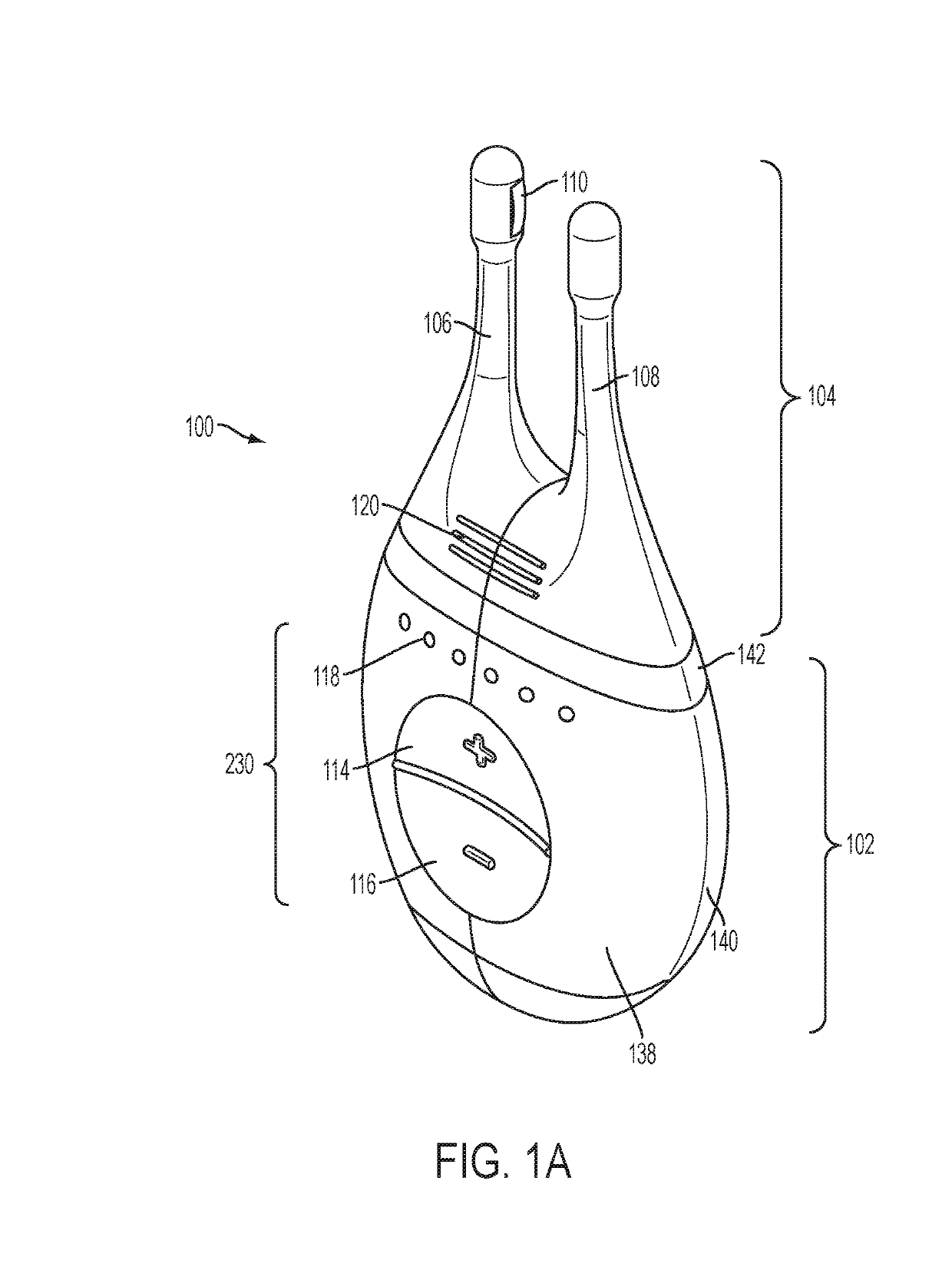
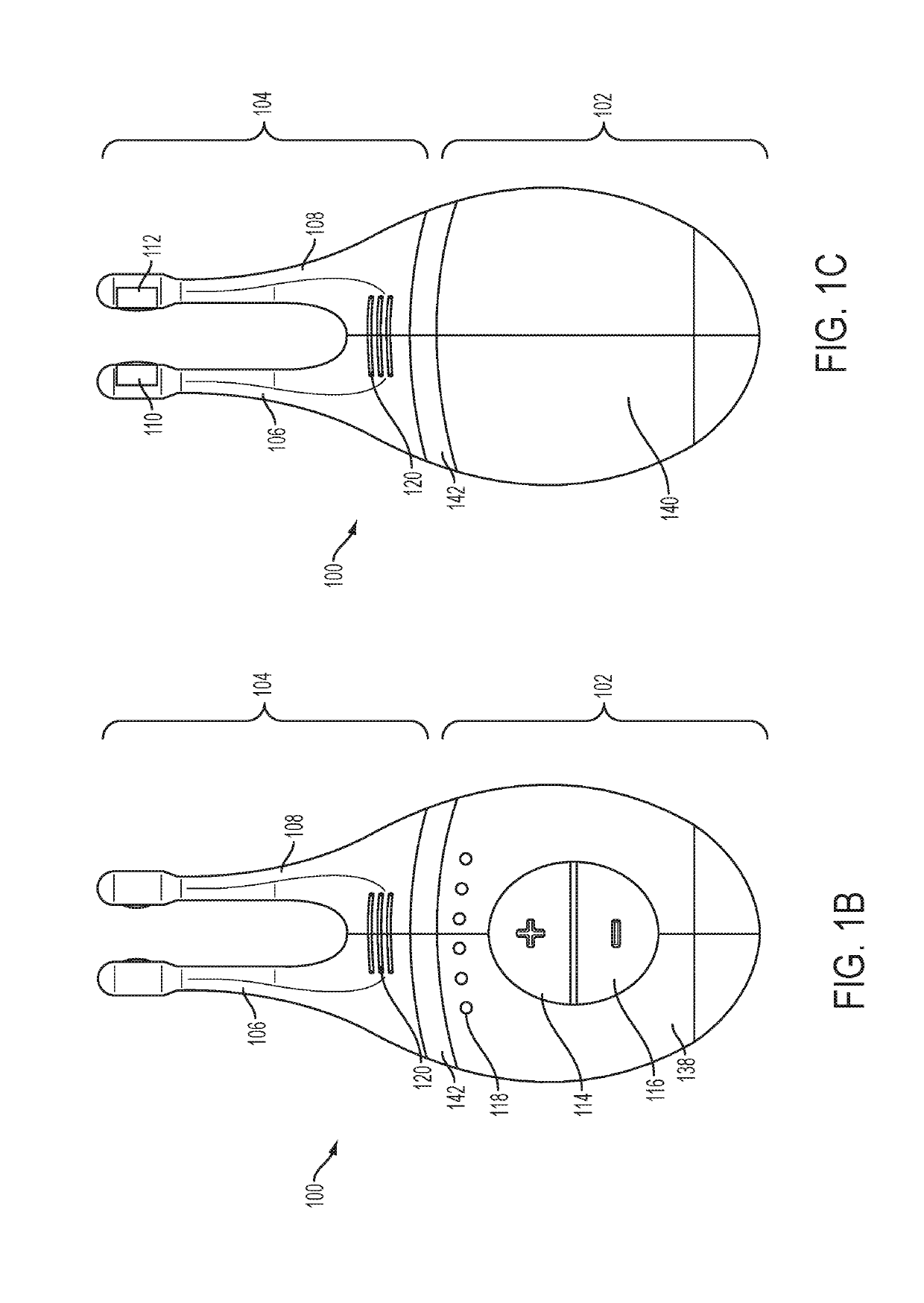
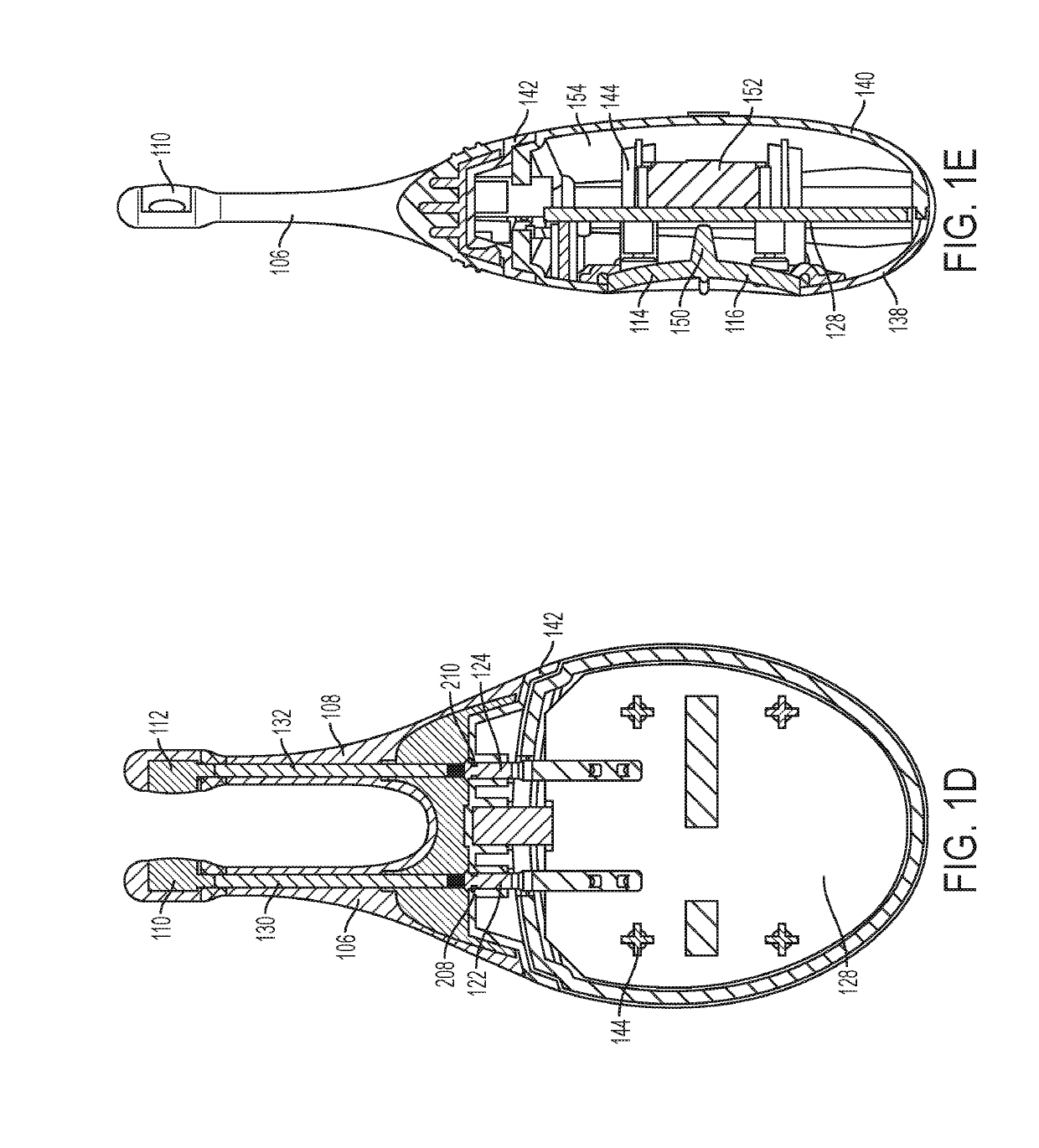
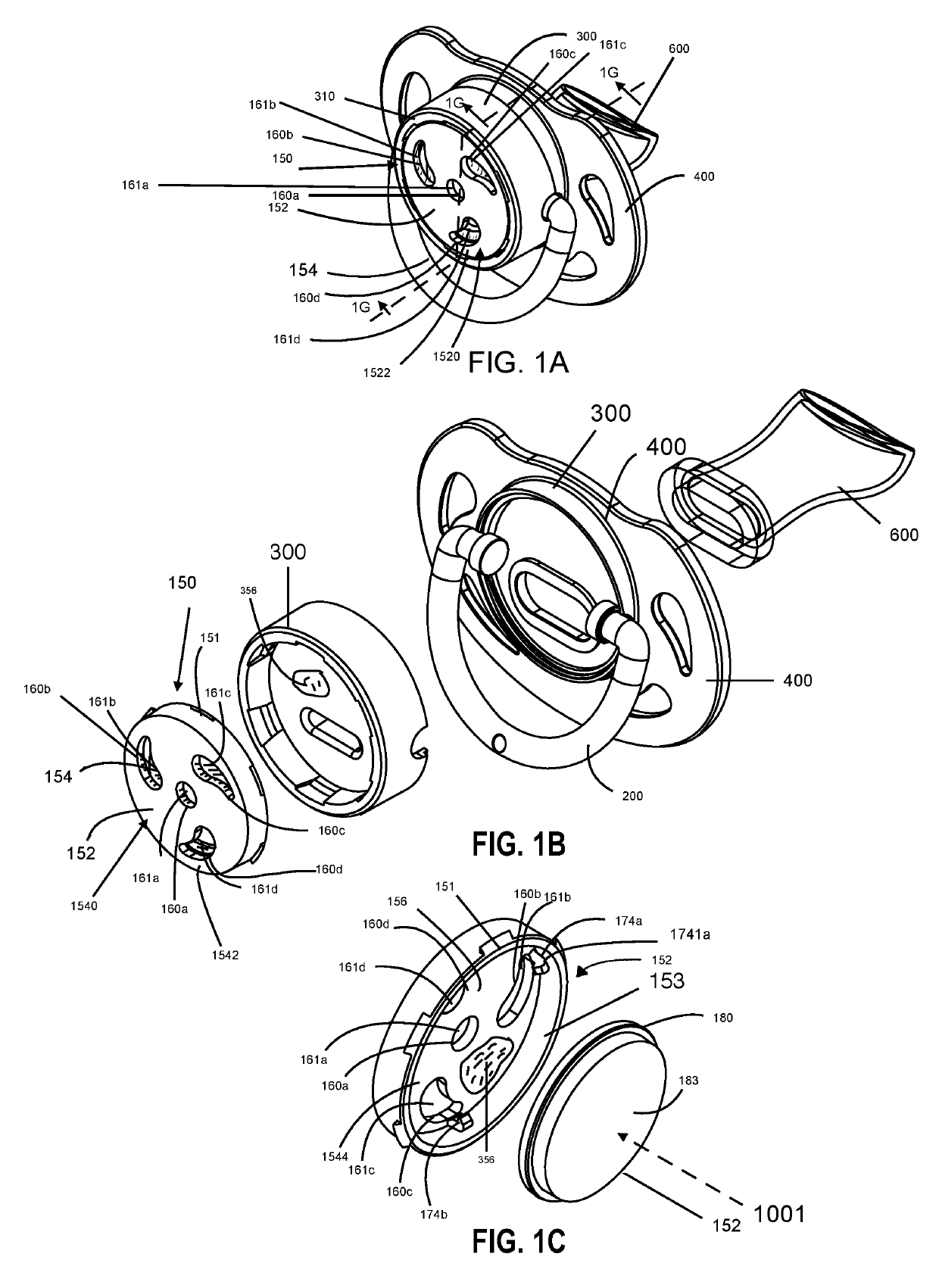
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
387 results about "Nasal congestion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Difficulty in breathing usually due to swelling of the mucous membrane lining of the nose.
