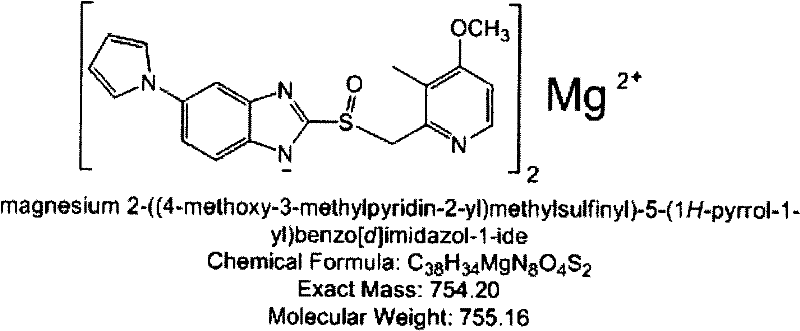
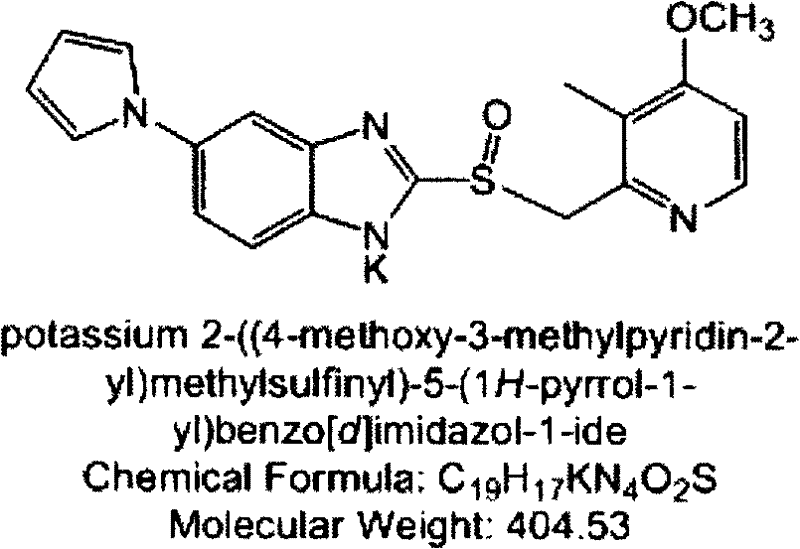
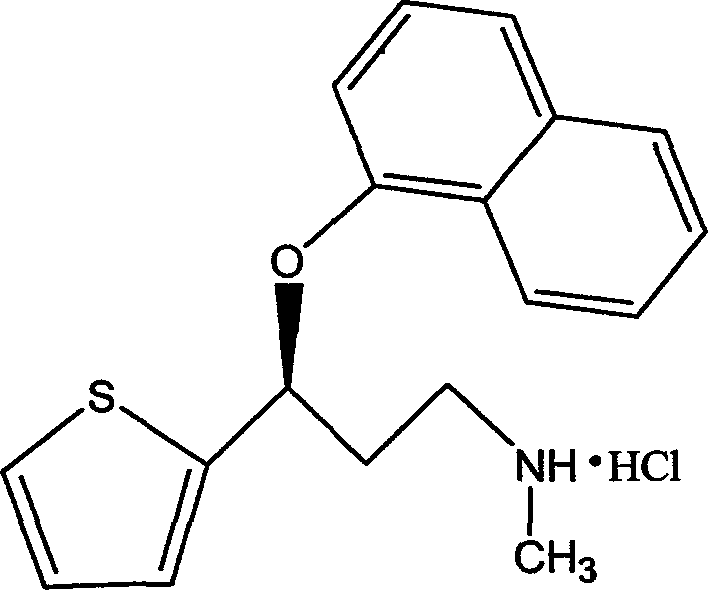
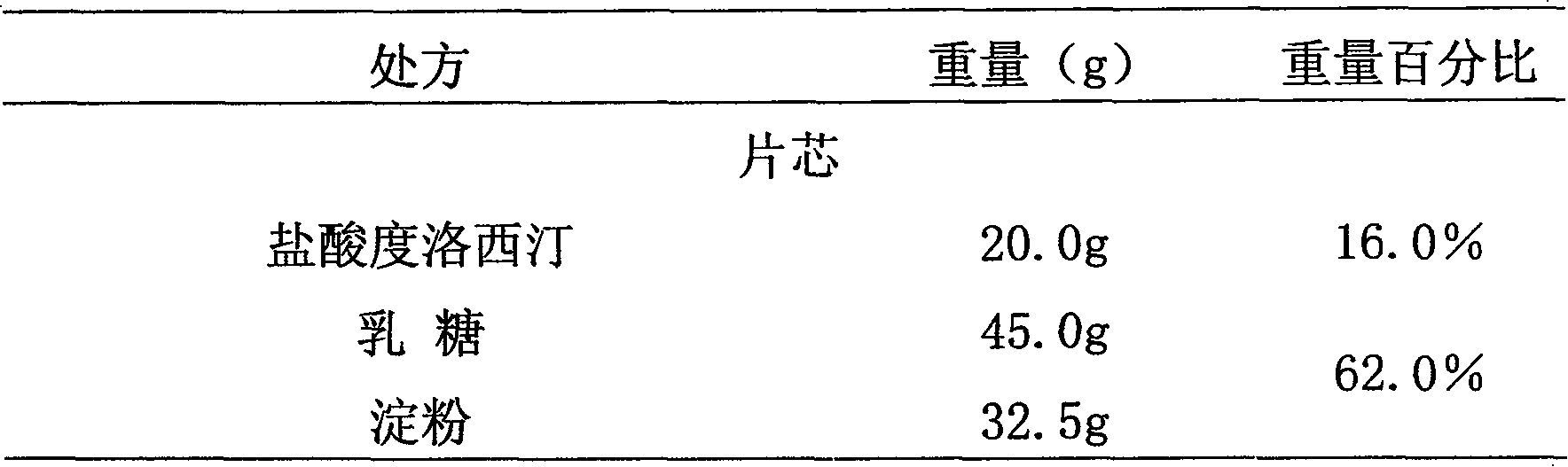
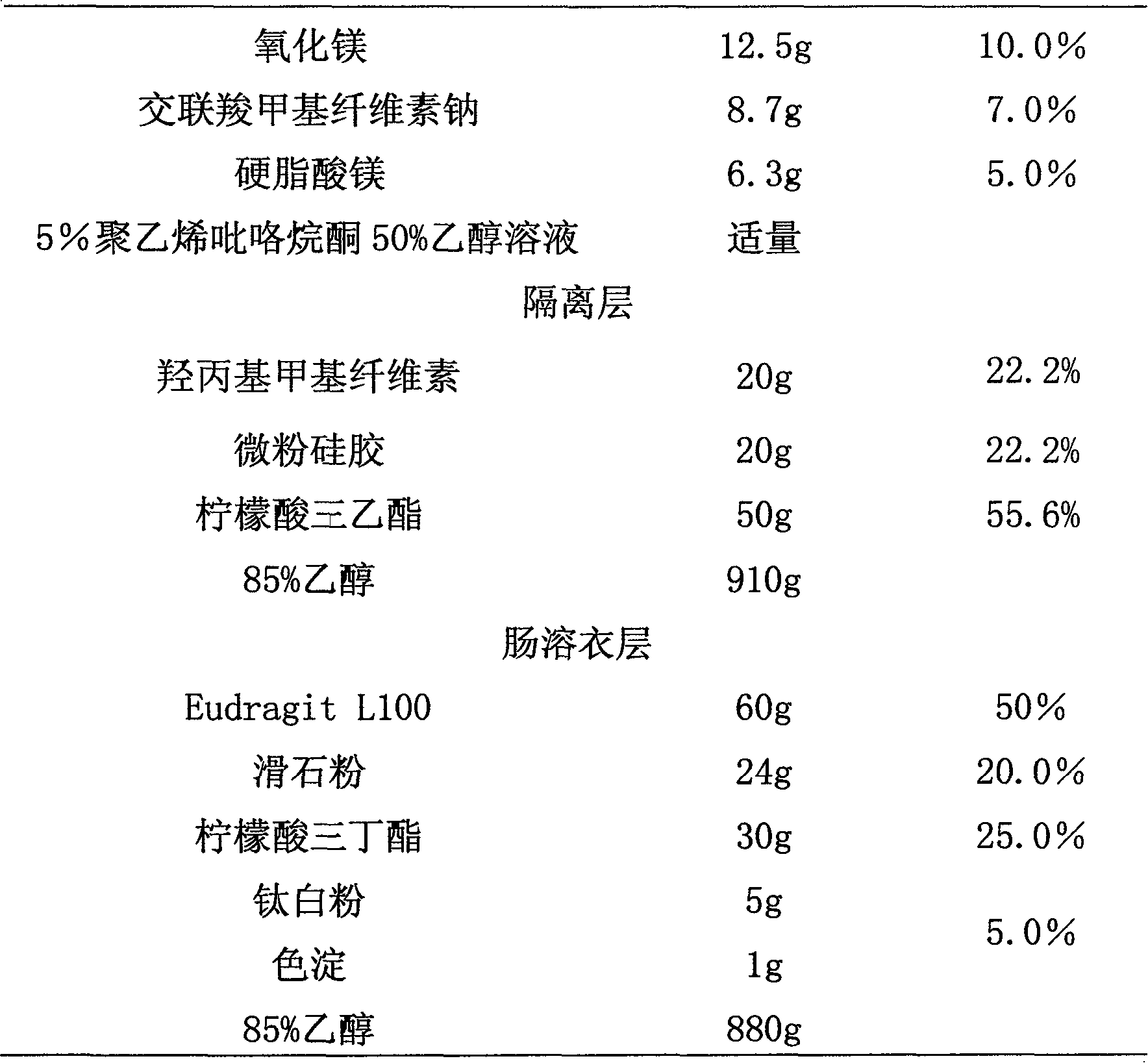
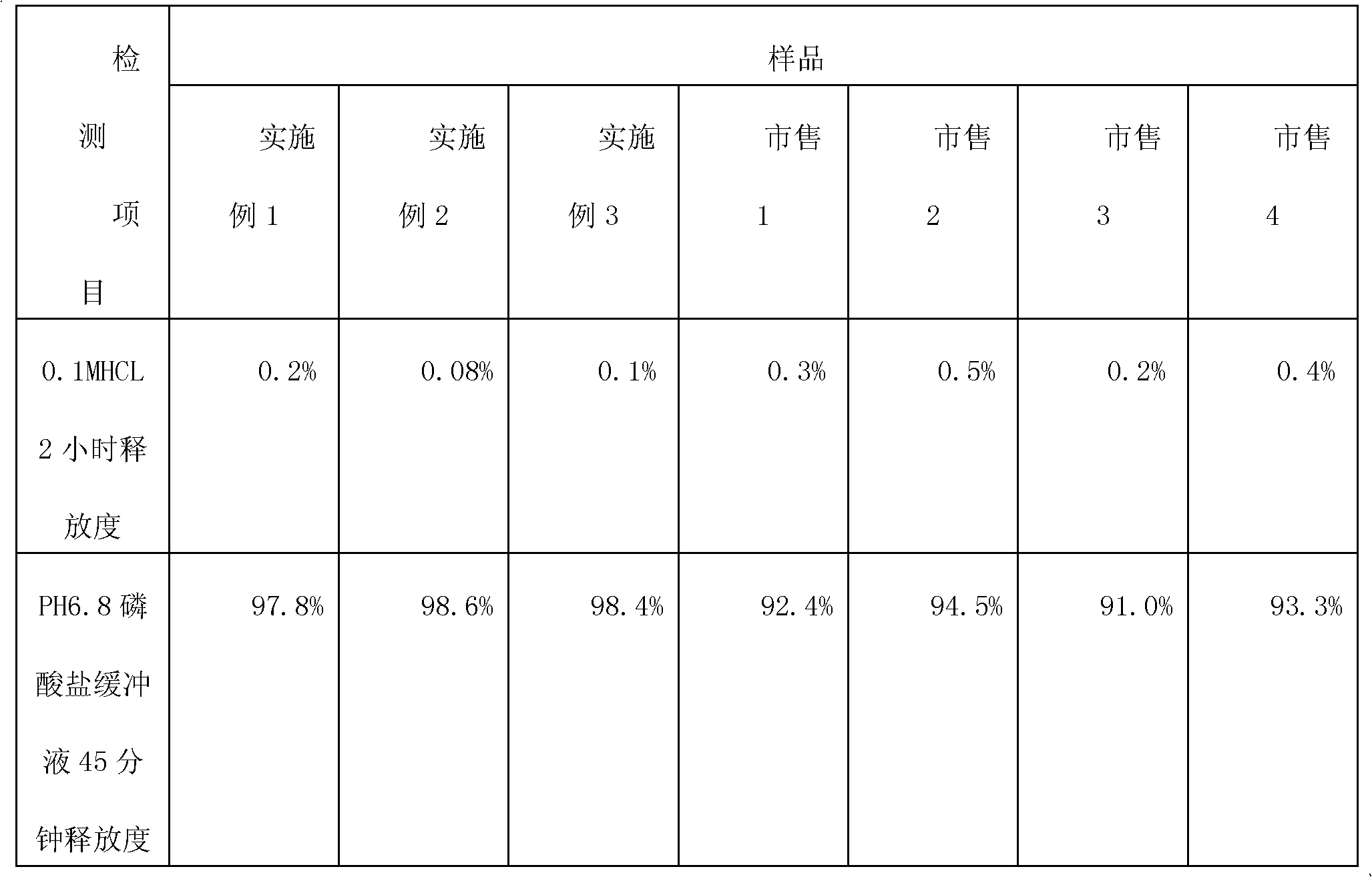
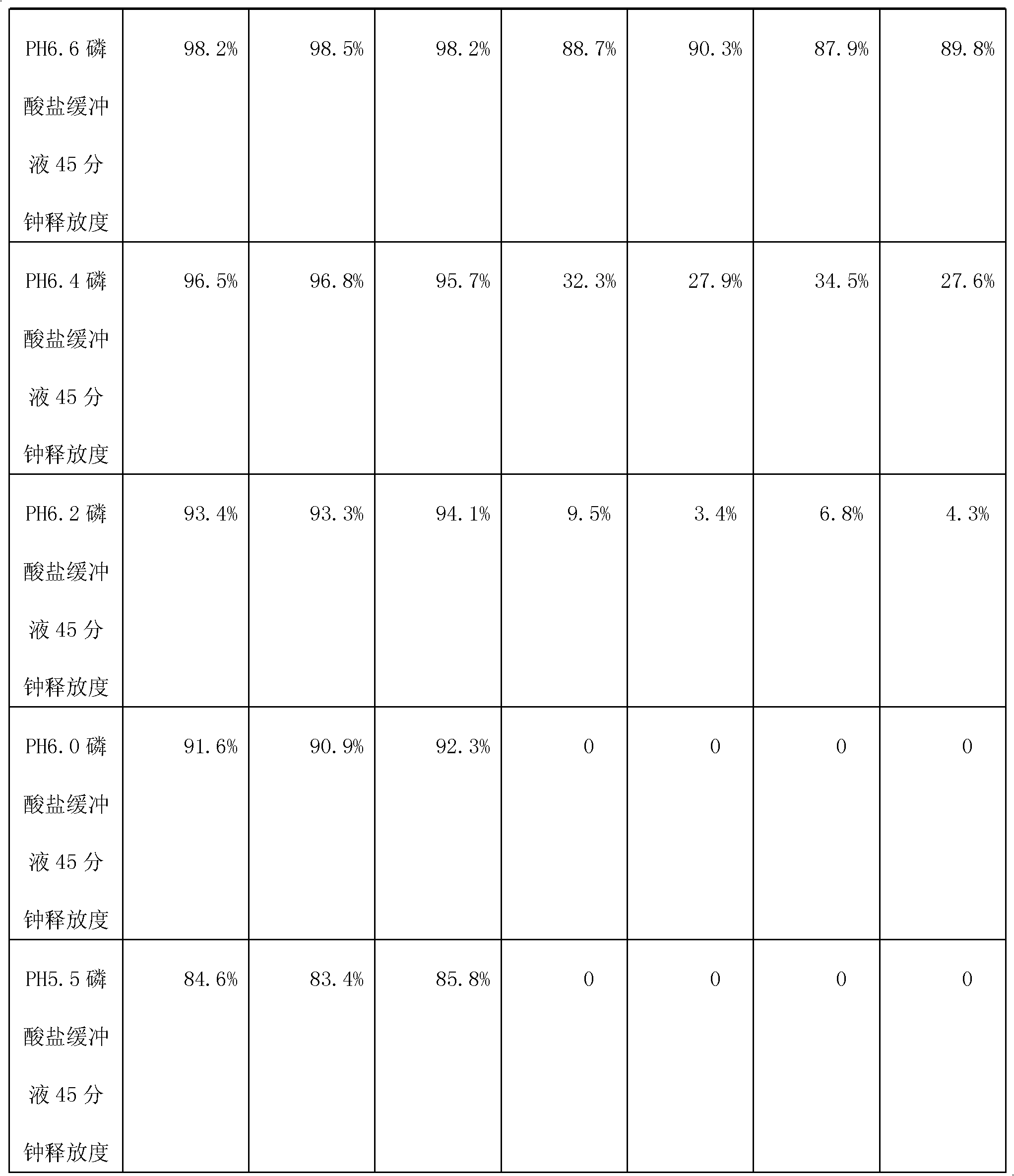
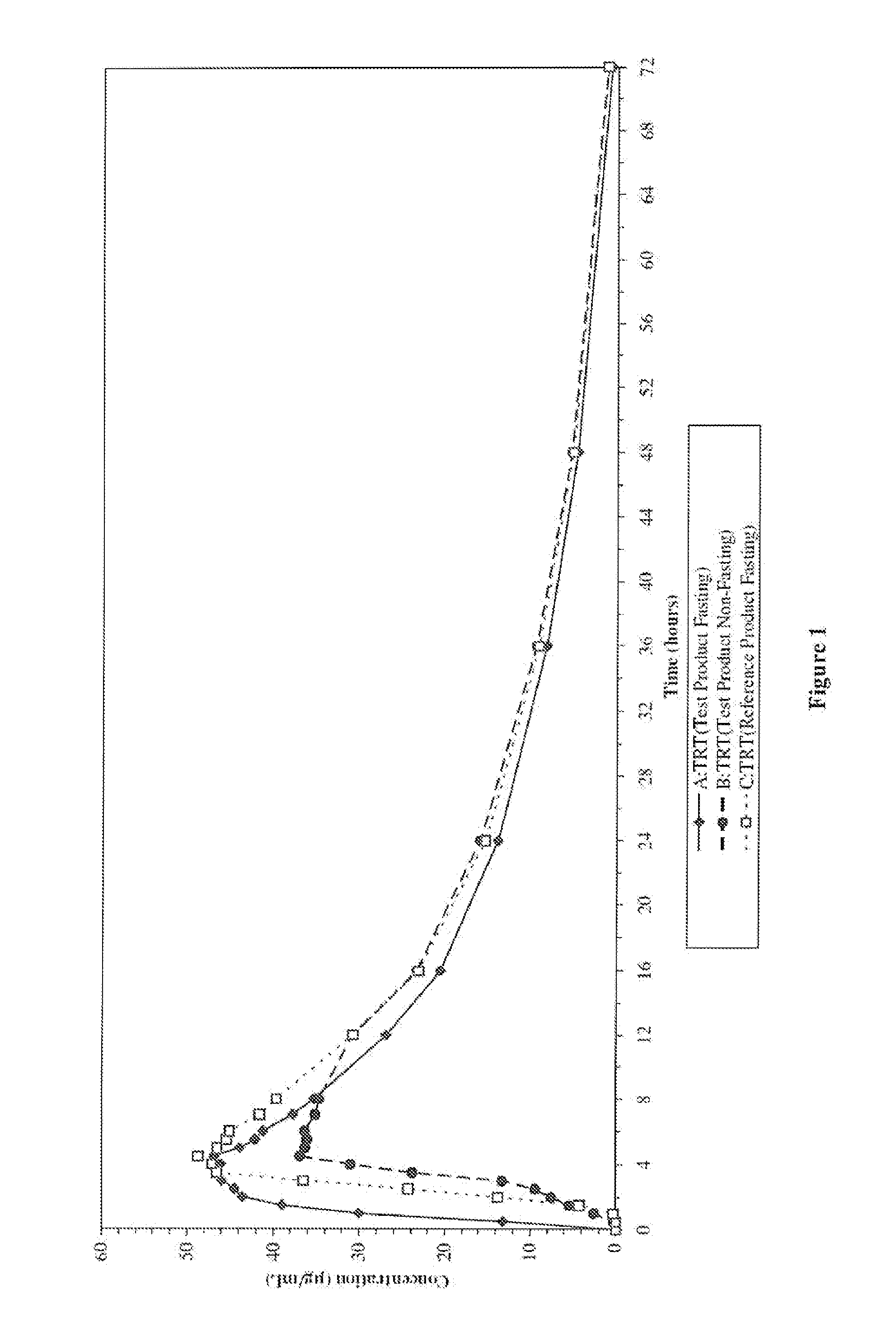
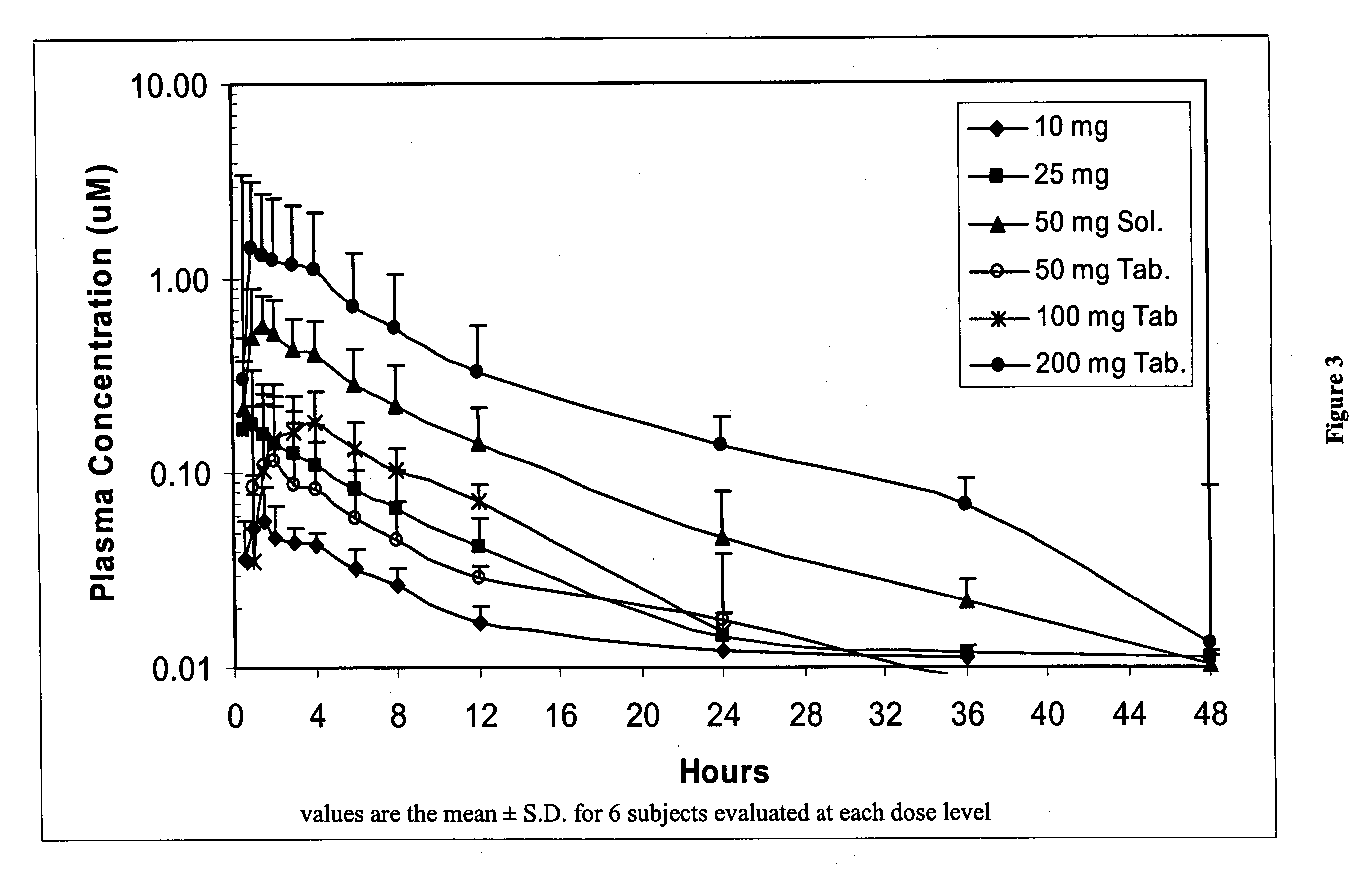
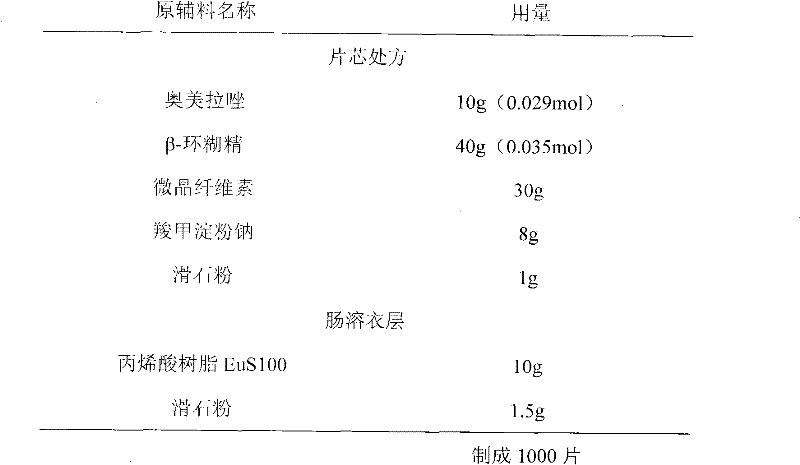
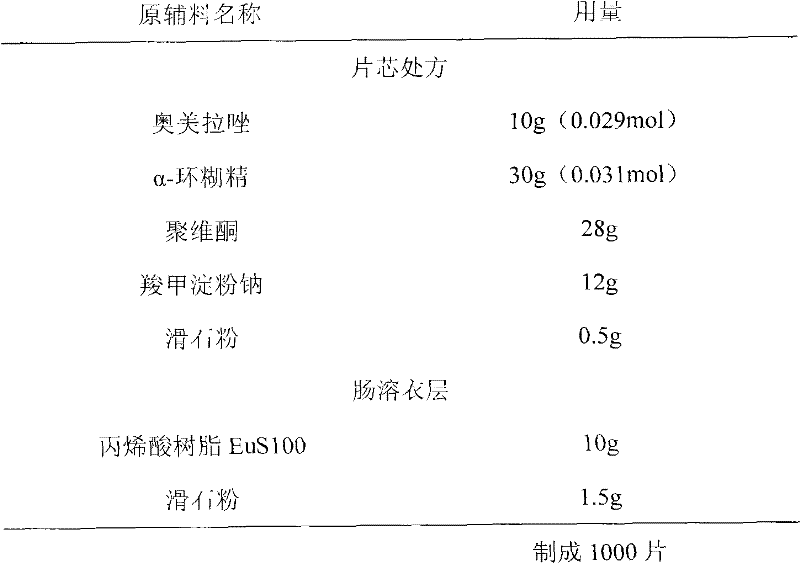
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
442 results about "Enteric coated tablets" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Enteric composition for the manufacture of soft capsule wall
ActiveUS8685445B2Loss of strengthLoss of viscosityOrganic active ingredientsPeptide/protein ingredientsHard CapsuleBiomedical engineering
Owner:PATHEON SOFTGELS INC
Synbiotics of bacillus licheniformis and oligosaccharide class prebiotics and composition and formulation thereof
