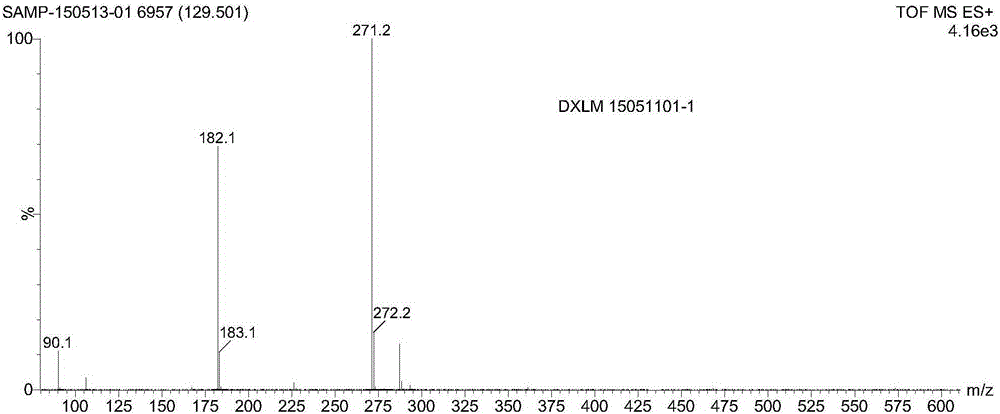
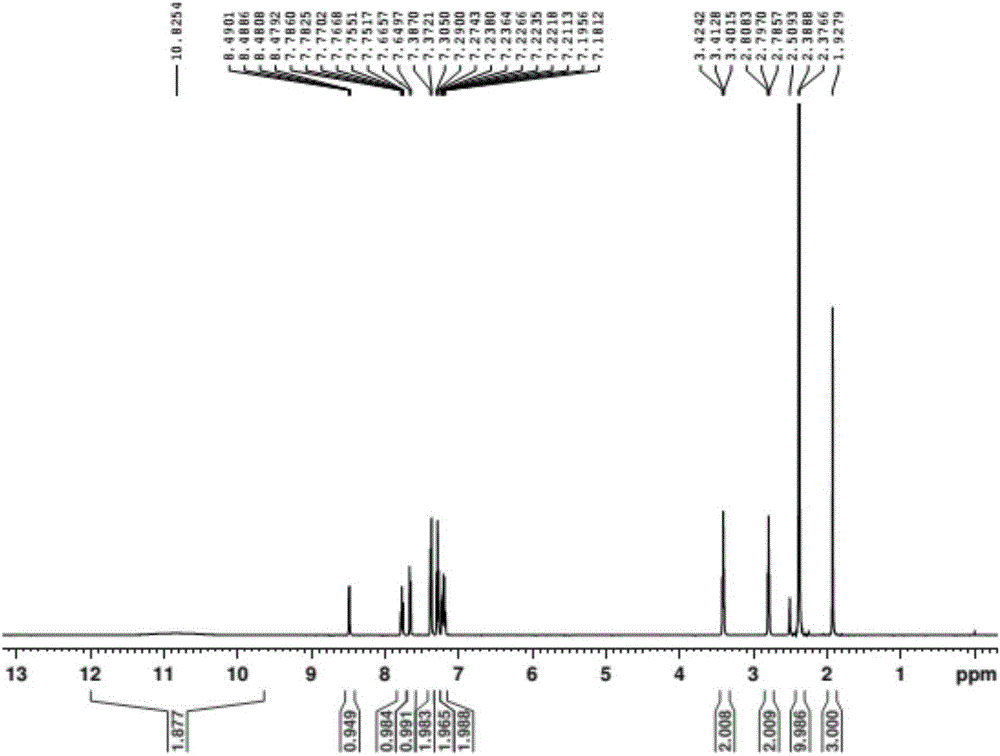
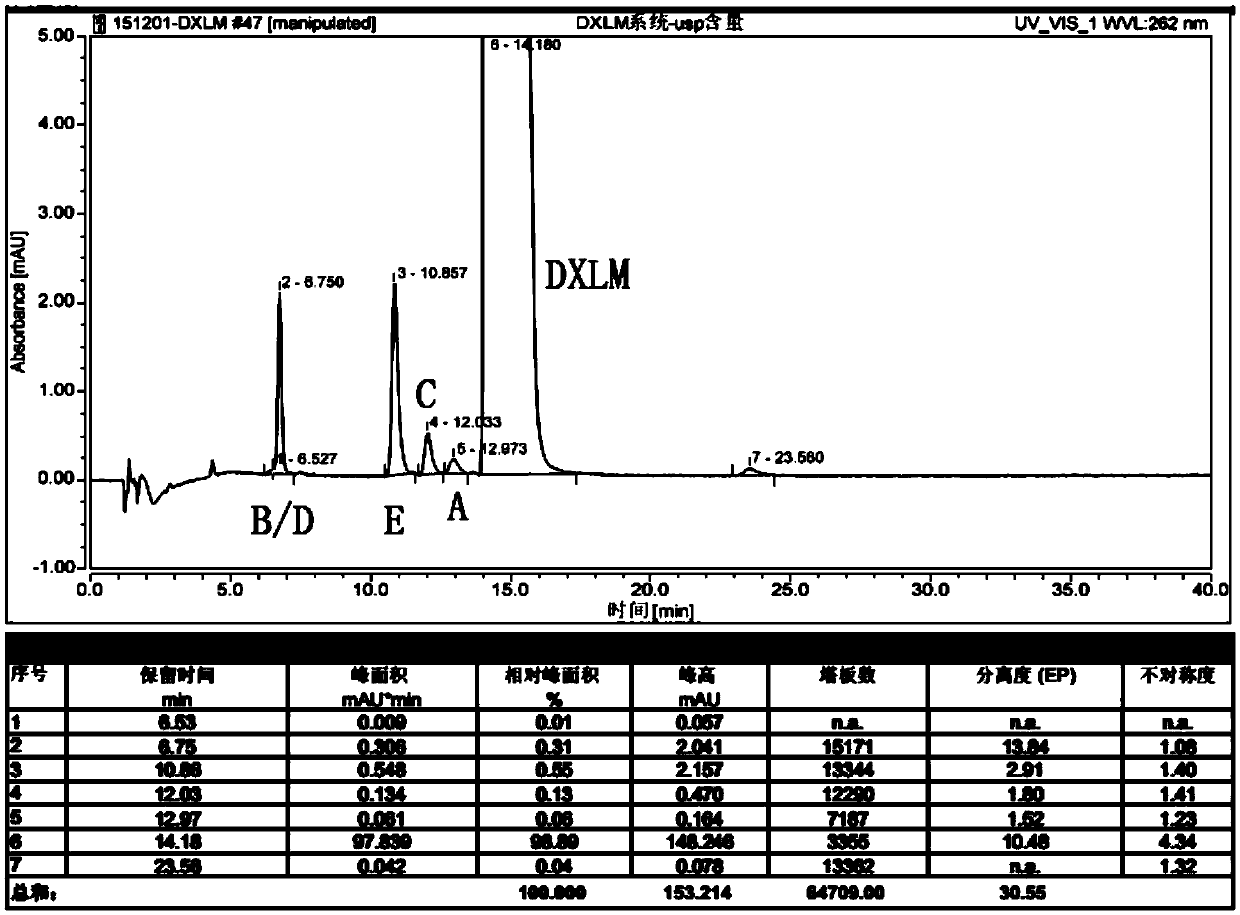
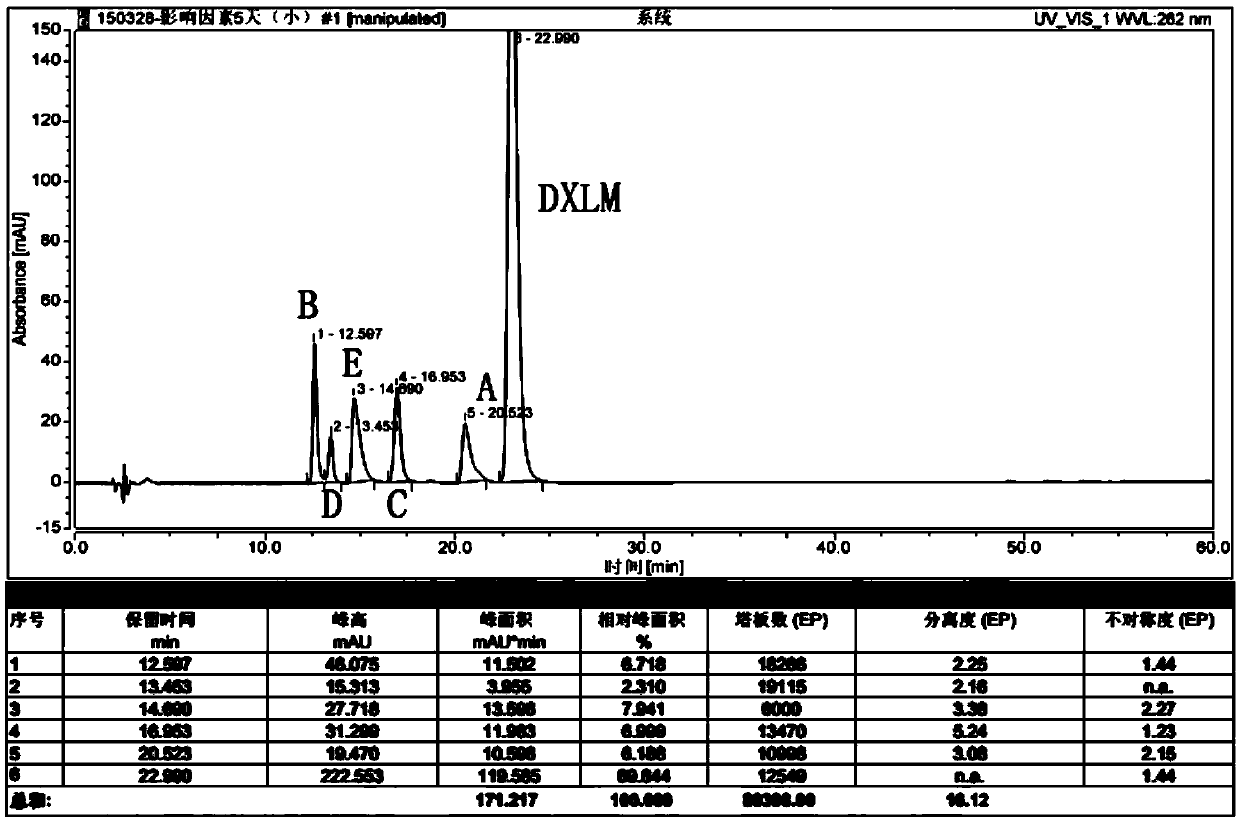
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Doxylamine Succinate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
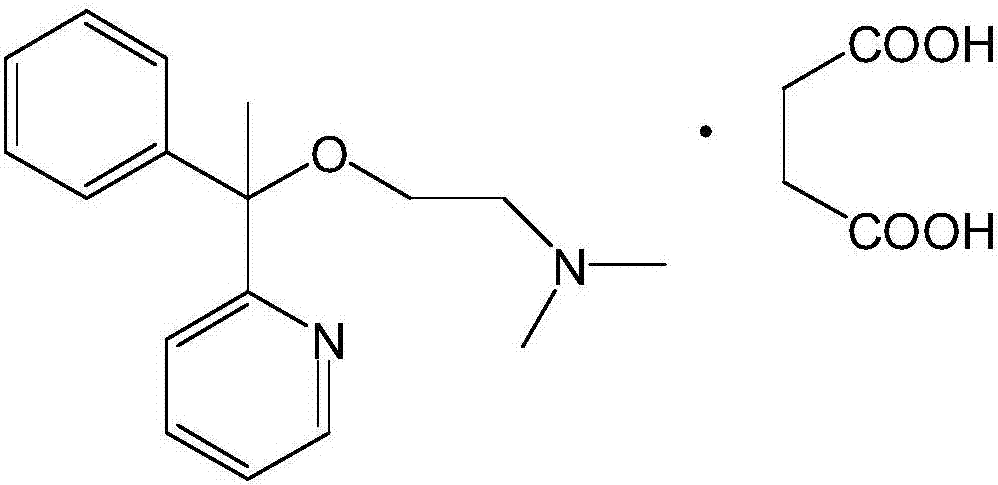
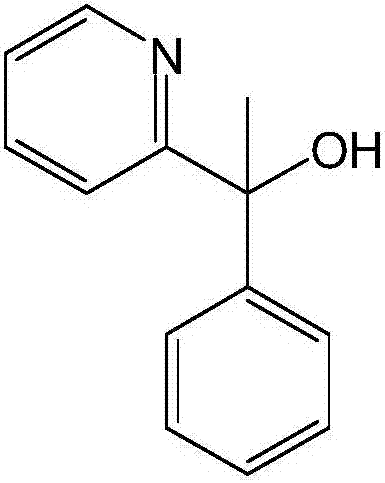
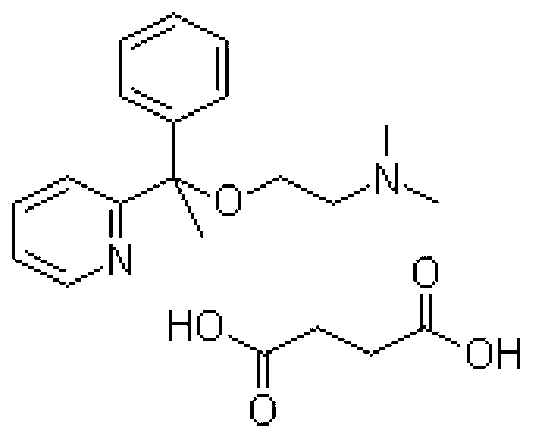
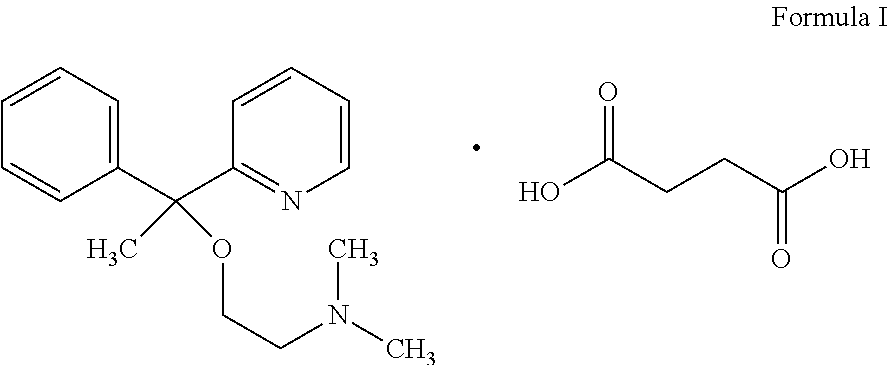
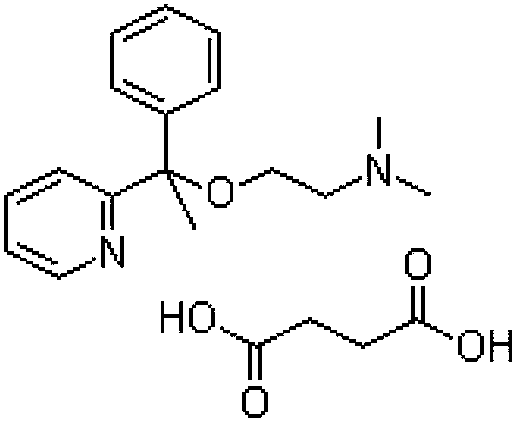
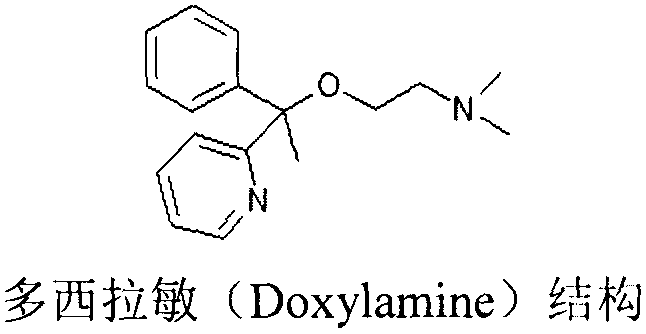
A pyridine derivate histamine H1 antagonist with pronounced sedative properties. Doxylamine succinate competitively blocks the histamine H1 receptor and limits the typical allergic and anaphylactic responses, including bronchoconstriction, vasodilation, increased capillary permeability, and spasmodic contraction of gastrointestinal smooth muscle, caused by actions of histamine on bronchial and gastrointestinal smooth muscles, and on capillaries. This drug also prevents histamine-induced pain and itching of the skin and mucous membranes.
