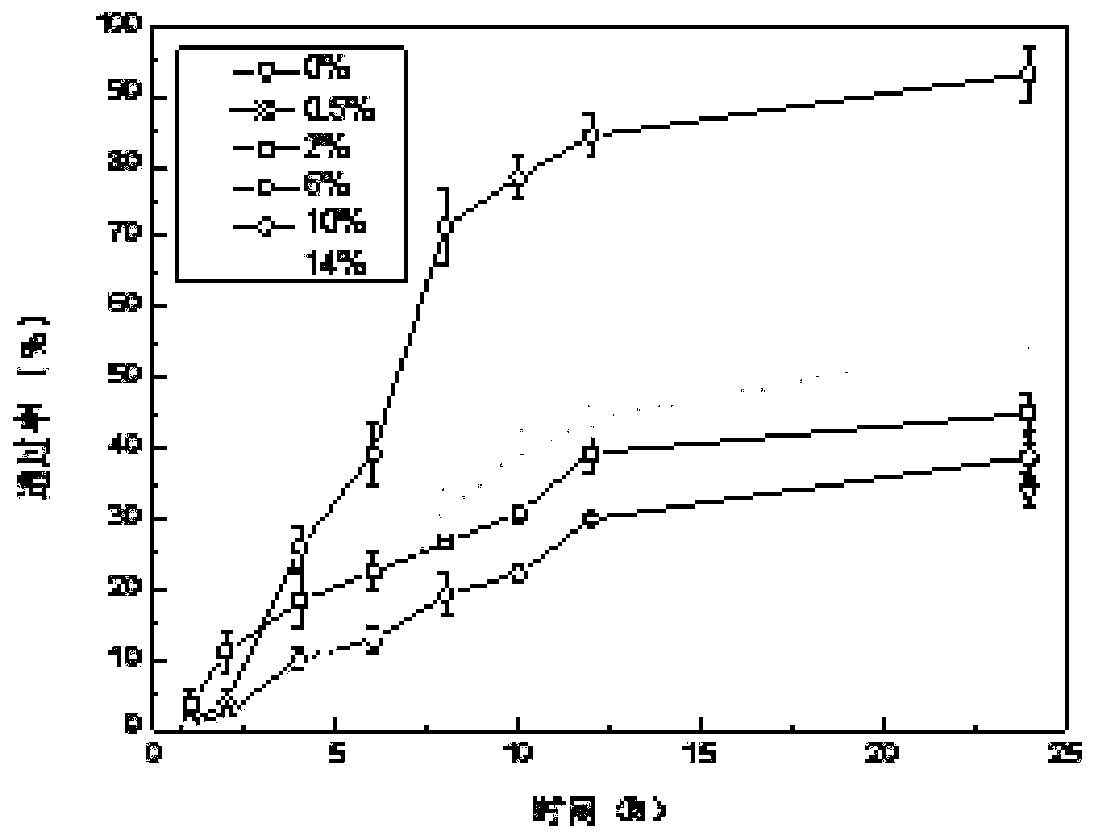
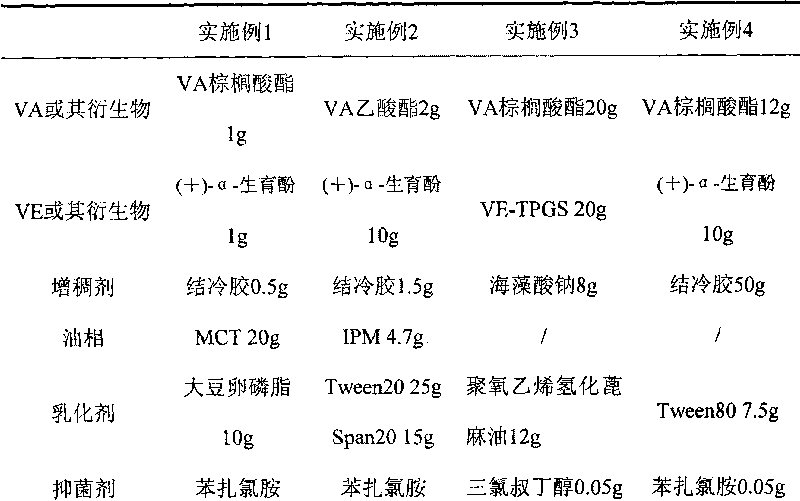
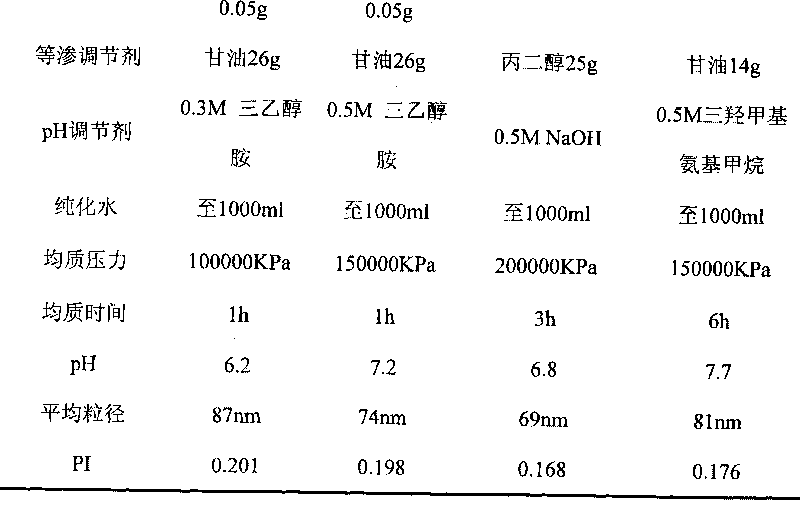
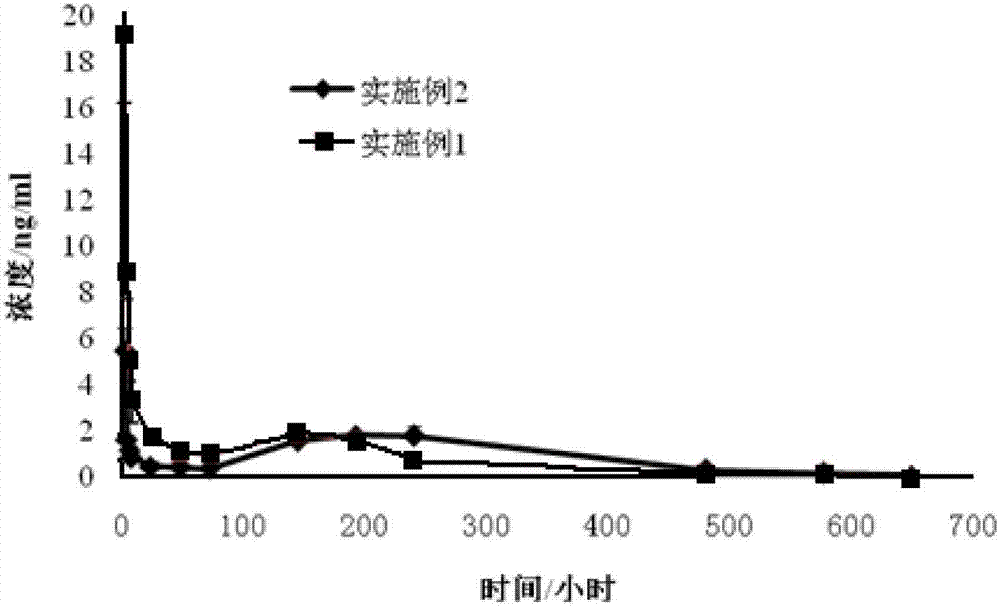
Micro-emulsion/submicro-emulsion in-situ gel preparation for eyes and its making method
InactiveCN101028240BNon-irritatingImprove comfortSenses disorderHydroxy compound active ingredientsGel preparationPharmaceutical drug
An in-situ microemulsion / sub-microemulsion gel for treating eye disease is prepared from VA or its derivative, VE or its derivative, oil phase, emulsifier, thickening agent, isotonic regulator, antibacterial agent, pH regulator and purified water through shearing-high-pressure homogenizing process. It has high flowability. If it is dropped in eye, it can become hydrogel.