ActiveCN101537020APromote growth and reproductionStrong antagonistic effectOrganic active ingredientsBacteria material medical ingredientsBacillus licheniformisSynbiotics
The invention relates to a synbiotics of bacillus licheniformis and oligosaccharide class prebiotics and a composite and a formulation thereof, being characterized in that the synbiotics comprises bacillus licheniformis medical live bacterial powder and the oligosaccharide class prebiotics, wherein the bacillus licheniformis medical live bacterial powder contains live bacteria about 30 billion / gram; the weight ratio of the bacillus licheniformis medical live bacterial powder to the oligosaccharide class prebiotics is 1:1 to 76; the weight ratio of the medical live bacterial powder of the composite, the oligosaccharide class prebiotics and auxiliary materials is as follows: 1.25-5 percent of the bacillus licheniformis medical live bacterial powder, 5-95 percent of the oligosaccharide class prebiotics, 20-50 percent of preferable oligosaccharide class prebiotics, 30-65 percent of diluting agents, 1-20 percent of bonding agents, 1-15 percent of disintegrating agents, 0.1-3 percent of lubricating agents, 1-10 percent of coating agents, 0.01-0.1 percent of flavoring agents, 0.0001-0.001 percent of coloring agents, and 0.1-15 percent of suspending agents. The synbiotics composite is prepared into oral common tablets, oral cavity disintegration tablets, dispersing tablets, enteric-coated tablets, granular formulation, enteric-coated granular formulation, capsules, enteric-coated capsules, dry supensoid agents and external tablets according to a conventional process; and an in vitro test shows that each formulation can promote the growth and the reproduction of the synbiotics of bacillus licheniformis, restrain the growth of harmful bacteria, enhance the immunizing power of parasitifers, reduce diarrhea and enhance the health care.