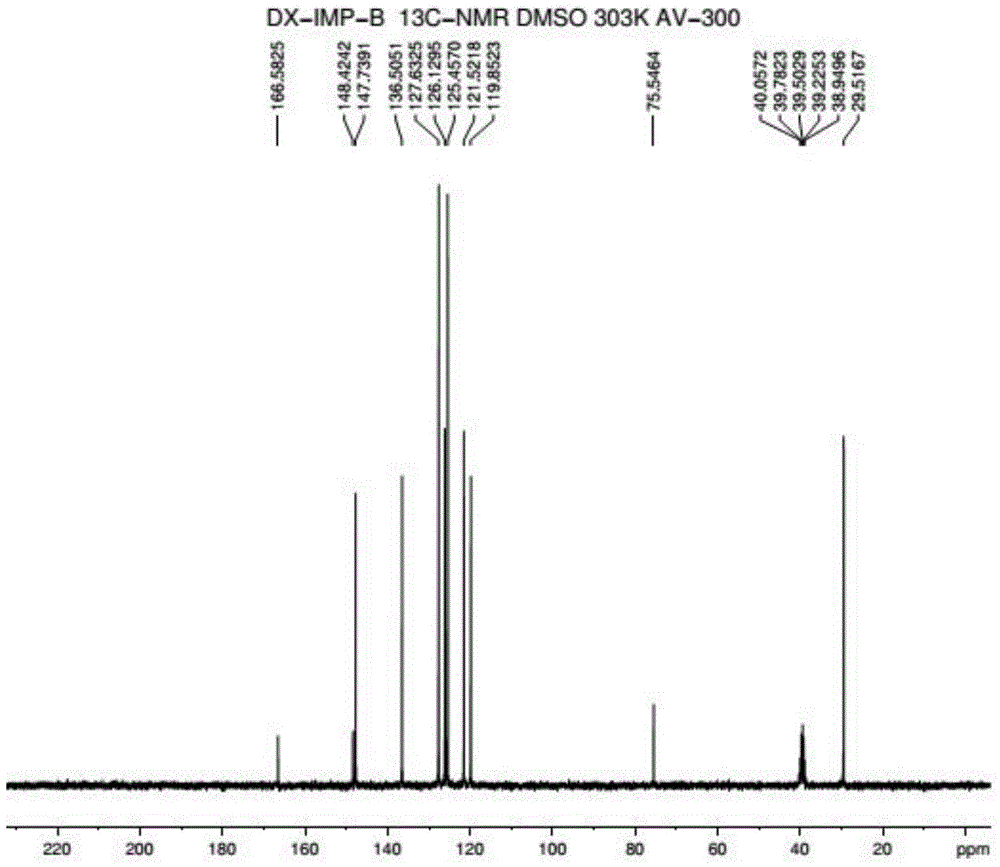
Method for synthesizing doxylamine succinate
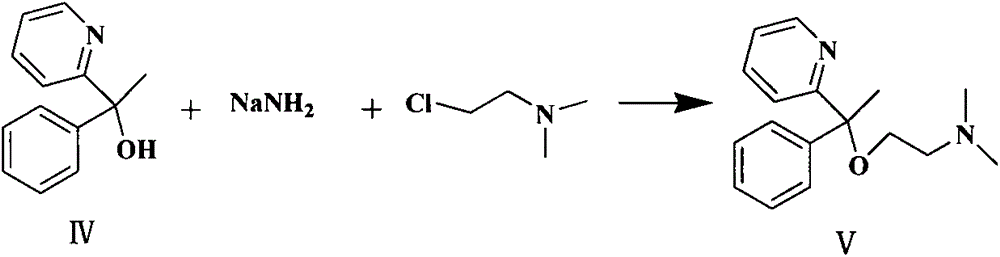
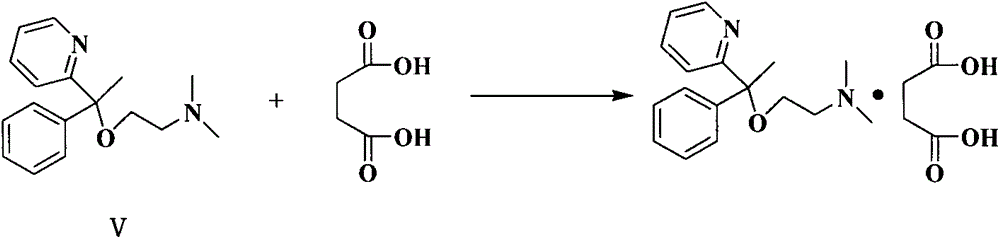
The invention discloses a method for synthesizing doxylamine succinate. 2-acetylpyridine is taken as an initiative material, and the method comprises the following steps of: reacting the 2-acetylpyridine with a Grignard reagent prepared from bromobenzene and magnesium to obtain 2-pyridyl phenyl methyl carbinol; reacting the 2-pyridyl phenyl methyl carbinol with sodium amide and 2-dimethylaminoethyl chloride in turn to obtain doxylamine; and performing salt-forming reaction on the doxylamine and succinic acid to obtain the doxylamine succinate. The synthesis route is orientation reaction without byproduct. The doxylamine succinate is an ethanol antihistamine, and has antihistaminic and cholinolytic effects and obvious tranquillizing effect.
Owner:HEFEI UNIV OF TECH +1
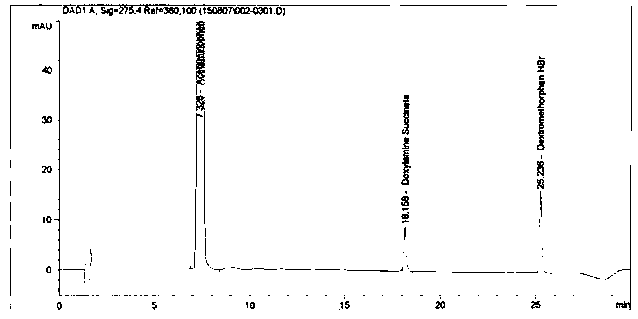
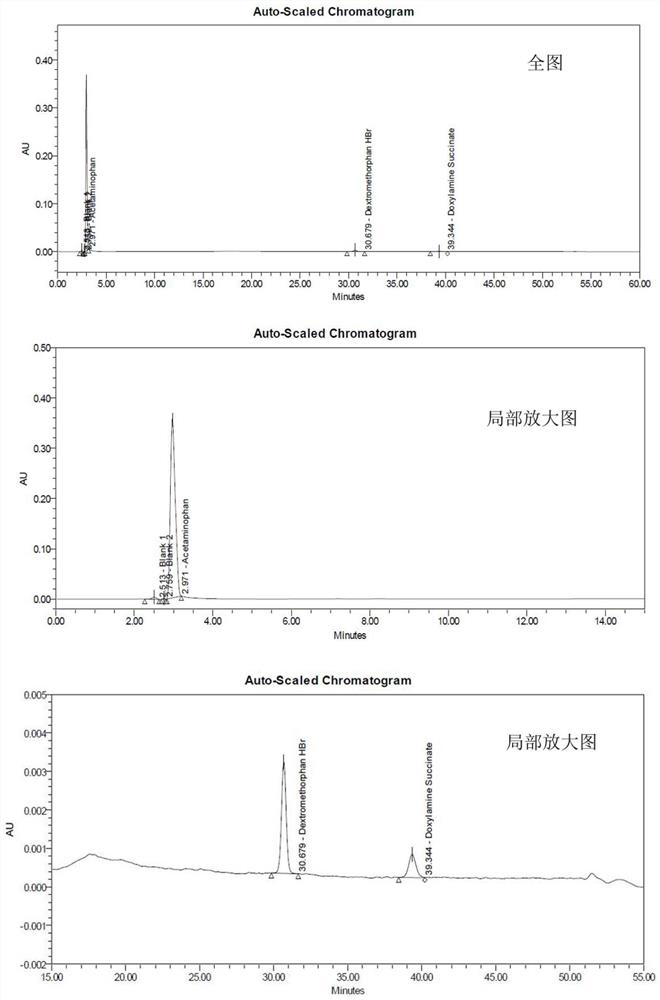
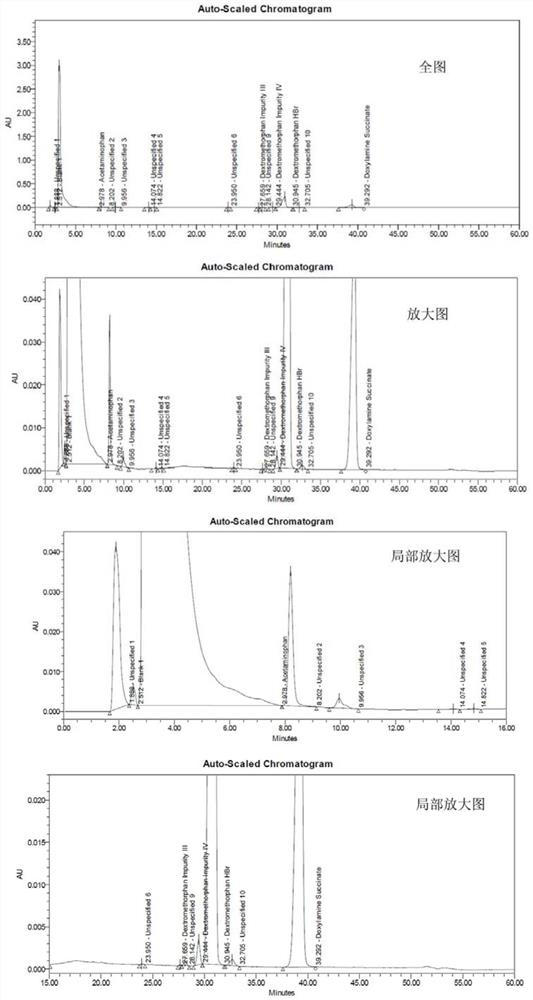
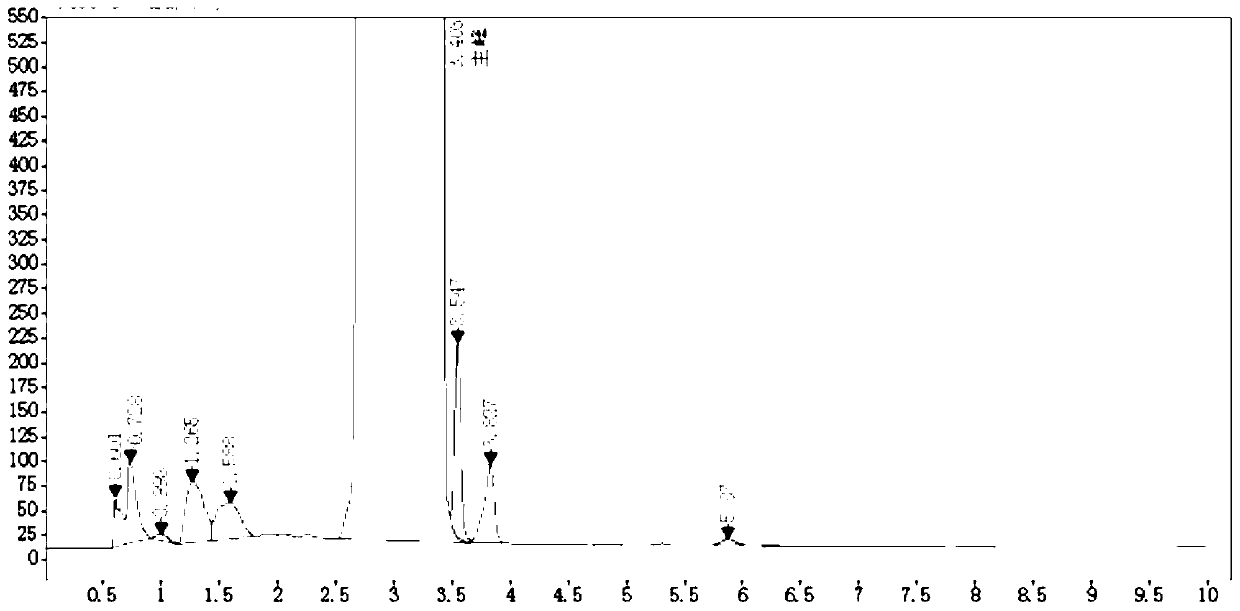
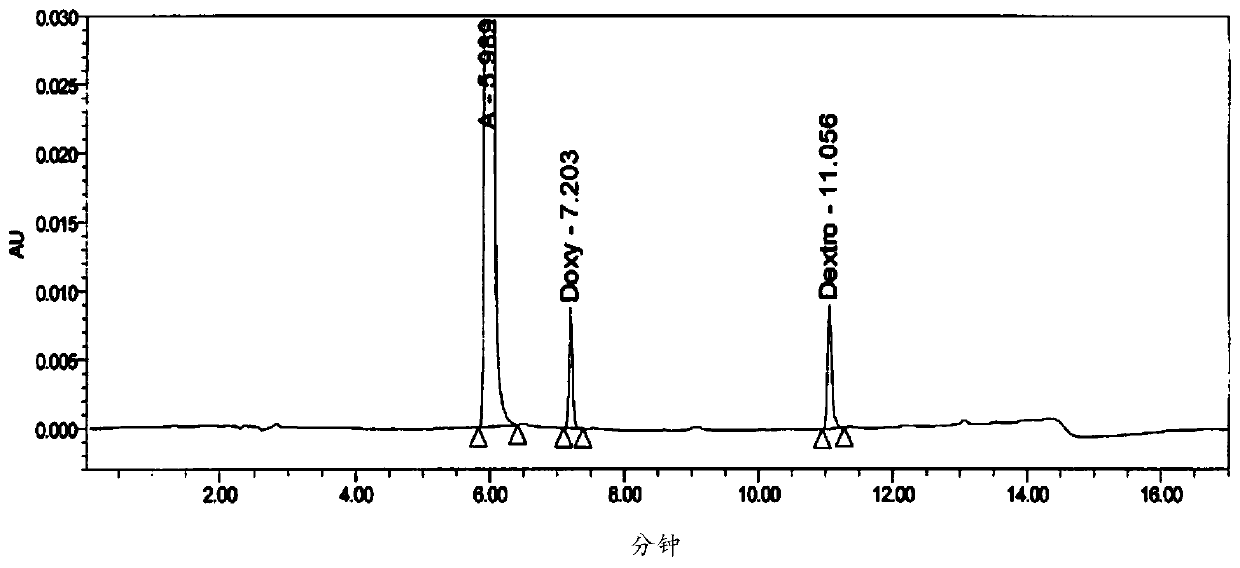
Method for analyzing night cold flu cough allergy capsule by utilizing HPLC (High Performance Liquid Chromatography)
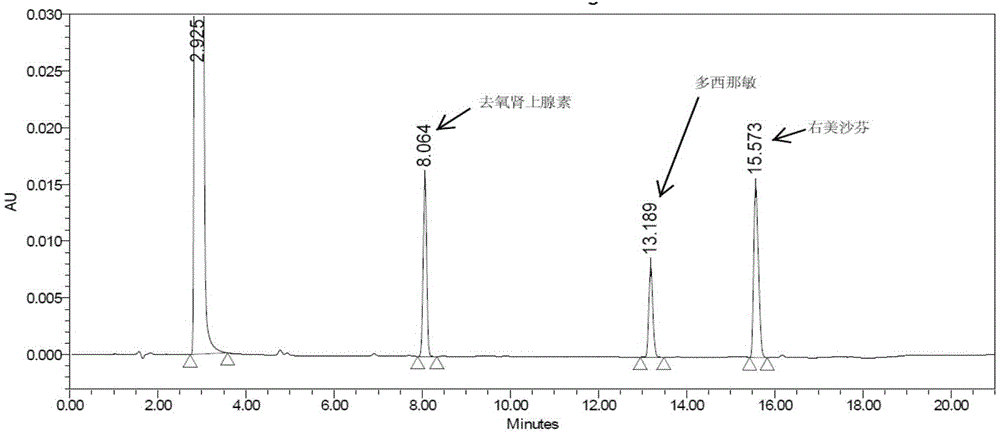
The invention discloses a method for analyzing a night cold flu cough allergy capsule by utilizing HPLC (High Performance Liquid Chromatography). The night cold flu cough allergy capsule contains acetaminophen, phenylephrine hydrochloride, succinic acid doxylamine and dextromethorphan hydrobromide. In the HPLC analysis, an octadecyl silane bonded silica gel column is adopted as a chromatographic column; a sodium 1-octanesulfonate-phosphate buffer solution with the pH of 2.0-3.0 acts as a mobile phase A; acetonitrile and a mixed solution of acetonitrile and methyl alcohol act as a mobile phase B. The method can be simultaneously and effectively used for detecting four effective ingredients in the night cold flu cough allergy capsule, is simple to operate, analyzes rapidly, is good in repeatability, has favorable specificity, and can effectively and comprehensively control the product quality of the night cold flu cough allergy capsule.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Preparation method of doxylamine succinate
The invention discloses a preparation method of doxylamine succinate. The preparation method comprises following steps: step 1, 2-pyridyl phenyl methyl carbinol is dissolved in an organic solvent, and is reacted with 2-dimethylaminoethyl chloride hydrochloride at high temperature, after reaction, target product N,N-dimethyl-2-[1-phenyl-1-(2-pyridine)oxethyl]ethylamine is obtained via quenching extracting separation; and step 2, N,N-dimethyl-2-[1-phenyl-1-(2-pyridine) oxethyl]ethylamine obtained in the step 1 and succinic acid are subjected to salt forming in an organic solvent; and finished product N,N-dimethyl-2-(1-phenyl-1-(2-pyridine)ethoxy)ethanamine succinate (doxylamine succinate) is obtained via cooling crystallization. The preparation method is simple, safe, and reliable, is high in doxylamine succinate yield, and is suitable for industrialized enlarged production; and post-treatment is simple and convenient.
