A kind of determination method of dissolution rate of pharmaceutical preparation containing paracetamol, dextromethorphan hydrobromide and doxylamine succinate
A technology of dextromethorphan hydrobromide and doxylamine succinate, applied in the field of medicine, achieves the effects of good accuracy and reproducibility, saving detection cost and saving detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
[0077] experimental method:
[0078] The dissolution assay method of the soft capsule containing paracetamol, dextromethorphan hydrobromide and doxylamine succinate, it comprises the steps:
[0079] (a) The dissolution medium is placed in a water bath, preheated to 41°C, and degassed by vacuum filtration for 10 minutes;
[0080] (b) 900 mL of the degassed dissolution medium was added to the dissolution vessel, and the temperature was kept at 37°C;
[0081] (c) drop the soft capsule containing paracetamol, dextromethorphan hydrobromide and doxylamine succinate into the dissolution vessel;
[0082] (d) running the dissolution apparatus, the rotation speed is set to 100rpm / min, at 45min, take 10mL of the dissolution solution, filter, and take the subsequent filtrate as the dissolution solution;
[0083] (e) adopt the HPLC method to measure the content of acetaminophen, dextromethorphan hydrobromide and doxylamine succinate in the leaching solution; chromatographic conditions in...
experiment example 1
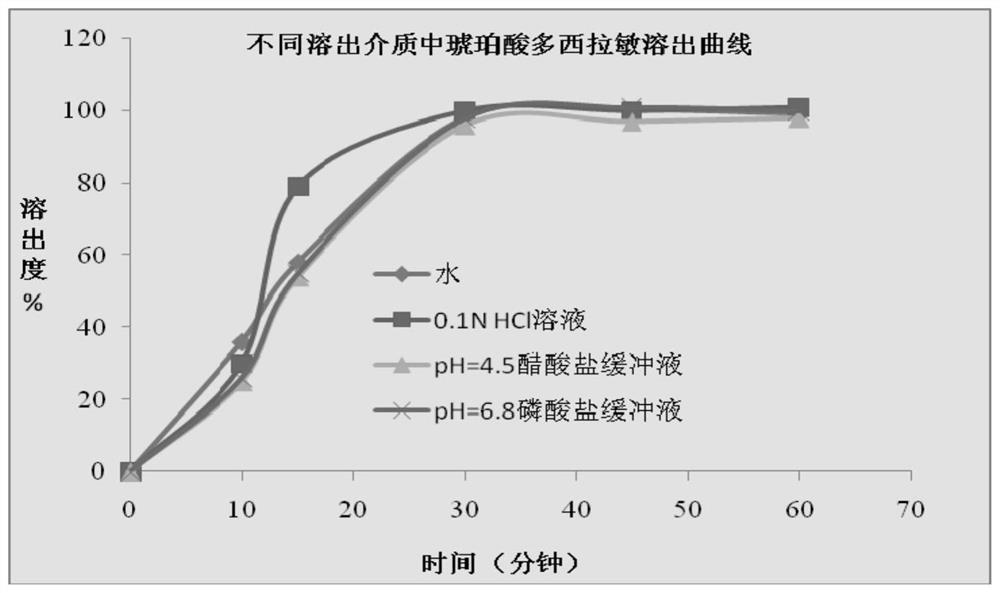
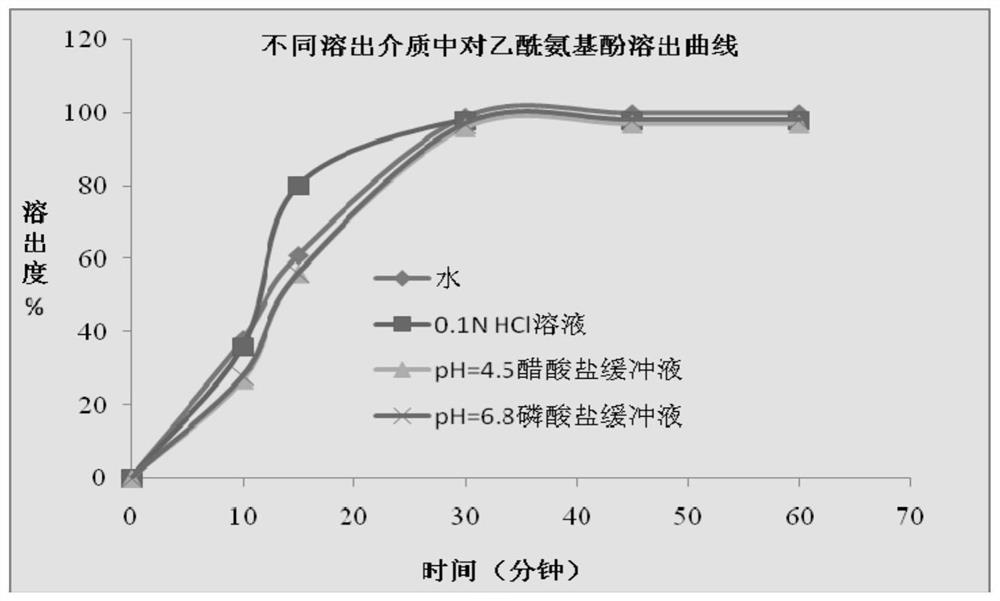
[0100] In this experimental example, a variety of dissolution media were compared to determine the optimal dissolution media. Specifically, purified water, 0.1N HCl solution, acetate buffer with pH=4.5, and phosphate buffer with pH=6.8 were used as dissolution media, and different dissolution media were used to determine the content of acetaminophen according to the operation of the above experimental method. Dissolution of soft capsules of phenol, dextromethorphan hydrobromide, and doxylamine succinate, the results are as follows Figures 2 to 4 and Tables 1 to 4.
[0101] Table 1 Dissolution with purified water as the dissolution medium
[0102]
[0103]
[0104] Table 2 The dissolution rate of 0.1N HCl solution as the dissolution medium
[0105]
[0106]
[0107] Table 3 dissolution medium is pH=4.5 dissolution rate of acetate buffer
[0108]
[0109]
[0110] Table 4. The dissolution medium is the dissolution rate of pH=6.8 phosphate buffer
[0111] ...
experiment example 2
[0115] In this experimental example, different rotational speeds are compared to determine the optimal rotational speed. According to the operation of the above-mentioned experimental method, the dissolution rate of the soft capsules containing paracetamol, dextromethorphan hydrobromide and doxylamine succinate was measured at different rotational speeds (50 rpm / min and 100 rpm / min), and the results are as follows Figures 5 to 7 and Tables 5-6.
[0116] Table 5 Dissolution at 50 rpm / min
[0117]
[0118]
[0119]Table 6 Dissolution at 100 rpm / min
[0120]
[0121] It can be seen from the above determination results that the soft capsules containing paracetamol, dextromethorphan hydrobromide and doxylamine succinate have better dissolution effect under the condition of 100rpm / min.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com