Owner:SHENYANG NO 1 PHARMA FACTORY DONGBEI PHARMA GRP
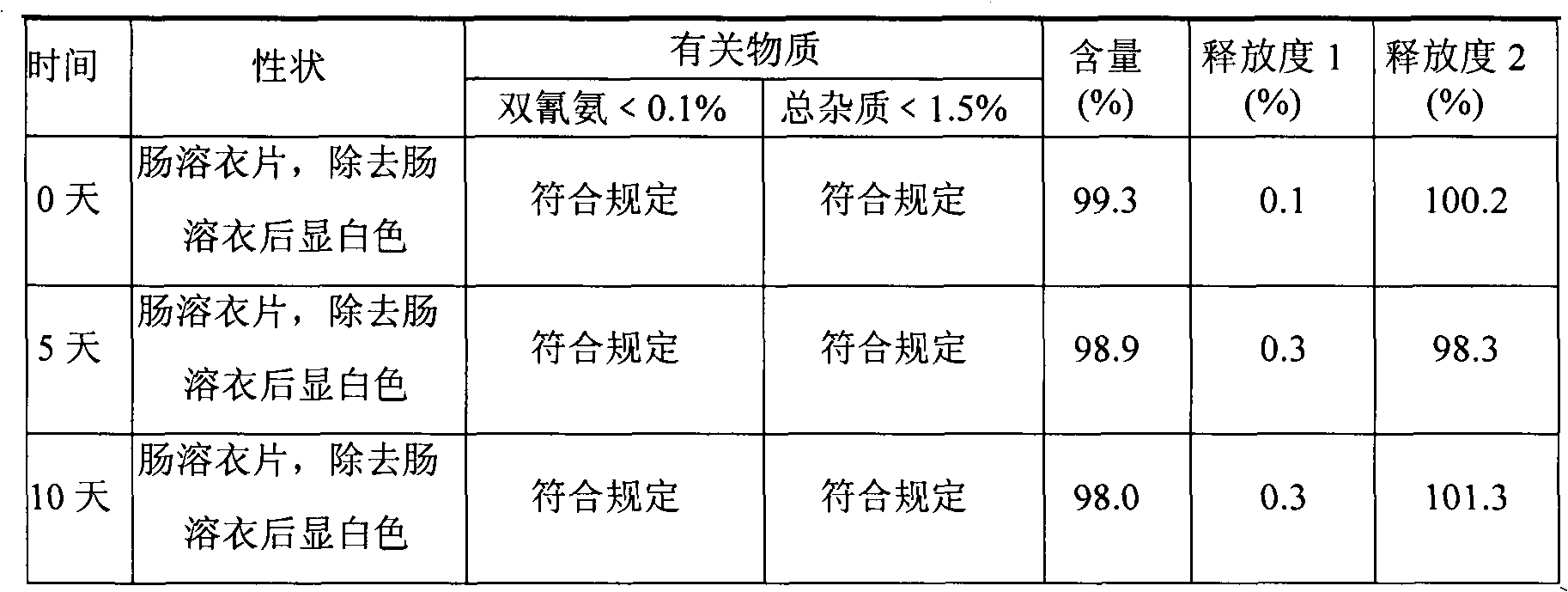
Metformin hydrochloride enteric-coated tablets quality control method
ActiveCN101339178AFacilitated releaseGuaranteed to dissolveComponent separationColor/spectral properties measurementsPhosphateMetformin Hydrochloride
The invention discloses a quality control method of metformin hydrochloride enteric coated tablet, comprising the aspects of character, identification, examination and content measurement; wherein, release examination comprises the release quantity examination of acid in hydrochloric acid solution of 0.1 mol / l and the release quantity examination in phosphate buffer with the pH value of 6.8; the examination of relevant substances comprises the following steps: dicyandiamide is taken as reference, sulfonic group cation exchange bonded silica is taken as filler, ammonium dihydrogen phosphate solution of 1.7 percent with the pH value of 3 is mobile phase and the high performance liquid chromatography is used for examining the relevant substances. The invention controls the release quantity of the metformin hydrochloride enteric coated tablet in gastric juice strictly, reduces the adverse reaction of patients effectively, improves the release quantity of the metformin hydrochloride enteric coated tablet in the buffer solution (simulated intestinal juice) and ensures the dissolution of the enteric coated tablet in the intestinal juice effectively; the invention also adds the examination of dicyandiamide impurity under the examination item and enhances the safety of the medicine.
Owner:贵州天安药业股份有限公司
Enteric solid preparation containing lycopene, resveratrol or melatonin and preparation method of enteric solid preparation
The invention relates to the field of medical preparations, in particular to an enteric solid preparation containing lycopene, resveratrol or melatonin and a preparation method of the enteric solid preparation. The enteric solid preparation comprises one or more of lycopene, resveratrol and melatonin as an active ingredient, water-soluble and / or enteric carrier adjuvants or other pharmaceutic adjuvants. The water-soluble carrier adjuvants can be used as water-soluble solid dispersoid