Owner:NANJING GRITPHARMA CO LTD
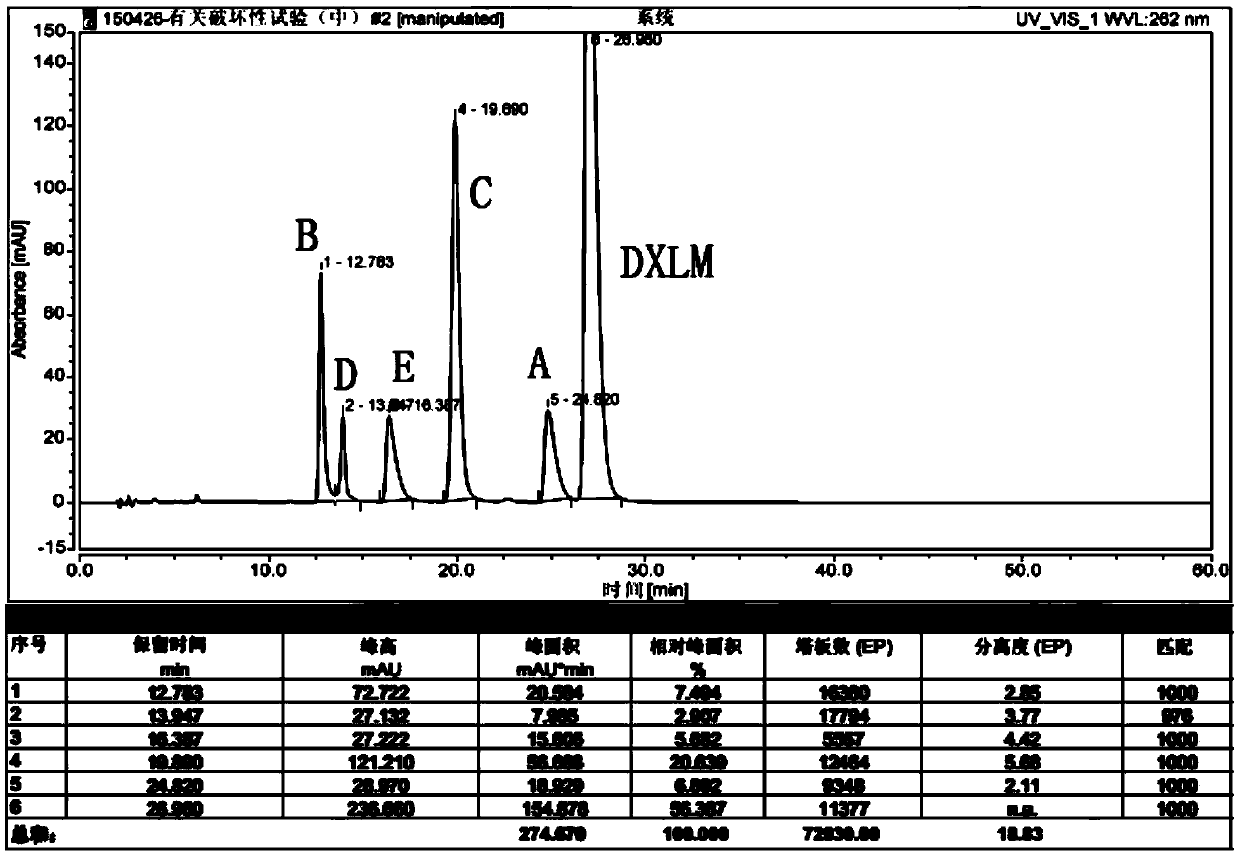
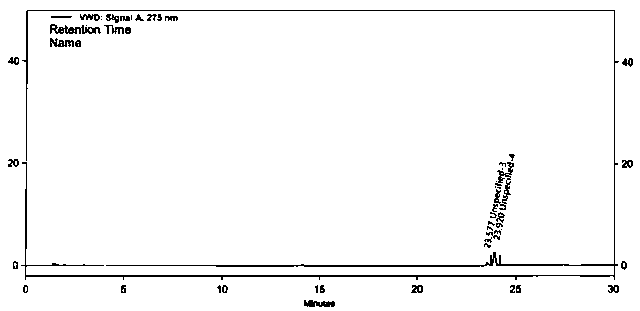
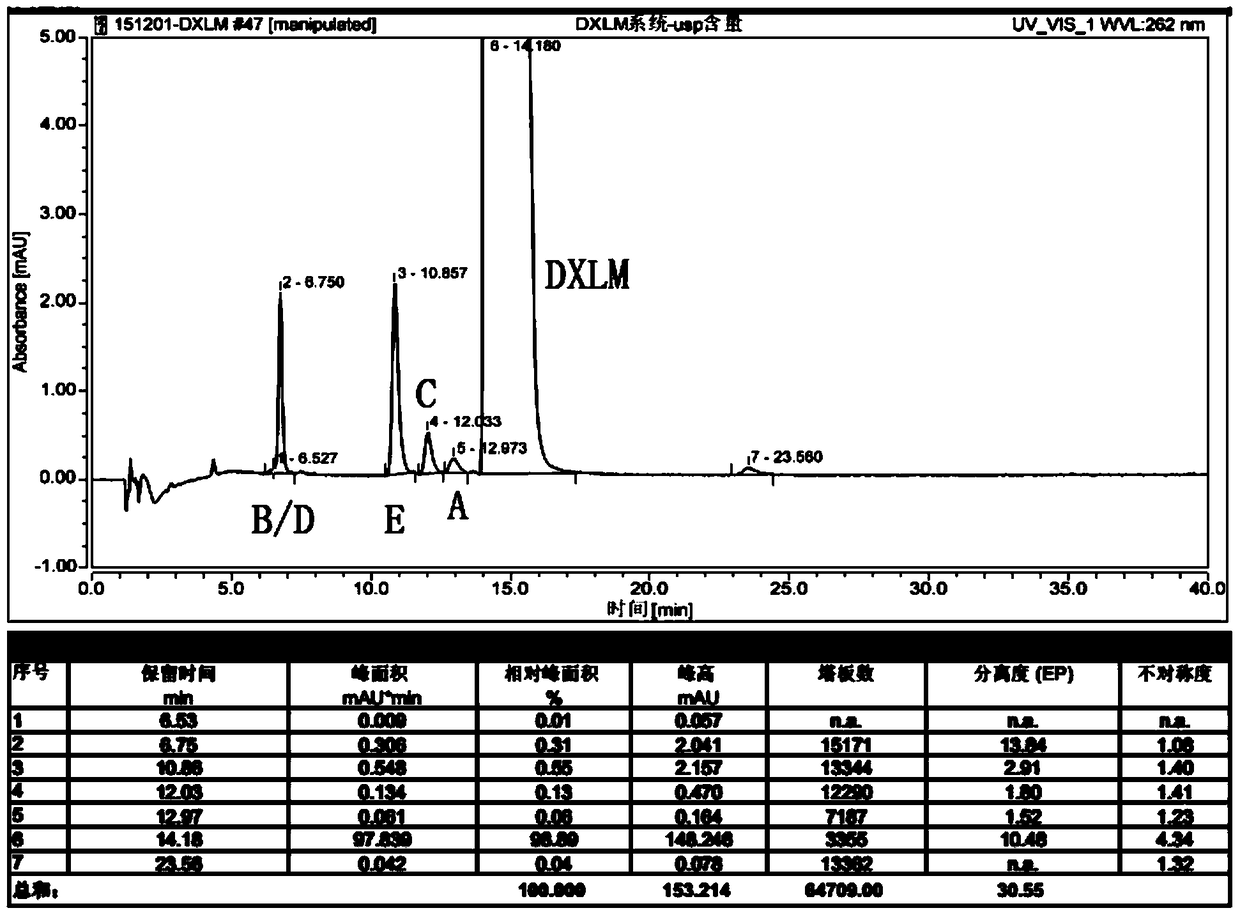
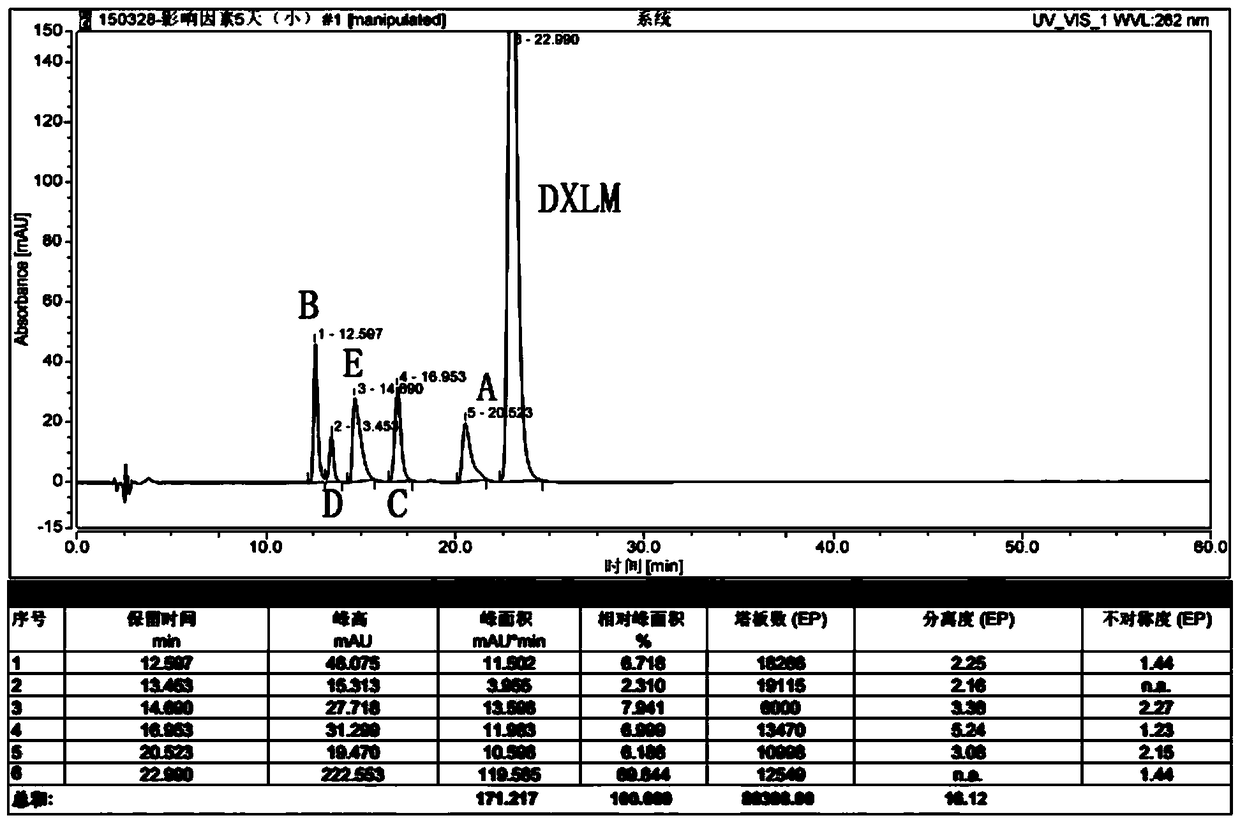
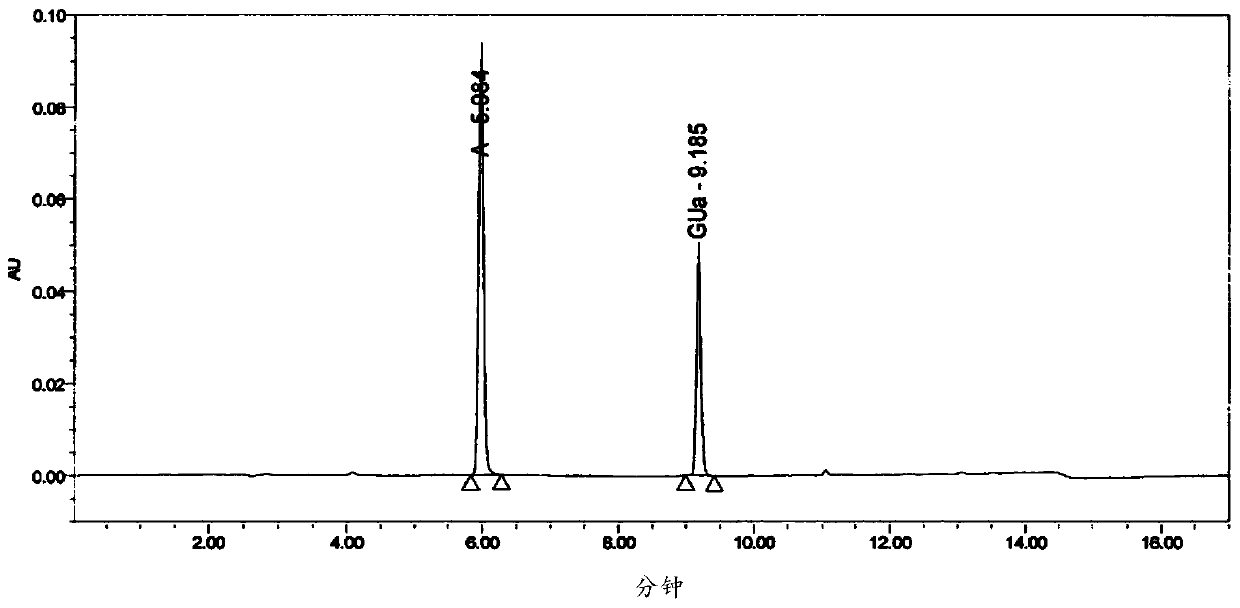
RT-HPLC detection method for related substances of doxylamine succinate
ActiveCN105510512AEasy to handleReduce dosageComponent separationRelated impuritiesAnalytical technique
The invention relates to the technical field of pharmaceutical analysis, in particular to a reversed-phase high-performance liquid chromatography detection method for related substances of doxylamine succinate and discloses an RT-HPLC detection method for the related substances of doxylamine succinate. The RT-HPLC detection method comprises the following steps of preparing an analyzing solution, setting chromatographic conditions and carrying out measurement on a machine. By means of the RT-HPLC detection method, various related impurities in a doxylamine succinate solution can be effectively measured, and separation of doxylamine succinate characteristic peaks and the related impurities in a spectrogram is especially achieved. Thus, it is ensured that the quality of products is controllable.
Owner:NANJING GRITPHARMA CO LTD
Synthetic method of doxylamine succinate
InactiveCN107056685ASimple processSafe and reliable processOrganic chemistrySuccinic acidAcetophenone
The invention discloses a synthetic method of doxylamine succinate. The synthetic method specifically comprises the following three reaction steps: (1) synthesizing 2-pyridylphenyl methyl methanol from acetophenone and 2-bromopyridine in the presence of butyl lithium; (2) reacting by virtue of 2-pyridylphenyl methyl methanol with 2-dimethylamino chloroethane under the action of organic alkali so as to generate doxylamine free alkali; and (3) carrying out salt formation reaction by virtue of doxylamine free alkali and succinic acid, so as to generate doxylamine succinate. The synthetic method has the beneficial effects that the raw materials are easily available, the yield is high, the cost is low, an acceptable product can be obtained without carrying out chromatographic column separation on the product of each step, the process is simple, the post-treatment is simple, the process is safe and reliable, and the synthetic method is suitable for large-scale production.
Owner:珠海市海瑞德生物科技有限公司
Drug composition for treating vomiting during pregnancy
InactiveCN103432126ADissolution rate is fastImprove complianceOrganic active ingredientsDigestive systemPregnancyMedicine
A drug composition for treating vomiting during pregnancy is formed by compositing doxylamine succinate or a composition of doxylamine succinate and pyridoxine hydrochloride as an active ingredient, and pharmaceutically acceptable auxiliary materials. The drug composition can be prepared into an oral preparation, including a tablet, a capsule and the like, by a certain preparation technology. The drug composition is used for treating women who have nausea and vomiting during the pregnancy and poor response to conservative treatment.
Owner:FUKANGREN BIO PHARMA
Synthetic method of doxylamine succinate intermediate
InactiveCN103058916AStrong response specificityRapid responseOrganic chemistryGrignard reagentPyridine
The invention discloses a synthetic method of a doxylamine succinate intermediate, which is characterized by comprising the following steps of: A, 2-pyridine-phenyl methyl methanol synthesis: dissolving acetophenone into methyl tertiary butyl ether, adding a catalyst, then agitating and dropwise adding a Grignard reagent, and obtaining a target product, i.e. 2- pyridine-phenyl methyl methanol after separation and desolvation; and B, 2-pyridine-phenyl methyl methanol purification: dissolving the 2-pyridine-phenyl methyl methanol in the step A into methanol, dropwise adding acetone slowly into solution till a large amount of 2-pyridine-phenyl methyl methanol is crystallized and separated out. The invention aims to overcome the defects in the prior art and provides the synthetic method of the doxylamine succinate intermediate, which has the advantages of simple, safe and reliable process, high efficiency and low production cost.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
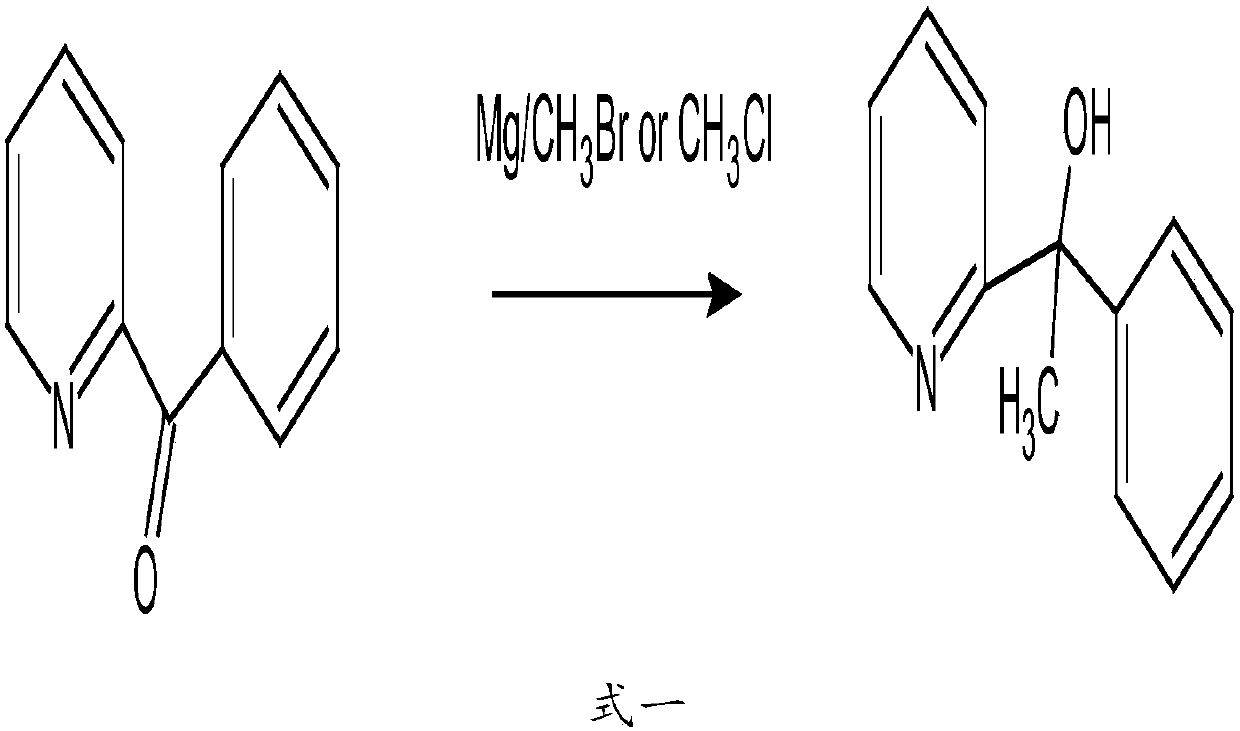
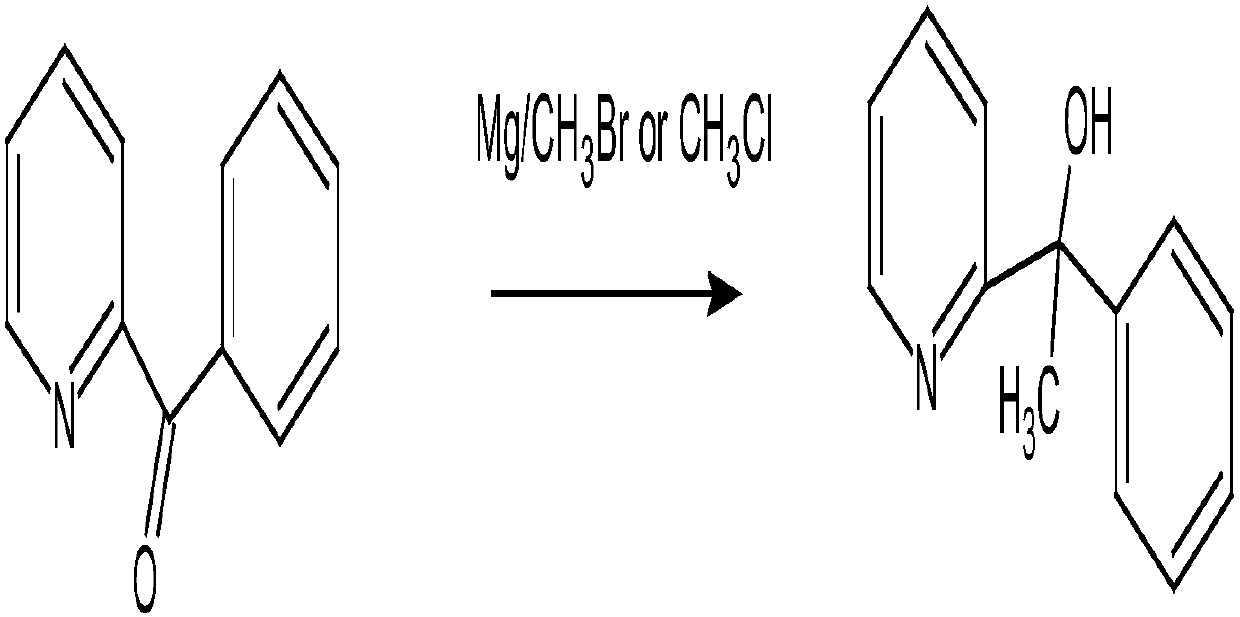
Method for preparing 2-pyridinemethanol-alpha-methyl-alpha-phenyl serving as intermediate of doxylamine succinate
The invention discloses a method for preparing 2-pyridinemethanol-alpha-methyl-alpha-phenyl serving as an intermediate of doxylamine succinate. The method includes the following steps: S1, benzoylpyridine is dissolved into organic solvents and reacted with methyl Grignard reagents at the low temperature, and after the reaction is completed, the target product 2-pyridinemethanol-alpha-methyl-alpha-phenyl is obtained through quenching separation; S2, the crude 2-pyridinemethanol-alpha-methyl-alpha-phenyl prepared in the step S1 is extracted and purified to obtain the fine 2-pyridinemethanol-alpha-methyl-alpha-phenyl. An acid-base inversion method in the step S2 includes the following specific steps: the crude 2-pyridinemethanol-alpha-methyl-alpha-phenyl is dissolved into purified water, the pH value is adjusted to enable the mixture to be faintly acid, impurity removal by extraction is carried out through ethyl acetate, the pH of a water layer is adjusted to enable the water layer to be faintly basic, the water layer is extracted through ethyl acetate, and an organic layer is desolvated to obtain the fine 2-pyridinemethanol-alpha-methyl-alpha-phenyl. The method is simple in technology, safe and reliable; the yield and the purity of the prepared 2-pyridinemethanol-alpha-methyl-alpha-phenyl serving as the intermediate of the doxylamine succinate are high, and the method is suitable for industrial large-scale production.
