Synthetic method of doxylamine succinate
A technique for the synthesis of doxylamine succinate, which is applied in the fields of medical technology and organic synthesis, can solve the problems of low melting point, insufficient purity, and toxic benzene by-products, and achieve safe and reliable processes, simple post-processing, and easy raw materials. The effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] A kind of synthetic method of doxylamine succinate of the present invention comprises the following steps:
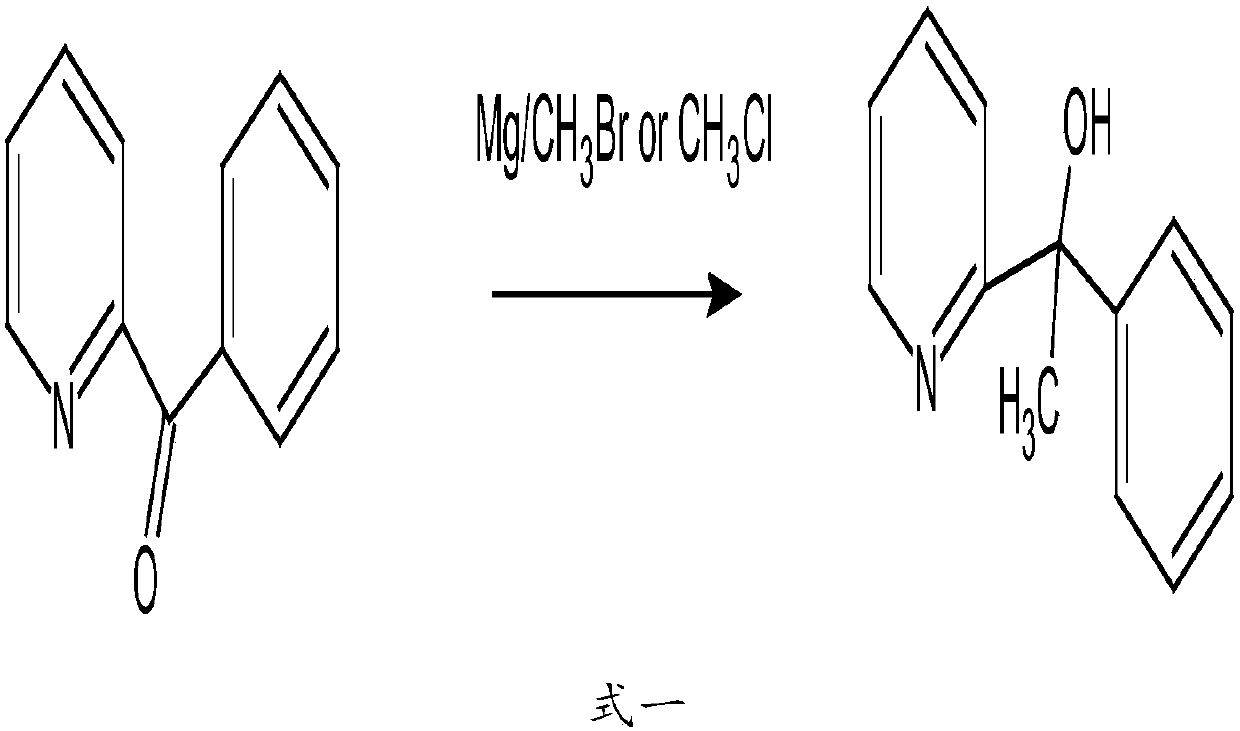
[0045] 1. Synthesis of 2-pyridylphenylmethylmethanol from acetophenone and 2-bromopyridine under the condition of butyllithium;
[0046]
[0047] 2. 2-pyridylphenylmethylcarbinol and 2-dimethylaminoethyl chloride generate doxylamine free base under the action of an organic base;
[0048]
[0049] 3. Doxylamine free base is finally salified with succinic acid to generate doxylamine succinate.