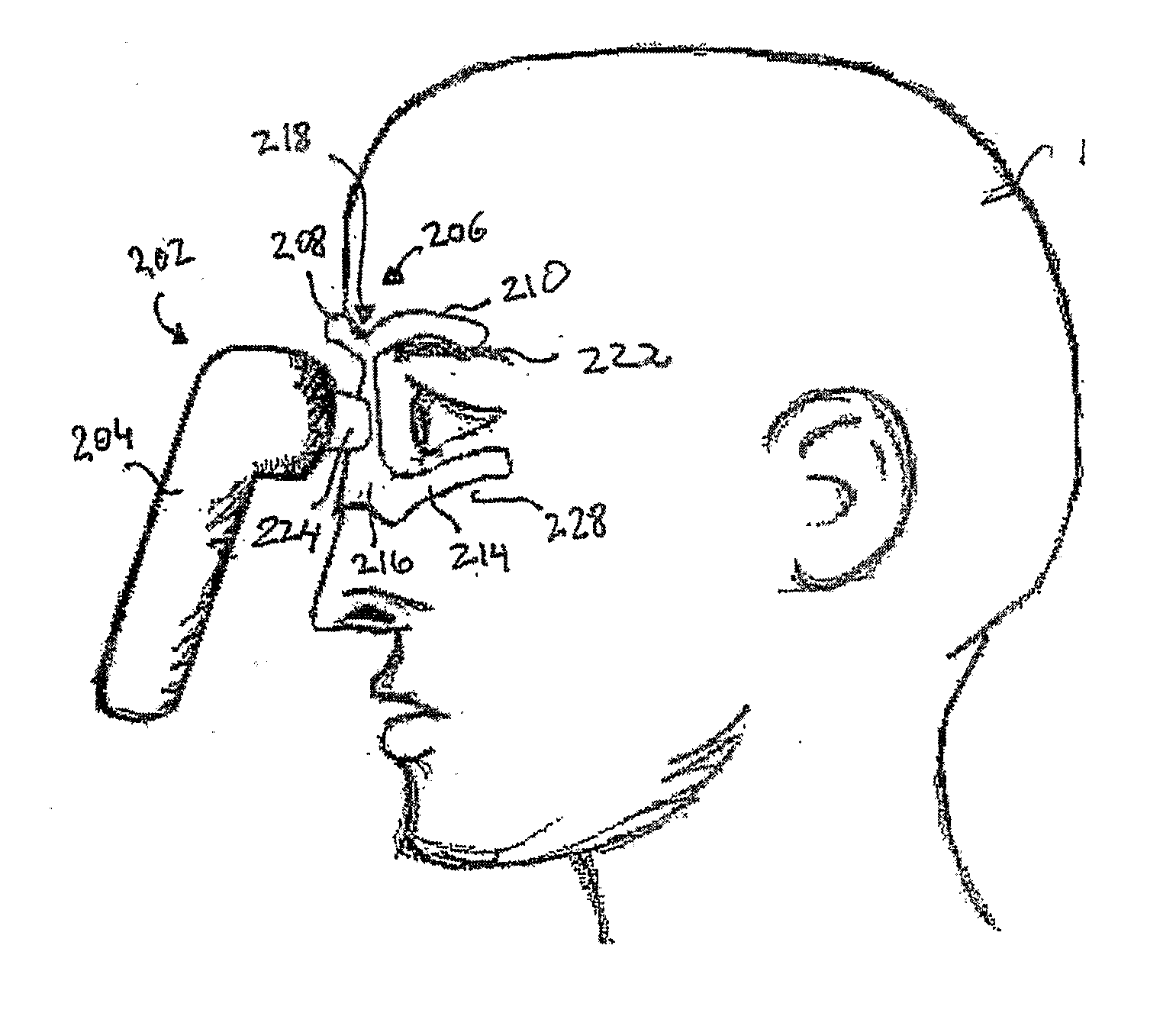
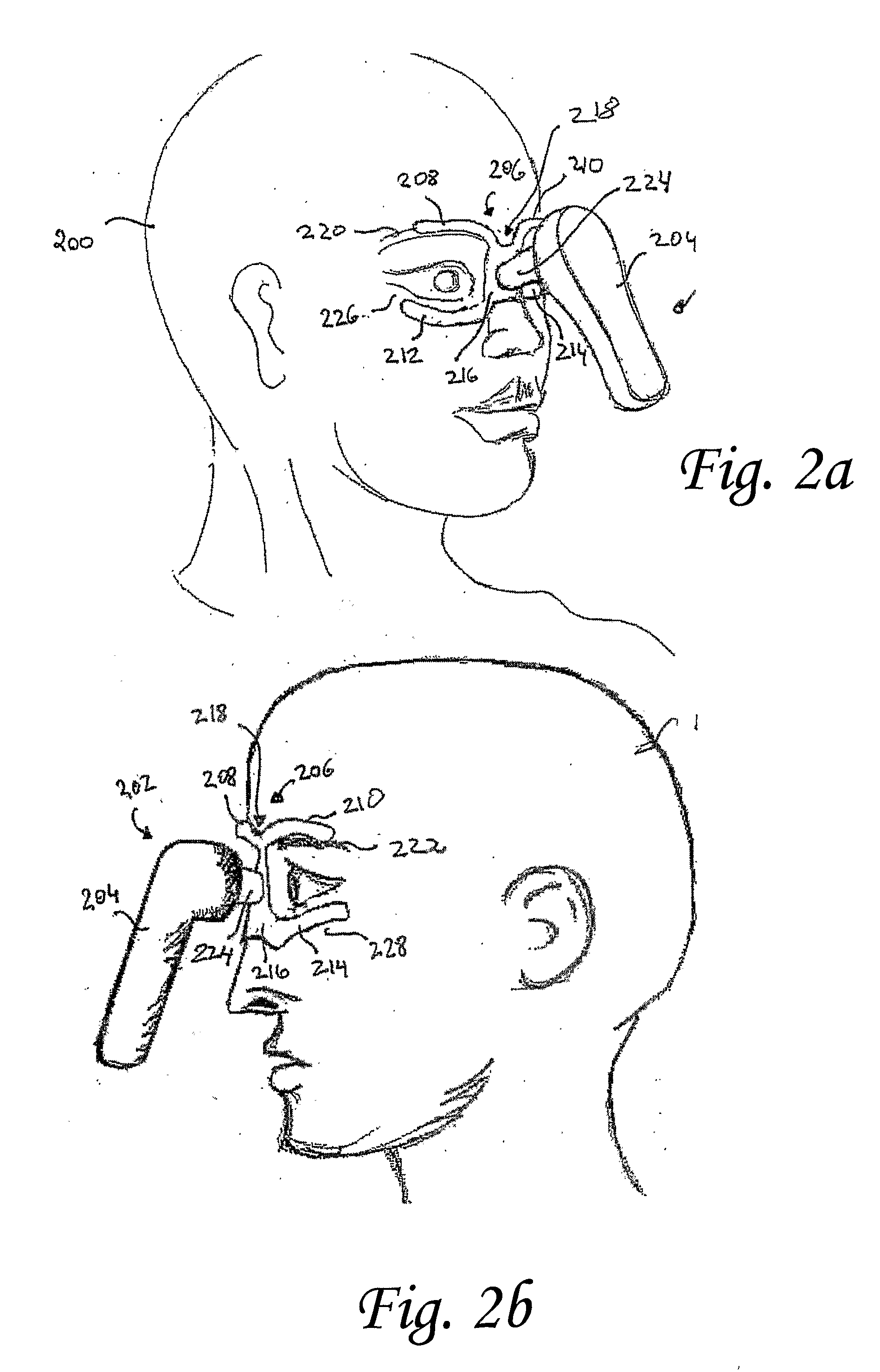
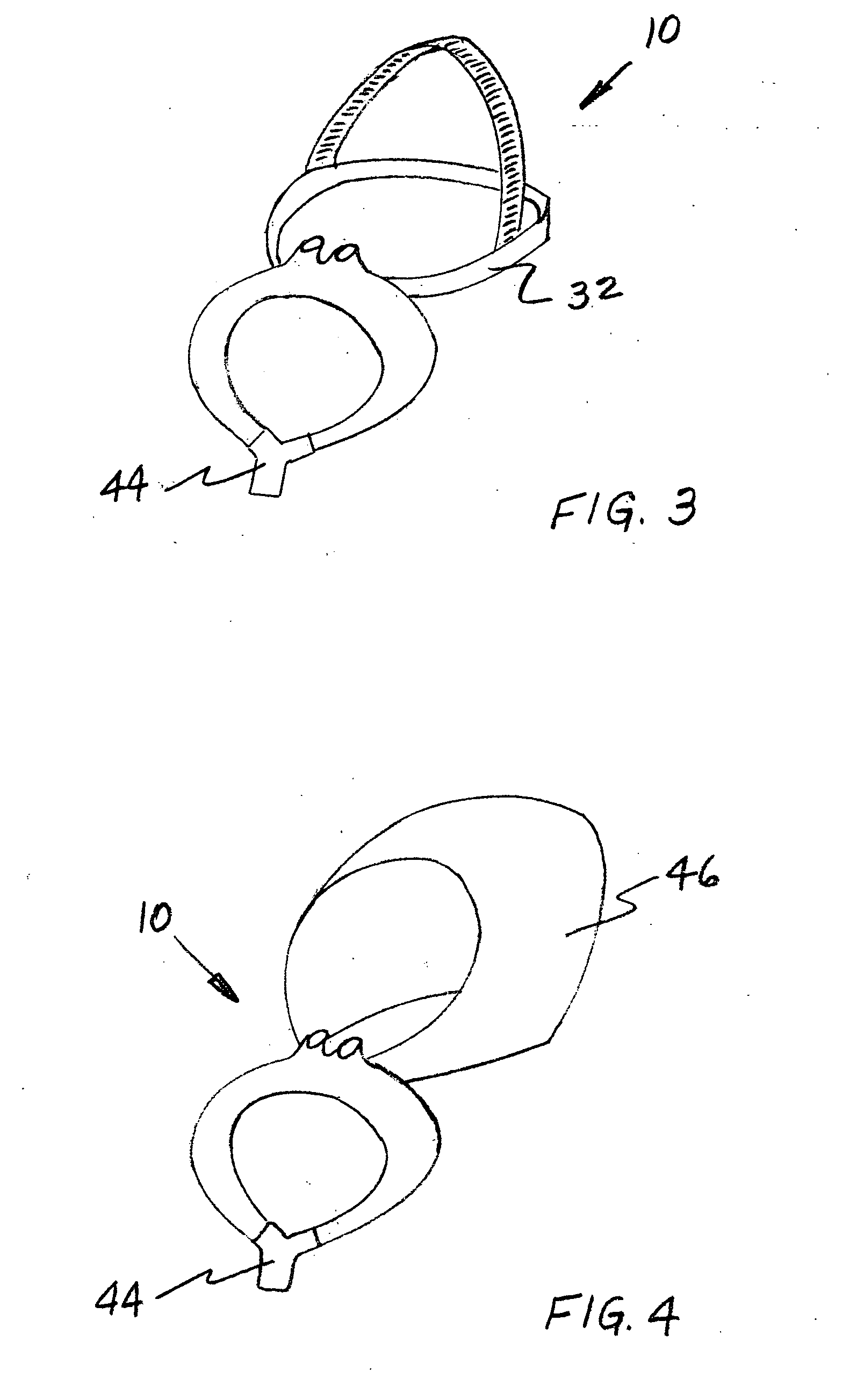
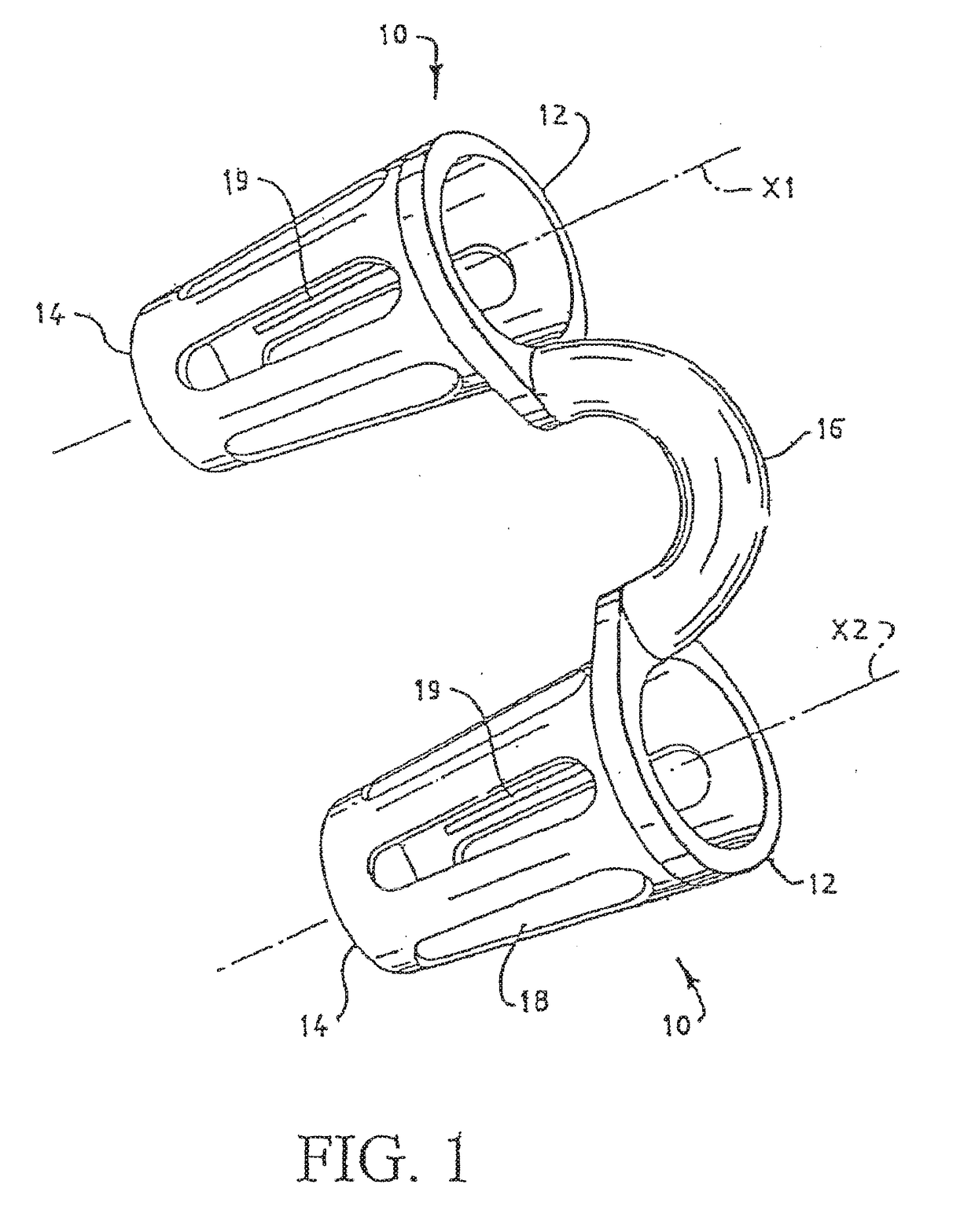
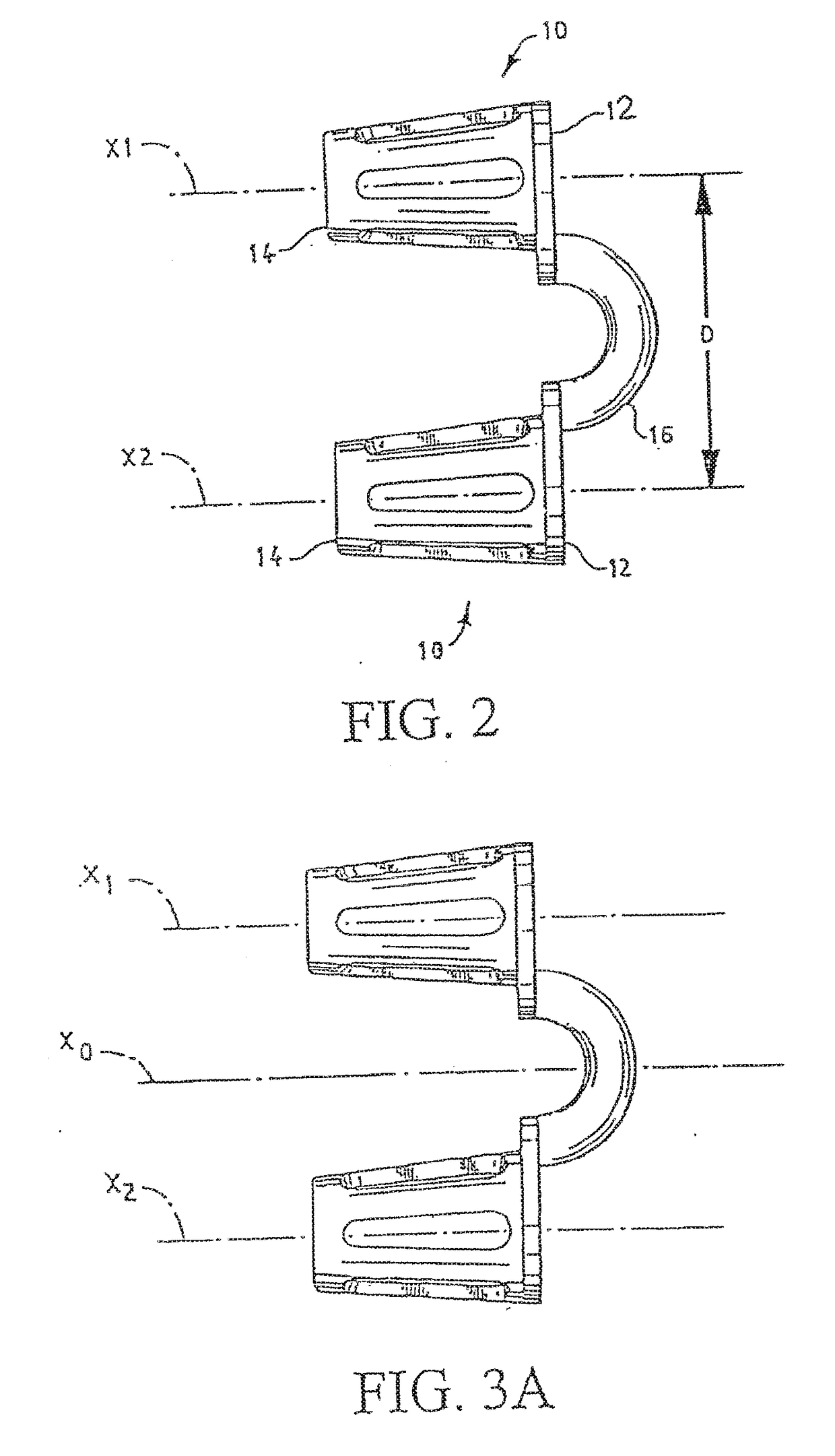
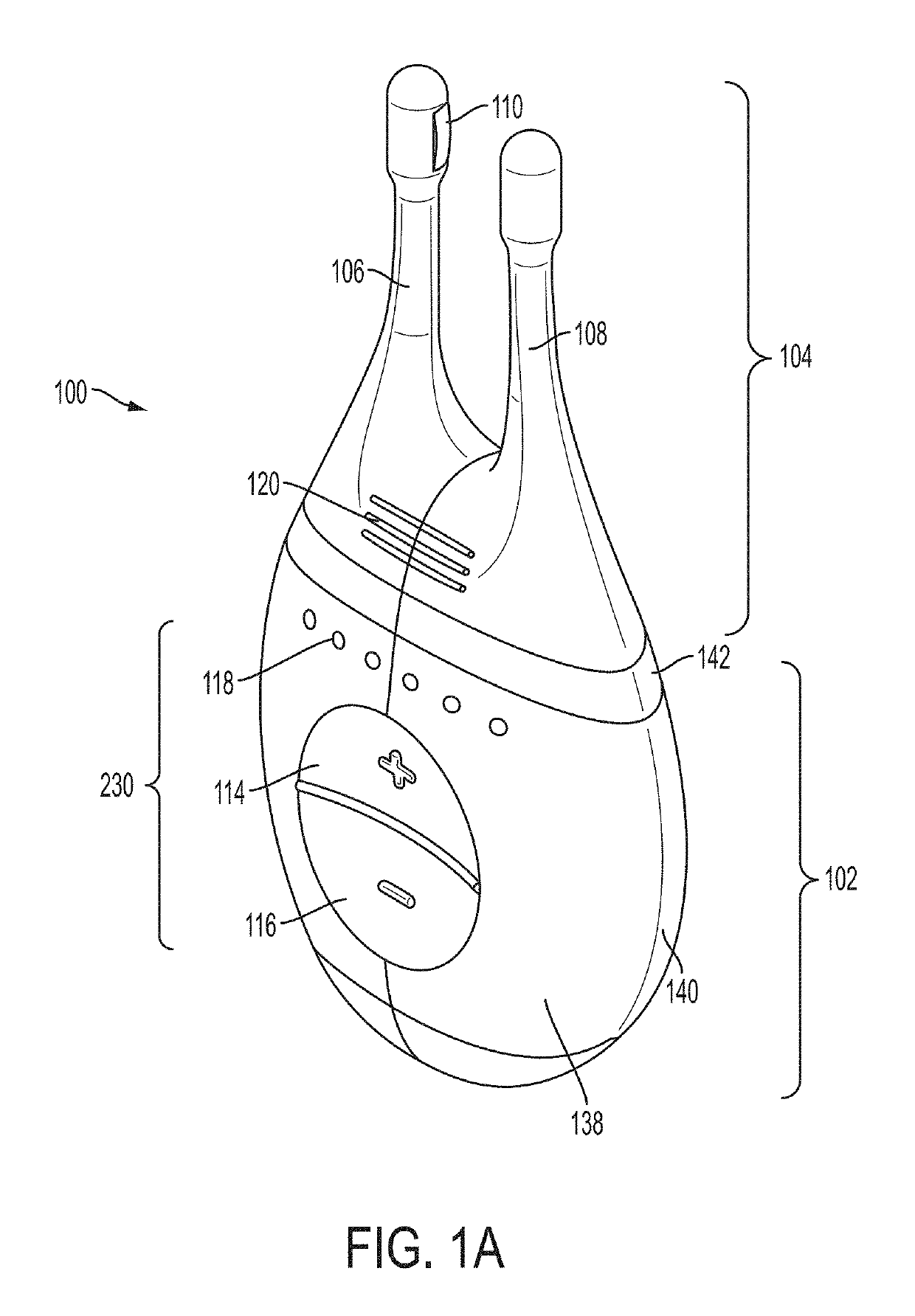
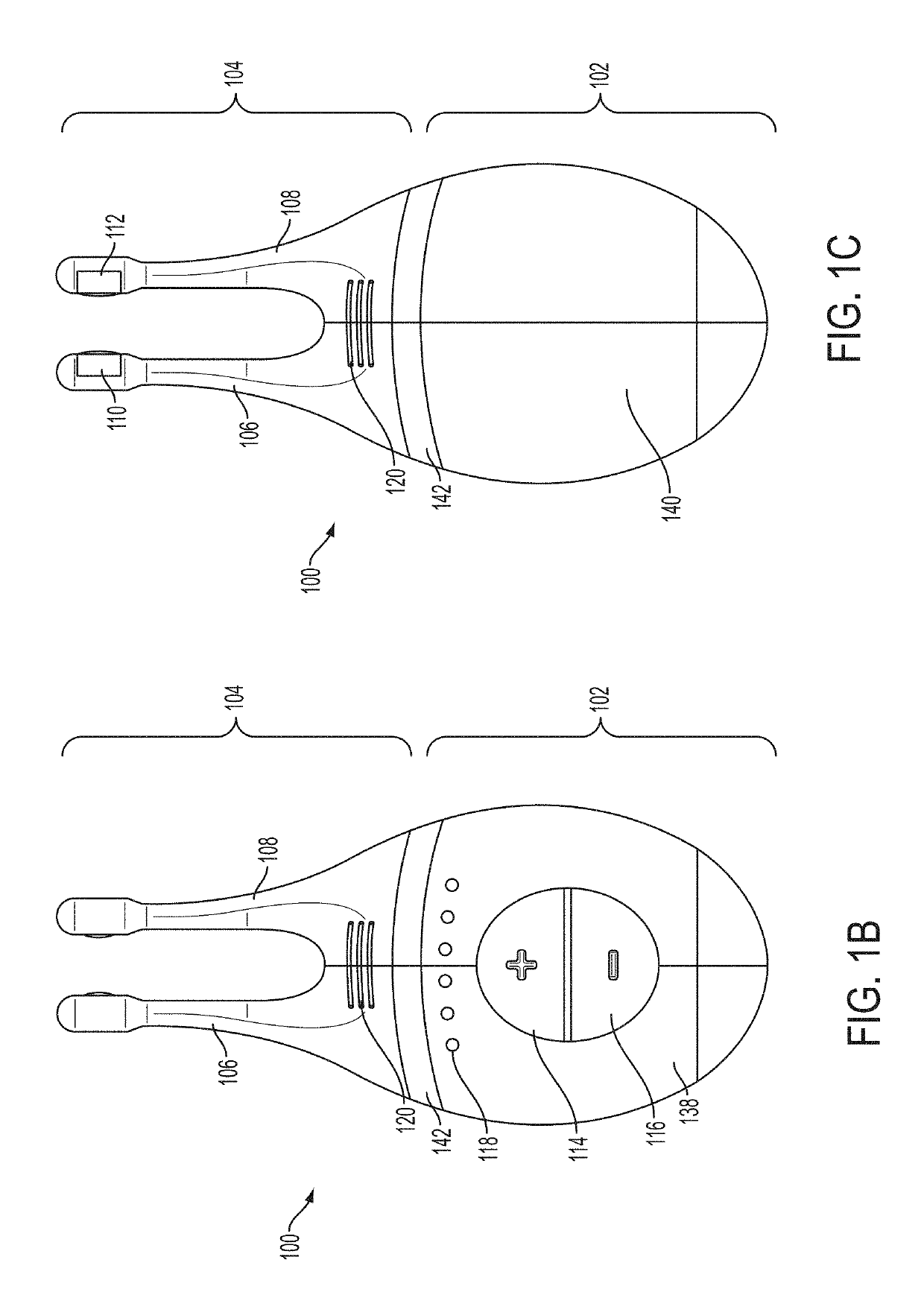
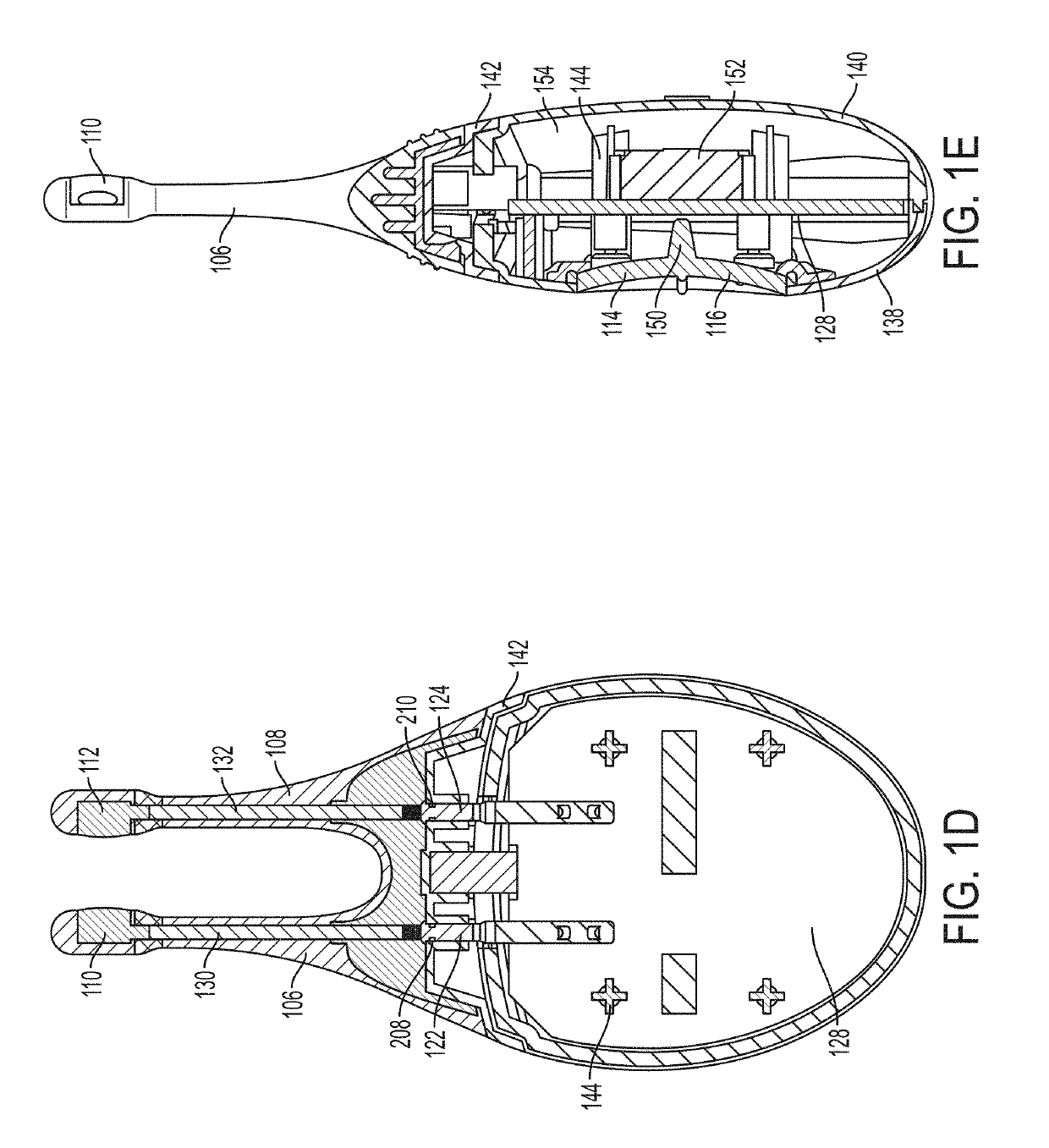
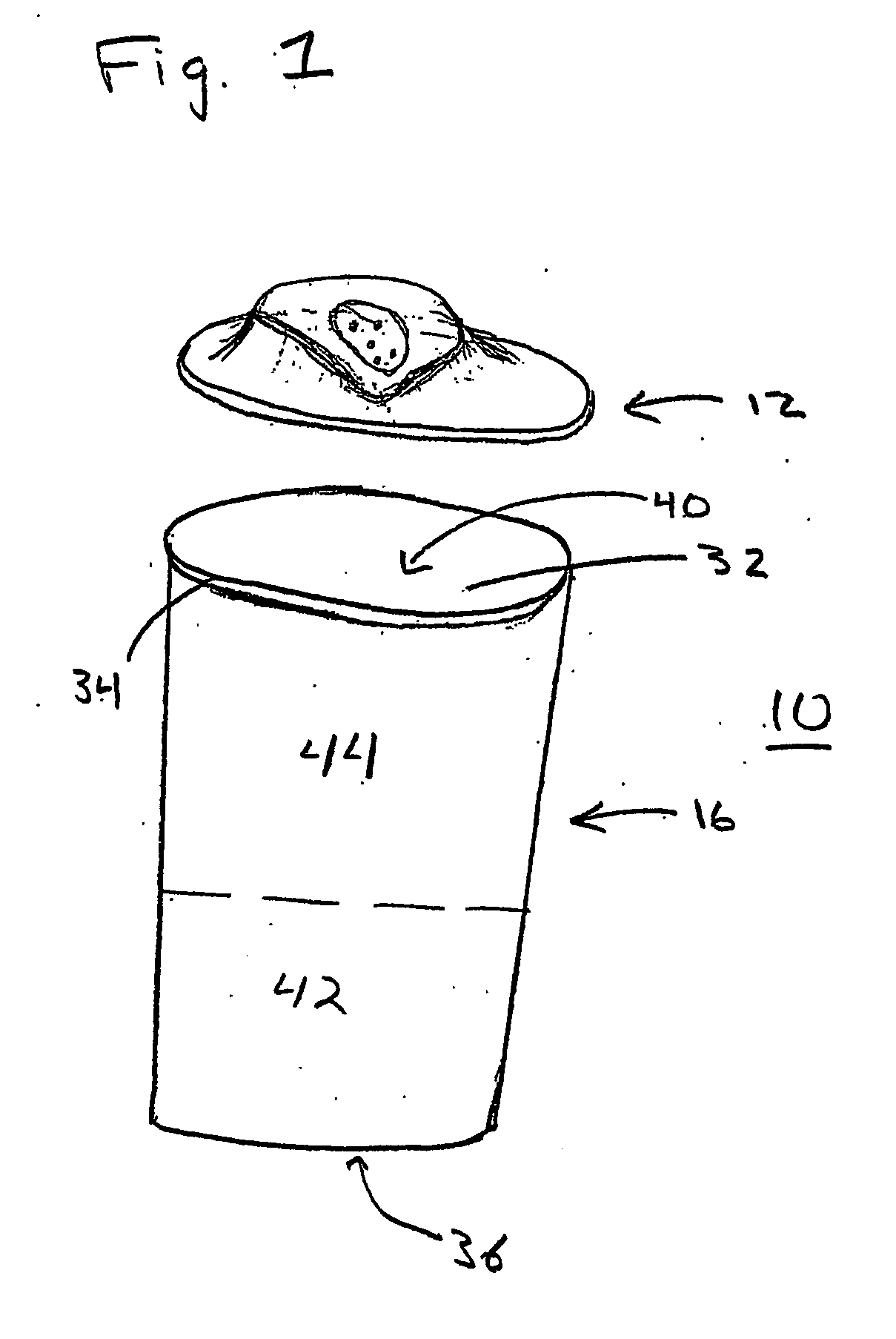
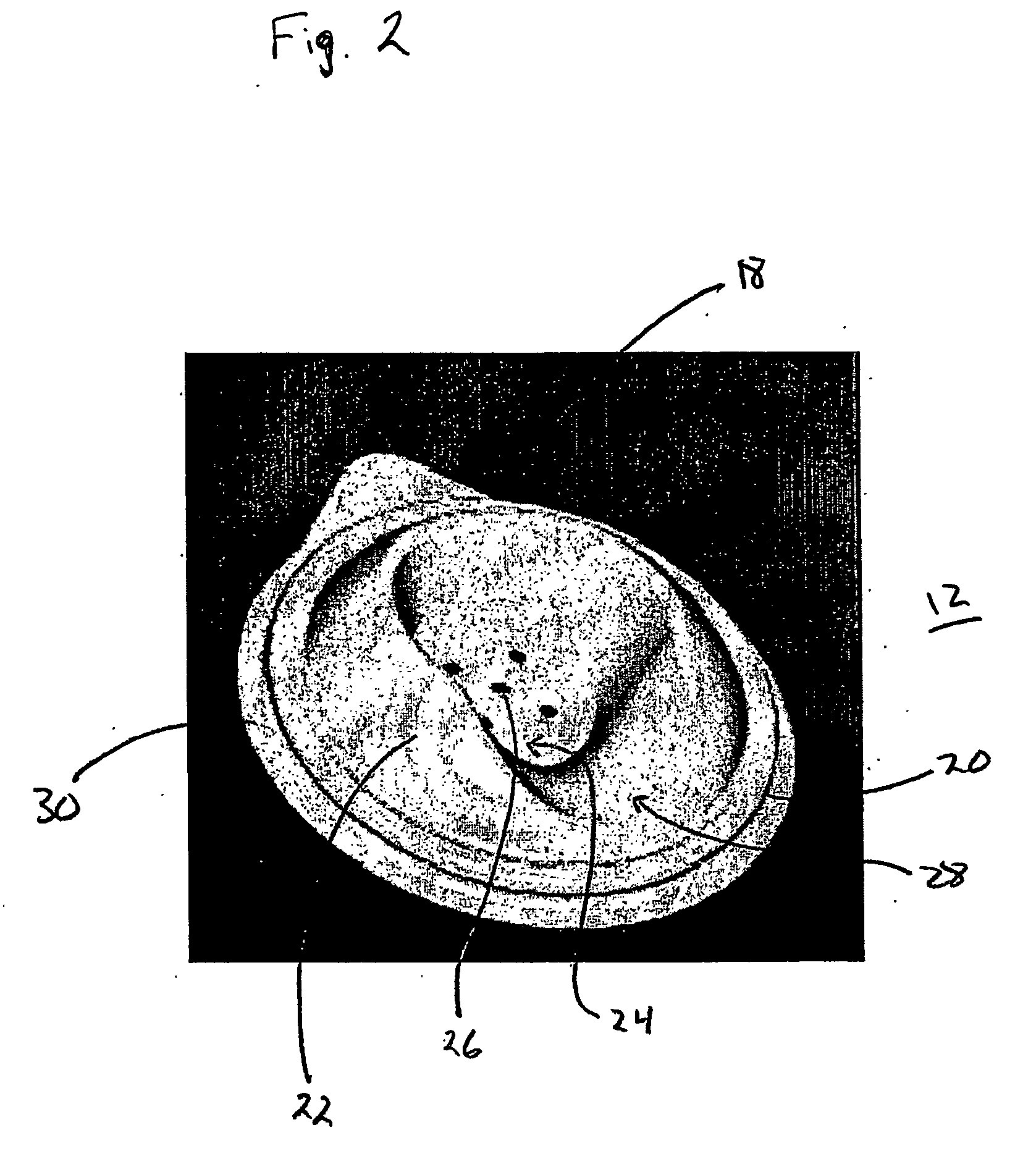
Vibrating Device For Treating Nasal Congestion and Sinusitis Symptoms and Method Thereof
The present invention discloses a method of treating nasal congestion and / or relieving sinusitis symptoms in a patient comprising attaching a vibration generating means to the patient head, at a location adjacent to sinuses to be treated, generating a vibration by said vibration generating means, and delivering the same to said patient. The present invention also discloses a device for treating nasal congestion and / or relieving sinusitis symptoms in a patient, comprising attaching means and a vibration generating means; said attaching means are in communication with the patient's head in a location adjacent to said nasal cavity, nasal passageway or sinuses to be treated; said generating means are adapted to vibrate said attaching means, to vibrate the location adjacent to said nasal cavity or sinuses and thus improved mucociliary clearance of secretions in nasal cavity or sinuses.