Owner:NANJING GRITPHARMA CO LTD
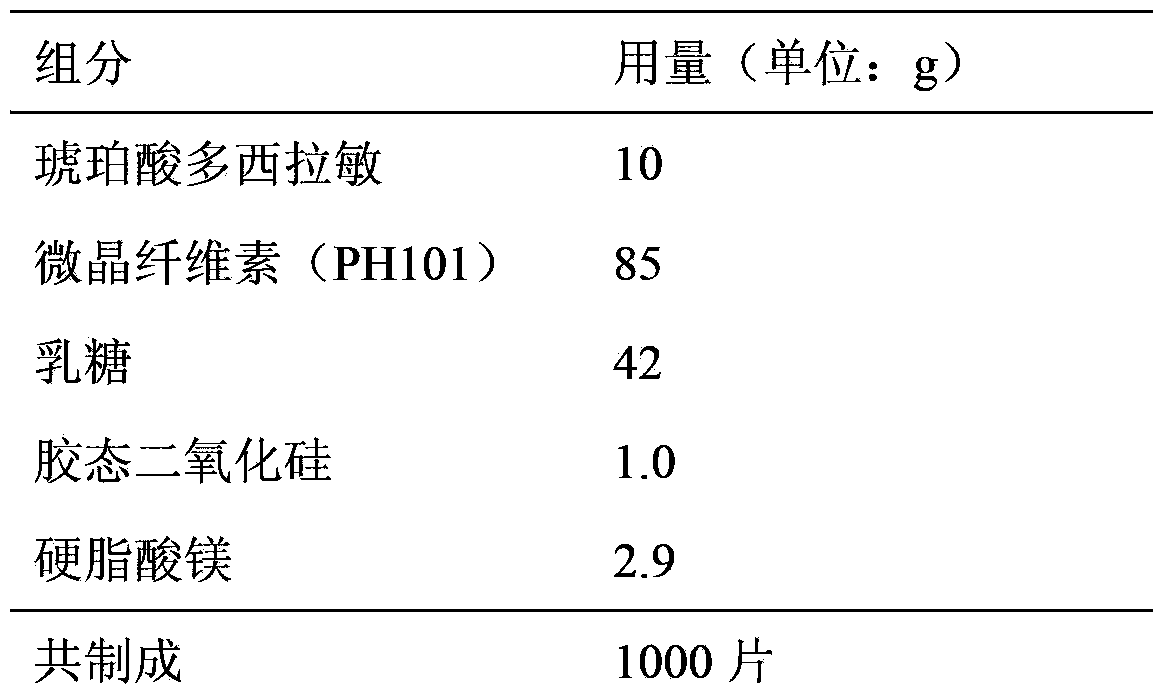
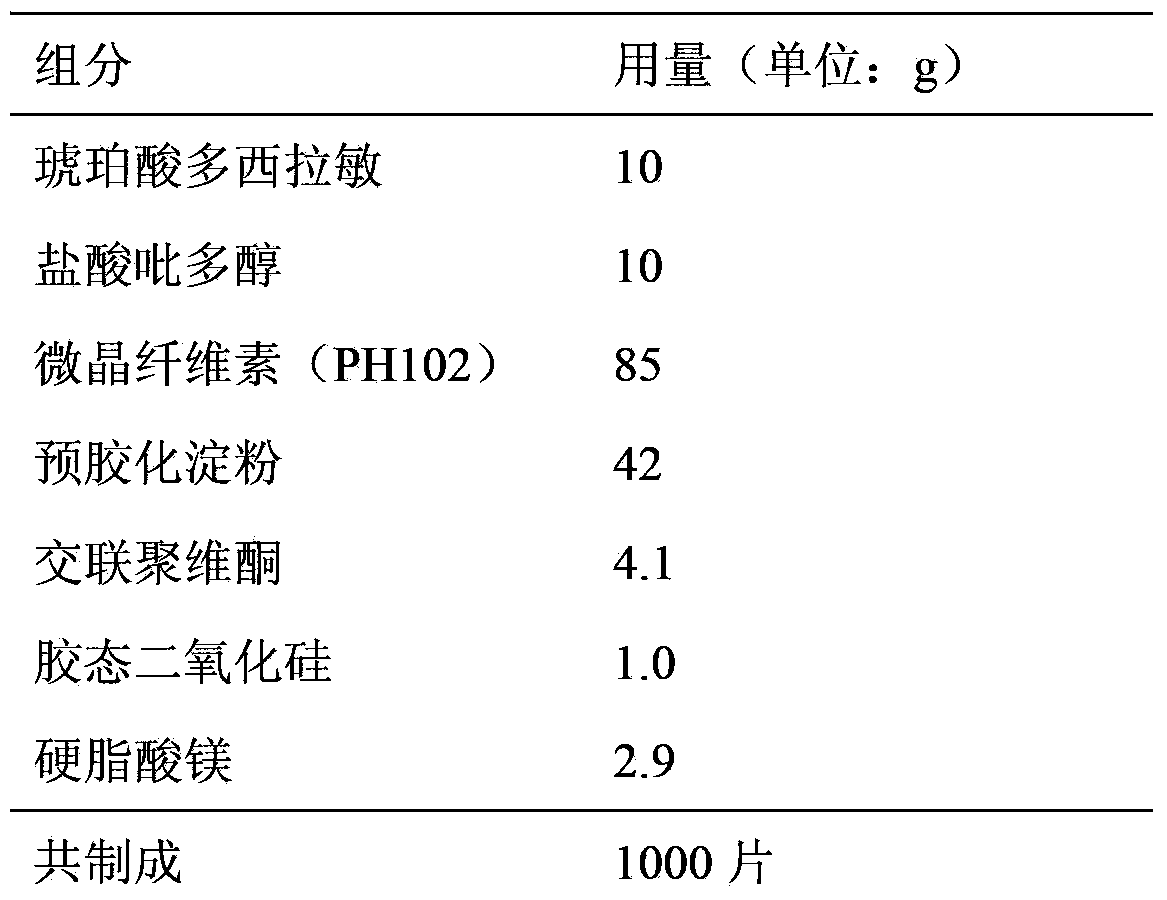
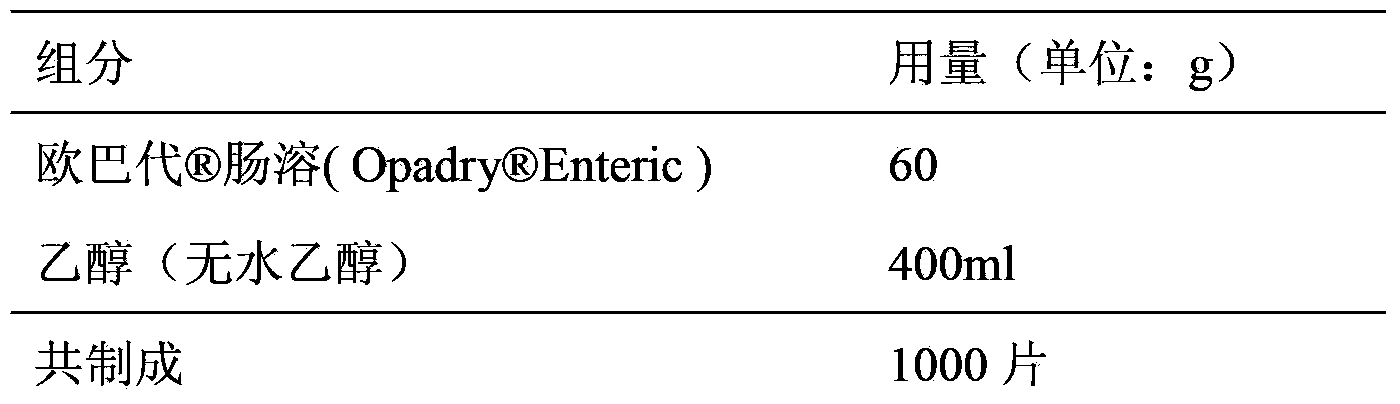
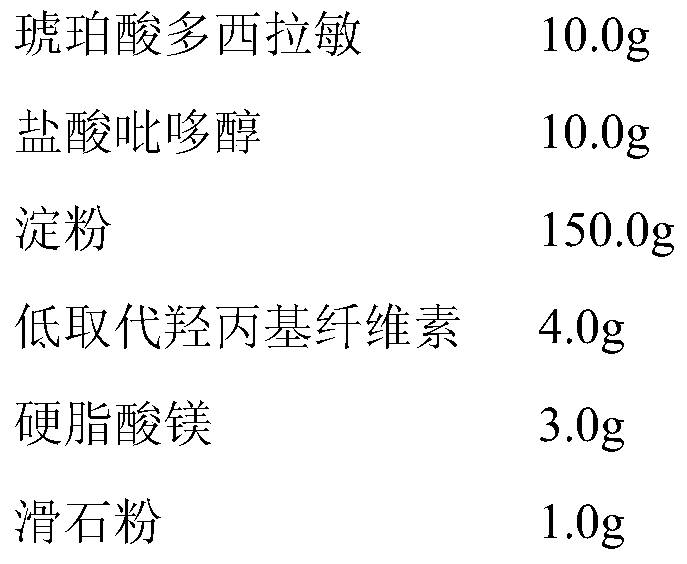
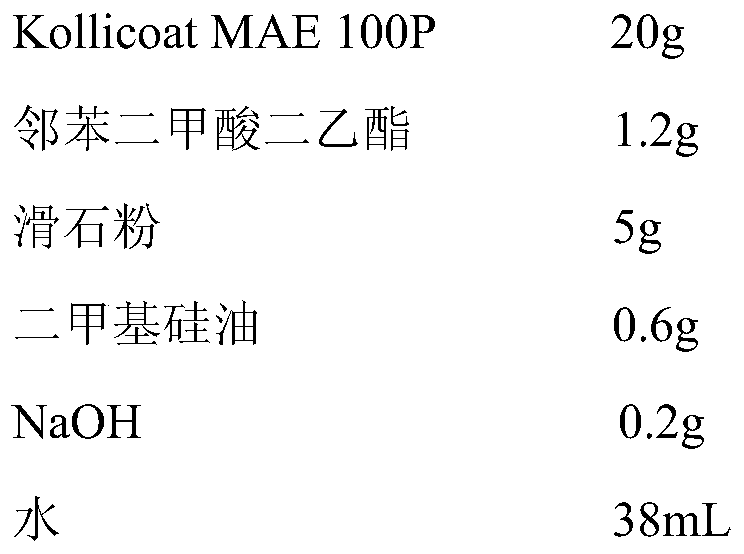
Doxylamine succinate-pyridoxine hydrochloride enteric-coated tablet pharmaceutical composition and preparation method thereof
ActiveCN106606502AImprove stabilityAvoid defectsOrganic active ingredientsDigestive systemFast releaseEnteric coating
The invention provides a compound doxylamine succinate-pyridoxine hydrochloride enteric-coated tablet pharmaceutical composition and a preparation method thereof. The composition comprises a tablet core containing doxylamine succinate and pyridoxine hydrochloride, an isolation coating, an enteric coating and other medicinal auxiliary materials. The preparation method has simple processes. The pharmaceutical composition has good stability, is insoluble in the stomach and can be fast released in the intestine so that the problem that the existing pharmaceutical composition has a reduced release rate when entering into an intra-intestinal neutral environment from a gastric acid environment.
Owner:SICHUAN HAISCO PHARMA CO LTD
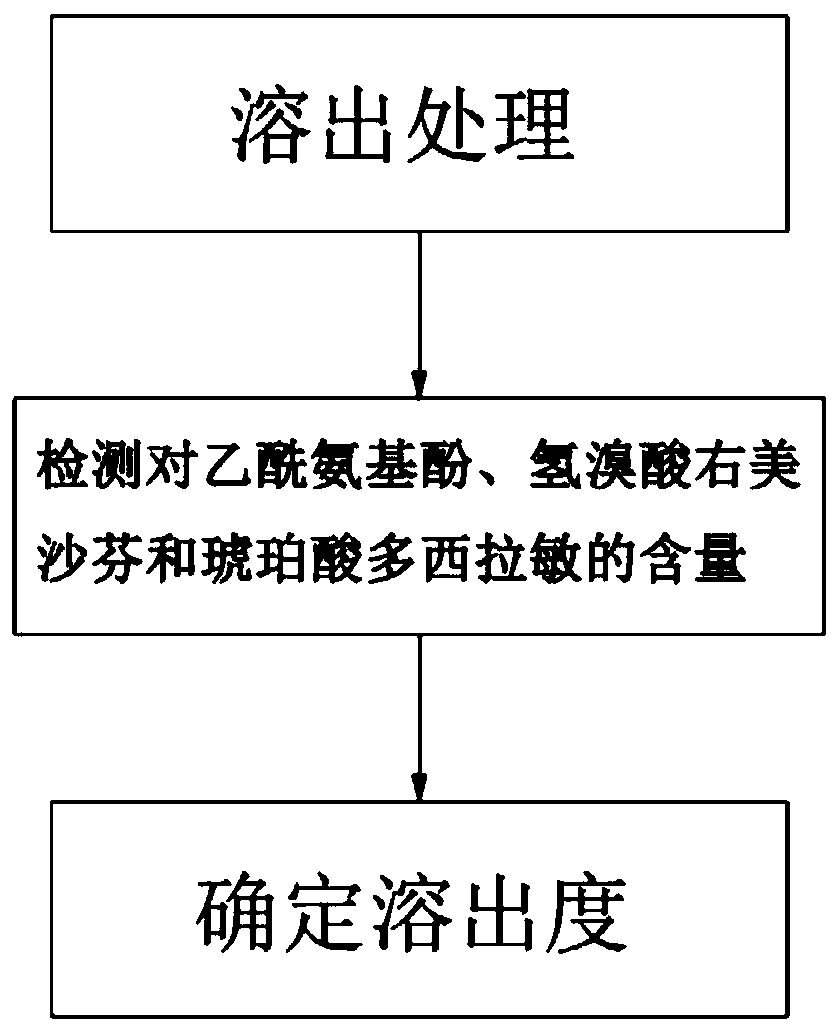
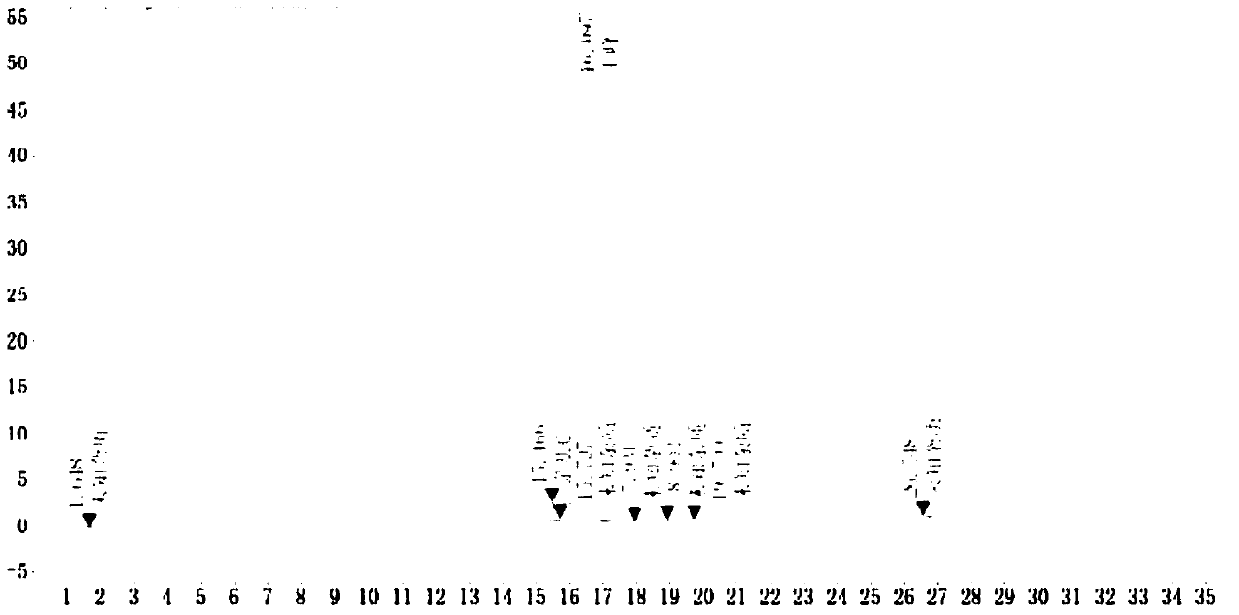
Method for determining dissolution rate of medicinal preparation containing acetaminophen, dextromethorphan hydrobromide and doxylamine succinate
ActiveCN110286162ASave time and costSave testing costComponent separationDextromethorphan HydrobromideQuality control
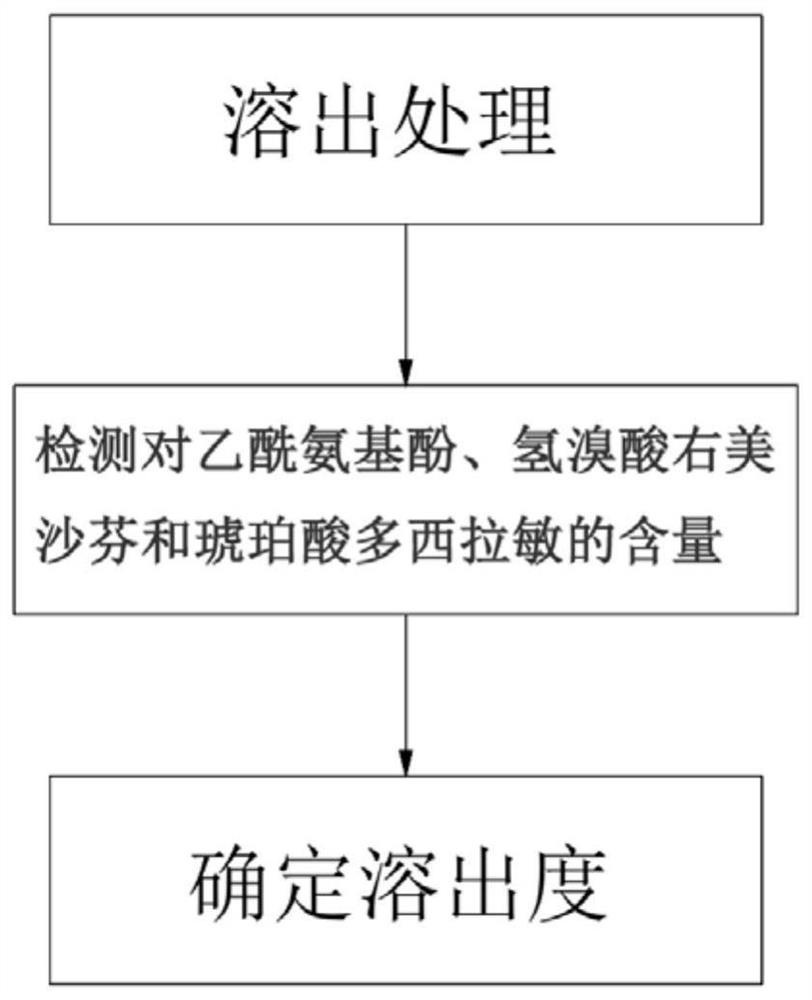
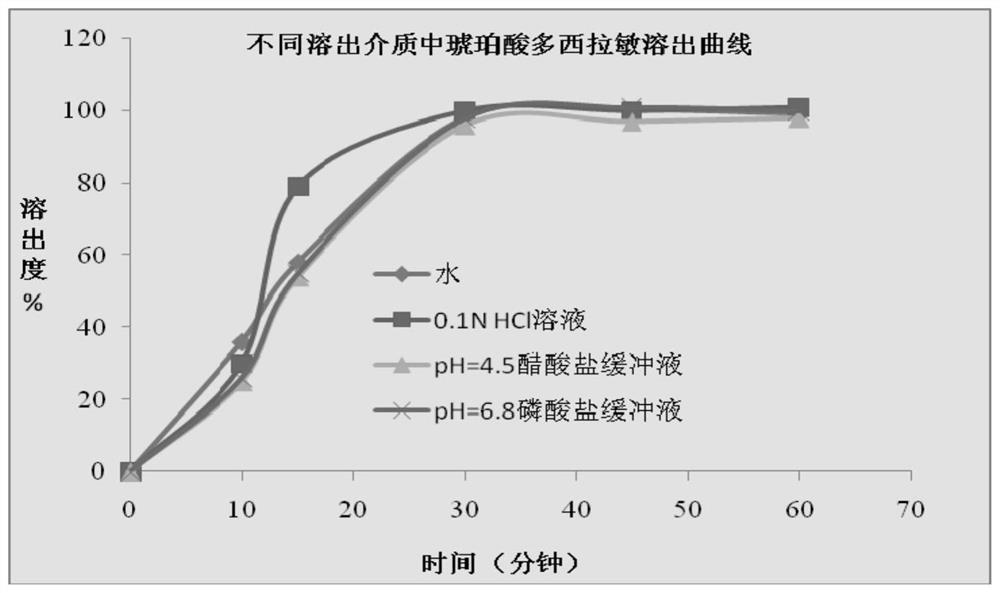
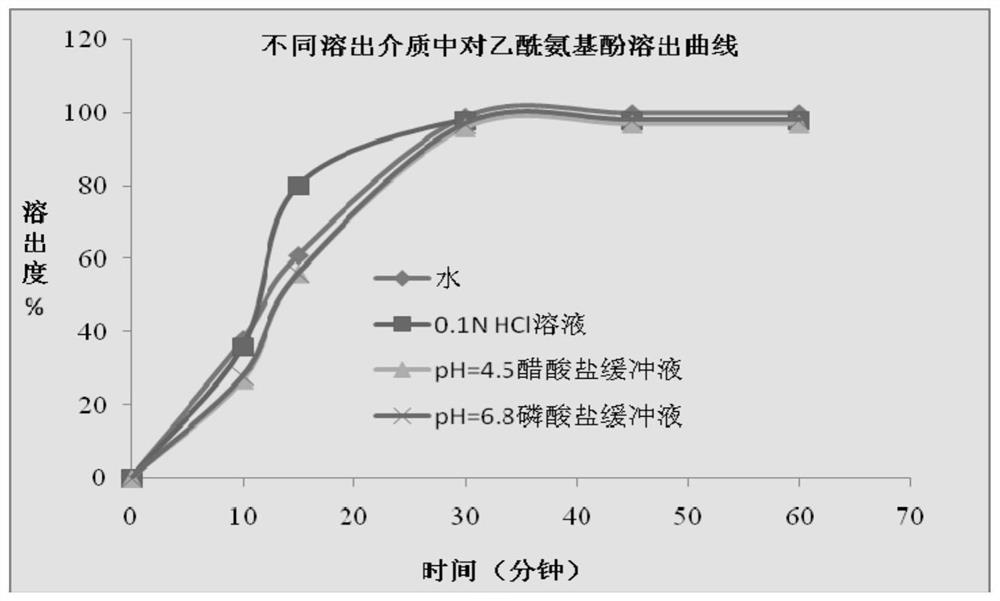
The invention discloses a method for determining a dissolution rate of a medicinal preparation containing acetaminophen, dextromethorphan hydrobromide and doxylamine succinate. The method comprises the following steps of: (1) adding medicinal preparation containing acetaminophen, dextromethorphan hydrobromide and doxylamine succinate into a dissolution medium for dissolution treatment, performing sampling and filtering to obtain dissolution liquid; (2) detecting the contents of acetaminophen, dextromethorphan hydrobromide and doxylamine succinate in the dissolution liquid; and (3) and determining the dissolution rate of the medicinal preparation according to the detection result of the step (2). The method can effectively simulate the dissolution behavior in vivo in vitro, can determine the dissolution rate of the soft capsule containing acetaminophen, dextromethorphan hydrobromide and doxylamine succinate, and provides technical support for the research and development and quality control of medicaments. The method can simultaneously determine the dissolution results of the three substances under the same condition, greatly saves the detection time and the detection cost, and has good accuracy and reproducibility.
Owner:安士制药(中山)有限公司
A kind of preparation method of doxylamine succinate