carriers; and the enteric carrier adjuvants are enteric polymers and can be used as enteric solid dispersoid carriers or enteric coating film materials. The lycopene, the resveratrol and the melatonin of the enteric solid preparation have favorable dissolubility in the intestinal tract, so that the medicament, namely, the enteric solid preparation, can be rapidly dissolved and released in the intestinal tract, and thus absorption and bioavailability of the lycopene, the resveratrol and the melatonin are increased. The enteric solid preparation containing the lycopene, the resveratrol and the melatonin can be suitable for application and industrial production of oral preparations, such as tablets, particles, pellets, capsules, enteric capsules, enteric coating tablets, enteric coating pellets, enteric coating particles and the like.
Owner:SINOTHERAPEUTICS
Metformin hydrochloride enteric-coated tablets and preparation method thereof
The invention belongs to the technical field of medicinal preparations, in particular relates to metformin hydrochloride enteric-coated tablets and a preparation method thereof, and provides stable metformin hydrochloride enteric-coated tablets. Each metformin hydrochloride enteric-coated tablet comprises a tablet core, an insulation coating and an enteric coating, wherein the tablet core is prepared from metformin hydrochloride, dextrin, hyprolose, magnesium stearate and talcpowder by adopting the ethanol aqueous solution of hypromellose as an adhesive; the insulation coating is prepared from a gastric soluble film coating premixed suspension agent and purified water; and the enteric coating is prepared from an enteric film coating premixed suspension agent and the purified water.
Owner:BEIJING JINGFENG PHARMA GRP
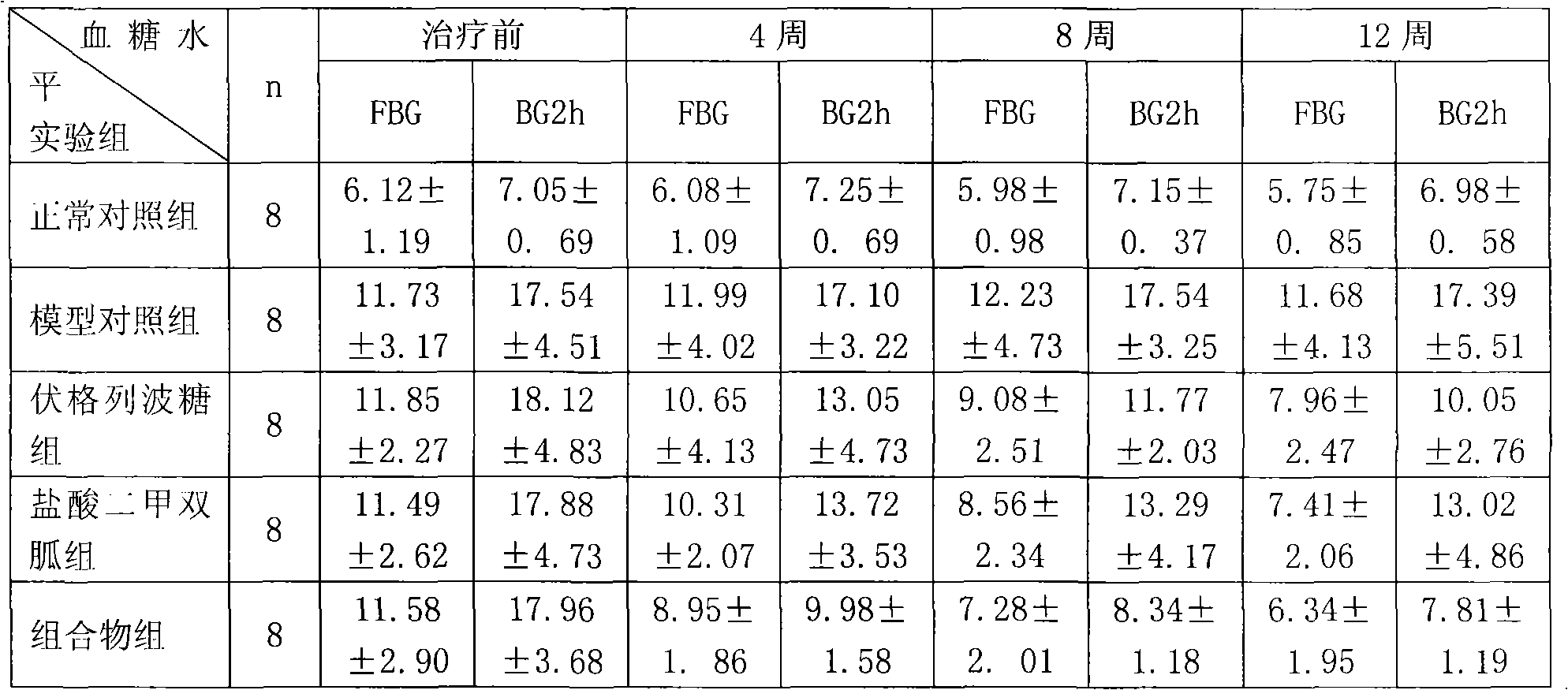
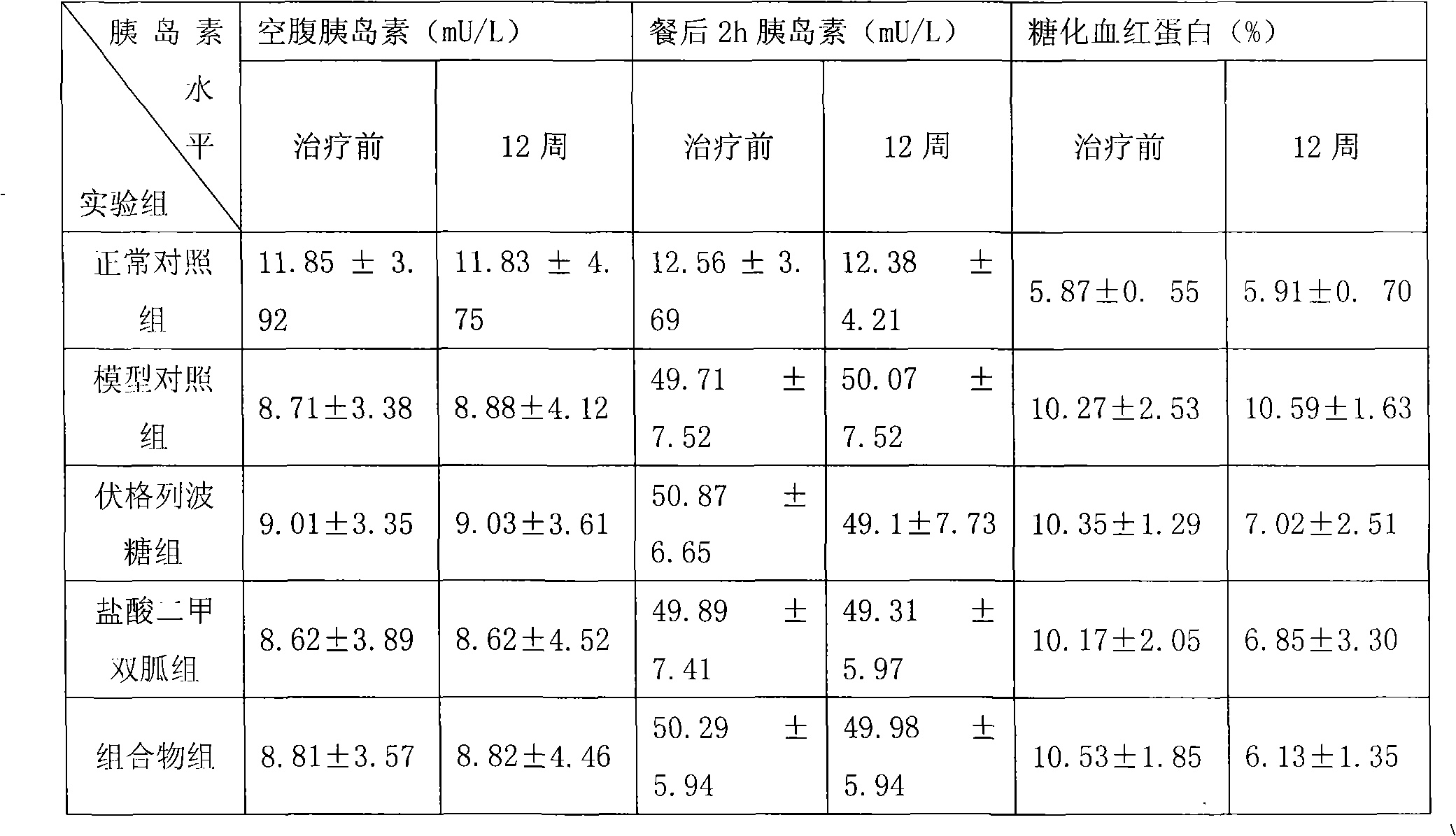
Metformin hydrochloride/voglibose sugar-lowering oral preparation composition and preparation method thereof
InactiveCN101590007AEnsure complianceConvenience guaranteedOrganic active ingredientsMetabolism disorderEnteric-coated granulesSecond-line therapy