Owner:ADS & B INVESTMENT FUND
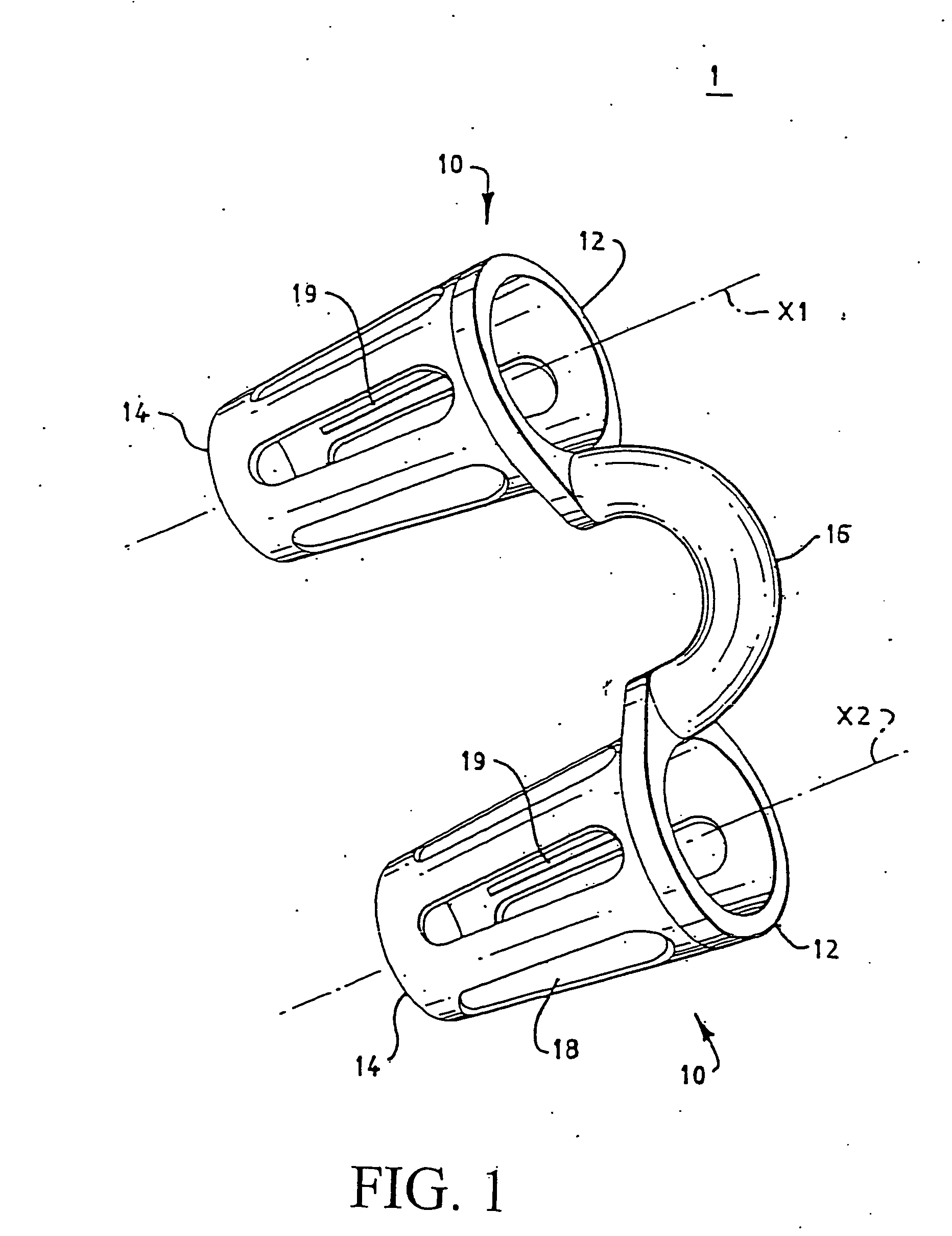
Method and apparatus for alleviating nasal congestion
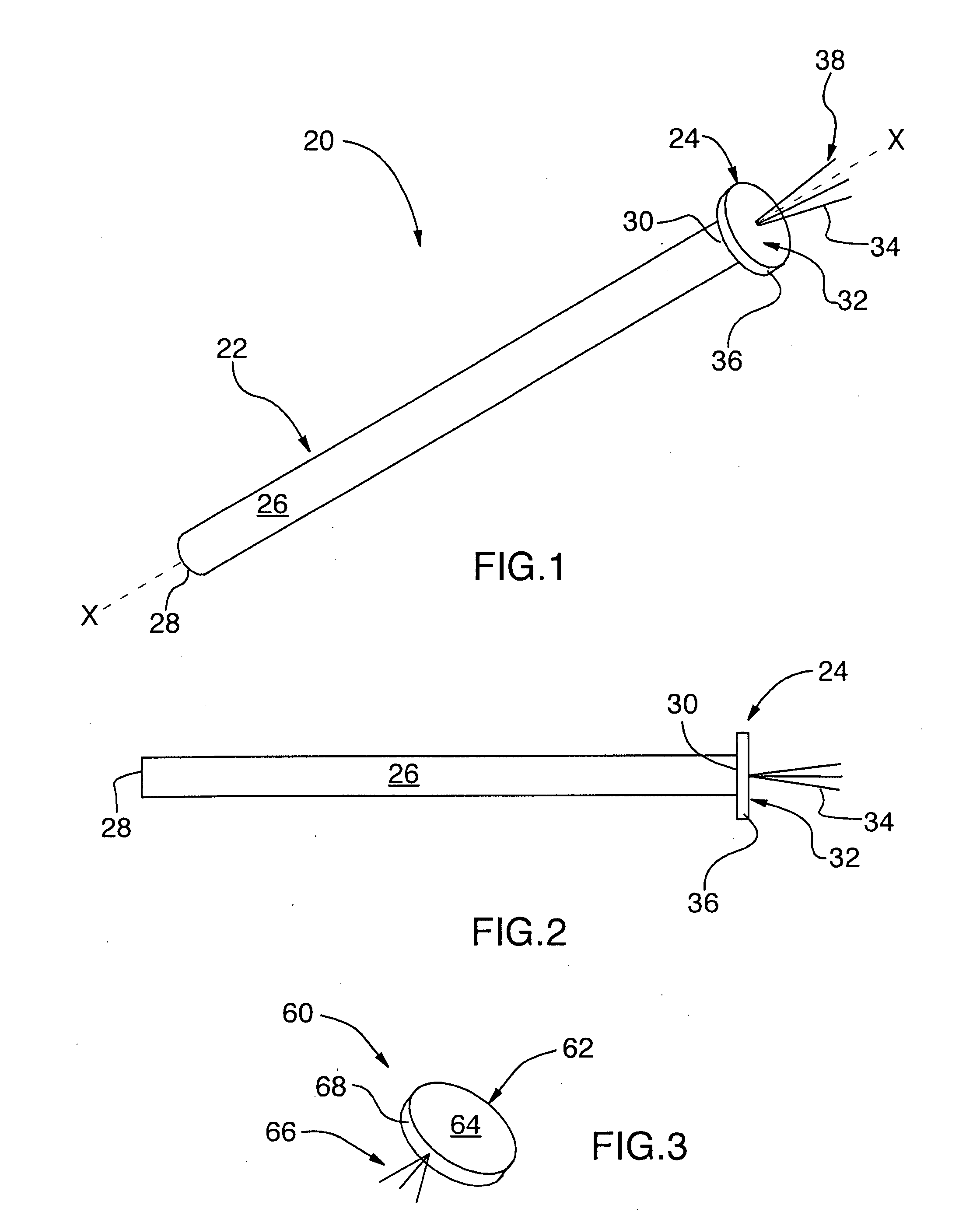
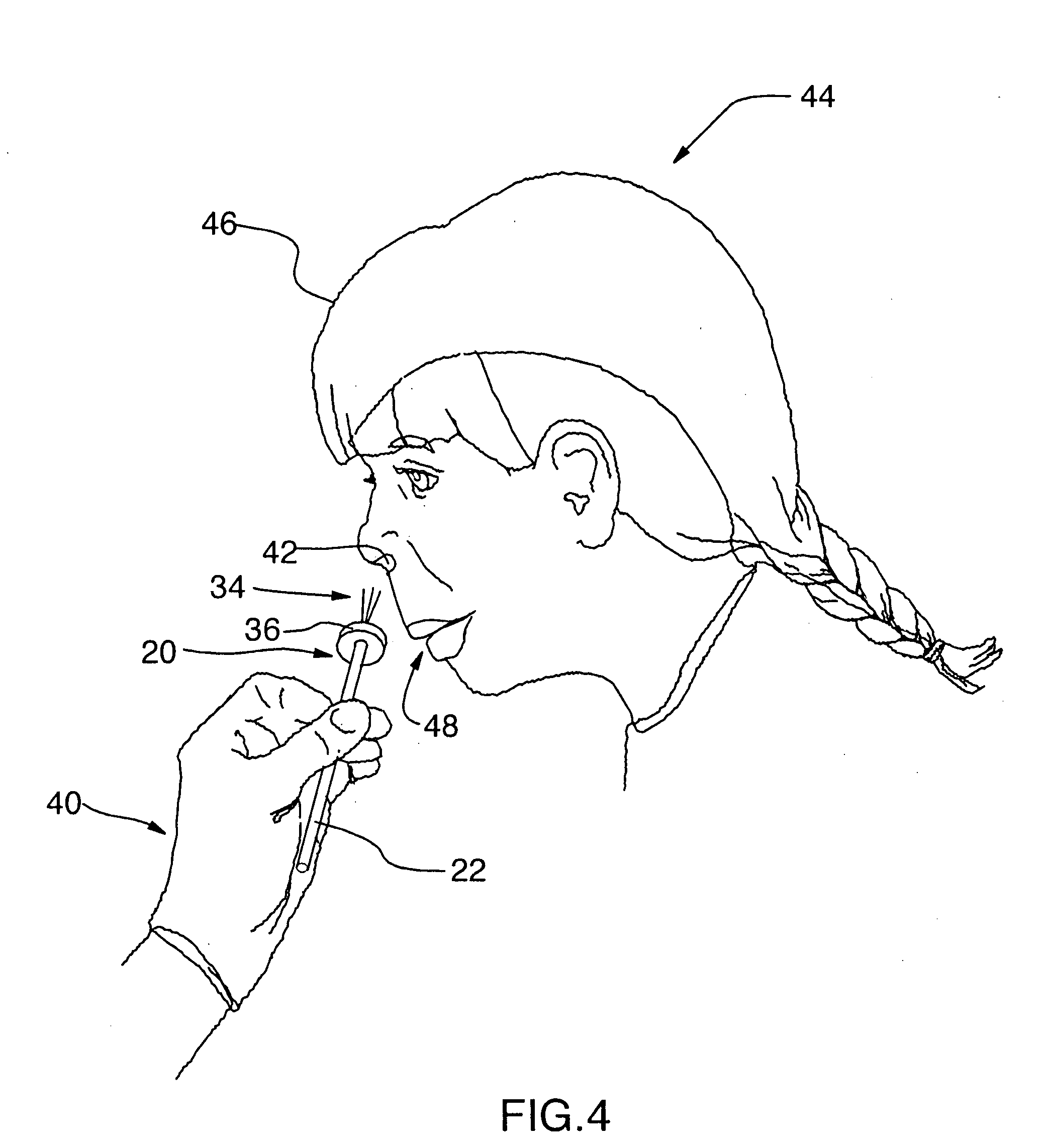
InactiveUS20090056709A1Reduce riskLimit insertionRespiratorsBreathing masksNasal passageNasal cavity
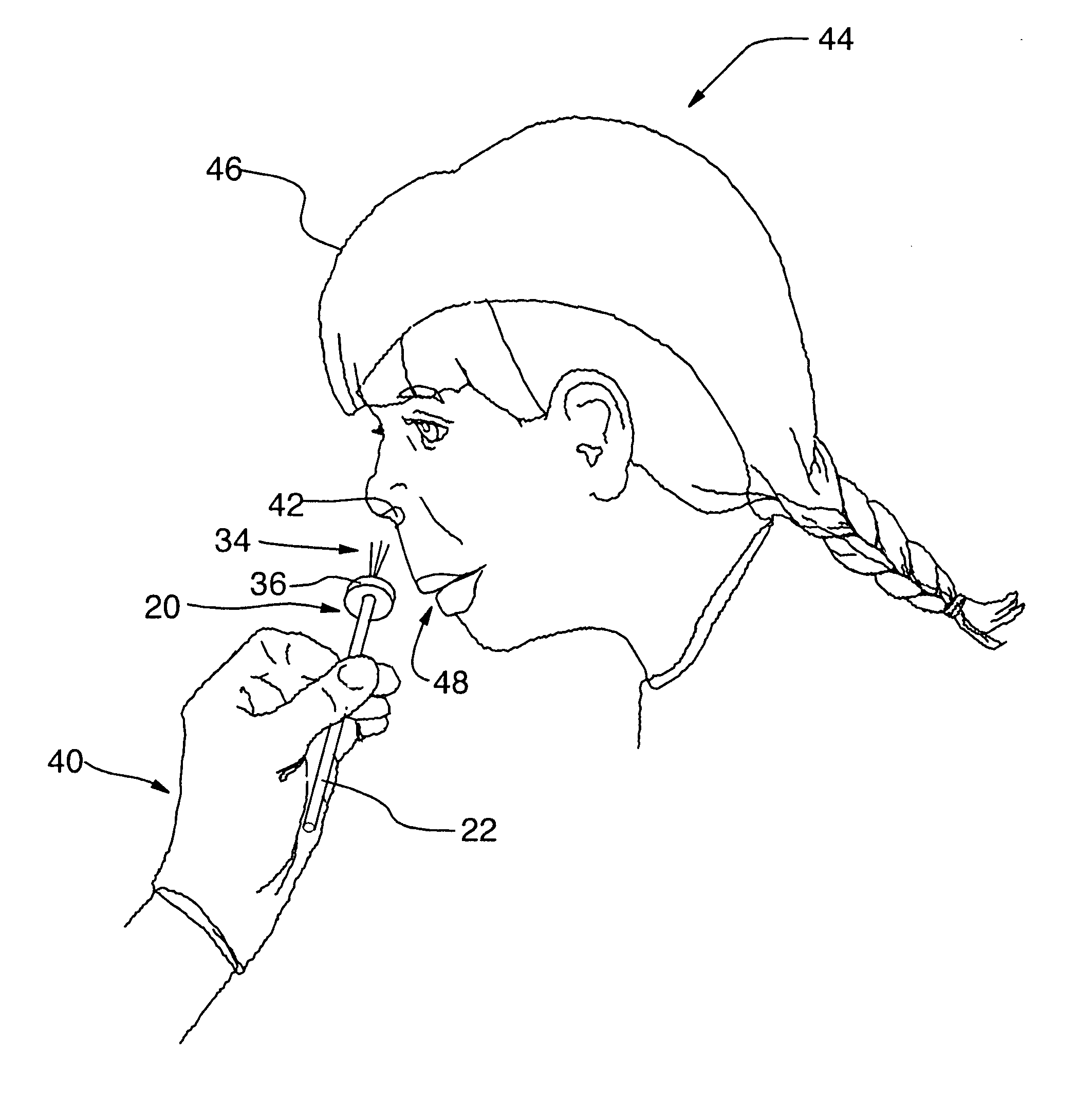
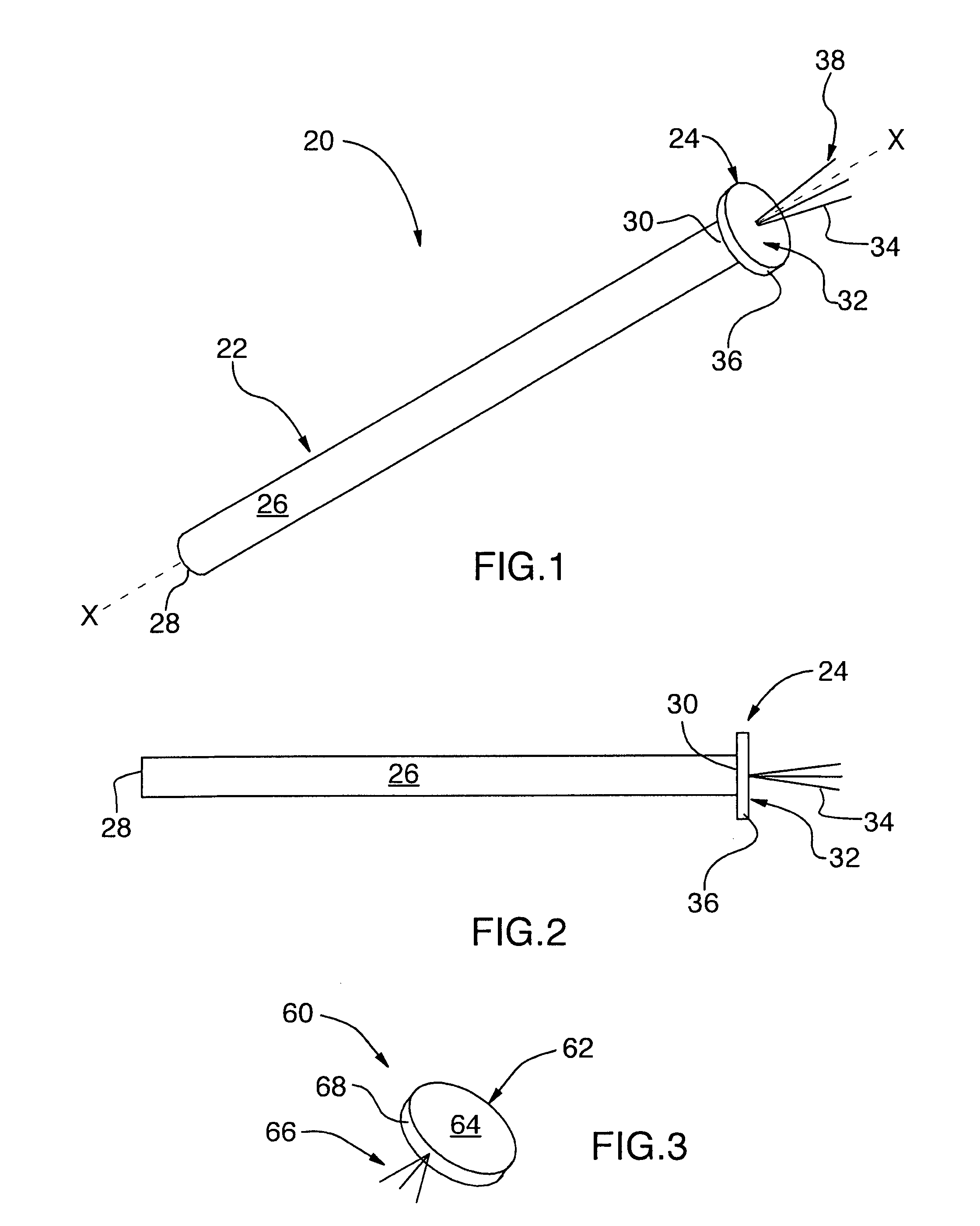
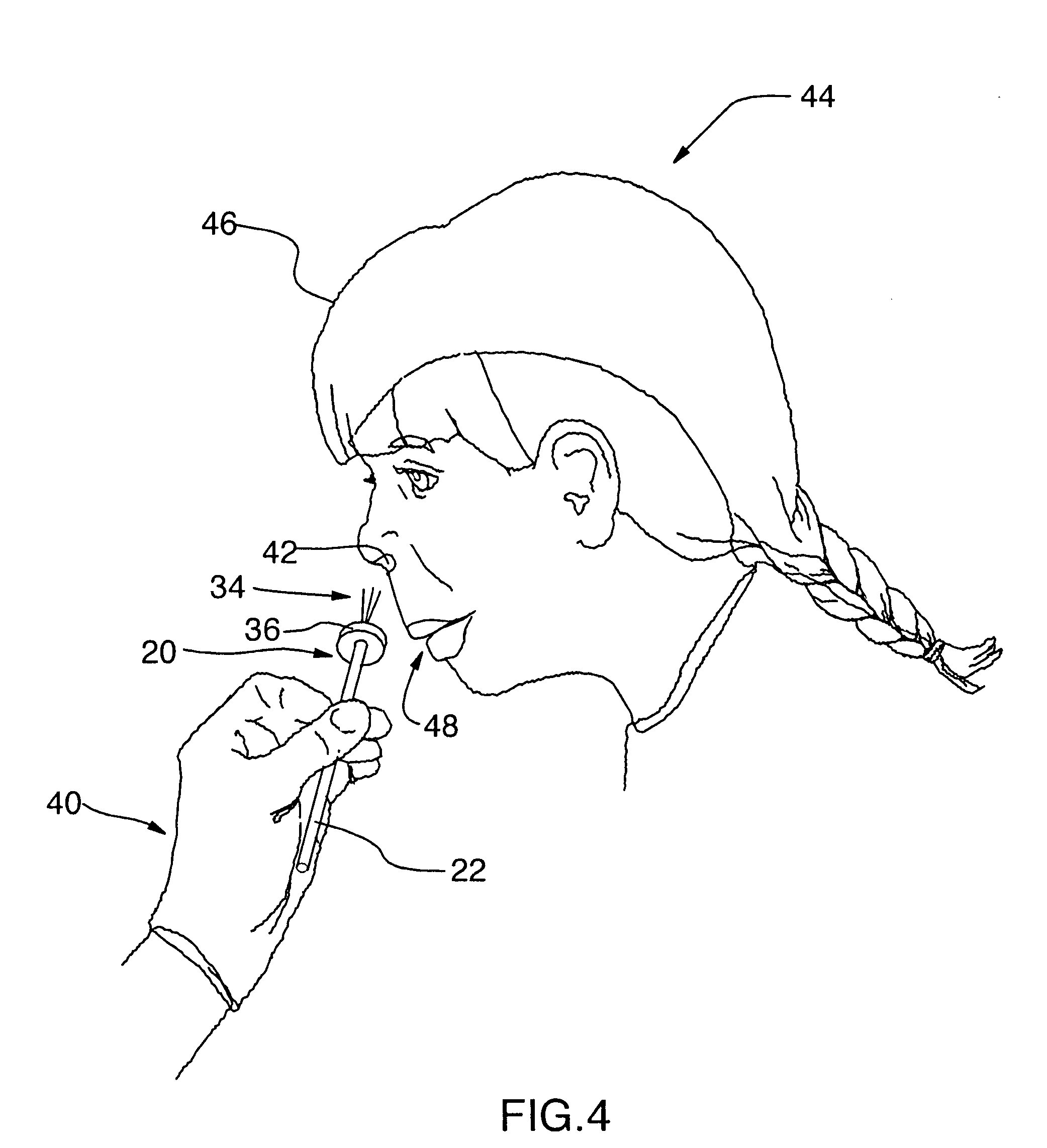
The present invention relates generally to the field of treatments for nasal congestion, and in particular, to a method and apparatus for alleviating nasal congestion in a patient by mechanically stimulating the sneezing reflex in the patient to urge drainage of the nasal passageways. The method includes inserting an instrument having a work tip portion provided with at least one filament into the nostril of the patient. The nasal mucosa of the patient is then probed with the at least one filament to stimulate in the patient the sneeze reflex. This probing action causes the patient to sneeze. With the patient's mouth closed, the sneeze urges at least one of mucus, fluid and debris in the nasal passages of the patient to be forcibly expelled through the nostrils of the patient thereby draining the nasal cavity of the patient. The instrument used in the performance of this method includes a handle portion upon which the work tip portion is carried. The handle portion may be integrally formed with the work tip portion or releasably attachable thereto. The instrument is further provided with a physical stop associated with one of the work tip portion and the handle portion to prevent injury resulting from the working tip portion being inserted too deeply into the nasal passages of the patient.