The invention discloses a preparation method of doxylamine succinate. The preparation method comprises following steps: step 1, 2-pyridyl phenyl methyl carbinol is dissolved in an organic solvent, and is reacted with 2-dimethylaminoethyl chloride hydrochloride at high temperature, after reaction, target product N,N-dimethyl-2-[1-phenyl-1-(2-pyridine)oxethyl]ethylamine is obtained via quenching extracting separation; and step 2, N,N-dimethyl-2-[1-phenyl-1-(2-pyridine) oxethyl]ethylamine obtained in the step 1 and succinic acid are subjected to salt forming in an organic solvent; and finished product N,N-dimethyl-2-(1-phenyl-1-(2-pyridine)ethoxy)ethanamine succinate (doxylamine succinate) is obtained via cooling crystallization. The preparation method is simple, safe, and reliable, is high in doxylamine succinate yield, and is suitable for industrialized enlarged production; and post-treatment is simple and convenient.
Owner:NANJING GRITPHARMA CO LTD
Preparation method of night cold-treating soft capsules
ActiveCN103463087ANice appearanceStrong complianceOrganic active ingredientsNervous disorderDrugs solutionMelting tank
The invention discloses a preparation method of night cold-treating soft capsules. The preparation method comprises the steps: A, preparing a gelatin liquid: dissolving a plasticizer in water, adding into a gelatin-melting tank and heating, adding gelatin into the gelatin-melting tank, stirring to dissolve, adding a colorant into water, stirring evenly to obtain a pigment solution, adding the pigment solution into the gelatin solution, mixing and vacuumizing to discharge air bubbles, and thereby obtaining the gelatin liquid; B, preparing contents: mixing polyethylene glycol and a polyol to obtain a mixed liquid, adding povidone into the mixed liquid, stirring to dissolve, adding acetaminophen, stirring into a mixed suspension, then adding the mixed suspension into a liquid mixing tank, vacuumizing and introducing inert gas, heating to dissolve, after acetaminophen is dissolved, cooling, adding dextromethorphan hydrobromide and doxylamine succinate, vacuumizing, carrying out heat preservation for dissolving, and thereby obtaining a content solution; and C, preparing the soft capsules: respectively adding the gelatin solution and the content drug solution into a pelleting press, pelleting, drying, wiping pills, and packaging. The invention aims to provide the preparation method of the night cold-treating soft capsules, wherein the preparation method has the advantages of simple process, convenient production and good product stability.
Owner:安士制药(中山)有限公司
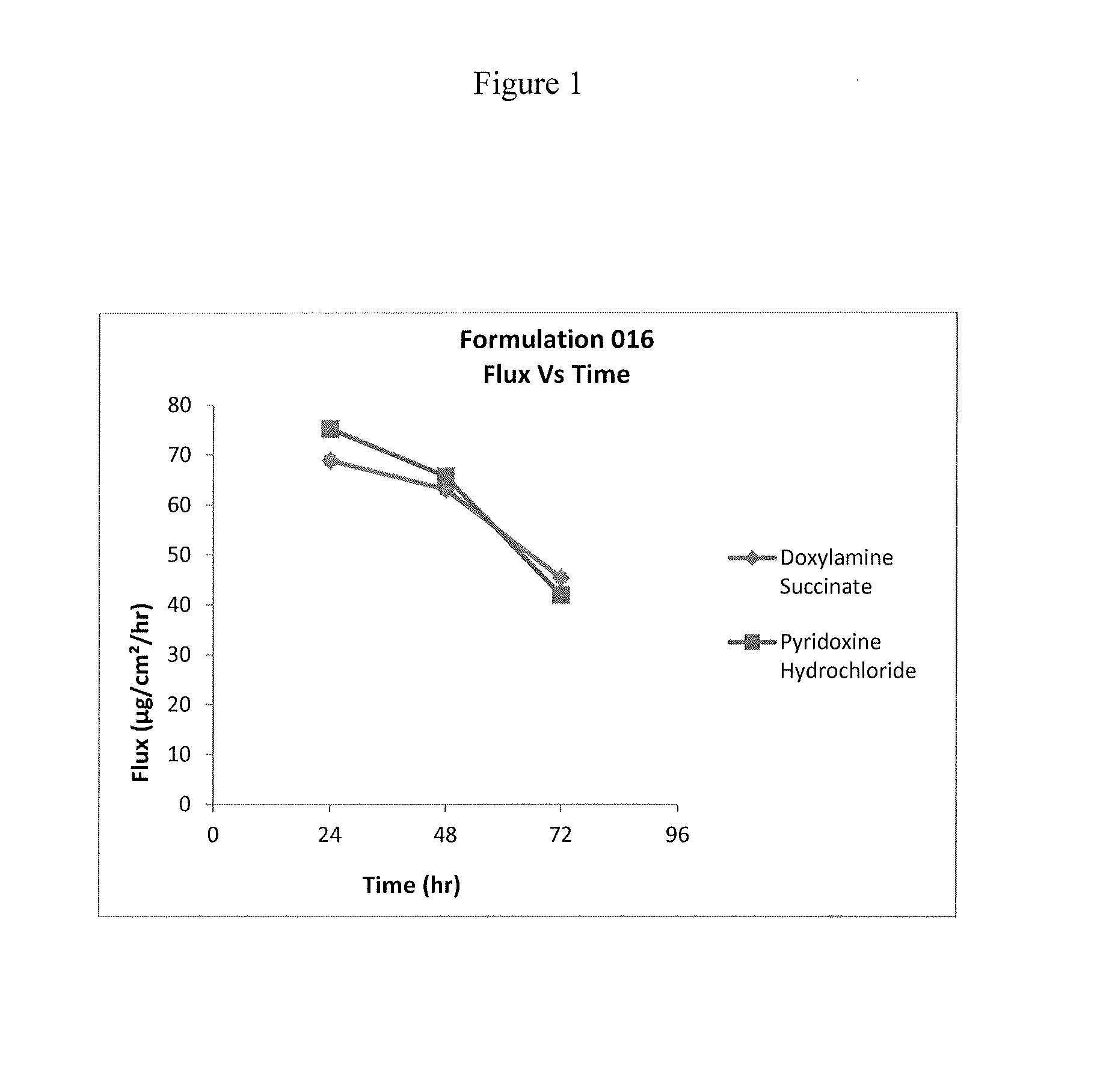
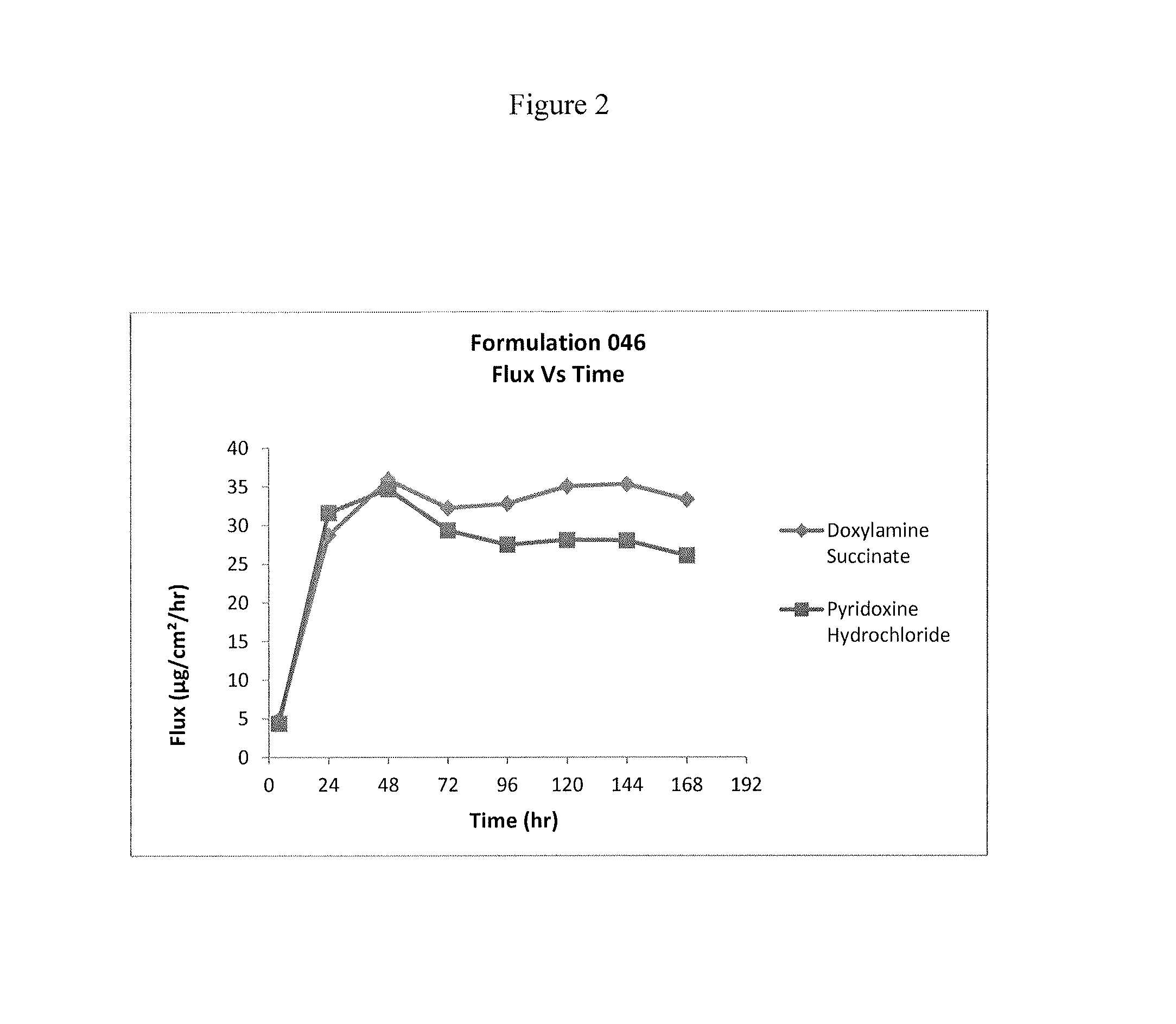
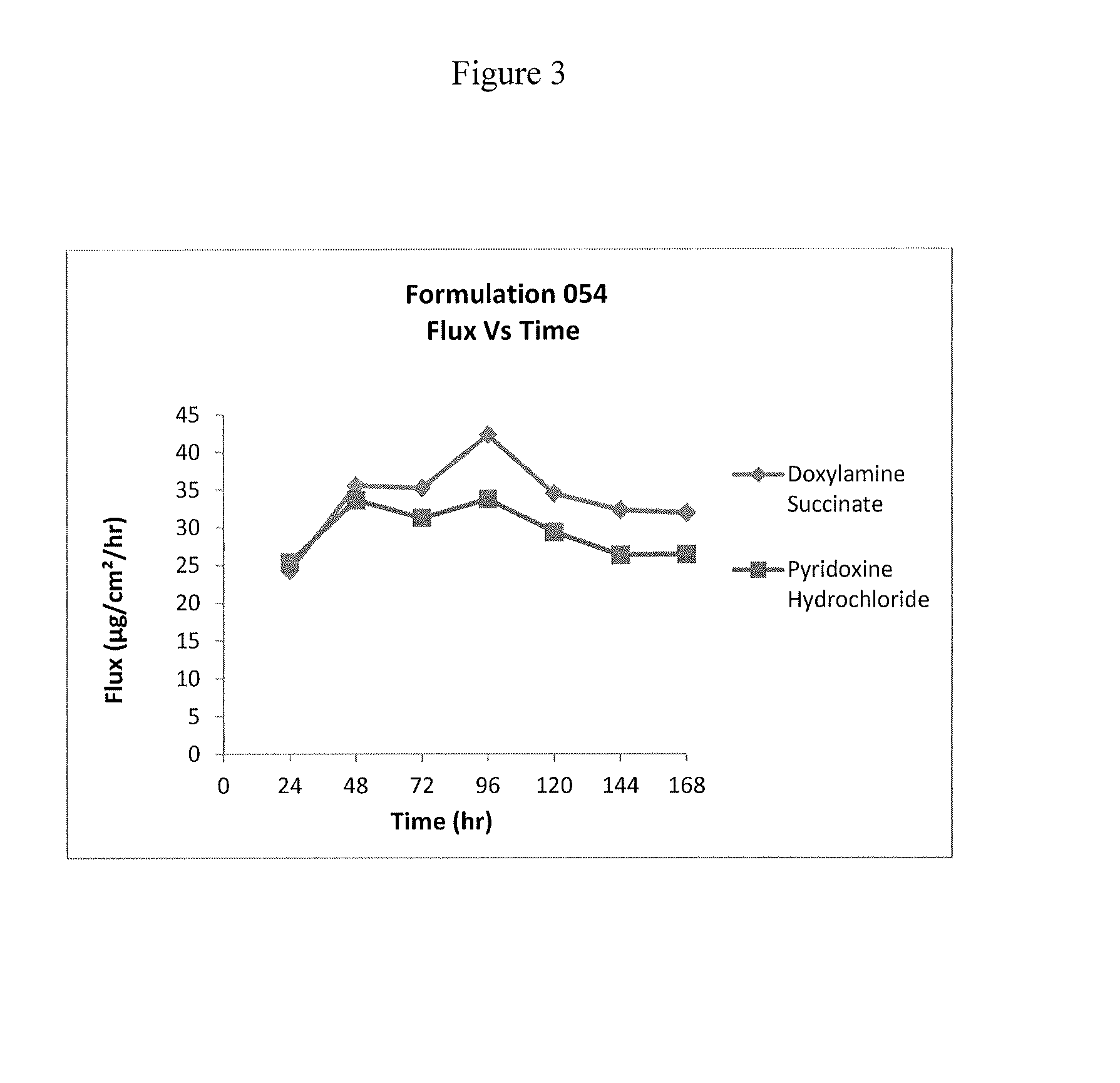
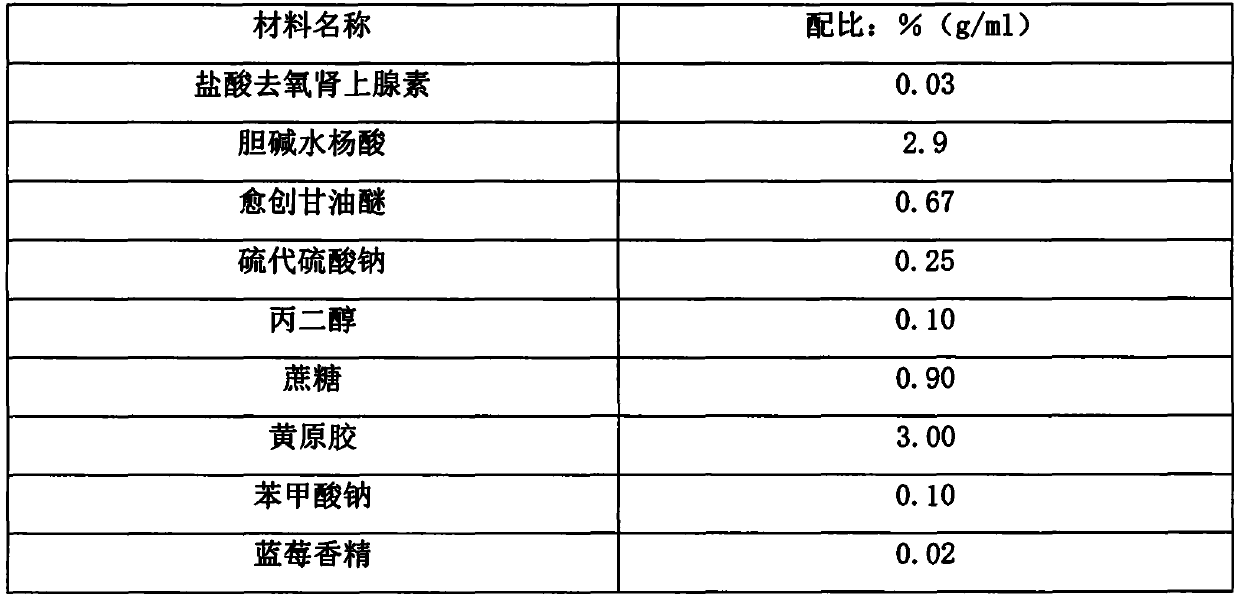
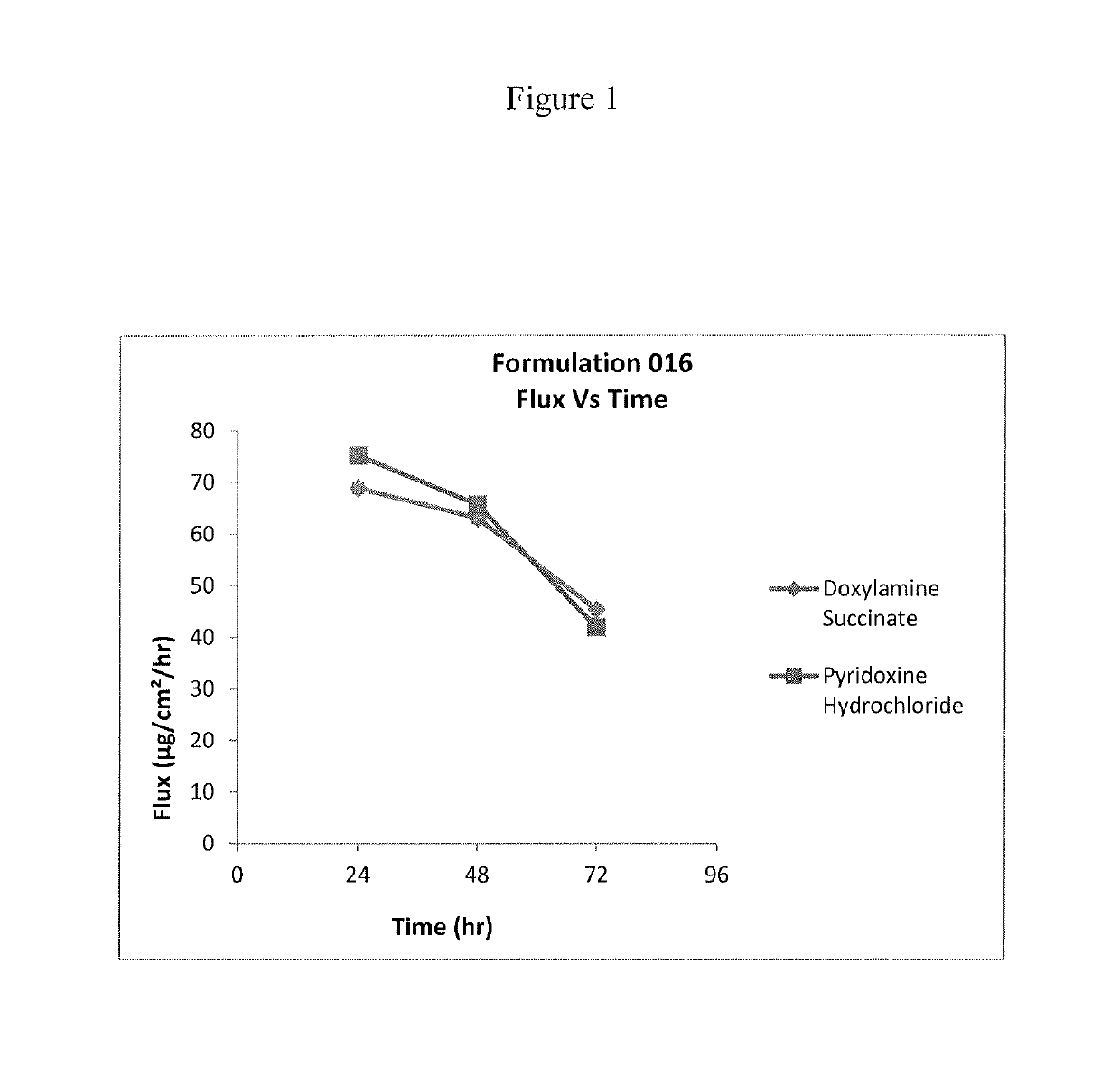
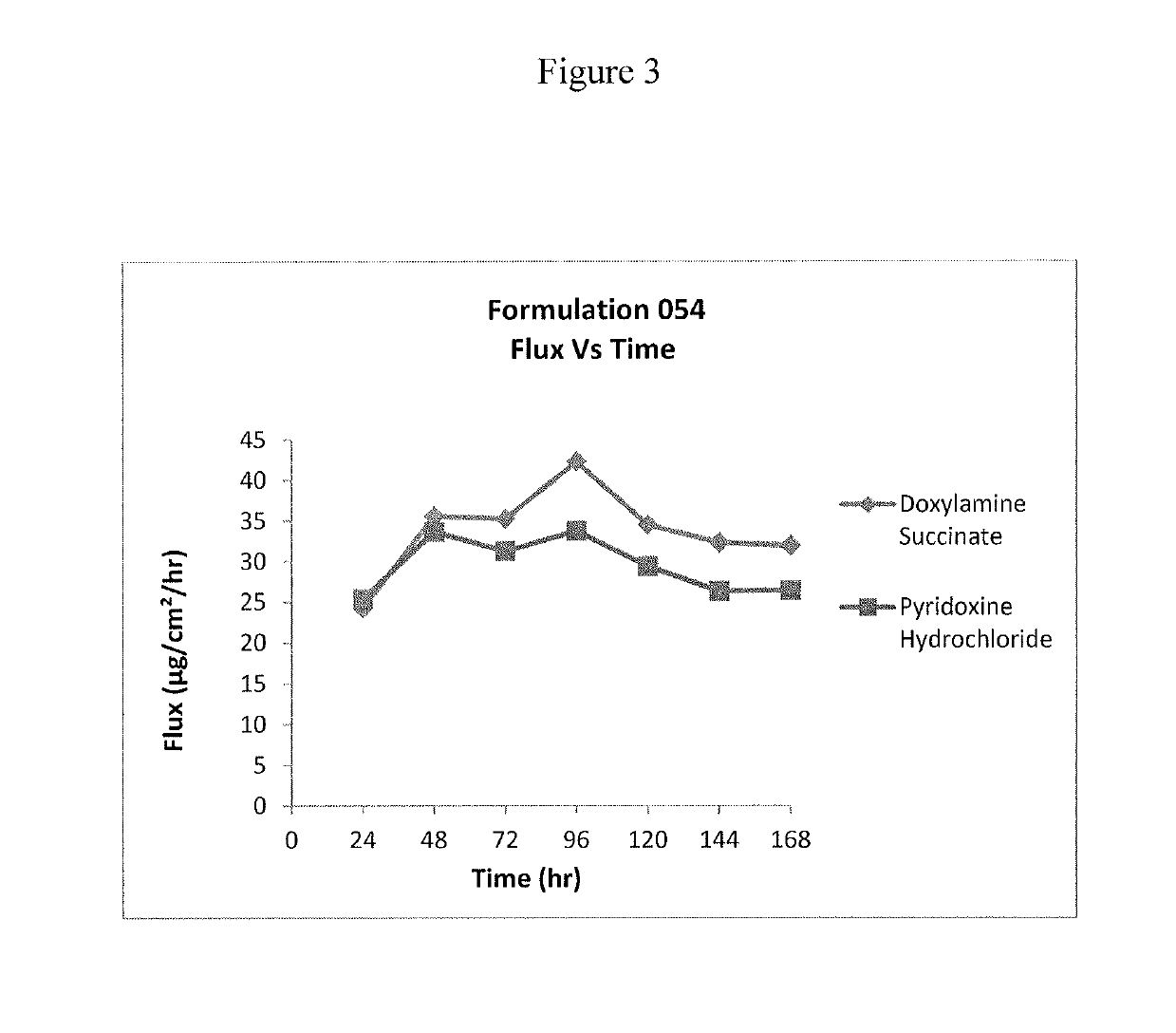
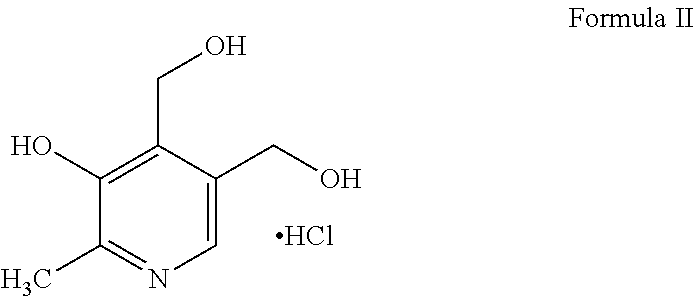
Transdermal and/or topical delivery systems composed of doxylamine succinate and pyridoxine hydrochloride in combination, or alone
ActiveUS20170049759A1Avoid nauseaTreating preventingOrganic active ingredientsAerosol deliveryTransdermal patchDelivery system