The invention provides a metformin hydrochloride / voglibose sugar-lowering oral preparation composition and a preparation method thereof. The weight ratio of two main medicines is 8000:1-375:1, preferably 2500:1-625:1. Except for the main medicines, the composition also can further contain commonly used medicine accessories, such as a binder, a filling agent, a disintegrating agent, a lubricant, a flavoring agent, a wetting agent and a flow agent, and the obtained composition can be prepared into tablets, granules, soft and hard capsules, sustained and controlled release preparations, optimum enteric-coated tablets, enteric-coated granules and enteric-coated soft and hard capsules by conventional methods. The composition provided by the invention has action mechanism complementation of the main medicines, multiple target points, good compliance of patients, and the like. The sugar-lowering oral preparation composition can be used for the first-line therapy of type 2 diabetes, or can be used for second-line therapy under the condition that the metformin hydrochloride or sulfonylurea medicines fail to singly and effectively control blood sugar; and the sugar-lowering oral preparation composition is especially suitable for the therapy of diabetic patients suffering from latent autoimmune diabetes in adults (LADA) and hyperinsulinemia.
Owner:北京瑞伊人科技发展有限公司 +1
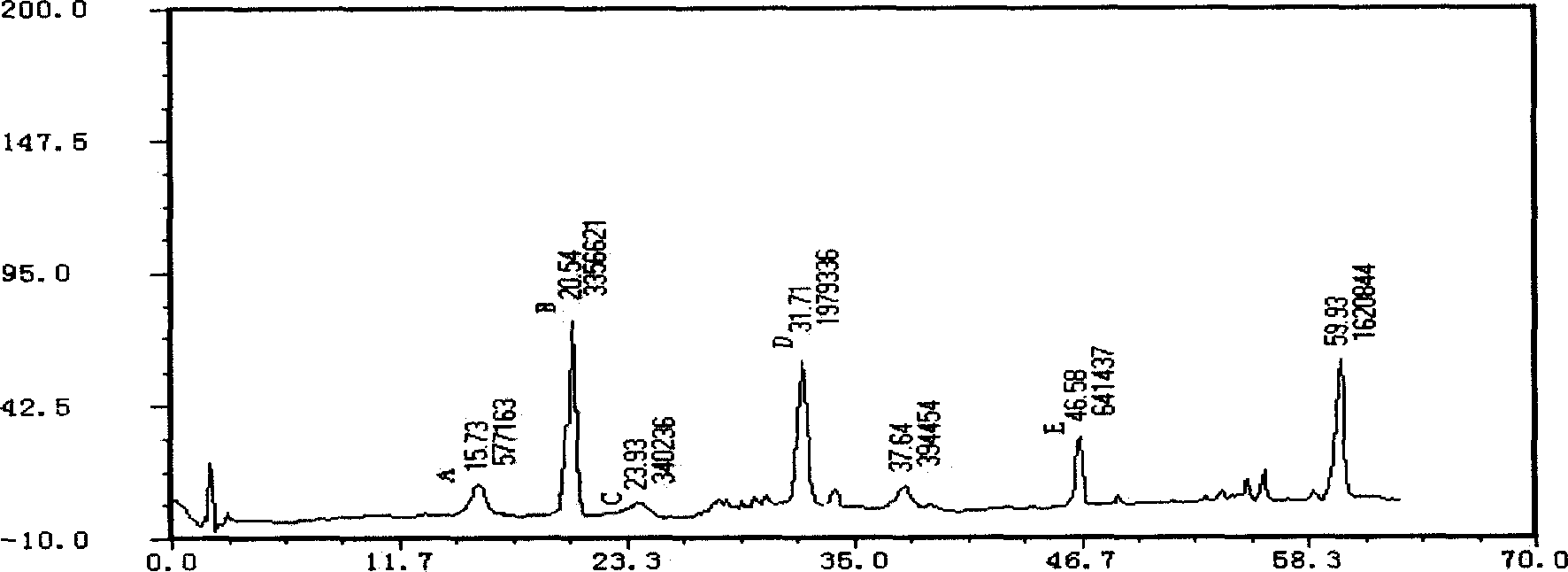
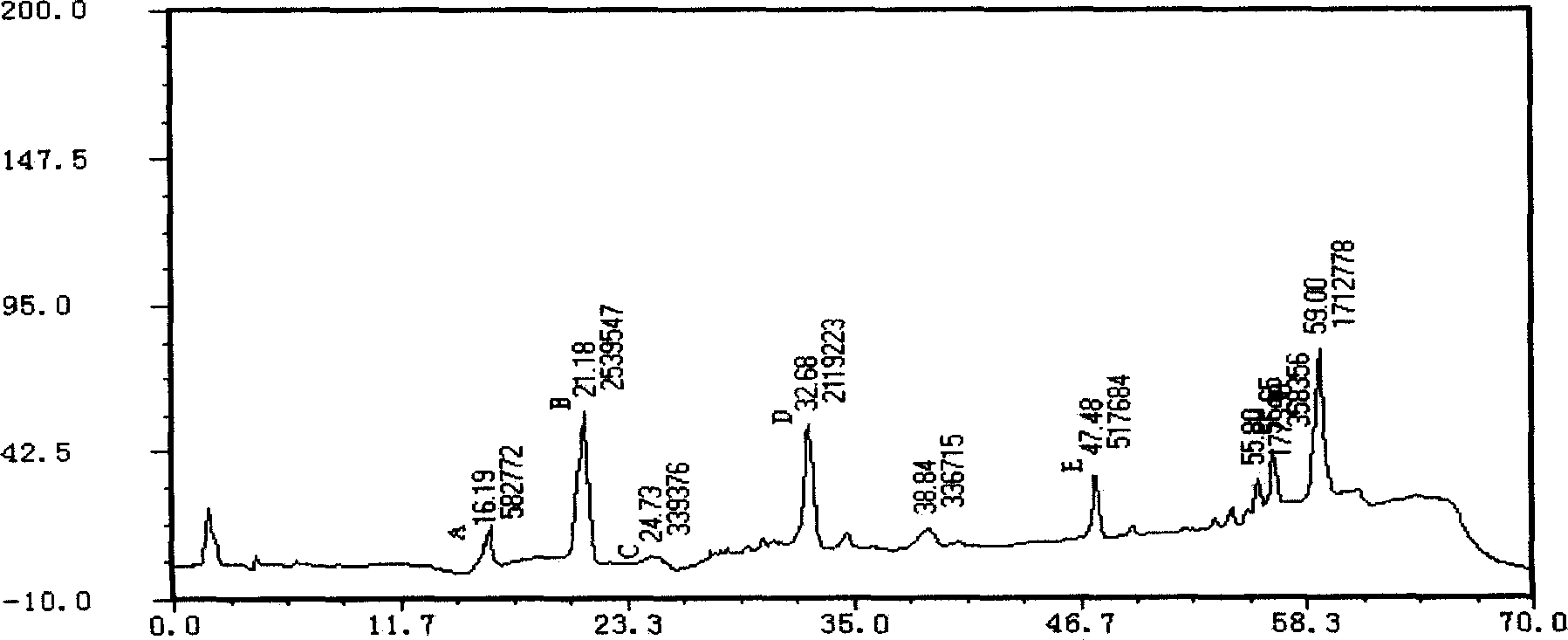
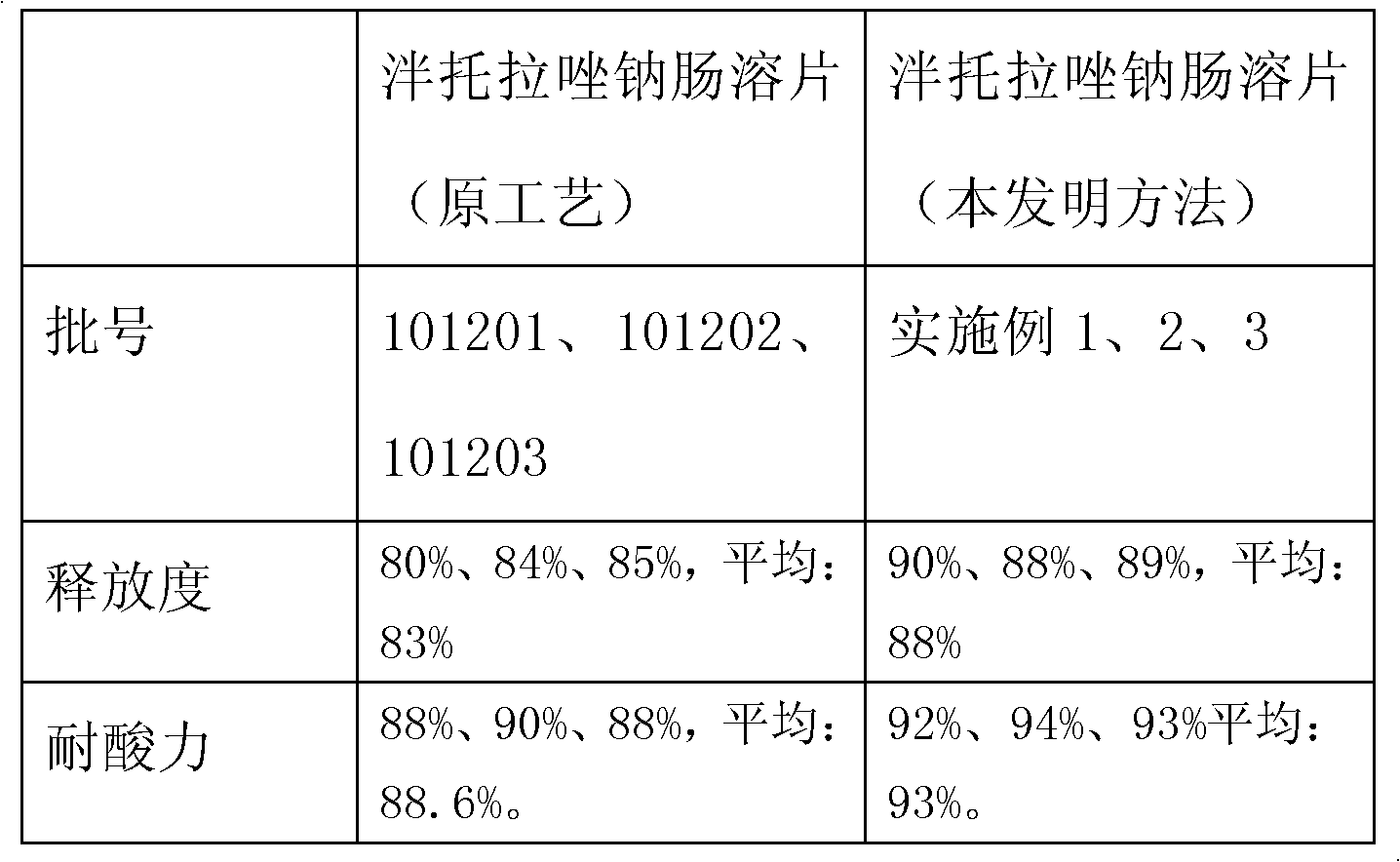
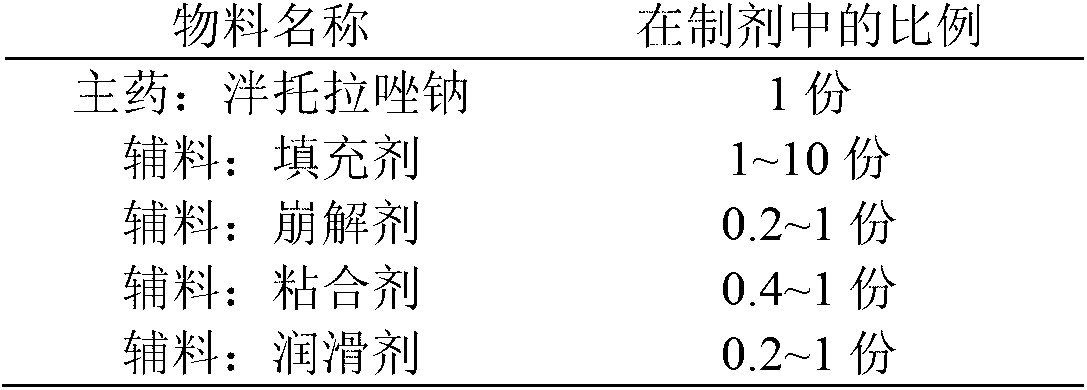
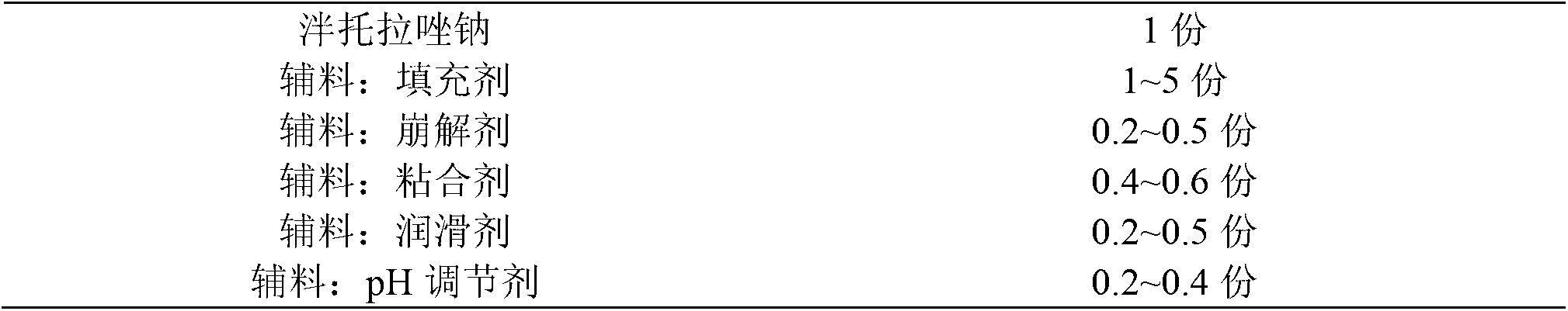
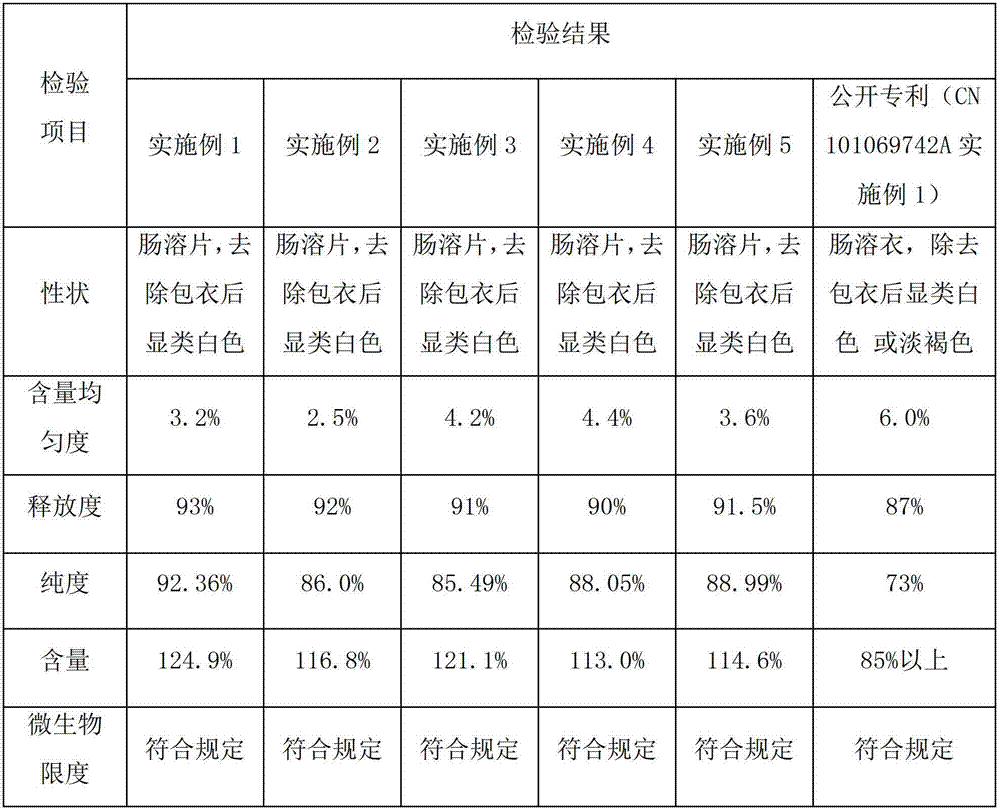
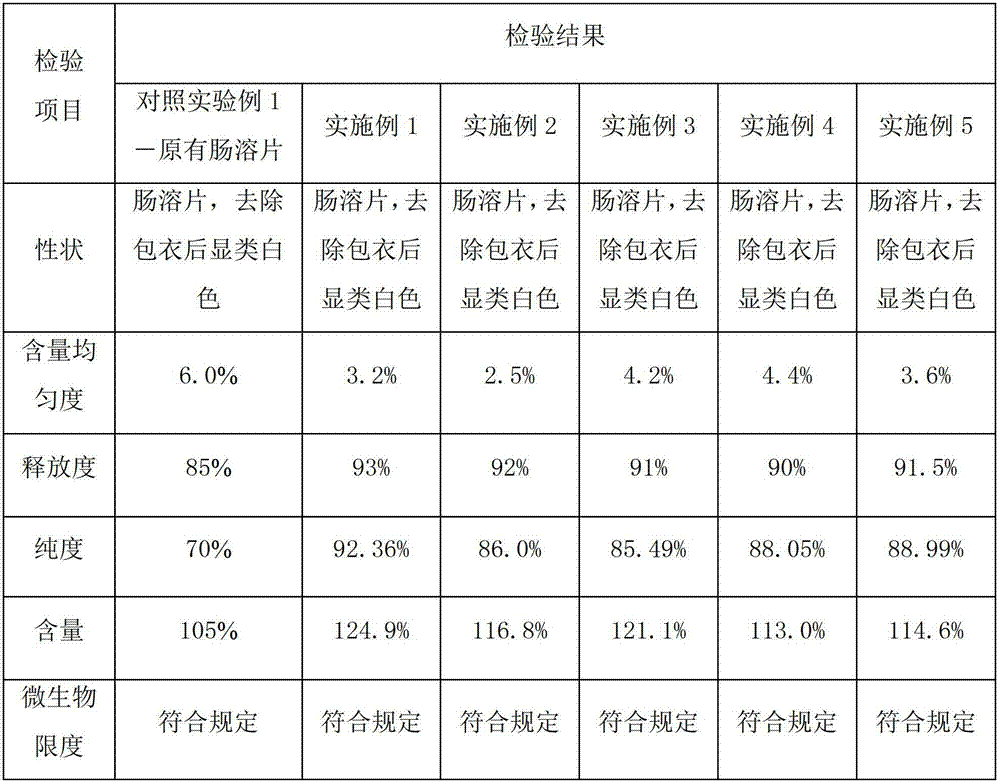
Pantoprazole sodium enteric tablet and preparation method thereof
InactiveCN101461809AOne-sided bright and tidyQuality improvementOrganic active ingredientsDigestive systemPantoprazole SodiumSilicon dioxide
The invention relates to a pantoprazole sodium enteric-coated tablet and a preparation method thereof. The enteric-coated tablet is prepared by a pantoprazole sodium plain tablet which is coated with an insulating layer and an enteric coating layer, and the pantoprazole sodium plain tablet contains 0.5 to 5 percent of silicon dioxide and 0.5 to 5 percent of talcum powder. The method solves the tablet pressing problem of extremely easy sticking during the pressing of the pantoprazole sodium plain tablet.