Owner:WORSOFF MAYA
Apparatus to provide continuous positive airway pressure
InactiveUS20070175479A1Good air tightnessImprove ventilationRespiratorsBreathing masksNasal cavityNasal prongs
Apparatus for communicating a positive fluid pressure to a patient's nasal passageways includes a pair of crush resistant nasal prongs disposed within a respective one of such patient's nasal passageways for delivering at least one of air, oxygen and a combination of air and oxygen to such patient's air passageways. There is a member engageable with at least a portion of said first open end of each one of said pair of crush resistant nasal prongs for substantially sealing them to at least a portion of an inner wall of such air passageways. A crush resistant fluid communication device engages with the second open end of each of the nasal prongs for communicating the at least one of air, oxygen and a combination of air and oxygen to the nasal prongs. Another device is disposed on such apparatus which is engageable with the fluid communication member for retaining the nasal prongs in position in such patient's air passageways during use.
Owner:CIRCADIANCE
Nasal congestion and obstruction relief and breathing assist devices
Owner:SANOSTEC CORP
Nasal stimulation for rhinitis, nasal congestion, and ocular allergies
Described here are devices, systems, and methods for treating one or more conditions, such as allergic rhinitis, non-allergic rhinitis, nasal congestion, ocular allergy, and / or symptoms associated with these conditions, by providing stimulation to nasal or sinus tissue. In some variations, the handheld devices may have a stimulator body and a stimulator probe having one or more nasal insertion prongs, and the nasal insertion prongs may be configured to deliver an electrical stimulus to the tissue.
Owner:OCULEVE
Method and apparatus for alleviating nasal congestion
InactiveUS8088120B2Reduce riskLimit insertionRespiratorsBreathing masksNasal cavityMechanical irritation
The present invention relates generally to the field of treatments for nasal congestion, and in particular, to a method and apparatus for alleviating nasal congestion in a patient by mechanically stimulating the sneezing reflex in the patient to urge drainage of the nasal passageways. The method includes inserting an instrument having a work tip portion provided with at least one filament into the nostril of the patient. The nasal mucosa of the patient is then probed with the at least one filament to stimulate in the patient the sneeze reflex. This probing action causes the patient to sneeze. With the patient's mouth closed, the sneeze urges at least one of mucus, fluid and debris in the nasal passages of the patient to be forcibly expelled through the nostrils of the patient thereby draining the nasal cavity of the patient. The instrument used in the performance of this method includes a handle portion upon which the work tip portion is carried. The handle portion may be integrally formed with the work tip portion or releasably attachable thereto. The instrument is further provided with a physical stop associated with one of the work tip portion and the handle portion to prevent injury resulting from the working tip portion being inserted too deeply into the nasal passages of the patient.
Owner:WORSOFF MAYA
Nasal formulation
InactiveUS20070286813A1Improve nasal airflowBiocideInorganic active ingredientsNasal passageNasal cavity
The present invention relates to a novel nasal formulation for the treatment and prophylaxis of nasal congestion. When an infant is born, its nasal passages are relatively sterile, in time the nasal passages are colonized by fungi, molds and other organisms which compromise the integrity of the nasal and paranasal mucosa. The presence of these organisms elicits a mild chronic inflammatory reaction of the nasal and paranasal mucosa, in some cases leading to congestion and excess production of mucus. This chronic inflammatory process compromises the integrity of the nasal and paranasal mucosa, making it more vulnerable to binding with allergens and other invading organisms such as the rhinovirus. The purpose of this invention is to establish a treatment and prophylaxis of nasal congestion, which will control the presence of the invading organisms in the nasal and paranasal mucosa, reducing congestion as well as mucus production. By reducing the mild chronic inflammation in the nasal passages, prophylaxis with this nasal formulation passively reestablishes the integrity of the nasal and paranasal mucosa, thereby reinforcing the body's defenses against the implantation of allergens and of organisms such as the rhinovirus.
Owner:TOUTOUNGHI CAMILLE
Chemical composition and method for cold and sinus relief
InactiveUS20060210482A1Safe and effective reliefSalicyclic acid active ingredientsBiocideSinusitisNasal passage
A chemical composition and method for treatment of the common cold, sinusitis and other conditions associated with nasal congestion is provided. The chemical composition is made of predetermined concentrations of thymol, alcohol, eucalyptol, menthol, methyl salicylate, a preservative, saline solution and a buffer. The method includes the step of administering the chemical composition of the invention via either intranasal instillation, oral inhalation or intermittent positive pressure breathing. Administration of the chemical composition via the method of the invention clears the nasal passages and alleviates the symptoms of the common cold, sinusitis, and related conditions.
Owner:CASSARA JOHN
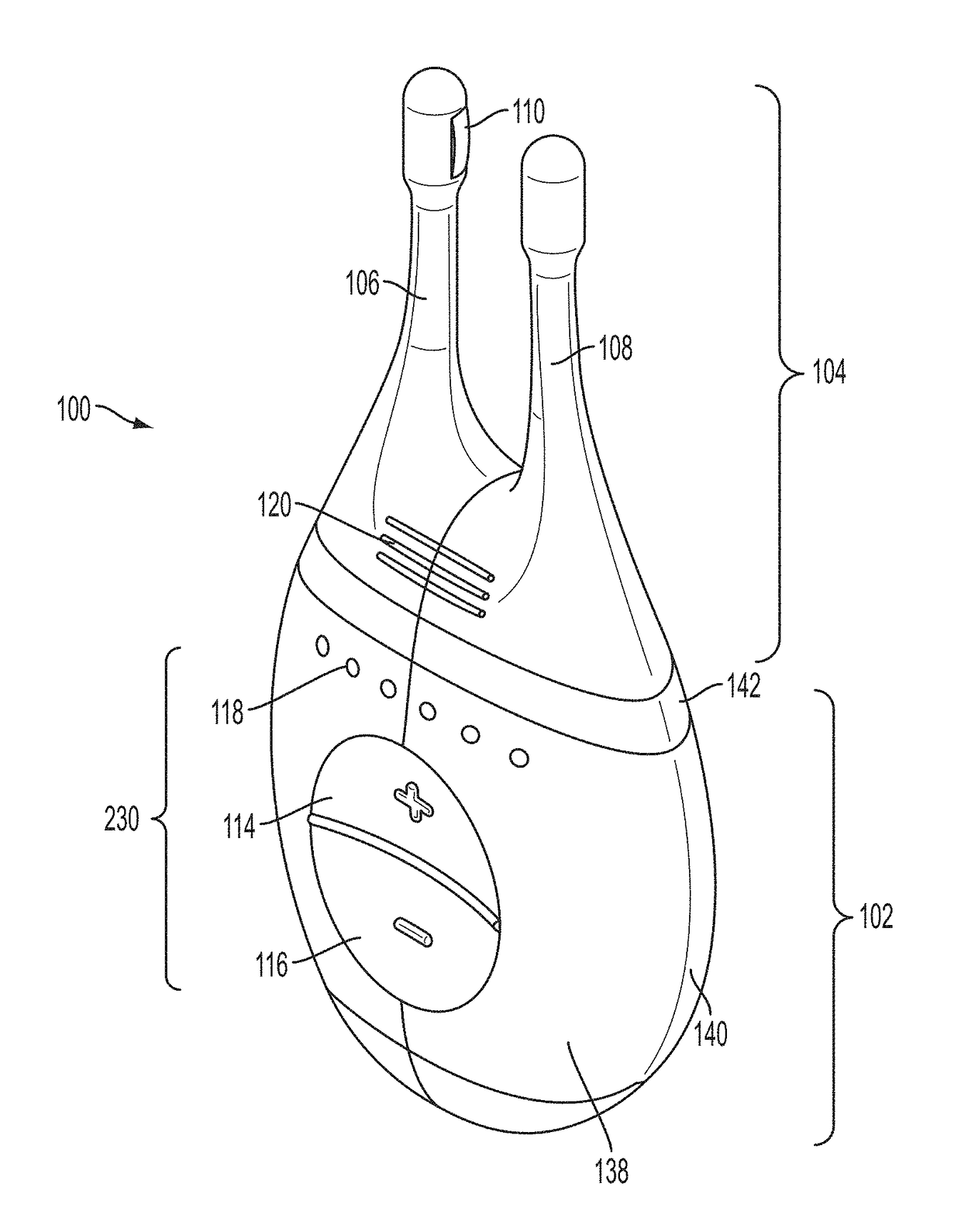
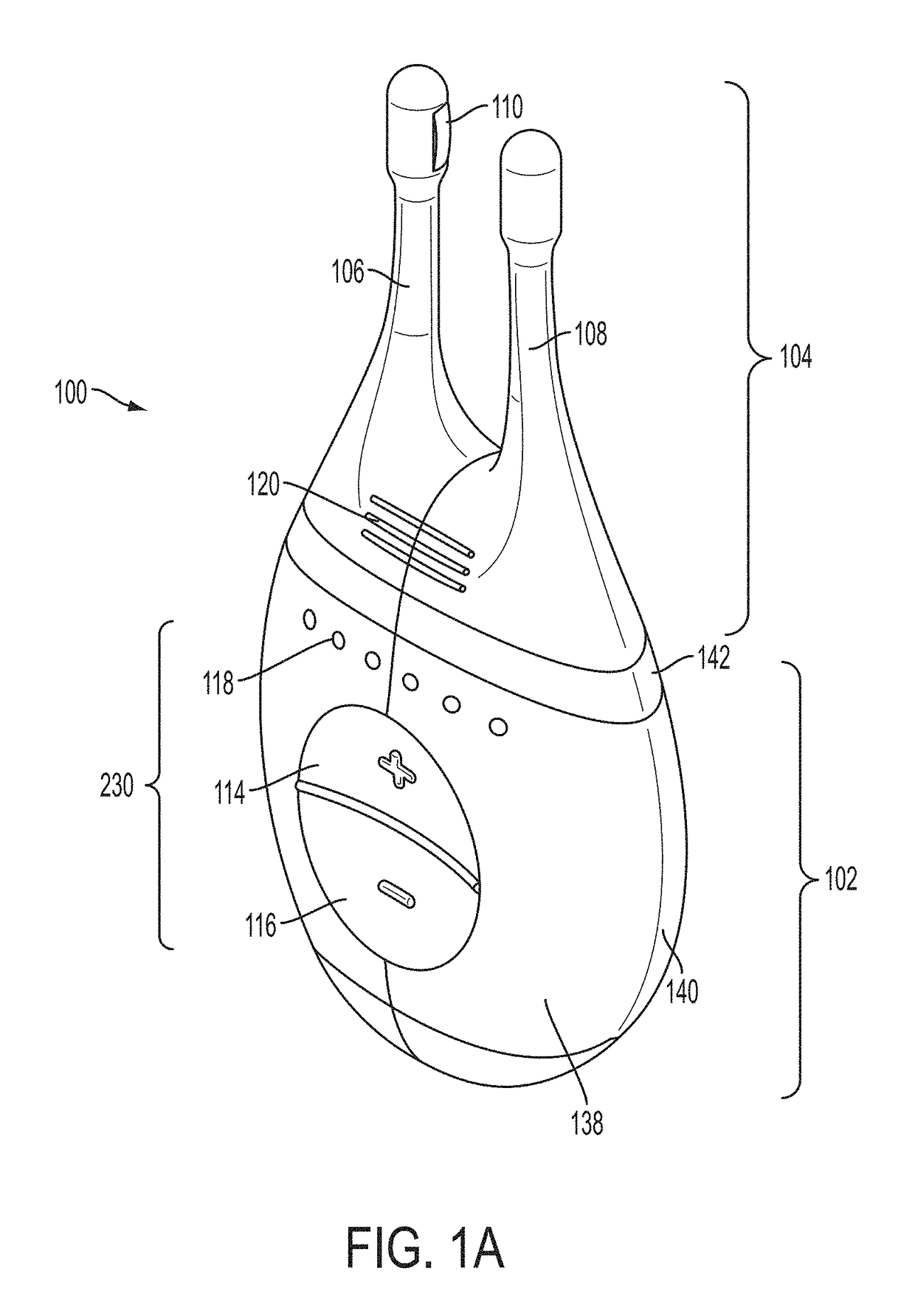
Nasal insert having one or more sensors
InactiveUS20170273626A1Increase airflowReduce or eliminate a wide variety of nasalMedical devicesRespiratory organ evaluationAirflowRespiratory health
The disclosed devices and methods provide one or more sensors associated with a nasal insert to provide data on respiratory health from within a patient's nasal passage. The nasal insert may simply be a vehicle for carrying one or more sensors, or the nasal insert itself may form part of the treatment. For example, the nasal insert may be used to increase airflow through the nasal passages. The nasal insert can contain a bio-sensor that may be affixed to an internal or external surface of the insert, be embedded partially or fully in an internal or external surface of the insert, or may pass through an internal or external wall of the nasal insert. In addition, the insert may include grooves that can be used to attach a sensor to the insert, for example, in place of or in conjunction with a filter.
Owner:SANOSTEC CORP
Vibrating device for treating nasal congestion and sinusitis symptoms and method thereof
A device for treating nasal congestion and / or relieving sinusitis symptoms in a patient comprises: means for generating vibrations, attaching means configured attach said generating means to a location adjacent to the patient's nasal cavity, nasal passageway or sinuses and means for generating a fluid stream and delivering the fluid stream to the respiratory tract of said patient. The vibration generating means further comprises a motor, an eccentric member rotatable by said motor and an impact member mechanically linked to the eccentric member such that the impact member is vibratable by the eccentric member.
Owner:VINCENT YUVAL R&D LTD
Nasal stimulation for rhinitis, nasal congestion, and ocular allergies
Described here are devices, systems, and methods for treating one or more conditions, such as allergic rhinitis, non-allergic rhinitis, nasal congestion, ocular allergy, and / or symptoms associated with these conditions, by providing stimulation to nasal or sinus tissue. In some variations, the handheld devices may have a stimulator body and a stimulator probe having one or more nasal insertion prongs, and the nasal insertion prongs may be configured to deliver an electrical stimulus to the tissue.
Owner:OCULEVE
Sodium pyruvate nasal spray and preparation method thereof
ActiveCN102657611AGood antibacterial effectLess irritatingAerosol deliveryPharmaceutical non-active ingredientsNasal passageNasal passages
The present invention relates to a nasal spray containing sodium pyruvate and a preparation method thereof, belonging to the field of medical technology, being characterized in that: the nasal spray containing sodium pyruvate comprises the following components: sodium pyruvate, pyruvic acid, an isoosmotic adjusting agent, a preservative and water. Because the sodium pyruvate is capable of alleviating nasal obstruction and inflammation caused by rhinitis, a medical fluid provided in the invention can be made into a nasal spray medical fluid which is capable of entering nasal cavities by adopting a portable spraying device. The nasal spray medical fluid is particularly suitable for cleaning and removing harmful pollutants in nasal passages, paranasal sinuses and mucosal cilia and is capable of being used in nursing after a nasal cavity operation, relieving rhinostegnosis and reducing respiratory tract irritation and risks of infection. By adding the pyruvic acid to form a buffer solution, tingling caused by using the flushing fluid to wash the cavities can be reduced, and inflammation is alleviated. Allergic symptoms can be prevented or gradually alleviated for the people of special professional who often use the product provided in the invention to wash and care nasal cavities.
Owner:JIANG SU PHARMAMAXCORP +1
Compositions and Methods for the Treatment of Migraine
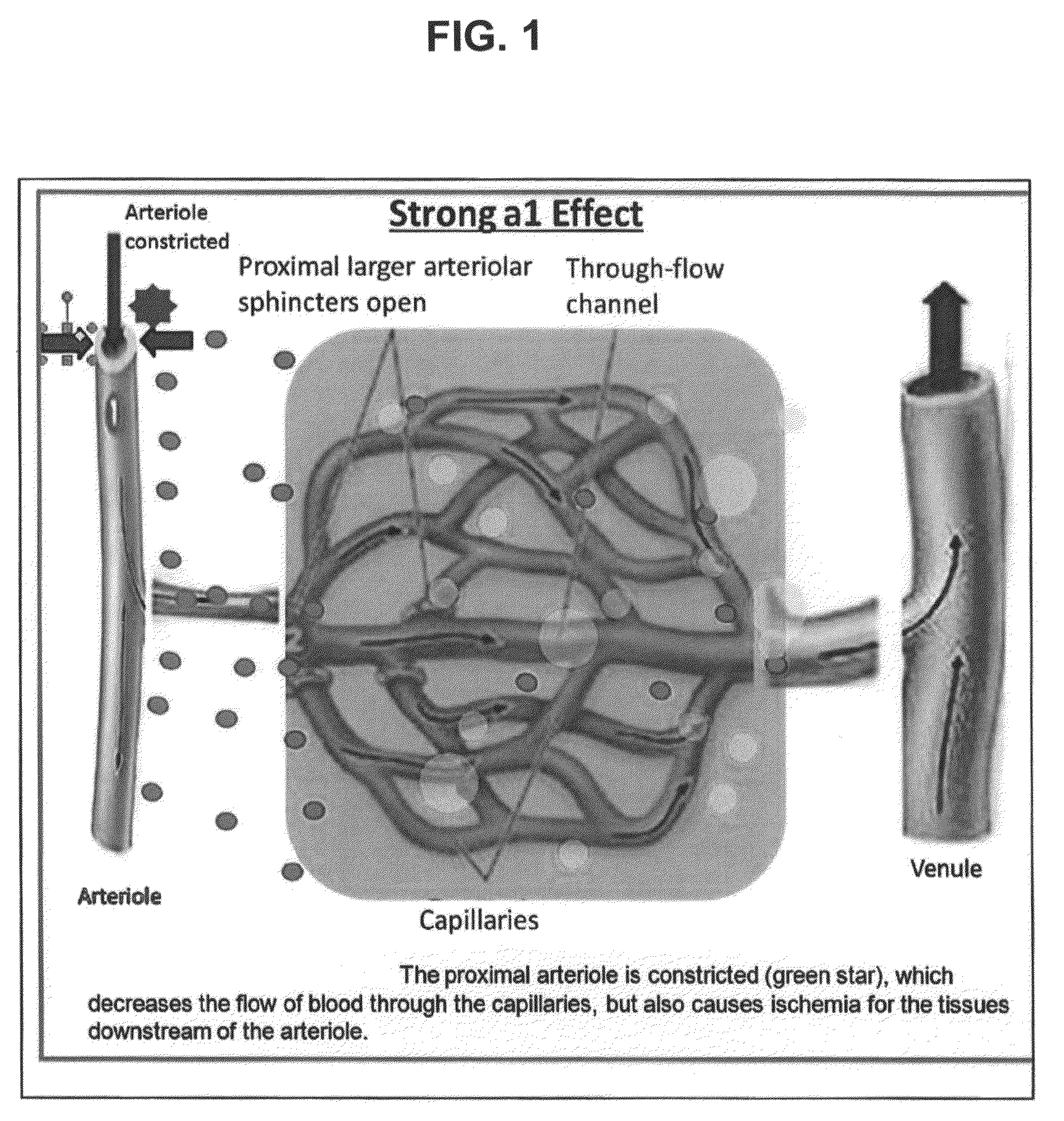
Pharmaceutical compositions for the treatment of nasal congestion or migraine, wherein the pharmaceutical compositions comprise low concentrations of a super-selective subclass of selective α-2 adrenergic receptor agonists.