Pharmaceutical compositions for simultaneous transdermal delivery of Doxylamine and Pyridoxine comprising Doxylamine or its salts, Pyridoxine or its salts or its active metabolites and a vehicle system wherein pharmaceutical compositions are liquid formulations, semisolid formulations and polymer matrices. Further pharmaceutical compositions can be incorporated into transdermal delivery systems or transdermal patches. The invention provides a method for treatment of nausea and vomiting in general, and for pregnant women in particular by continuous and simultaneous transdermal delivery of Doxylamine and Pyridoxine. This is to be accomplished through topical application of pharmaceutical compositions or by application of a transdermal delivery system or transdermal patch to the surface of the skin wherein the duration of application is once in a day, once every two days, once every three days, once every four days, once every five days, once every six days, or once in a week.
Owner:AEQUUS PHARMA INC
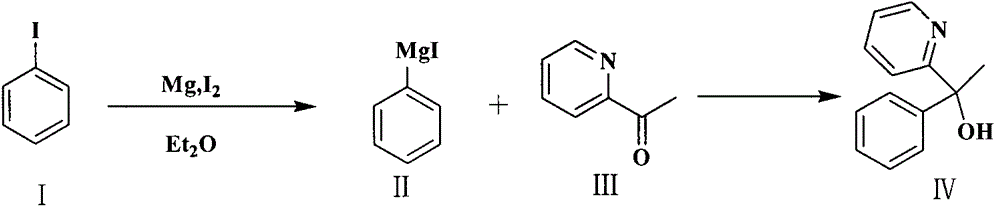
A kind of preparation method of doxylamine succinate
The invention relates to a preparation method of doxylamine succinate. The preparation method comprises the steps as follows: firstly, a Grignard reagent generated by iodobenzene and magnesium reacts with 2-acetylpyridine to generate 2-pyridyl phenyl methyl alcohol, the 2-pyridyl phenyl methyl alcohol is recrystallized to be purified, then the 2-pyridyl phenyl methyl alcohol reacts with sodium amide and 2-dimethylamino chloroethane sequentially, doxylamine is obtained and has a salt forming reaction with succinic acid finally, and the doxylamine succinate is obtained. The preparation method is high in reaction efficiency, lower in cost and applicable to industrial mass production.
Owner:JIANGSU LEEWAY BIOLOGICAL TECH
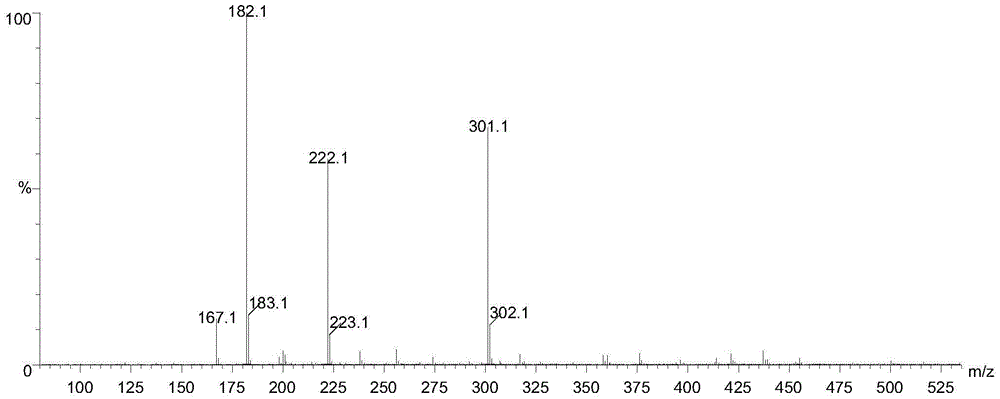
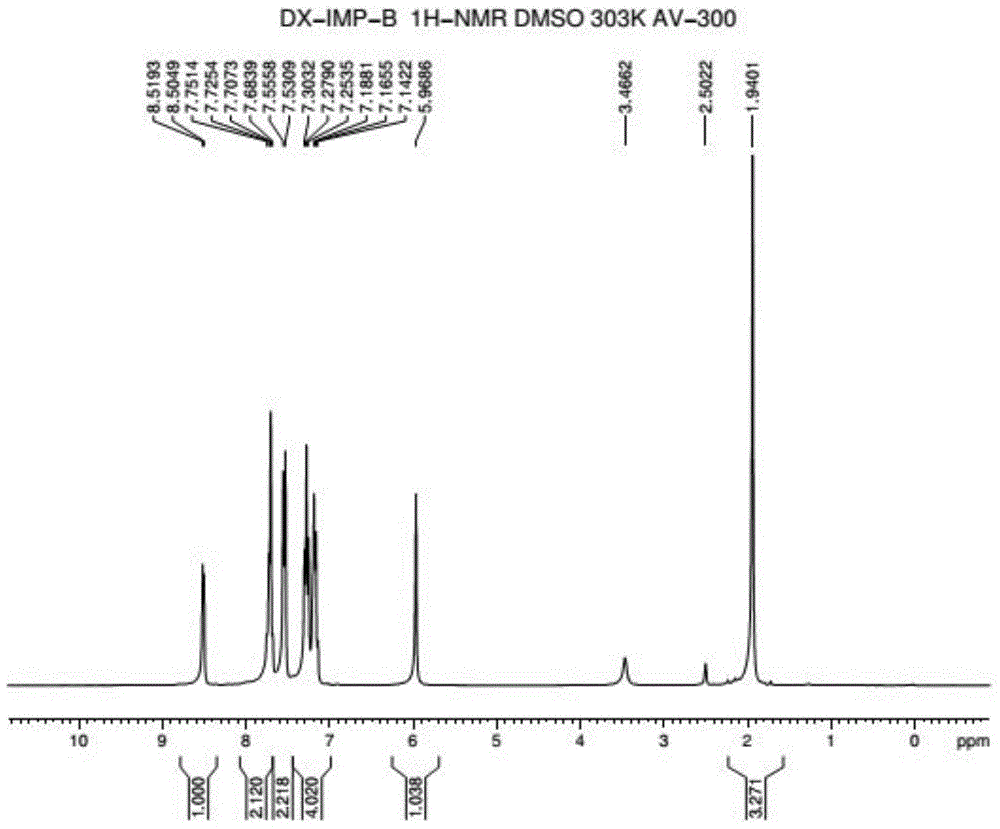
Preparation method of doxylamine succinate impurity C
InactiveCN107382832ARaise quality standardsMild reaction conditionsOrganic chemistryOrganic solventImpurity
The invention discloses a preparation method of a doxylamine succinate impurity C. The preparation method comprises steps as follows: alpha-phenyl-2-pyridinemethanol and an organic solvent are added to a reactor, stirred and dissolved, after air in the reactor is subjected to N2 replacement, alkali liquor is added, a 2-dimethylaminoethyl chloride solution is dropwise added during stirring, a mixed solution is heated to produce backflow and is cooled after the reaction, water is added to a reaction product, a mixture is layered and purified, and a target product is obtained. According to the preparation method, alpha-phenyl-2-pyridinemethanol is taken as a raw material and subjected to an elimination reaction with 2-dimethylaminoethyl chloride under the alkaline condition, and the target product is obtained; the method has the advantages of adopting mild reaction conditions and short synthesis route and being simple and convenient to operate and can be applied to qualitative and quantitative analysis of impurities in doxylamine succinate production, and therefore, quality standard of doxylamine succinate can be improved; the product is high in yield and purity.