Owner:YAOPHARMA CO LTD
Ilaprazole enteric-coated tablets and preparation method thereof
InactiveCN102525990AImprove acid resistanceWidely distributedOrganic active ingredientsDigestive systemActive agentSurface-active agents
The invention provides ilaprazole enteric-coated tablets and a preparation method thereof. Each enteric-coated tablet contains an enteric-coated pellet and pharmaceutically acceptable tablet excipients, wherein the enteric-coated pellet contains a pellet layer, a medicine loading layer, an isolation layer and an enteric coating layer; and the medicine loading layer contains ilaprazole or pharmaceutically acceptable salt thereof and a stabilizer. The enteric-coated pellet tablets prepared from the ilaprazole have good acid resistance; barrier substances such as an antiacid, a surfactant, an organic solvent, a hydrophobic substance and the like are not contained in the prescription, so that the enteric-coated pellet tablets have health benefits and are safe; the preparation method is easy to operate, the organic solvent is not used, and an active substance is quickly and stably released; and in addition, the pellets in the tablets can be widely and uniformly distributed in an intestinal tract after being taken, the dose dumping is dispersed, and the distribution area of a medicine on the surface of the intestinal tract is increased, so the irritation of the medicine to the intestinal tract can be reduced or eliminated, and the bioavailability of the medicine can be improved.
Owner:LIVZON PHARM GRP INC
Esomeprazole magnesium contained enteric-coated tablet and preparation method thereof
ActiveCN102940611AHigh safety complianceImprove stabilityOrganic active ingredientsDigestive systemCoated tabletsInsulation layer
The invention provides an esomeprazole magnesium contained enteric-coated tablet and a preparation method thereof. The enteric coated tablet is composed of an inner tablet core layer which uses esomeprazole as an active ingredient, an intermediate insulation layer and an enteric coating protection layer. According to the esomeprazole magnesium contained enteric-coated tablet, the conditions that basic remedies are instable and prone to be oxidized and decomposed are overcome, the prepared enteric-coated tablet is even in coating, compact in coating layer, stable and reliable in quality and capable of meeting large-scale production requirements of enterprises at the present stage and has good market development prospects, the releasing rate can reach over 90%, the bioavailability is obviously improved, and safe and effective drug using is guaranteed.
Owner:KAMP PHARMA
Medicinal preparations containing duloxetine hydrochloride and preparation method thereof
ActiveCN101190208AImpact releaseImprove toleranceOrganic active ingredientsNervous disorderIsolation layerPharmaceutical formulation
The invention relates to an enteric-coated tablet containing duloxetine hydrochloride and a preparation method thereof. The enteric-coated tablet of duloxetine hydrochloride consists of three parts: a tablet core, a gastric-coated isolation layer and an enteric-coated layer. The technical proposal of the invention can effectively avoid the cross reaction between the medicine and enteric coating material occurring during the releasing process of the medicine and affecting the release of the medicine; therefore, the stability of the medicine can be effectively improved. The preparation method is simple and is easy to be operated, which is suitable for industrialized production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Metformin hydrochloride enteric-coated tablet
InactiveCN102357088AAvoid situations of rapid aggregate denaturationReduce use costOrganic active ingredientsMetabolism disorderManufacturing cost reductionClinical efficacy
The invention discloses a metformin hydrochloride enteric-coated tablet, which comprises a tablet core and an enteric-coated layer. The tablet core is prepared by the following raw materials by weight: 100 parts of metformin hydrochloride, 3-5 parts of povidone K30, 8-12 parts of 75% ethanol water containing 10% povidone K30 and 0.5-1.0 part of magnesium stearate. The enteric-coated layer is prepared by the following raw materials by weight: 100 parts of eudragit L30D-55, 10-30 parts of talcum powder, 2-4 parts of polyethylene glycol 4000, 4-6 parts of 4% sodium hydroxide solution and 80-120 parts of purified water. The preparation process includes the steps of weighing all raw materials according to proportion, preparing the tablet core, preparing enteric-coated solution and then obtaining the metformin hydrochloride enteric-coated tablet. The metformin hydrochloride enteric-coated tablet simplifies preparation technology, effectively reducing manufacture cost, can simultaneously control releasing speed, and effectively guarantees clinical effects of products.
Owner:HEBEI JAMESHILL PHARMA
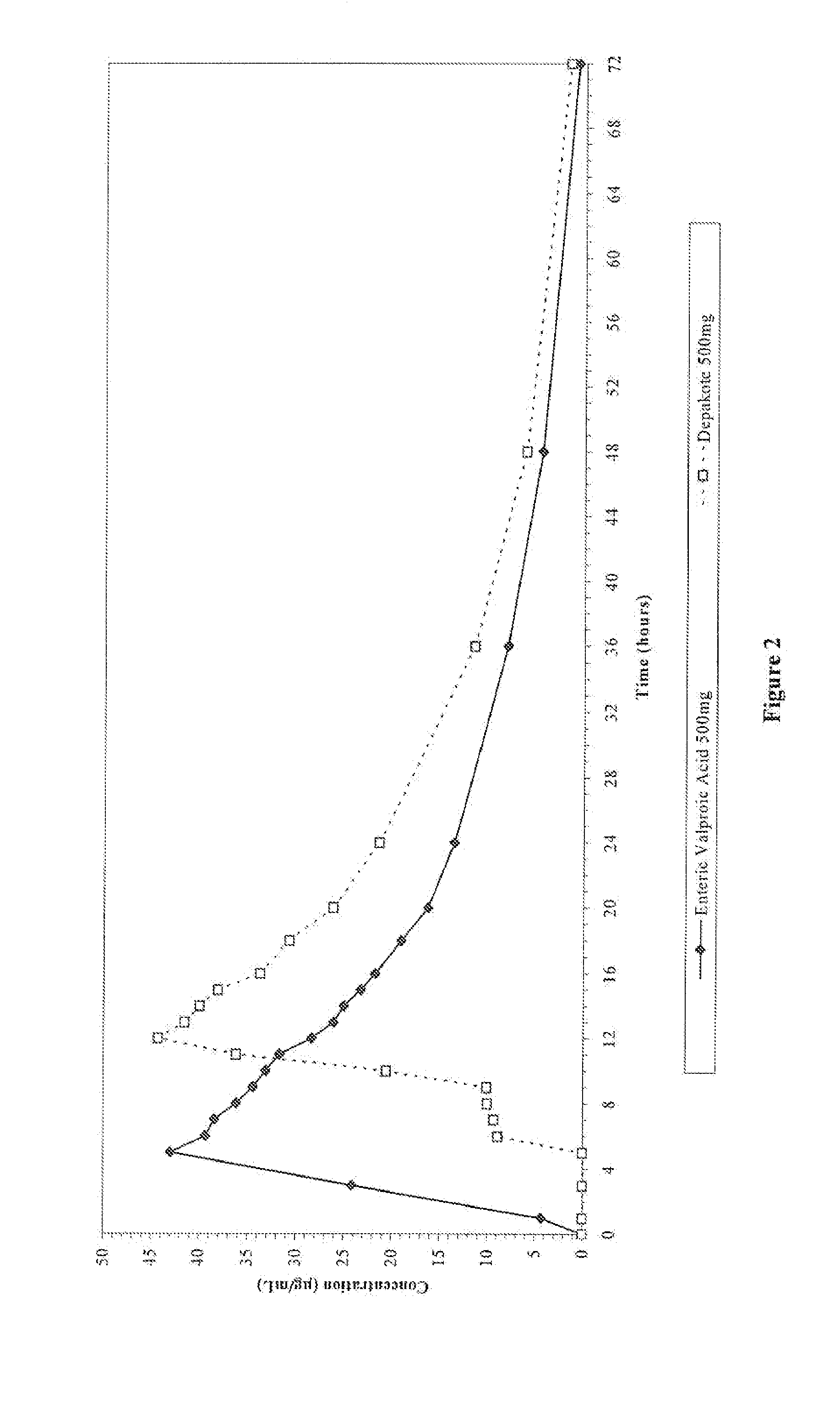
Enteric valproic acid
An enteric valproic acid soft gelatin capsule, in which the enteric polymer is a component of the capsule shell rather than a coating, has been developed. The fill material comprises valproic acid or divalproex sodium and, optionally, one or more pharmaceutically acceptable excipients such as corn oil. The capsule shell is prepared from a mass comprising a film-forming polymer, an acid insoluble polymer, an aqueous solvent, and optionally a plasticizer. Suitable film-forming polymers include gelatin. Suitable acid-insoluble polymers include acrylic-acid / methacrylic acid copolymers. The acid-insoluble polymer is present in an amount from about 8% to about 20% by weight of the wet gel mass. The weight ratio of acid-insoluble polymer to film-forming polymer is from about 25% to about 50%. The aqueous solvent is water or an aqueous solution of alkalis such as ammonia or diethylene amine or hydroalcoholic solutions of the same. Suitable plasticizers include glycerin and triethylcitrate. The enteric soft gelatin capsule does not require an enteric coating and thus is not susceptible to the processing problems associated with enteric coated dosage forms. Enteric valproic acid soft gelatin capsules may be smaller in size and thus easier to swallow than currently available enteric coated tablets due to the presence of fewer ingredients, as well as smaller amounts of ingredients in the capsule shell.