Owner:EYE THERAPIES
Use of hypertonic saline to draw fluid out of swollen tissue and relieve nasal congestion
InactiveUS20140356460A1Less discomfortReduce congestionBiocideInorganic non-active ingredientsNarrow rangeSaline water
The optimum concentration of salt in solutions to combat congestion of the mucosa is a relatively narrow range. This use of in hypertonic saline solutions for treatment of congestion of the mucosa having a concentration within the range of 2.3% to and including 2.7% w / v sodium chloride / water with the most preferred concentration being 2.4% to and including 2.6% w / v of sodium chloride in water brings maximum relief of congestion and for cleansing membranes without causing irritation or discomfort.
Owner:LUTIN MATTHEW
Nasal Compositions and Uses Thereof
Pharmaceutical compositions for the treatment of nasal congestion, wherein the pharmaceutical compositions comprise low concentrations of a super-selective subclass of selective α-2 adrenergic receptor agonists. Methods of using the compositions for the treatment of nasal congestion, cerebrovascular disease or systemic conditions, and as delivery vehicles to deliver other active agents to treat systemic or cerebrovascular diseases or conditions.
Owner:PS THERAPIES LTD
Nasal formulation
InactiveUS20070286812A1Improve nasal airflowImproving nasalBiocideBacteria material medical ingredientsNasal cavityOral hygiene
The present invention relates to a novel nasal formulation for nasal hygiene and to improve nasal airflow of people suffering from nasal congestion.
Owner:TOUTOUNGHI CAMILLE
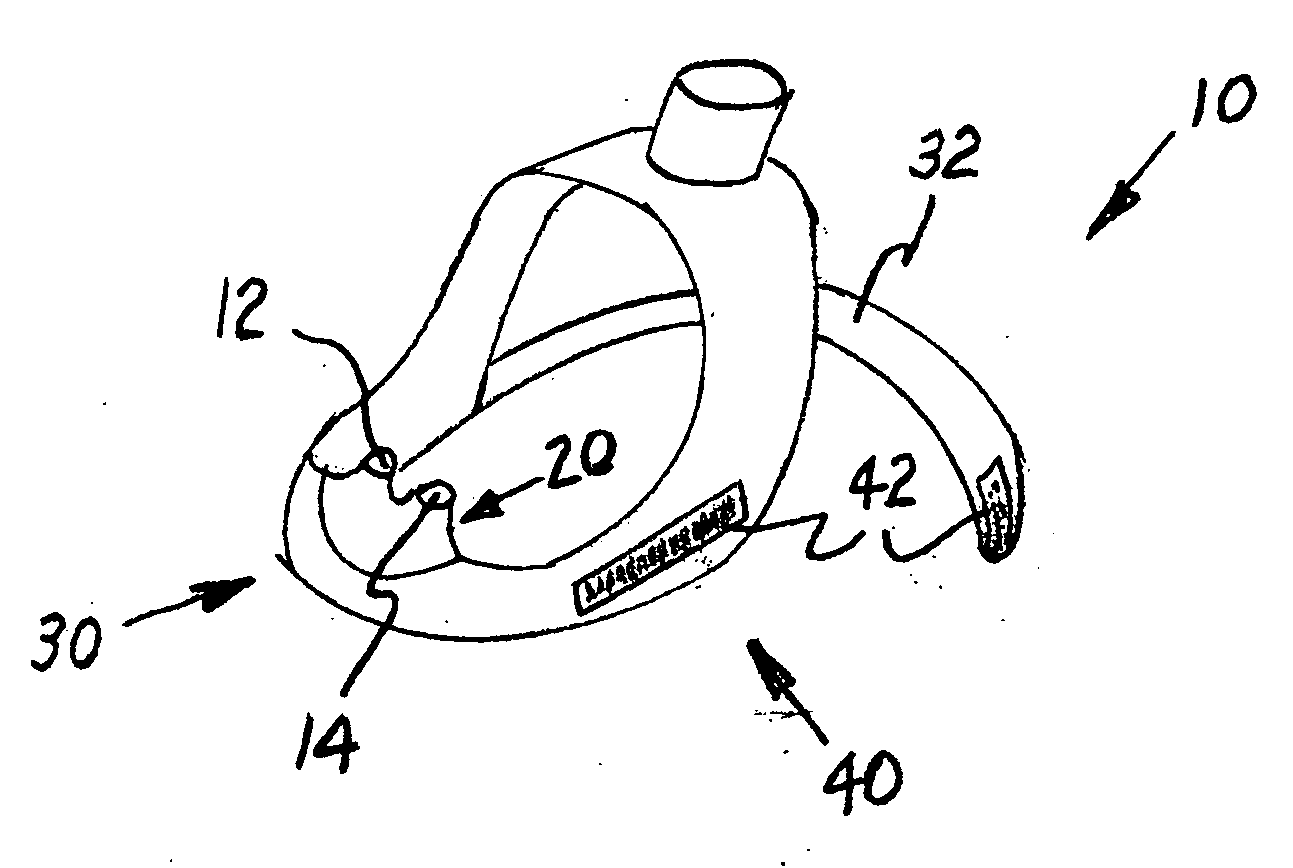
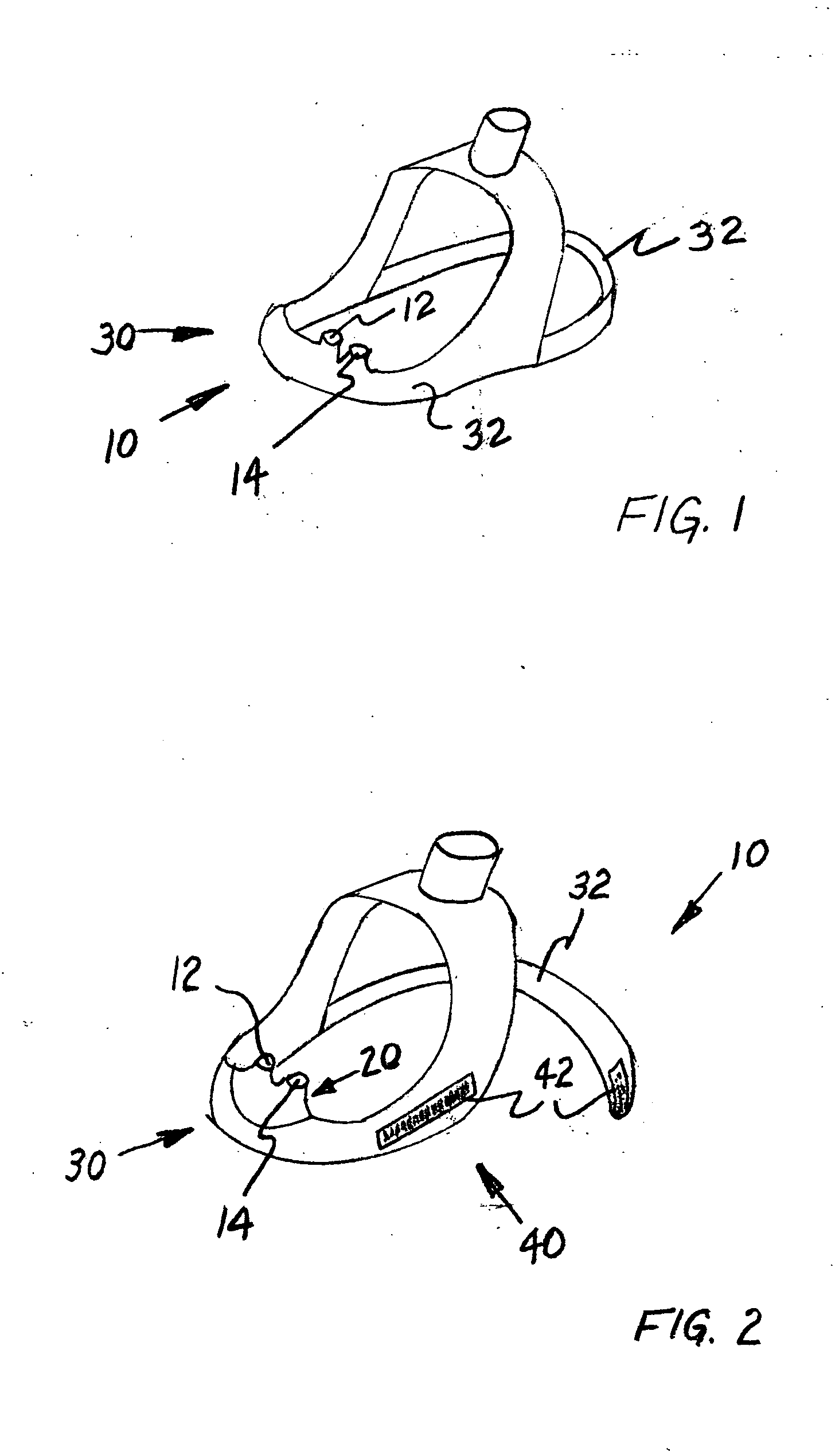
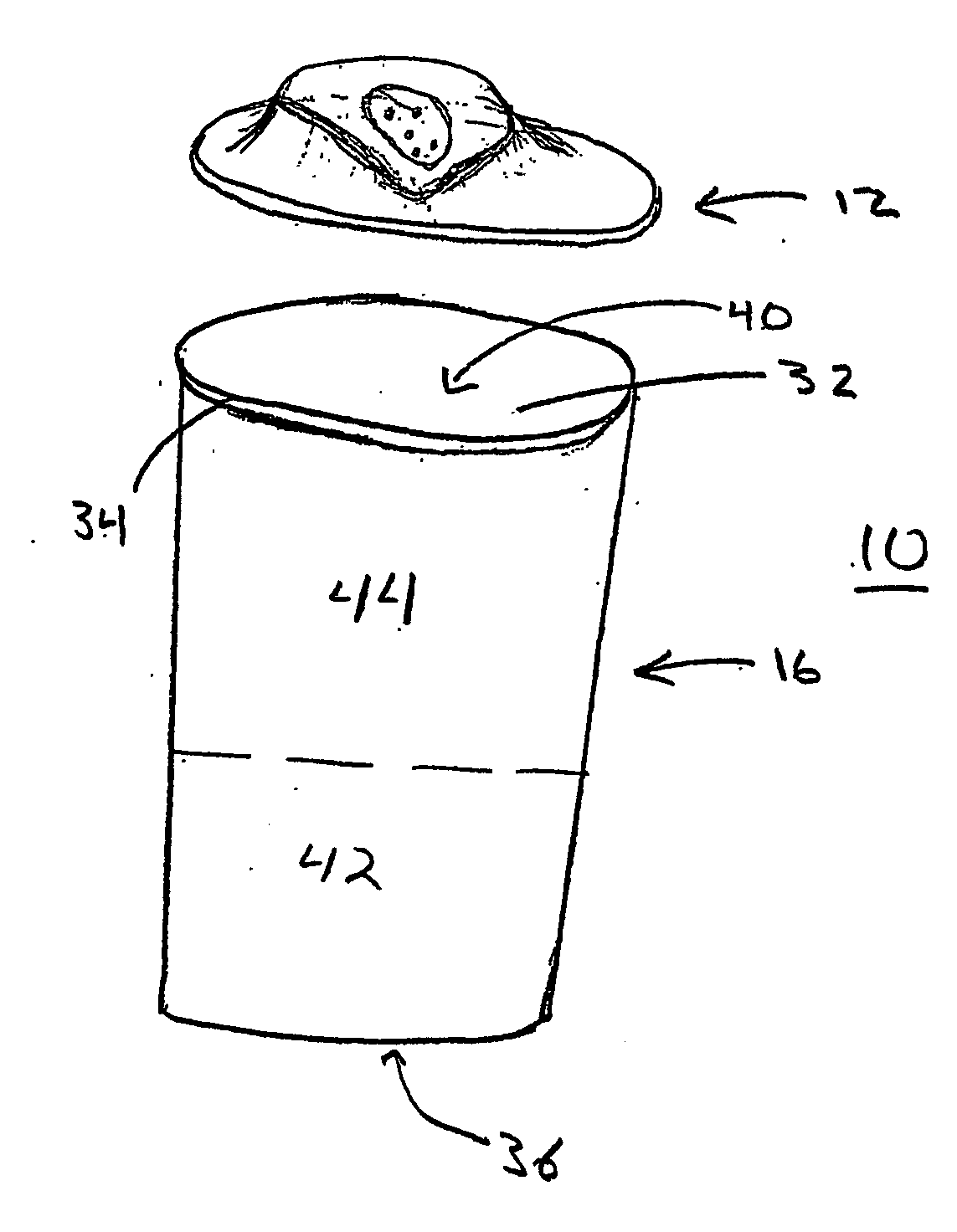
Portable vapor inhaler
InactiveUS20060112958A1Easy to breatheGood effectRespiratorsOhmic-resistance heatingMedicinePositive pressure
The present invention is a portable vapor inhaler (10) for improving the breathing of those suffering from nasal congestion. The invention includes a vapor-concentrating lid (12) and an effervescent composition (14). In further embodiments the invention may include a reservoir. Hot water is placed in the reservoir along with the effervescent composition and the vapor-concentrating lid is placed thereon. The hot water creates a humid vapor in the reservoir and beneath the vapor-concentrating lid. The positive pressure created by the effervescent composition then forces the humid air out through the vapor-concentrating lid and is then inhaled by the user.
Owner:CNS INC
Pharmaceutical compositions comprising plant extracts and methods for reducing duration of a common cold using same
ActiveUS20150231191A1Shorten the construction periodShorten the durationInorganic non-active ingredientsPharmaceutical delivery mechanismWatery eyeGalphimia glauca
The present disclosure describes pharmaceutical compositions and methods for reducing duration, intensity, and / or bothersomeness of common colds in humans and for reducing severity or duration of common cold symptoms such as nasal congestion, runny nose, watery eyes, dry / scratchy throat and sneezing in humans exhibiting such symptoms. The compositions herein comprise extracts of at least one of Luffa Operculata (L. operculata), S. officinale (V. sabadilla), and Galphimia Glauca (G. glauca) in a pharmaceutically acceptable carrier, and in various embodiments, comprise a mixture of all three Luffa Operculata (L. operculata) 10% extract MT, S. officinale (V. sabadilla) 3× extract, and Galphimia Glauca (G. glauca) 10% extract MT in a pharmaceutically acceptable carrier.
