Method for synthesizing doxylamine succinate through base catalysis
A technique for the synthesis of doxylamine succinate and its synthesis method, which is applied in the field of raw material drug synthesis, and can solve the problems of sodium amide being dangerous, not easy to store, and the price of 2-chloroethyldimethylamine is expensive.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
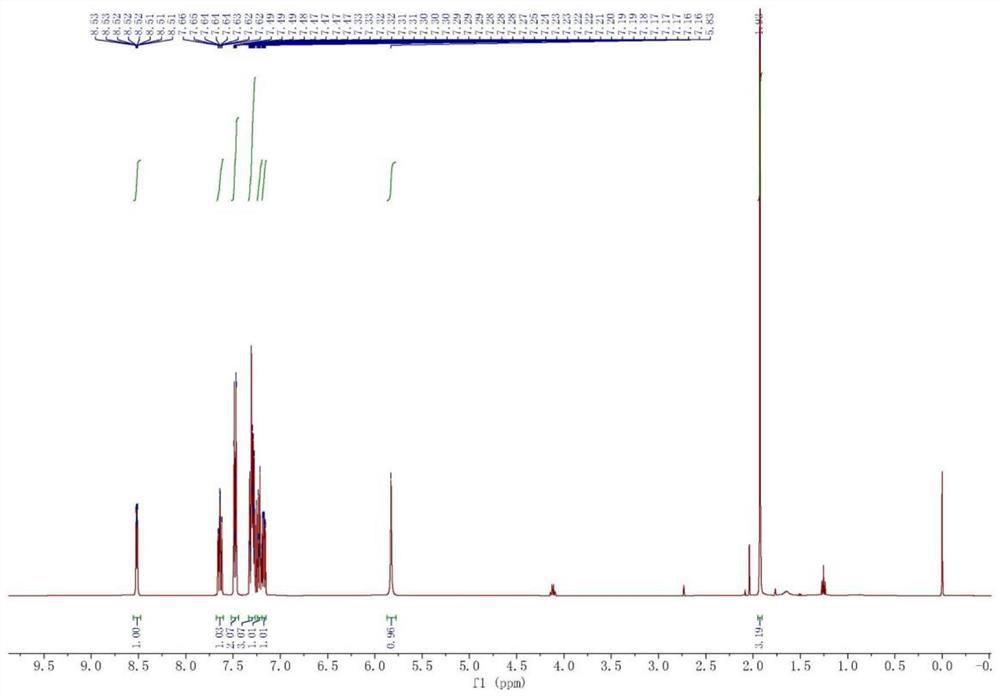
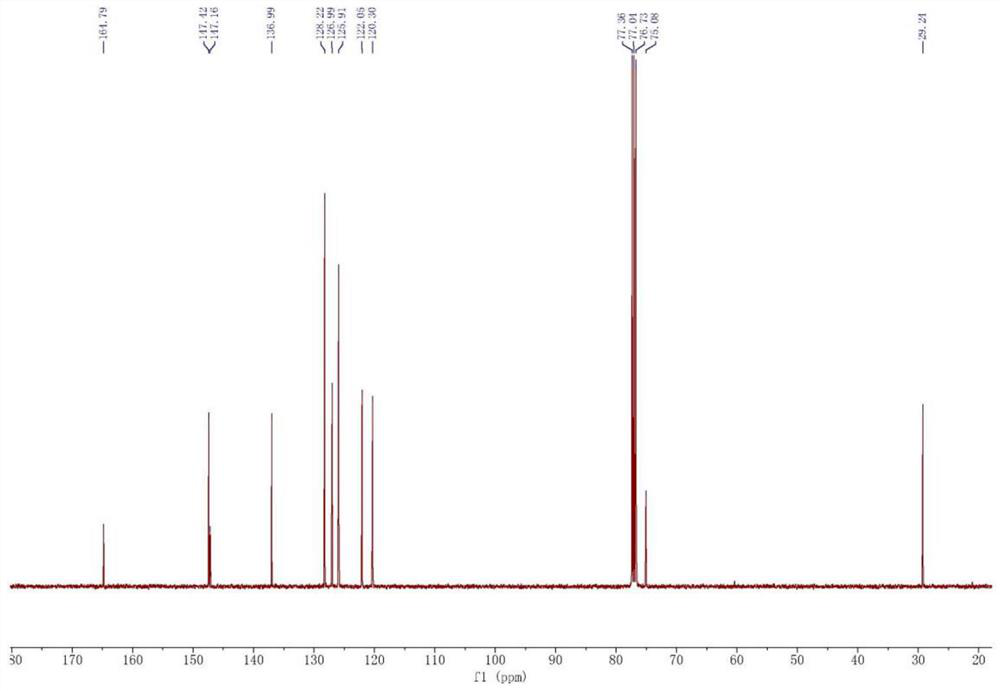
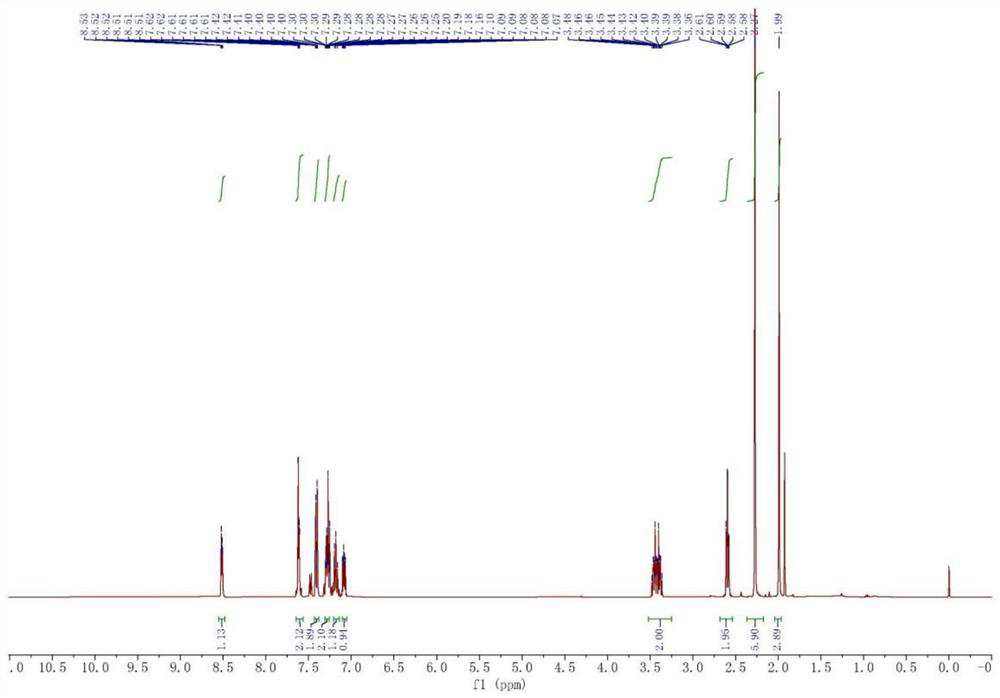
[0031] Synthesis of 1, 2-pyridylphenyl methyl methanol
[0032]Weigh 34.2g (1.42mol) magnesium chips and 50mg (0.20mmol) iodine into a 2L four-mouth bottle, add 1.2L anhydrous tetrahydrofuran, nitrogen protection, the reaction solution is heated to reflux. Weigh 195.6 g (1.24 mol) bromobenzene mixed with 195 g of tetrahydrofuran, slowly added to the reaction solution. About two hours in drops. After the reaction is initiated, the tetrahydrofuran is refluxed in large quantities, and the reaction liquid becomes turbid and the color gradually becomes metallic gray. Keep the tetrahydrofuran backflow for two hours. Weigh 100 g (0.83 mol) 2-acetylpyridine mixed with 100 g of tetrahydrofuran, slowly added to the above Grigald reagent, about three and a half hours. The mixture is immediately produced by the white milk when the mixture is dripped into the Grignard reagent, and a large amount of exothermic, anhydrous tetrahydrofuran reflux. After the drop-added, the solution becomes clear, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com