Owner:合肥创新医药技术有限公司
Liquid composition containing phenylephrine hydrochloride as well as preparation and application thereof
PendingCN111588694AImprove stabilityReduce solubilitySalicyclic acid active ingredientsAntipyreticSalicylic acidSuccinic acid
The invention relates to a liquid composition containing phenylephrine hydrochloride as well as a preparation and application thereof. The composition comprises three compositions: the first is a combination of the phenylephrine hydrochloride, choline salicylic acid and guaifenesin; the second is a combination of the phenylephrine hydrochloride, acetaminophen and pheniramine maleate; and the thirdis a combination of the phenylephrine hydrochloride, the acetaminophen, dextromethorphan hydrobromide and doxylamine succinate. The composition disclosed by the invention can be prepared into a liquid preparation, and a preferable dosage form is suspension.
Owner:北京博智绿洲医药科技有限公司
Transdermal and/or topical delivery systems composed of doxylamine succinate and pyridoxine hydrochloride in combination, or alone
Owner:TRANSDERMAL RES PHARM LAB
Method for synthesizing doxylamine succinate through base catalysis
PendingCN114524765AOrganic compound preparationCarboxylic acid salt preparationAlkaneGrignard reagent
The invention relates to a synthesis method of doxylamine succinate, which takes 2-acetylpyridine as an initial raw material, comprises the following unit processes: synthesis of doxylamine, salt forming reaction of doxylamine, separation and purification of doxylamine, and is characterized in that the synthesis of doxylamine comprises the following steps: firstly, reacting 2-acetylpyridine with a Grignard reagent generated by bromobenzene and magnesium to generate 2-pyridyl phenyl methyl methanol; then reacting 2-pyridyl phenyl methyl methanol with 2-chloroethyl dimethylamine hydrochloride under the catalysis of potassium tert-butoxide (or other alkane alkoxide potassium or sodium salt or potassium hydroxide or sodium hydroxide) to generate doxylamine; and finally salifying doxylamine and succinic acid to obtain the target product doxylamine succinate.
Owner:GUANGXI BOTANICAL GARDEN OF MEDICINAL PLANTS
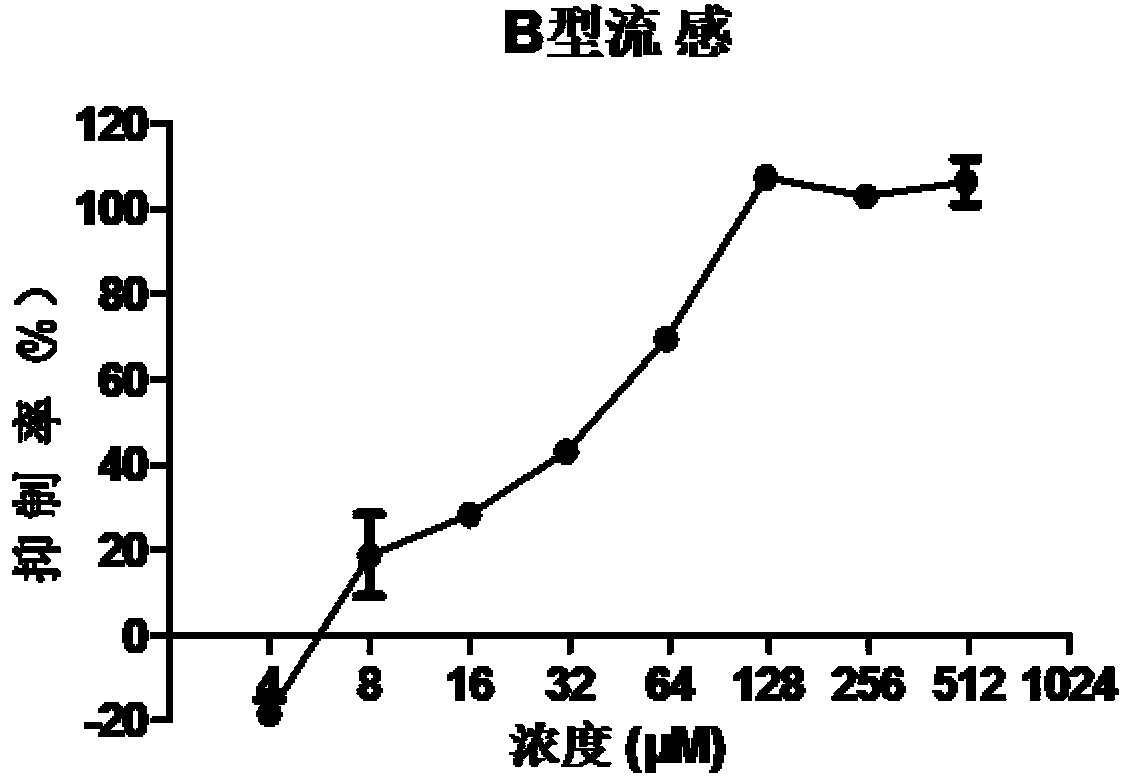
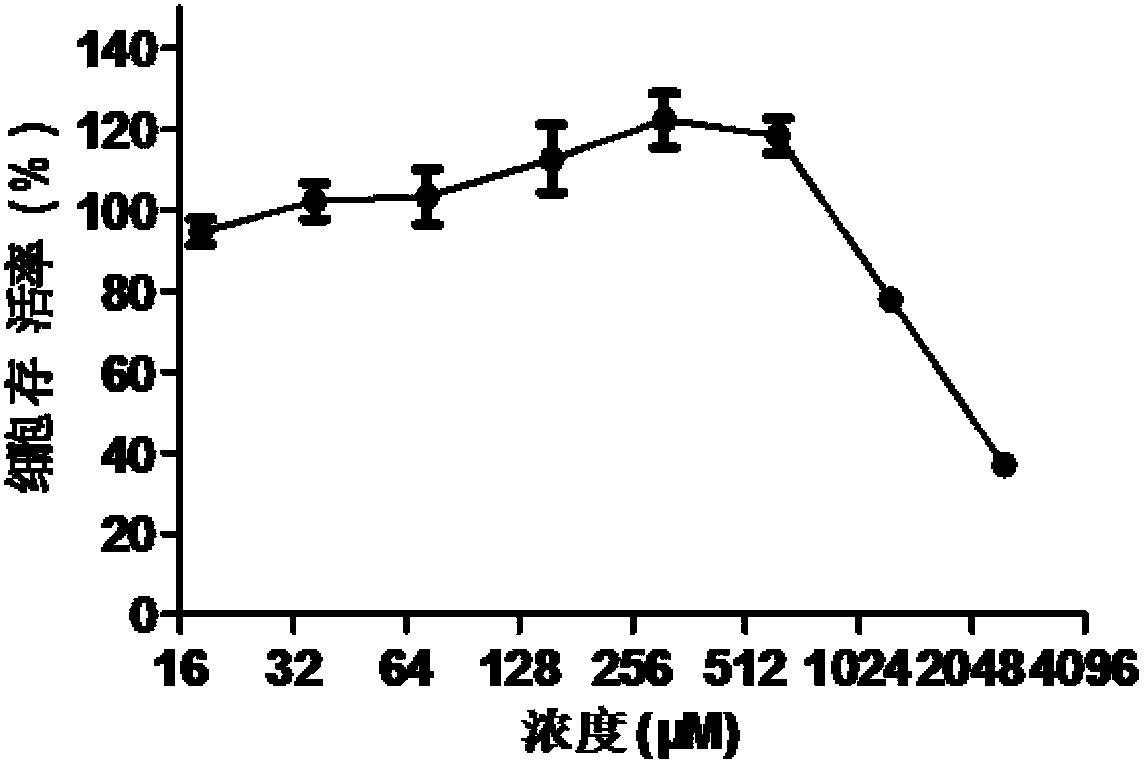
Application of doxylamine succinate in preparing drug for treating or preventing influenza virus
InactiveCN103251590AInhibition of replicationBroad-spectrum antiviral activityOrganic active ingredientsAntiviralsMedicineVirus strain
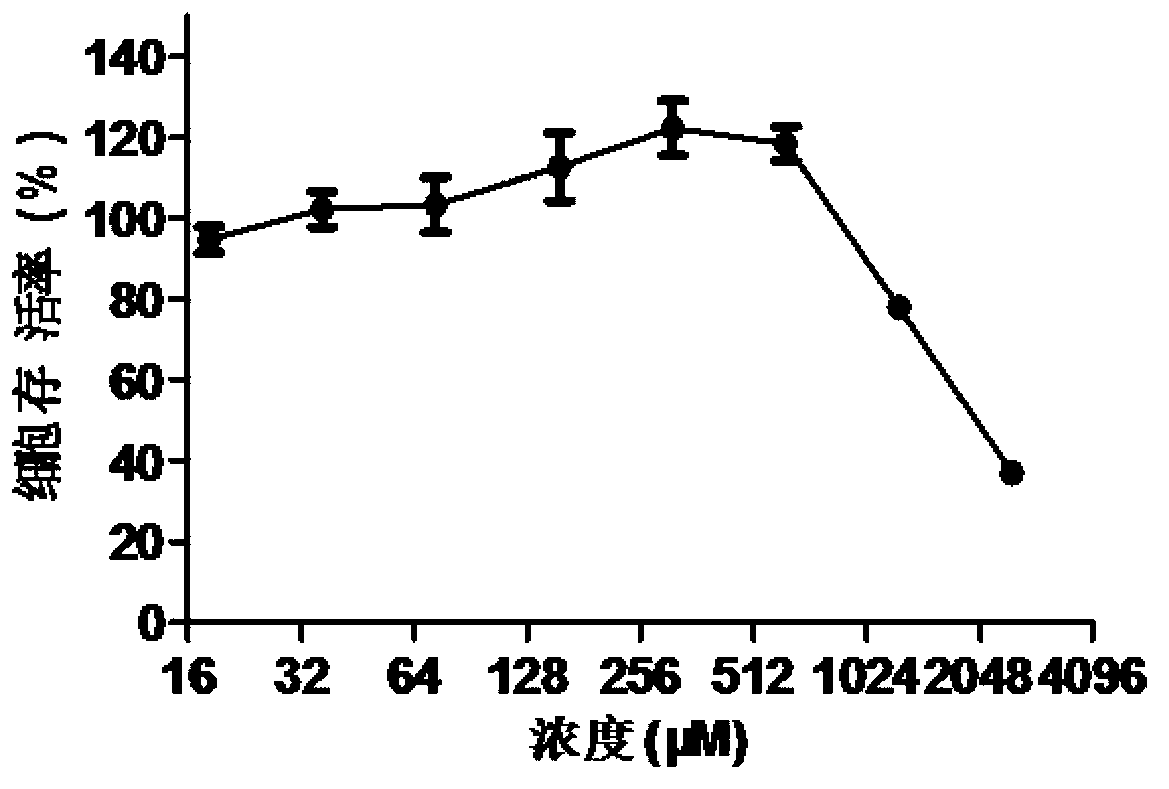
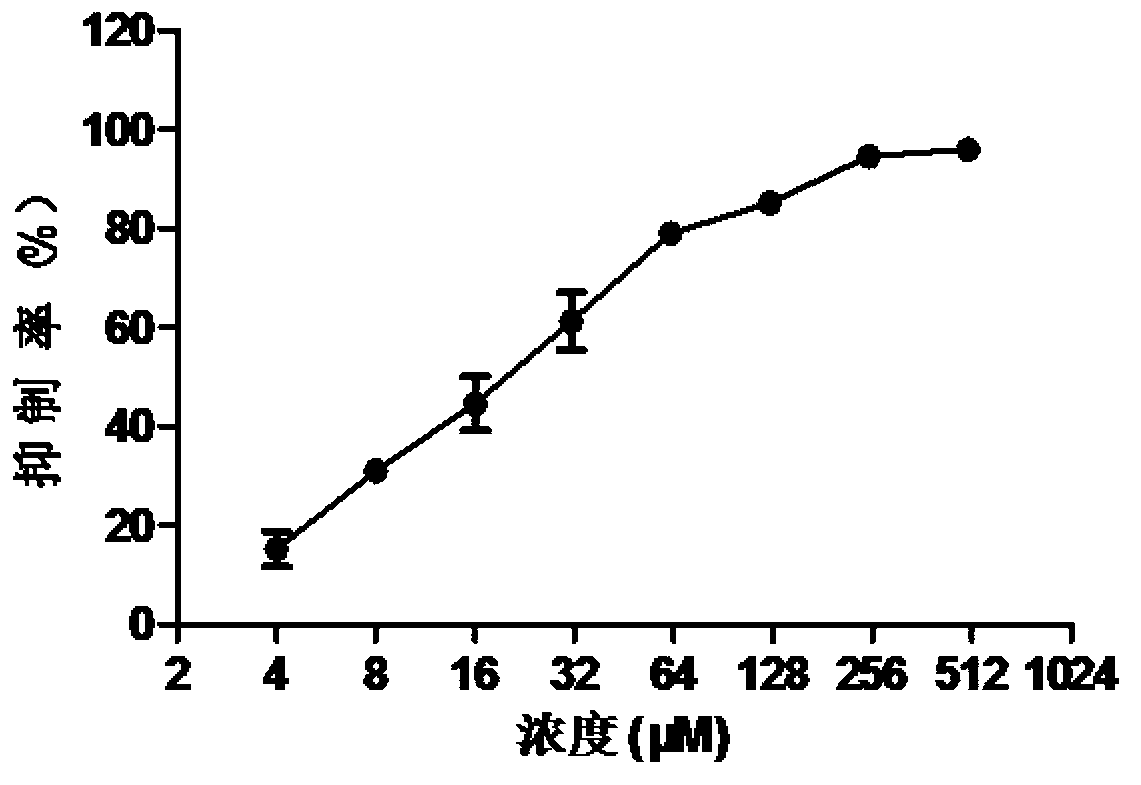
The invention discloses application of doxylamine succinate in preparing a drug for treating or preventing influenza virus. The application comprises the following steps of: selecting the doxylamine succinate with complete non-toxic concentration to carry out antiviral experiment, wherein the result shows that the micromolecule compound has remarkable antiviral activity and is dose-dependent; and detecting the antiviral activity of the doxylamine succinate to different types and subtypes of influenza viruses, wherein the result indicates that the doxylamine succinate has activity for detecting virus strain, a dose-dependent effect, as well as a certain broad-spectrum to the anti-influenza virus activity. Therefore, the doxylamine succinate disclosed by the invention can be developed as a novel anti-influenza virus drug, so that a novel way and means is provided for treating the influenza.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Transdermal delivery of doxylamine succinate and pyridoxine hydrochloride
Pharmaceutical compositions for simultaneous transdermal delivery of Doxylamine and Pyridoxine comprising Doxylamine or its salts, Pyridoxine or its salts or its active metabolites and a vehicle system wherein pharmaceutical compositions are liquid formulations, semisolid formulations and polymer matrices. Further pharmaceutical compositions can be incorporated into transdermal delivery systems or transdermal patches. The invention provides a method for treatment of nausea and vomiting in general, and for pregnant women in particular by continuous and simultaneous transdermal delivery of Doxylamine and Pyridoxine. This is to be accomplished through topical application of pharmaceutical compositions or by application of a transdermal delivery system or transdermal patch to the surface of the skin wherein the duration of application is once in a day, once every two days, once every three days, once every four days, once every five days, once every six days, or once in a week.
Owner:AEQUUS PHARMA INC
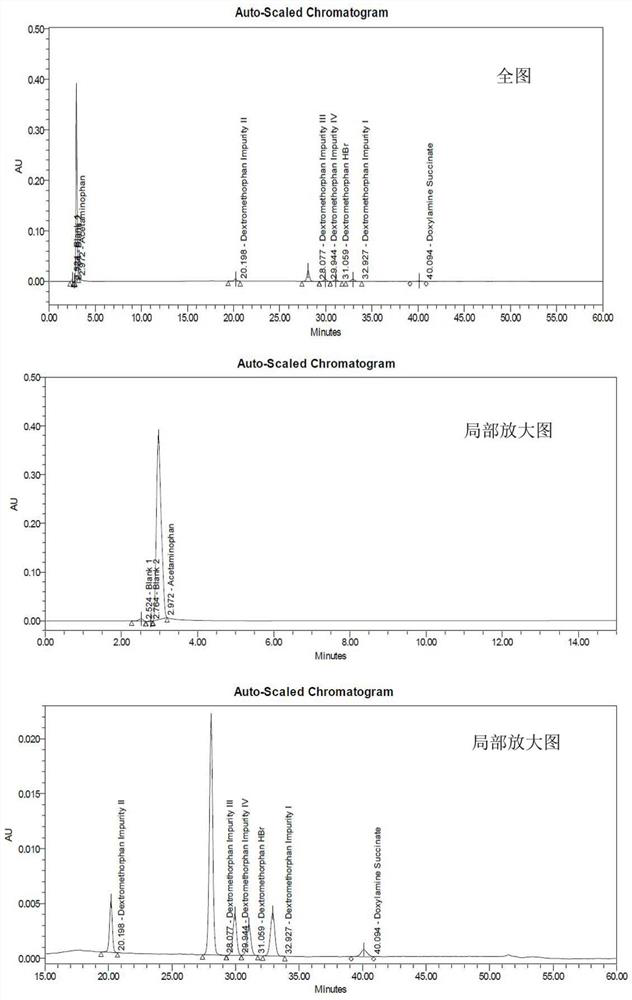
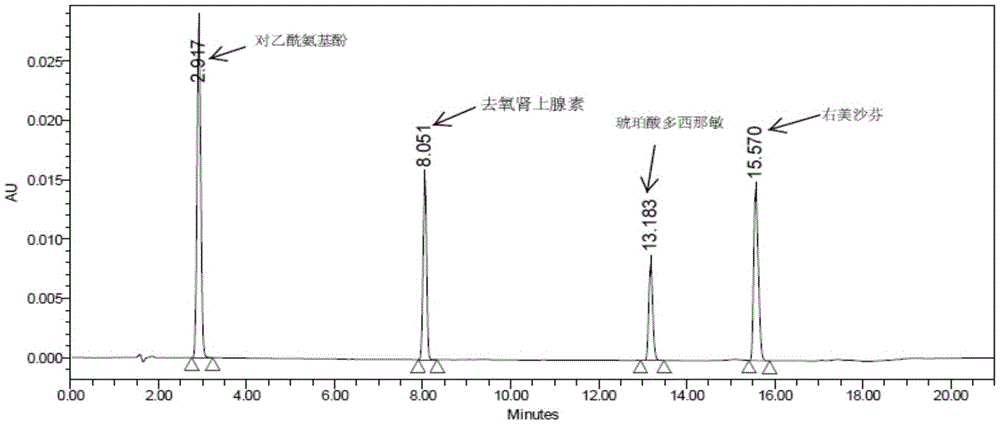
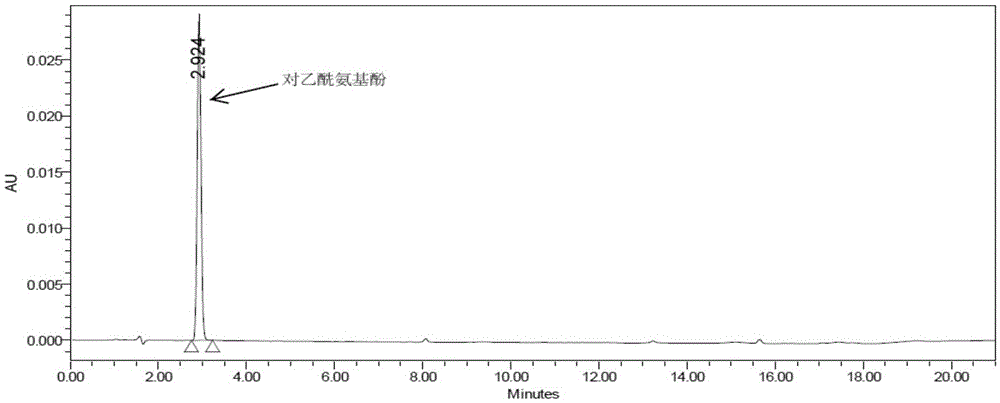
Method for determining related substances of pharmaceutical preparation containing acetaminophen, dextromethorphan hydrobromide and doxylamine succinate
ActiveCN111721855AEfficient separationSave time and costComponent separationDextromethorphan HydrobromideSuccinic acid
The invention discloses a method for determining related substances of a pharmaceutical preparation containing acetaminophen, dextromethorphan hydrobromide and doxylamine succinate. The determinationmethod disclosed by the invention is carried out by adopting a high performance liquid chromatography; proper HPLC chromatographic conditions are selected, four related substances including a dextromethorphan impurity I, a dextromethorphan impurity II, a dextromethorphan impurity III and a dextromethorphan impurity IV in a pharmaceutical preparation of acetaminophen, dextromethorphan hydrobromideand doxylamine succinate can be simultaneously determined under the same condition; effective separation of various impurities can be realized, and the detection time and the detection cost are greatly saved. The method can be used for quality research and quality control of pharmaceutical preparation products containing acetaminophen, dextromethorphan hydrobromide and doxylamine succinate.