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
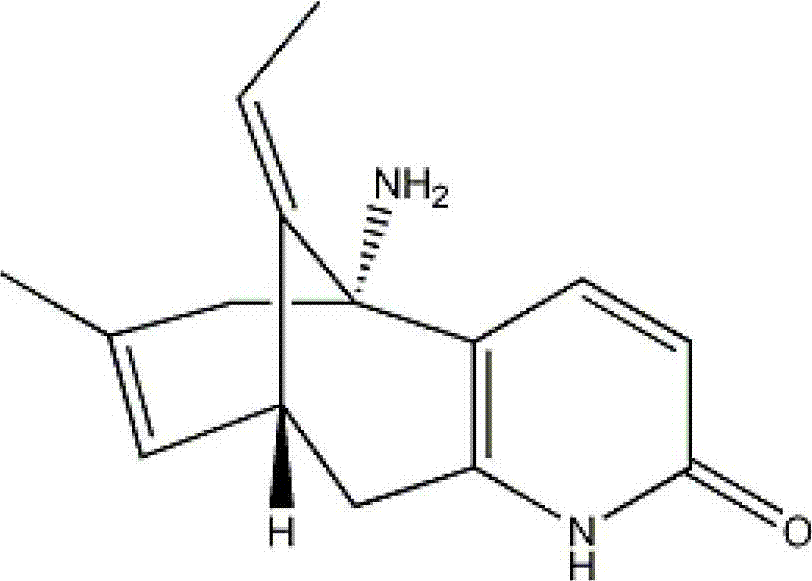
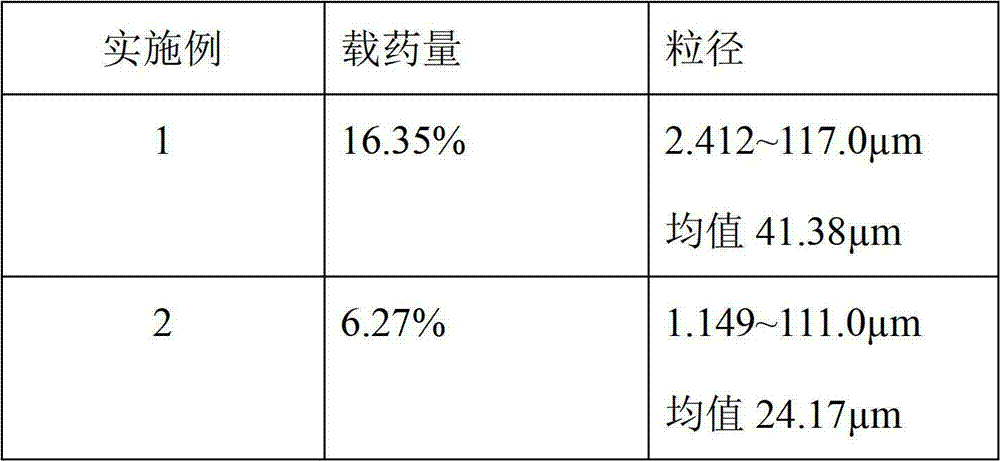
Huperzine-A particle long-acting injection and preparation method thereof
ActiveCN103505413BReduce voidsSlow drug releaseOrganic active ingredientsNervous disorderSide effectBlood concentration
The invention discloses a huperzine-A particle long-acting injection and a preparation method thereof. The huperzine-A particle long-acting injection is composed of huperzine-A and a biodegradable polymer, wherein the percentage by weight of huperzine-A is 9-30%. An emulsification step is not required in the preparation method disclosed by the invention, and the preparation method is simple in preparation process and low in requirements on equipment. The prepared huperzine-A microspheres have not obvious burst release effect and can release medicine for 30 days, thus avoiding the side effects caused by a blood concentration peak-valley phenomenon occurring in case of multiple dosing; a curative effect can be kept for half a month to a month by one-time dosing, thus being beneficial to treatment for patients with dementia; moreover, the huperzine-A microspheres has the characteristic of convenience in dosing.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com