[0050]
Embodiment 1
[0051] The synthetic method of embodiment 1 doxylamine succinate
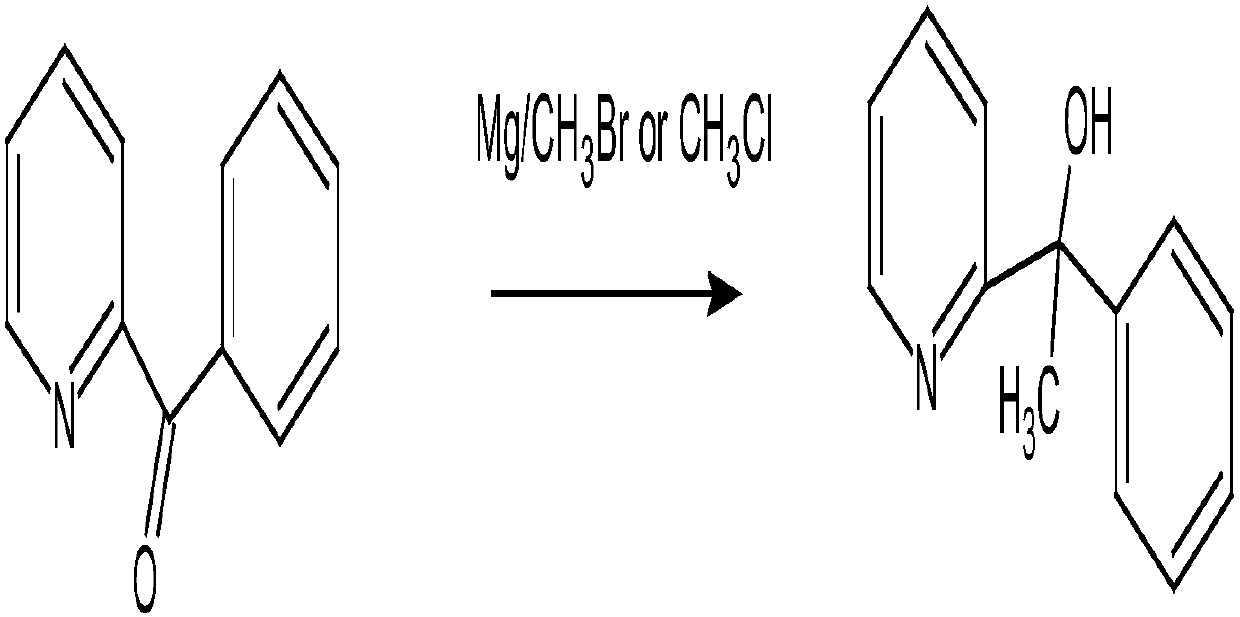
[0052] The synthetic method reaction formula of the doxylamine succinate of the present embodiment is as follows:
[0053]
[0054] Including the following specific synthetic steps:
[0055] A. Add anhydrous ether 1850G and 2-bromopyridine 370G (moisture content 0.05%, GC purity 99.50%, 2.34MOL) in anhydrous reactor, cool down to -65°C and replace with nitrogen three times to ensure that the reaction system is free of oxygen , then add 983.54ML (about 670G, 2.46MOL) 2.5M butyllithium n-hexane solution (about 4-6 hours) dropwise at the temperature of the reaction solution at -65~-55°C, complete the reaction at the same temperature for 1 hour . Then dropwise add a solution (about 3 hours) composed of 270.0G acetophenone (2.25MOL) and 400G anhydrous isopropyl ether under the condition of -65~-55°C. After completion, after 0.5 hours of reaction at the same temperature, the temperature is raised to - Continue ...
Embodiment 2
[0063] The synthetic method of embodiment 2 doxylamine succinate
[0064] The synthetic method reaction formula of the doxylamine succinate of the present embodiment is as follows:
[0065]
[0066]
[0067] Including the following specific synthetic steps:
[0068]A. Add anhydrous tetrahydrofuran 1450G and 2-bromopyridine 370G (moisture content 0.05%, GC purity 99.50%, 2.34MOL) into anhydrous reaction kettle, cool down to -65°C and replace with nitrogen three times to ensure that the reaction system is free of oxygen , then add 983.54ML (about 670G, 2.46MOL) 2.5M butyllithium n-hexane solution (about 4-6 hours) dropwise at the temperature of the reaction solution at -65~-55°C, complete the reaction at the same temperature for 1 hour . Then a solution (about 3 hours) consisting of 270.0G acetophenone (2.25MOL) and 400G anhydrous isopropyl ether was added dropwise at -65 to -55°C. After completion, the temperature was raised to - Continue to stir at 40 to -35°C for 3 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com