Owner:安士制药(中山)有限公司
Application of doxylamine succinate in preparing drug for treating or preventing influenza virus
InactiveCN103251590BInhibition of replicationBroad-spectrum antiviral activityOrganic active ingredientsAntiviralsVirus strainInfluenza a
The invention discloses application of doxylamine succinate in preparing a drug for treating or preventing influenza virus. The application comprises the following steps of: selecting the doxylamine succinate with complete non-toxic concentration to carry out antiviral experiment, wherein the result shows that the micromolecule compound has remarkable antiviral activity and is dose-dependent; and detecting the antiviral activity of the doxylamine succinate to different types and subtypes of influenza viruses, wherein the result indicates that the doxylamine succinate has activity for detecting virus strain, a dose-dependent effect, as well as a certain broad-spectrum to the anti-influenza virus activity. Therefore, the doxylamine succinate disclosed by the invention can be developed as a novel anti-influenza virus drug, so that a novel way and means is provided for treating the influenza.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
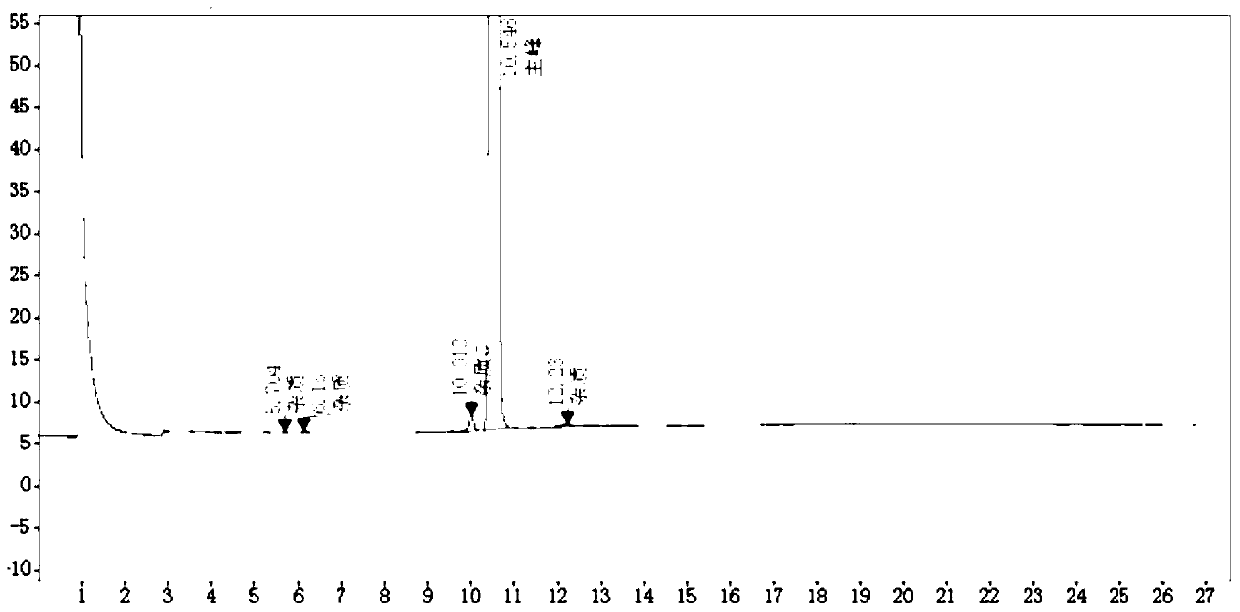
A kind of rt-hplc detection method of related substances of doxylamine succinate
The invention relates to the technical field of pharmaceutical analysis, in particular to a reversed-phase high-performance liquid chromatography detection method for related substances of doxylamine succinate and discloses an RT-HPLC detection method for the related substances of doxylamine succinate. The RT-HPLC detection method comprises the following steps of preparing an analyzing solution, setting chromatographic conditions and carrying out measurement on a machine. By means of the RT-HPLC detection method, various related impurities in a doxylamine succinate solution can be effectively measured, and separation of doxylamine succinate characteristic peaks and the related impurities in a spectrogram is especially achieved. Thus, it is ensured that the quality of products is controllable.
Owner:NANJING GRITPHARMA CO LTD
A kind of doxylamine succinate hydrochloride pyridoxine enteric-coated tablet medicinal composition and preparation method thereof
ActiveCN106606502BImprove stabilityAvoid defectsOrganic active ingredientsDigestive systemFast releaseEnteric coated tablets
The invention provides a compound doxylamine succinate-pyridoxine hydrochloride enteric-coated tablet pharmaceutical composition and a preparation method thereof. The composition comprises a tablet core containing doxylamine succinate and pyridoxine hydrochloride, an isolation coating, an enteric coating and other medicinal auxiliary materials. The preparation method has simple processes. The pharmaceutical composition has good stability, is insoluble in the stomach and can be fast released in the intestine so that the problem that the existing pharmaceutical composition has a reduced release rate when entering into an intra-intestinal neutral environment from a gastric acid environment.
Owner:SICHUAN HAISCO PHARMA CO LTD
Synthesis method of doxylamine succinate
PendingCN110498764AEasy to operateSimplify investmentOrganic compound preparationCarboxylic acid salt preparationSynthesis methodsSuccinic acid
The invention discloses a synthesis method of doxylamine succinate. The method includes: (1) adding 2-pyridylphenylmethylcarbinol into an organic solvent I, adding a catalyst, strong base or alkali metal and 2-dimethylaminochloroethane respectively, performing heating to 100-110DEG C and carrying out reaction for 1-2h; then conducting cooling and filtering; subjecting an aqueous phase to backwashing with the organic solvent I, then performing heating to 70-80DEG C, adding activated carbon for decolorization, then performing filtering, then adding a low-boiling-point organic solvent for extraction, drying the extracted organic phase, and finally conducting reduced pressure concentration at 50-60DEG C to dryness to obtain doxylamine; and (2) dissolving the doxylamine in an organic solvent II, adding succinic acid at 50-60DEG C for reaction, then employing medicinal activated carbon for decolorization and filtering, conducting cooling to a temperature at or below 5DEG C, carrying out stirring crystallization, then performing pumping filtering, and conducting washing with the precooled organic solvent II, and finally performing vacuum drying to obtain the doxylamine succinate. The synthesis method provided by the invention simplifies operation, lowers energy consumption, and has high reaction conversion rate and high yield.
Owner:深圳沃兰德药业有限公司
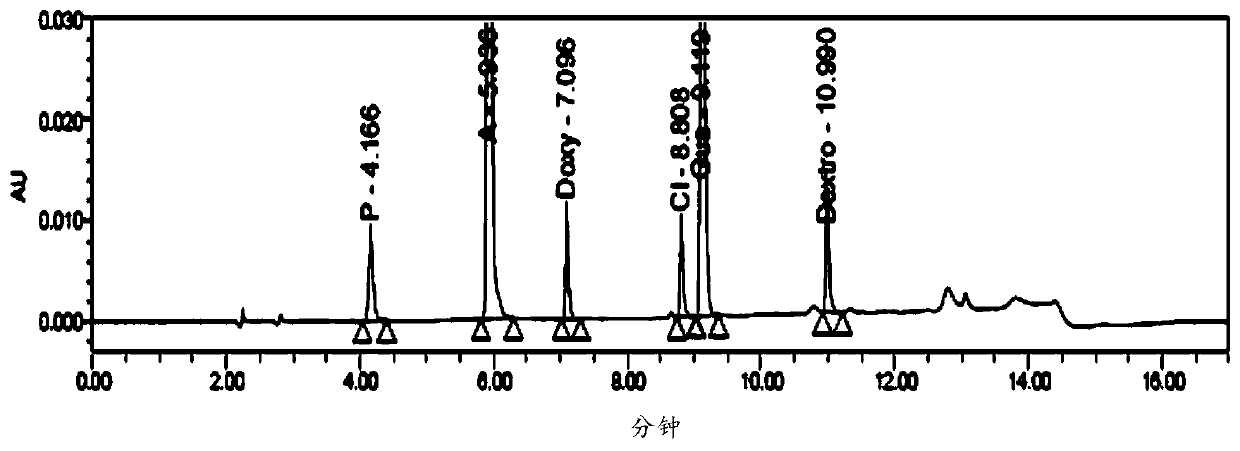
Method for the detection of six active ingredients in pharmaceutical samples
The invention provides a method for six active components in a drug sample. In the method, the six active components comprise paracetamol, phenylephrine hydrochloride, doxylamine succinate, dextromethorphan hydrobromide, guaifenesin and chlorpheniramine maleate; the method uses high performance liquid chromatography (HPLC) to detect the drug sample, wherein the mobile phase of HPLC comprises a mobile phase A which is an aqueous solution containing trifluoroacetic acid of which the concentration is 0.1v / v% and a mobile phase B which is a mixed solution of acetonitrile and methanol in a volume ratio of 60: 40. The detection method provided by the invention can simply and quickly detect the six active components in the drug sample simultaneously, and the six active components can be separatedeffectively, and the detection method is simply operated, and quickly analyzed, suitable for detection of most cold medicines and wide in detection application range.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Analysis of Amemixamine Ⅱ Soft Capsules by High Performance Liquid Chromatography
The present invention proposes a method for using high-performance liquid chromatography to analyze Amilamine II soft capsules, which contain acetaminophen, phenylephrine hydrochloride, doxylamine succinate, and dextromethorphan hydrobromide. For methorphan, HPLC analysis: using octadecylsilane bonded silica gel column as chromatographic column; using sodium octane sulfonate-phosphate buffer solution with pH 2.0-3.0 as mobile phase A; and using acetonitrile or acetonitrile The mixed solution with methanol was used as mobile phase B. This method can simultaneously and effectively detect the four active ingredients in Amimethamine II Soft Capsules, and is simple to operate, fast in analysis, good in repeatability, and has good specificity. It can more effectively and comprehensively control Amimethamine Min Ⅱ soft capsule product quality.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
A kind of determination method of dissolution rate of pharmaceutical preparation containing paracetamol, dextromethorphan hydrobromide and doxylamine succinate
ActiveCN110286162BSave time and costImprove accuracyComponent separationDextromethorphan HydrobromideDrugs preparations
The invention discloses a method for determining the dissolution rate of a pharmaceutical preparation containing paracetamol, dextromethorphan hydrobromide and doxylamine succinate. The steps are as follows: (1) The pharmaceutical preparations of dextromethorphan and doxylamine succinate are added to the dissolution medium for dissolution treatment, sampling and filtration to obtain a dissolution liquid; (2) Detecting paracetamol, dextromethorphan hydrobromide and succinate in the dissolution liquid content of doxylamine acid; (3) determining the dissolution rate of the pharmaceutical preparation according to the detection result of step (2). The invention can effectively simulate the dissolution behavior in vivo in vitro, determine the dissolution rate of soft capsules containing paracetamol, dextromethorphan hydrobromide and doxylamine succinate, and provide technical support for drug research and development and quality control . The invention can simultaneously measure the dissolution results of the three substances under the same condition, greatly saves the detection time and the detection cost, and has good accuracy and reproducibility.
Owner:安士制药(中山)有限公司
Doxylamine succinate preparation method
InactiveCN107098851ASolving the defects of high-purity doxylamine succinateEasy to operateCarboxylic acid salt preparationRe crystallizationCrystallization
The present invention provides a method for preparing high-purity doxylamine succinate by adding an auxiliary alkali in a doxylamine succinate crude product re-crystallization process. According to the present invention, the method has characteristics of simple operation, economy and environmental protection, and is suitable for industrial production.
Owner:NANJING NONGKANG BIOTECH CO LTD
Composition and soft capsule for treating cold at night
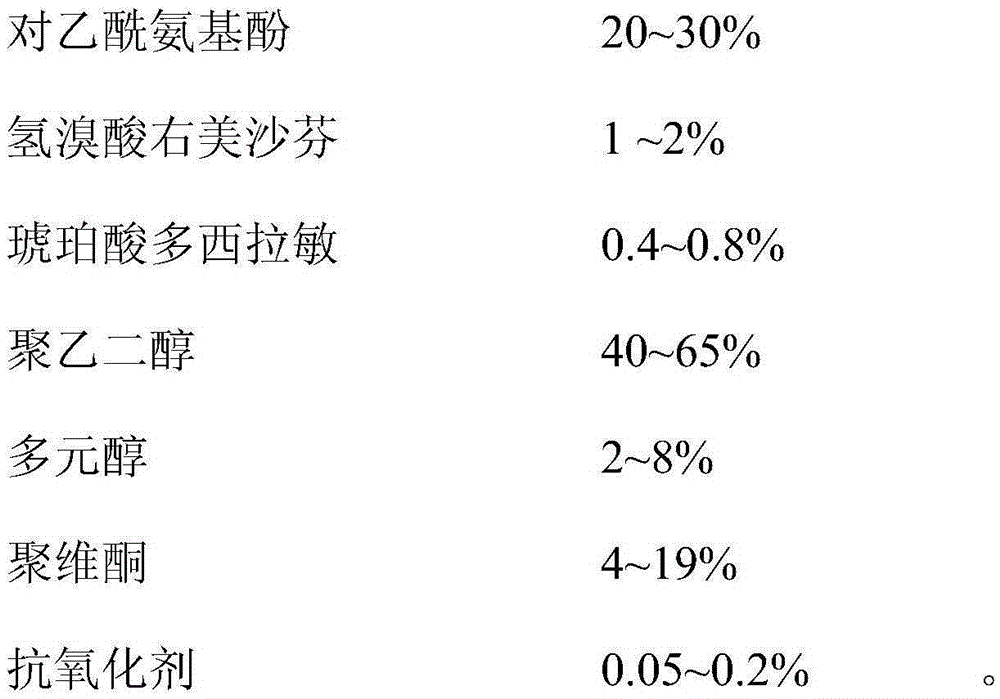
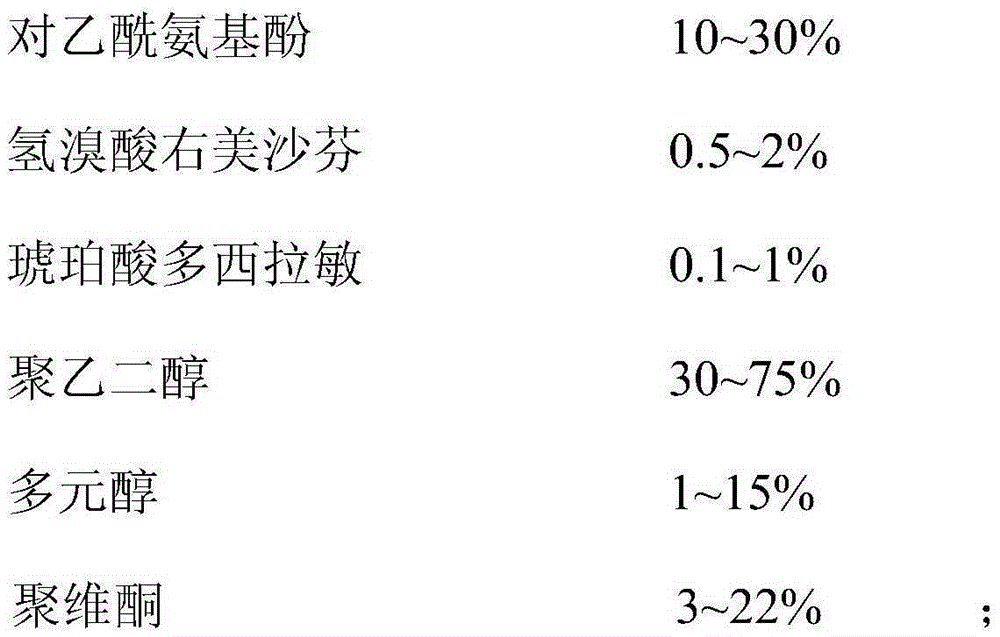
ActiveCN103446153BDefinite curative effectNice appearanceOrganic active ingredientsAntipyreticSimple componentPolyethylene glycol
The invention discloses a composition and a soft capsule for treating cold for night use. The composition comprises acetaminophen, dextromethorphan hydrobromide, succinic acid doxylamine, polyethylene glycol, polyhydric alcohols and povidone; the soft capsule comprises a soft capsule shell and content in the soft capsule shell, wherein the content comprises acetaminophen, dextromethorphan hydrobromide, succinic acid doxylamine, polyethylene glycol, polyhydric alcohols and povidone, and the soft capsule shell comprises the following components: gelatin, plasticizer, purified water and colorant. Aiming at overcoming the defects of the prior art, the invention provides the composition for treating cold for night use, and the composition has simple components and is stable and effective. The invention also aims at providing the soft capsule comprising the composition, and the soft capsule is applicable to relieving cold symptoms such as headache, fever, cough, rhinobyon, rhinorrhea, irritability, and the like.
Owner:安士制药(中山)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com