Owner:CHURCH & DWIGHT CO INC
Apparatus for Facilitating Respiration During Nasal Congestion, and Related Methods
InactiveUS20110186054A1Efficient and inexpensiveMeet needsRespiratorsRestraining devicesNasal congestionIntensive care medicine
Owner:BOYD BRYAN
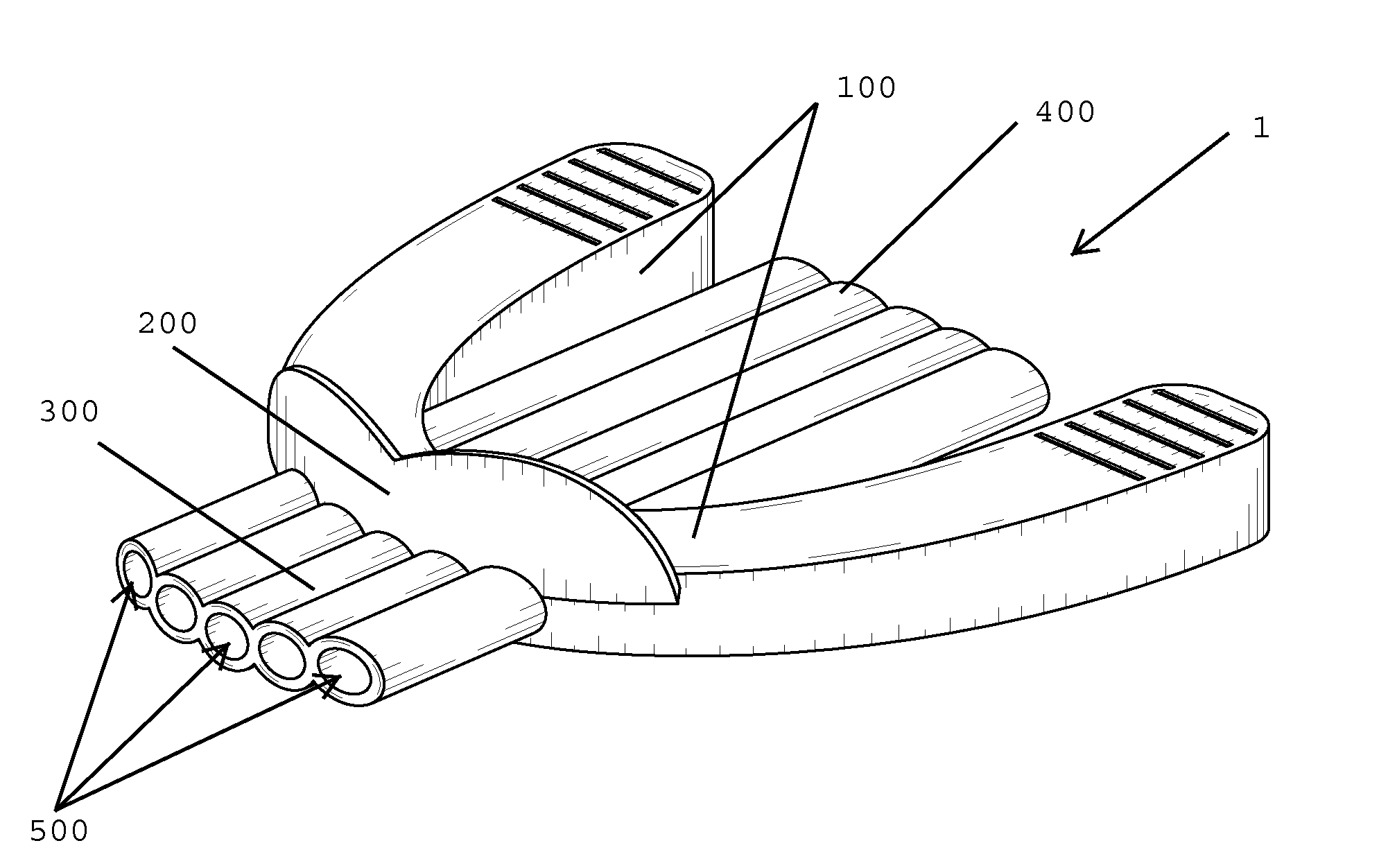
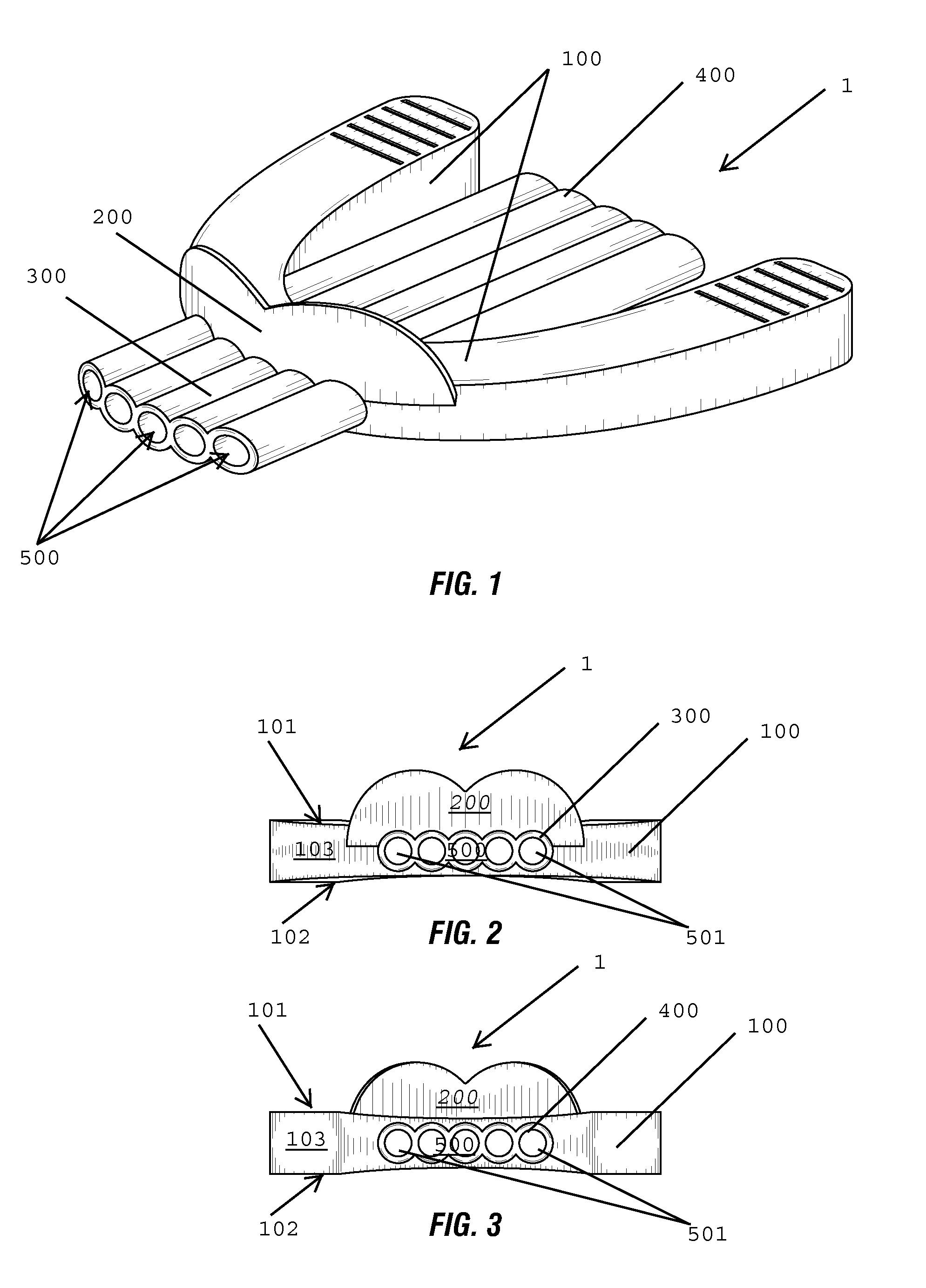
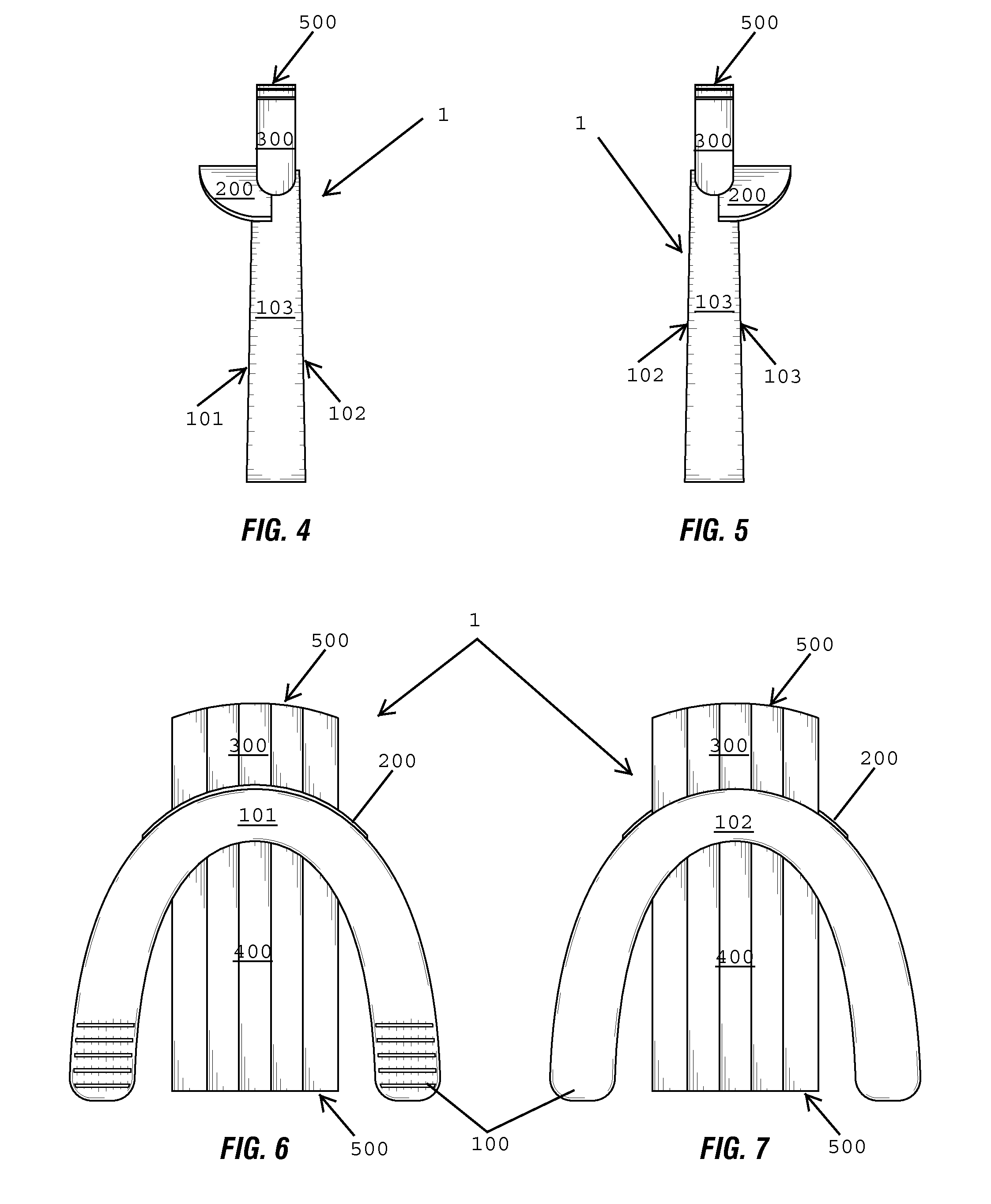
Nasal congestion, obstruction relief, and drug delivery
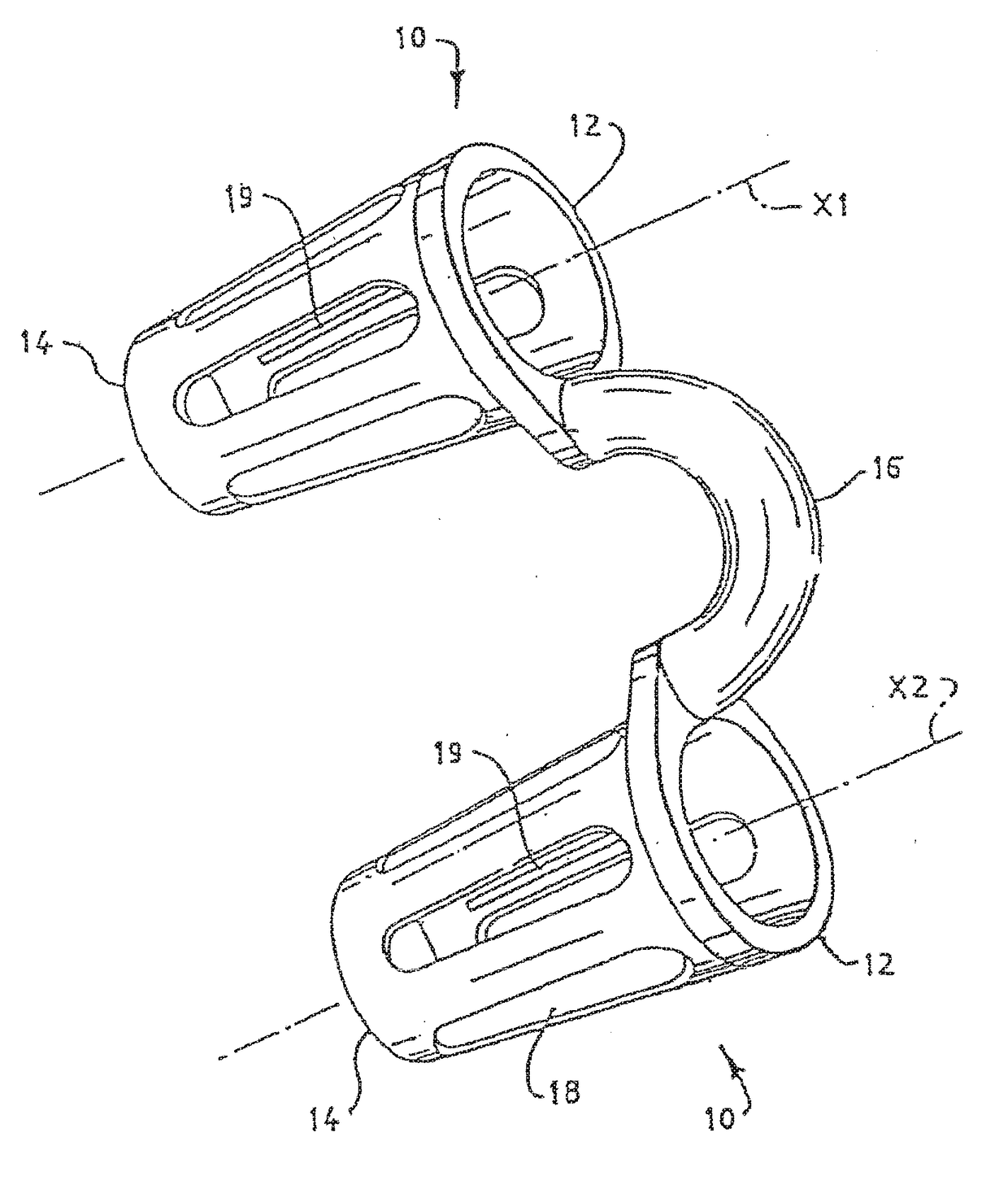
A nasal insert may include a wall in the shape of a tube, the wall including a first end defining a first orifice and a second end defining a second orifice. The first end may have a diameter, diagonal measurement, or cross-sectional area larger than that of the second end. The first end may define at least one break in the wall, so that the first end incompletely encircles the first orifice. Thesecond end may completely encircle the second orifice.
Owner:SANOSTEC CORP
Pharmaceutical compositions comprising plant extracts and methods for reducing duration of a common cold using same
ActiveUS9034401B1Shorten the construction periodShorten the durationBiocideInorganic non-active ingredientsWatery eyePharmaceutical medicine
The present disclosure describes pharmaceutical compositions and methods for reducing duration, intensity, and / or bothersomeness of common colds in humans and for reducing severity or duration of common cold symptoms such as nasal congestion, runny nose, watery eyes, dry / scratchy throat and sneezing in humans exhibiting such symptoms. The compositions herein comprise extracts of at least one of Luffa Operculata (L. operculata), S. officinale (V. sabadilla), and Galphimia Glauca (G. glauca) in a pharmaceutically acceptable carrier, and in various embodiments, comprise a mixture of all three Luffa Operculata (L. operculata) 10% extract MT, S. officinale (V. sabadilla) 3× extract, and Galphimia Glauca (G. glauca) 10% extract MT in a pharmaceutically acceptable carrier.
Owner:CHURCH & DWIGHT CO INC
Compositions and methods for ophthalmic delivery of nasal decongestants
InactiveUS20110152271A1Relieve nasal congestionReduce nasal decongestionBiocideOrganic chemistryNoseAdrenergic receptor agonists
The invention provides compositions and methods for treating nasal congestion through ophthalmic delivery. The provided compositions and methods utilize low concentrations of selective α-2 adrenergic receptor agonists. The compositions preferably include brimonidine.
Owner:HORN GERALD
Therapeutic formulations for the treatment of cold and flu-like symptoms
A pharmaceutical formulation of therapeutically effective amounts of acetaminophen, ibuprofen, and a sympathomimetic drug, such as pseudoephedrine (or its prodrug), or phenylephrine used in the treatment of cold and flu-like symptoms. Such symptoms may include fever, pain, nasal congestion, sinus congestion, runny nose, sore throat, myalgia, ear pressure and fullness, and headache. The formulation further includes various excipients used in the formulation process.
Owner:KINGSWAY PHARMA
Traditional Chinese medicine preparation for treating cough and preparation method thereof
ActiveCN103656410ASimple compositionSimple processUnknown materialsRespiratory disorderMedicineCurative effect
The invention discloses a traditional Chinese medicine preparation for treating cough and a preparation method thereof. The traditional Chinese medicine preparation consists of the following twelve Chinese herbal medicines: ephedra, radix stemonae, negundo chastetree fruit, affine cudweed, loquat seed dried powder, bezoar, radix polygonati officinalis, momordica grosvenori, radix puerariae, herb of savatier monochasma, manglietia fordiana and fig. The preparation method comprises the following steps: uniformly mixing a thick paste and dextrin of the medicine, taking ethanol as a wetting agent to prepare wet granules, drying, cooling, spraying volatile oil, and uniformly mixing, thereby obtaining the traditional Chinese medicine preparation. The preparation has good curative effects on symptoms such as cough, coughing up phlegm, fever with aversion to cold, headache without sweat, body ache and severe nasal obstruction caused by moderate and medium exogenous cold and unsmooth lung qi. Compared with the other process technologies, the medicine is simple and clear in composition and process, and the quality is easily controlled.
Owner:广西桂西制药有限公司
Aromatic Pacifier Assembly
An aromatic pacifier assembly for an infant gives off a predetermined scent by evaporation of volatile essential oils or similar substances. The aroma may be, for example, mint, menthol or eucalyptus, which may help relieve nasal congestion. The aromatic pacifier assembly is constructed to prevent evaporation of the essential oil before activation, so that the nipple does not become contaminated with a flavor the infant may find unpalatable, and so that the oil is conserved until it is desired to administer it to the infant.
Owner:BECKER STEPHENIE
Preparation of capsules against rhinitis
InactiveCN1456268AAdd TLC DiscriminationThe production process is perfect and reasonableUnknown materialsCapsule deliveryMentholAlcohol
A process for preparing Chinese medicine "Biyanling capsule" for treating chronic nasosinusitis, rhinitis, nasal congestion, etc includes such steps as extracting from xanthium fruit by alcohol, decocting dregs, concentrating, drying, extracting volatile oil of magnolia flower and asarum herb, pulverizing 4 Chinese-medicinal materials including scutellaria root, mixing, granulating, adding menthol and additive, mixing all together, and loading in capsules. Its advantage is high curative effect and safety.
