Doxylamine succinate-pyridoxine hydrochloride enteric-coated tablet pharmaceutical composition and preparation method thereof
A technology of pyridoxine hydrochloride and doxylamine succinate, which is applied in the field of medicine, can solve the problems that the release rate can only reach about 50%, affect the rapid effect of the drug, and is not easy to dissolve, so as to avoid adverse reactions , low cost, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Preparation of doxylamine succinate pyridoxine hydrochloride tablets of the present invention
[0047] 1. Tablet core preparation:
[0048] Prepare materials according to the following formula (1000 pieces):
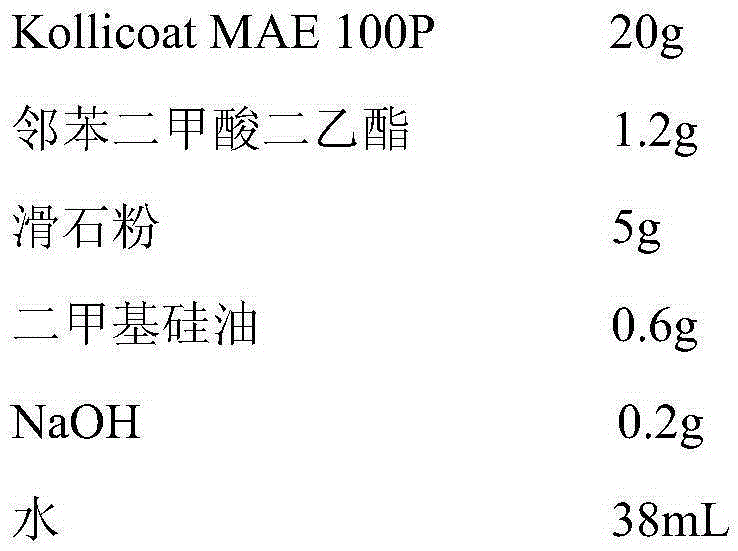
[0049]
[0050] Preparation steps:
[0051] (1) Dry the starch until the moisture content is less than 1.5% and the talcum powder to the moisture content less than 5% at 80°C;
[0052] (2) Crush doxylamine succinate and pyridoxine hydrochloride, pass through a 100-mesh sieve, and set aside;
[0053] (3) Mix doxylamine succinate and talc, pass through a 40-mesh sieve to disperse and mix to obtain a mixture ①;
[0054] (4) Add pyridoxine hydrochloride to mixture ① and mix to obtain mixture ②;
[0055] (5) Mix the low-substituted hydroxypropyl cellulose twice with the starch equal dilution method, then add it to the mixture ②, and mix to obtain the mixture ③;
[0056] (6) Add the remaining starch and mixture ③ into the multi-directional motion mixer, rotate at 10 rpm, an...
Embodiment 2
[0078] Example 2 Preparation of doxylamine succinate pyridoxine hydrochloride tablets of the present invention
[0079] 1. Tablet core preparation:
[0080] Prepare materials according to the following formula (1000 pieces):
[0081]
[0082] Preparation steps:
[0083] (1) Dry the microcrystalline cellulose PH102 at 80°C until the moisture content is less than 3% and silica to the moisture content less than 5%;
[0084] (2) Crush doxylamine succinate and pyridoxine hydrochloride, pass through a 100-mesh sieve, and set aside;
[0085] (3) Mix doxylamine succinate and silicon dioxide, pass through a 40-mesh sieve to disperse and mix to obtain mixture ①;
[0086] (4) Add pyridoxine hydrochloride to mixture ① and mix to obtain mixture ②;
[0087] (5) Mix the sodium carboxymethyl starch with mannitol equal dilution method twice, then add it to the mixture ②, and mix to obtain the mixture ③;
[0088] (6) Add the remaining mannitol, mixture ③, and microcrystalline cellulose PH102 into the multi-d...
Embodiment 3
[0110] Example 3 Preparation of doxylamine succinate pyridoxine hydrochloride tablets of the present invention
[0111] 1. Tablet core preparation:
[0112] Prepare materials according to the following formula (1000 pieces):
[0113]
[0114] Preparation steps:
[0115] (1) Dry microcrystalline cellulose PH102 at 80°C to moisture less than 3%, lactose to moisture less than 1.5%, and silicon dioxide to moisture to less than 5%;
[0116] (2) Crush doxylamine succinate and pyridoxine hydrochloride, pass through a 100-mesh sieve, and set aside;
[0117] (3) Mix doxylamine succinate and silicon dioxide, pass through a 40-mesh sieve to disperse and mix to obtain mixture ①;
[0118] (4) Add pyridoxine hydrochloride to mixture ① and mix to obtain mixture ②;
[0119] (5) Mix the croscarmellose sodium with lactose equal dilution method twice, then add it to the mixture ②, and mix to obtain the mixture ③;
[0120] (6) Add the remaining lactose, mixture ③, and microcrystalline cellulose PH102 to the mu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com