Owner:毛友昌
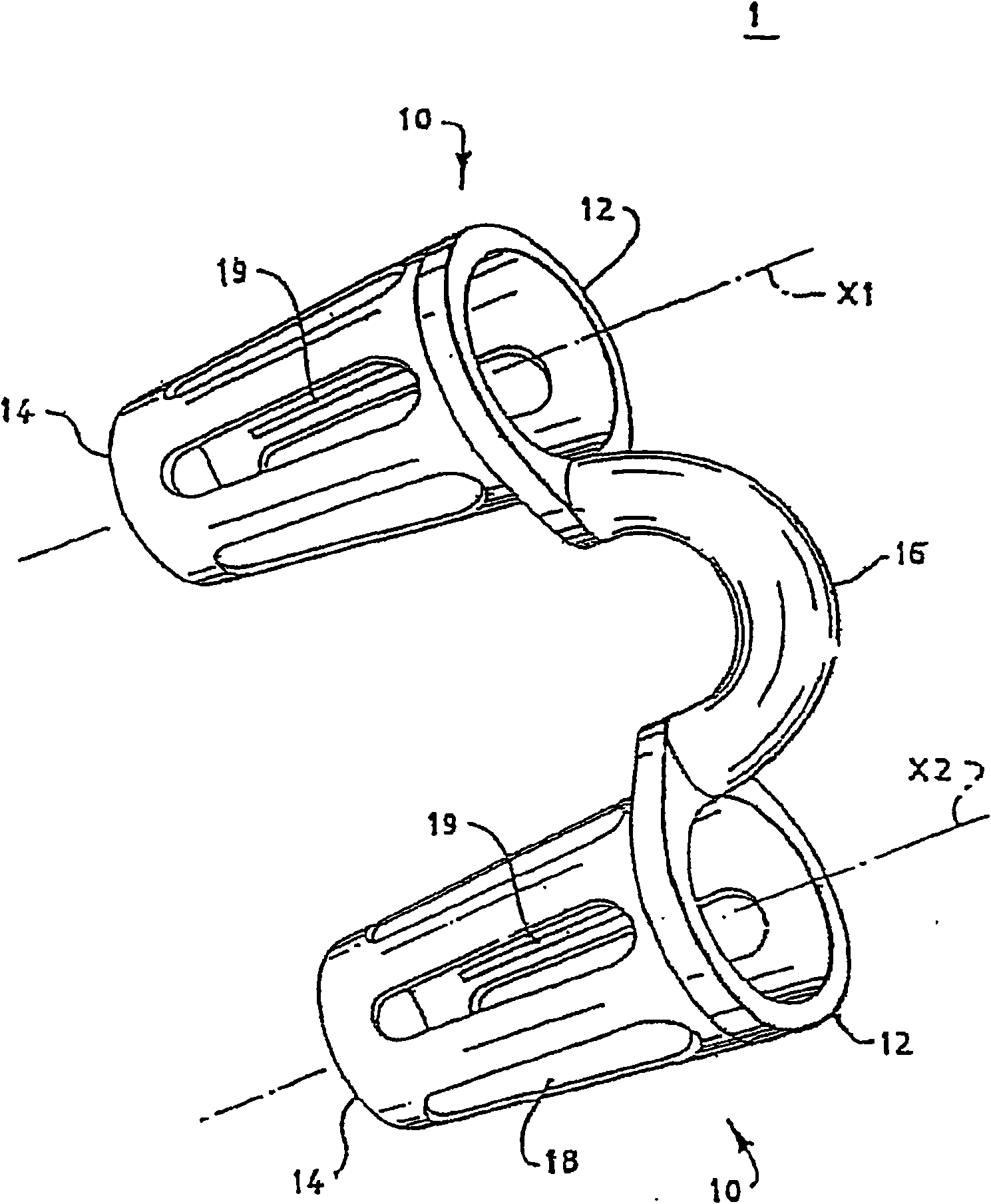
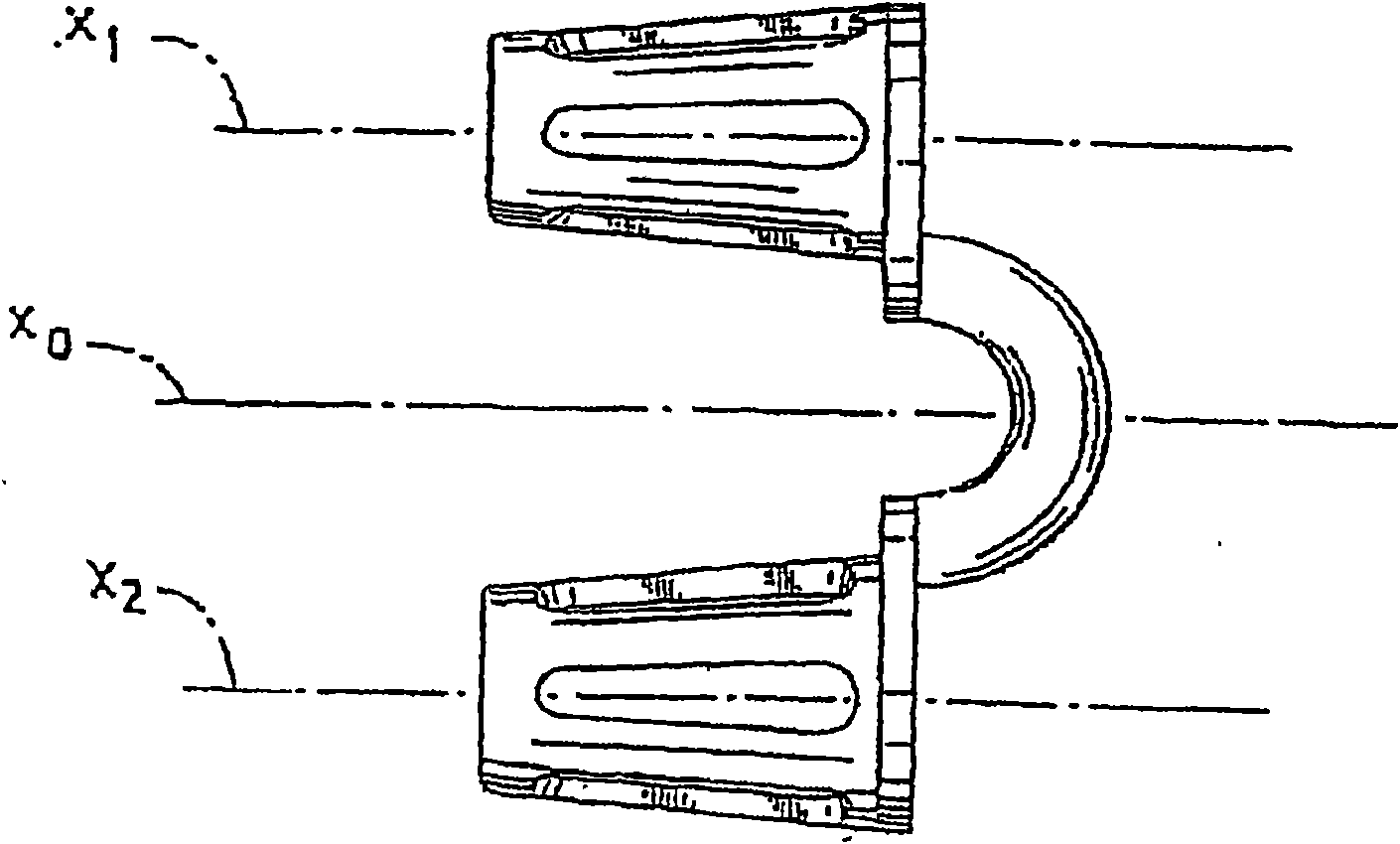
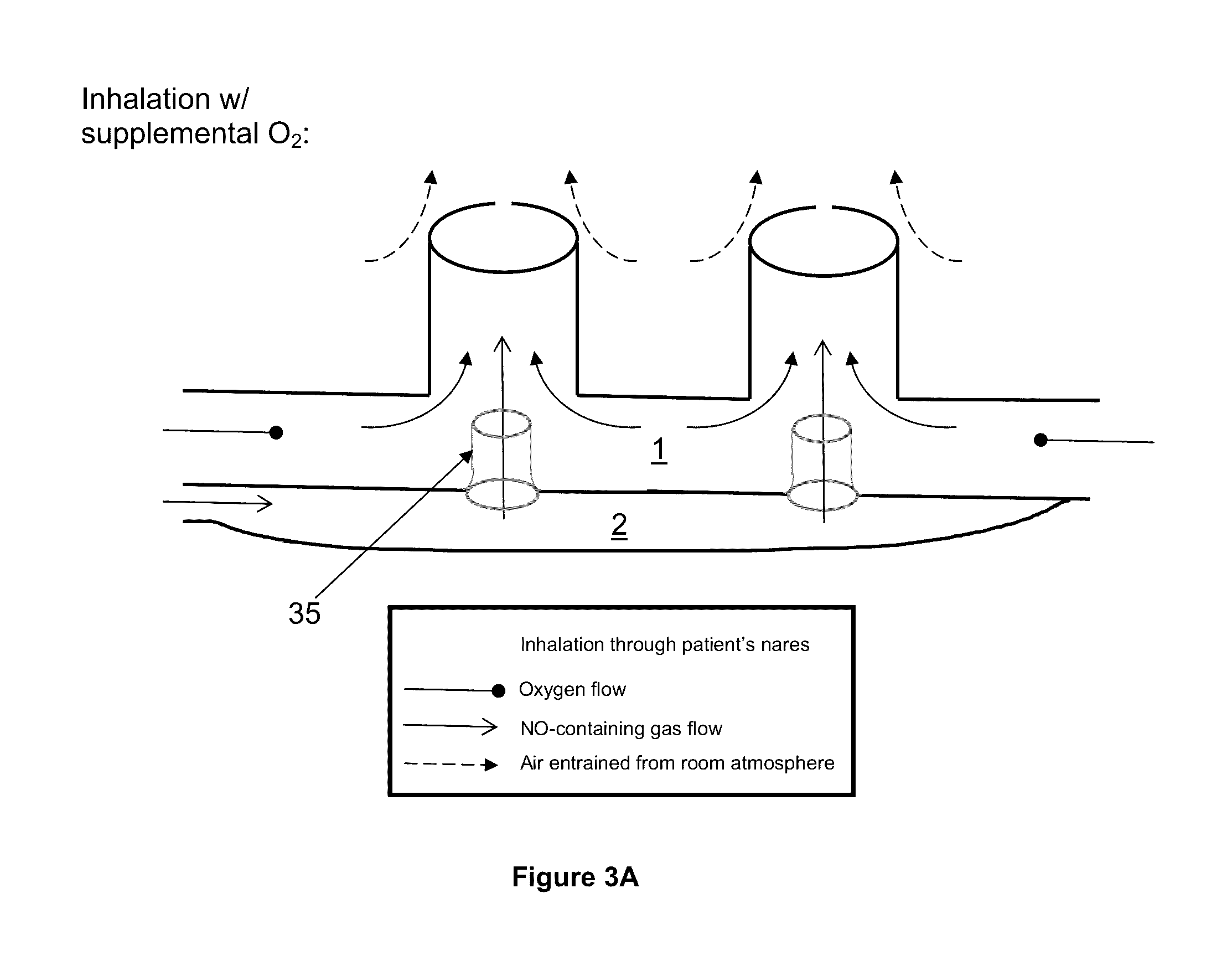
Breathing assistance apparatus for delivery of nitric oxide to a patient by means of a nasal cannula assembly with flow control passage
ActiveUS9522248B2Efficient managementMinimizes deliveryRespiratory masksMedical devicesNasal prongsNose
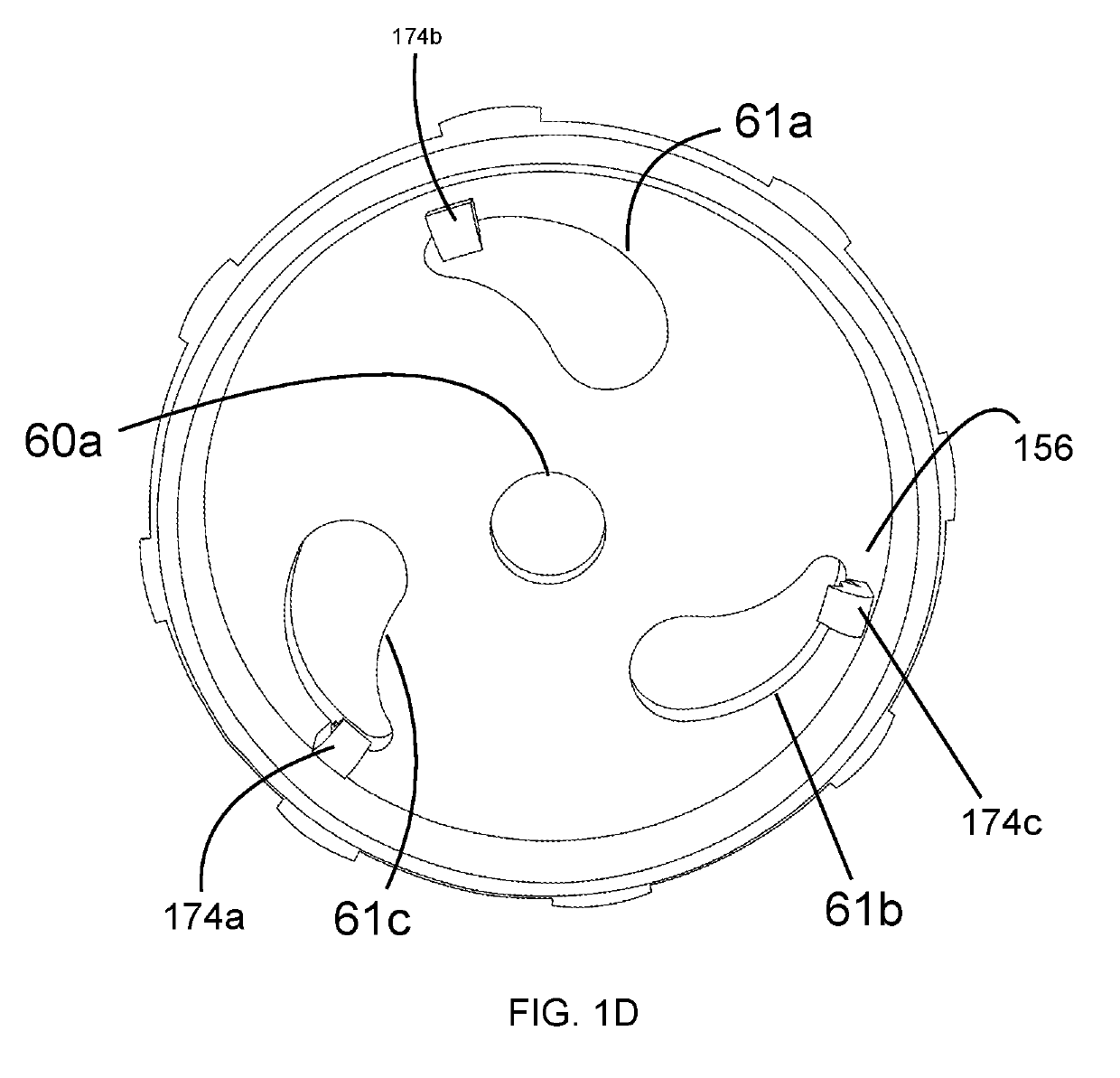
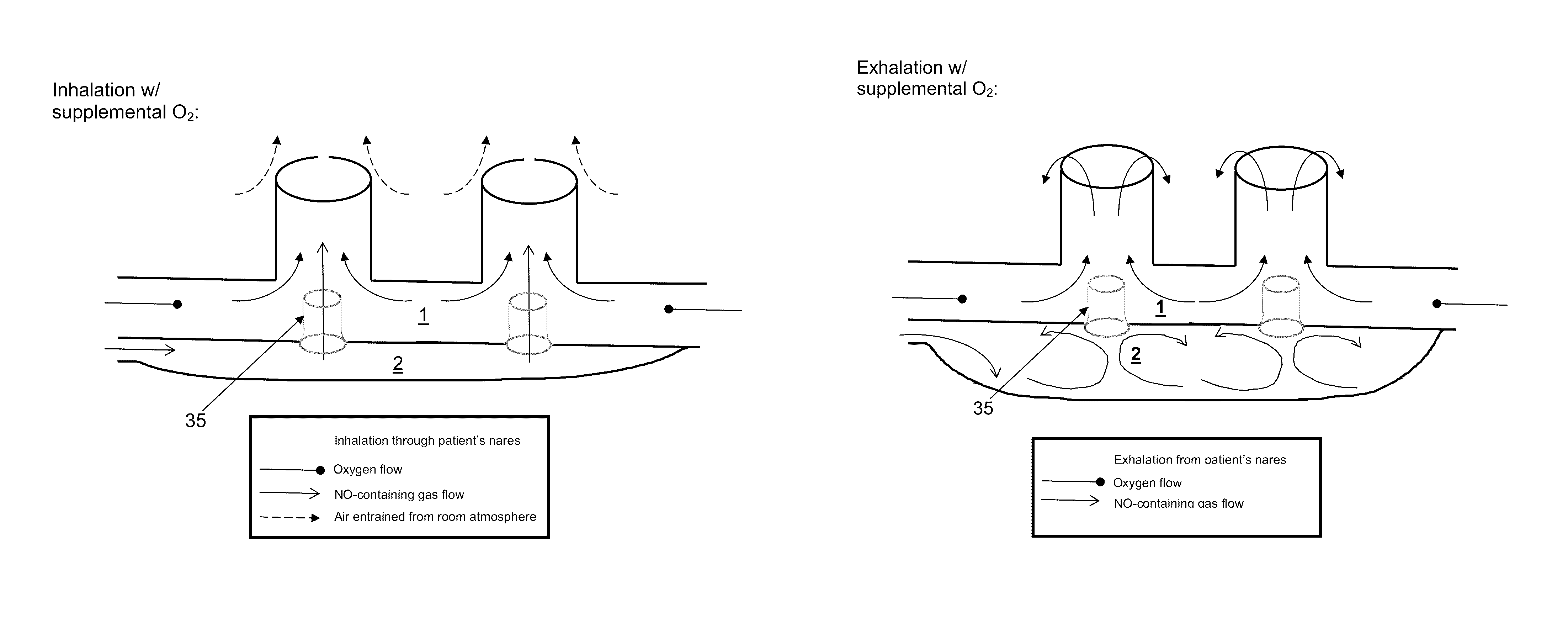
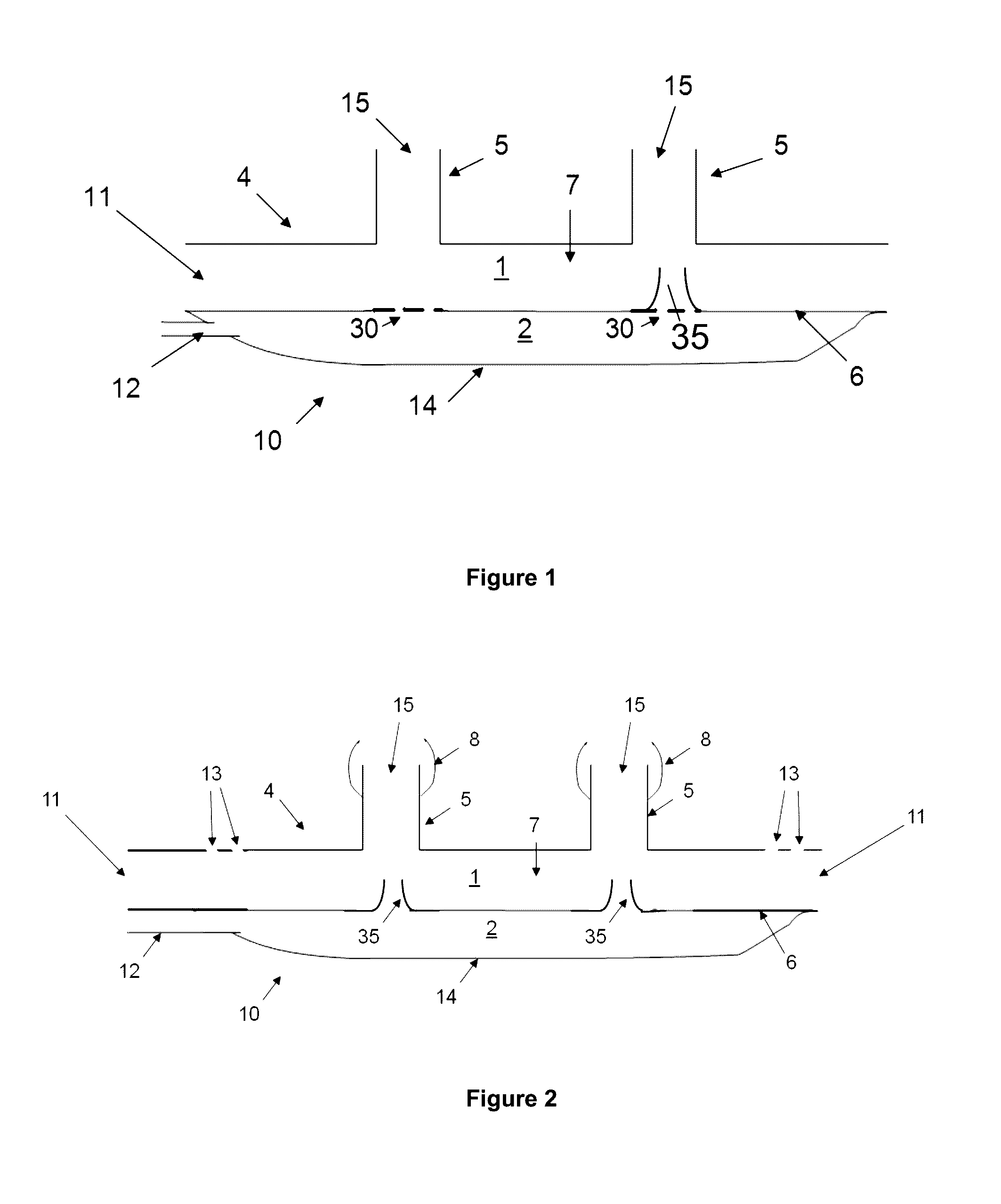
The invention concerns a breathing assistance apparatus having a source of Nitric Oxide in fluid communication with a nasal cannula assembly (10) adapted to deliver gases to a patient comprising a first compartment (1) and a second compartment (2) separated by a separation wall (6); a pair of nasal prongs (5) in fluid communication with the first compartment (1); the first compartment (1) comprising a first inlet (11) for introducing a first gas into said first compartment (1); the second compartment (2) comprising a second inlet (2) for introducing a second gas into said second compartment (2); and the separation wall (6) comprising at least one flow restriction element (35) for controlling the passage of gas from the second compartment (2) to the first compartment (1).
Owner:LAIR LIQUIDE SA POUR LETUDE & LEXPLOITATION DES PROCEDES GEORGES CLAUDE
Nasal rinse additive
InactiveUS20090069433A1Good effectAvoid symptomsBiocidePharmaceutical delivery mechanismPreventing painNose
A nasal rinse additive composition includes oleoresin capsicum containing capsaicin, dihydrocapsaicin, nordihydrocapsaicin, homodihydrocapsaicin, and homocapsaicin as active ingredients. The nasal rinse additive composition can further include vegetable glycerin, purified water, spearmint oil, lemongrass oil, sea salt, and / or ascorbic acid. The composition can be used to relieve and / or prevent pain, dryness, and / or inflammation associated with chronic and occasional nasal congestion.
Owner:DYNOVA LAB
Compound formula dextro methaphen oral disintegration tablet and its preparation method
InactiveCN1830442AShort disintegration timeGreat tasteOrganic active ingredientsPill deliveryMANNITOL/SORBITOLPseudoephedrine
An oral disintegrating tablet of dextromethorphan for treating the cold caused cough, nasal congestion and rhinorrhea is proportionally prepared from dextromethorphan, chlorphenamine, pseudoephedrine, beta-cyclodextrin or ion exchange resin, mannitol or lactose starch, and disintegrant through sieving, flavouring, mixing and die pressing.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com