Owner:PATHEON SOFTGELS INC
Notoginseng medicine composition for treating cardiac and cerebral vascular diseases
The present invention relates to a kind of total arasaponin composition, which has the active component of total arasaponin comprising arasaponin R1, ginsenoside Rg1, ginsenoside Re and ginsenoside Rb1 not less than 55.0 wt% and ginsenoside Rd not more than 2.5 wt%. The total arasaponin composition is prepared into injection, powder for injection, enteric coated tablet, bolus, medicine powder and other preparation forms. The medicine of the present invention has the functions of promoting blood circulation to disperse blood clots and activating collateral flow, and is used in treating blood stasis to block collateral channels, apoplexy, hemiplegia and other cardiac and cerebral vascular diseases.
Owner:GUANGXI WUZHOU PHARMA GRP
Pantoprazole sodium enteric-coated tablet and preparation method thereof
InactiveCN102626398AImprove acid resistanceFacilitated releaseOrganic active ingredientsDigestive systemAcrylic resinDissolution
The invention provides a pantoprazole sodium enteric-coated tablet, which consists of the following components in parts by weight: 1 part of a pantoprazole sodium plain film, 0.1-0.5 part of an isolating layer and 0.5-1 part of an enteric-coated layer, wherein the isolating layer consists of hydroxypropyl methylcellulose and an alkali in the weight part ratio of 1:5-5:1; and the enteric-coated layer is prepared from 0.5-1 part of acrylic resin. The invention provides a preparation method of the pantoprazole sodium enteric-coated tablet. Parameters such as spray speed, spray pressure, the rotating speed of a coating pan and the like are improved through process parameters of the isolating layer, an enteric liquid and a coating, so that the prepared pantoprazole sodium enteric-coated tablet has high acid tolerance and dissolution rate, the acid tolerance of a finished product is over 90 percent, the dissolution rate is over 75 percent, and the pantoprazole sodium enteric-coated tablet is accordant with and superior to the requirements of the Chinese Pharmacopoeia on the enteric tablet. The product has the advantages of stable quality, convenience for storing and transporting and contribution to clinical application. The method is simple, is suitable for industrial production, and has high application value.
Owner:SHANGHAI TENRY PHARMCEUTICAL CO LTD
Treatment of allergic conditions
Orally administered sodium cromoglycate has been found to be effective in the treatment of allergic conditions such as asthma, general food allergies, ulcerative colitis, atopic eczema, chronic urticaria and irritable bowel syndrome if it is presented such that the sodium cromoglycate becomes bioavailable within 10 minutes of exposure to intestinal fluid. The sodium cromoglycate may be presented as enteric-coated tablets or individually enteric-coated pellets or microgranules packaged with disintegrant in a ratio of at least 1.2:1 disintegrant:sodium cromoglycate (w:w). Optionally, the patients are first selected to have a total serum IgE level of at least 150 iu / ml.
Owner:THORNTON & ROSS
Oral preparation containing andrographolide and preparation method thereof
InactiveCN103070843AImprove toleranceAvoid direct accessOrganic active ingredientsAntiviralsEnteric-coated granulesSide effect
The invention relates to an oral preparation containing andrographolide and a preparation method thereof, belonging to the field of medicines. The oral preparation containing andrographolide is in dosage forms of enteric-coated granules, enteric-coated tablets, enteric-coated capsules and enteric-coated dispersible tablets which are prepared by mixing andrographolide and pharmaceutically acceptable auxiliary materials. The prepared oral preparation can reduce incidence of adverse reactions and side effects thereof and improve the medication safety while ensuring the therapeutic effect, and uses the andrographolide more widely.
Owner:司鹏 +1
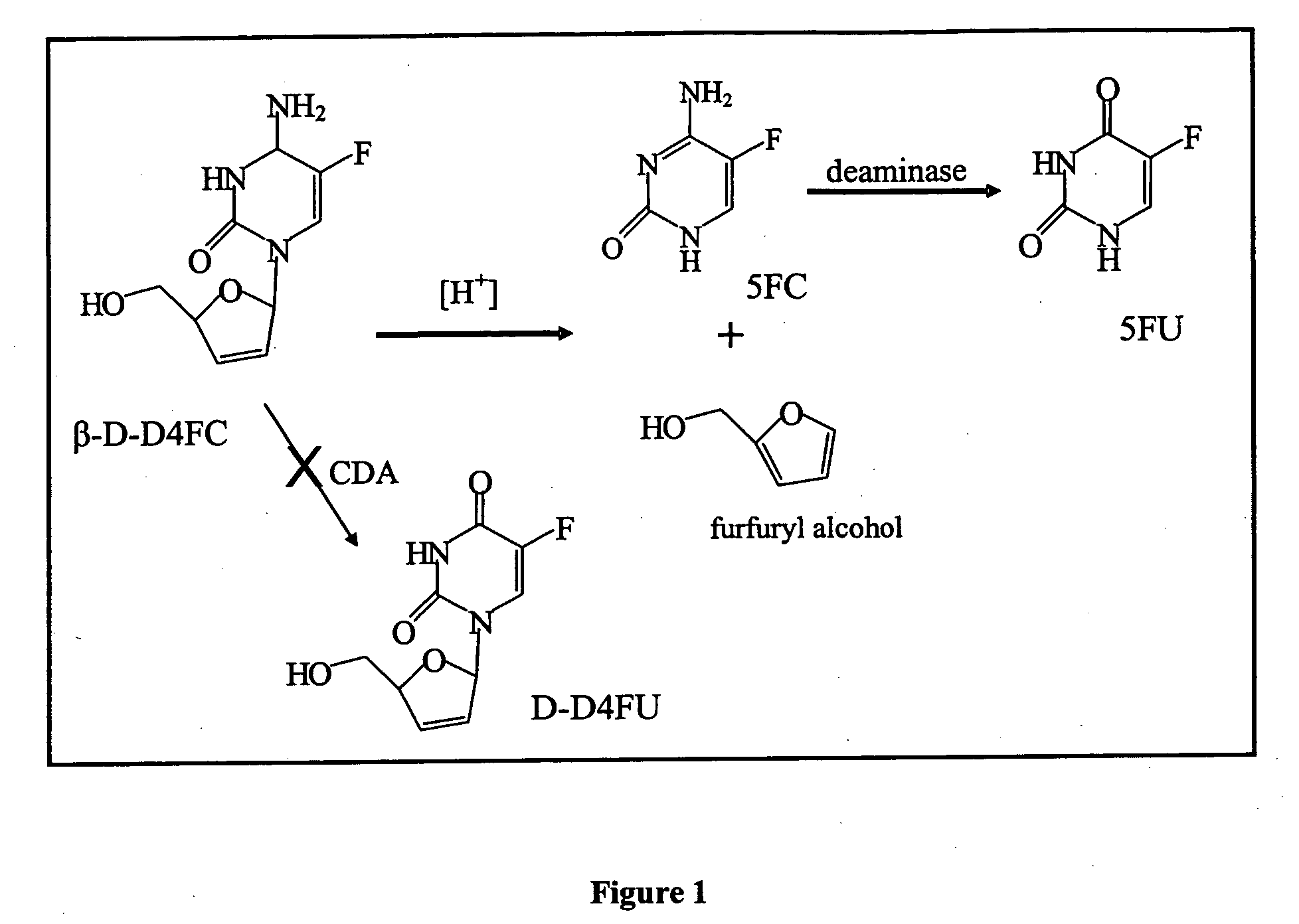
Dosing methods for beta-D-2',3'-dideoxy-2',3'-didehydro-5-fluorocytidine antiviral therapy
InactiveUS20050244490A1Improve oral bioavailabilityLow pill burdenBiocideCarbohydrate active ingredients5-fluorocytidineIn vivo
The disclosed invention is a composition for and a method of treating a HIV infection in a host, such as a human, using a single, once a day, oral dose of β-D-D4FC in an enteric-coated tablet. The enterically coated β-D-D4FC increases the amount of the drug that remains in active form for use in inhibiting the HIV virus in vivo.
Owner:PHARMASSET
Erythromycin micro pill enteric coatel tablet and its preparation method
InactiveCN1589807AInhibition releaseImprove stabilityAntibacterial agentsPowder deliveryMedicineEnteric coated tablets
A micropill-type enteric tablet of erythromycin is proportionally prepared from erythromycin, filler, adhesive, lubricant, flowing aid, isolating coating and enteric coating.
Owner:范敏华
Preparation method of proton pump inhibitor enteric-coated tablet
ActiveCN104922086AImprove product qualityDigestive systemPharmaceutical delivery mechanismActive agentDissolution
The invention discloses a preparation method of a proton pump inhibitor enteric-coated tablet. The proton pump inhibitor enteric-coated tablet consists of a medicated tablet core, an isolating layer and an enteric-coated layer, wherein the medicated tablet core consists of active ingredients, filling agents, disintegrating agents, stabilizing agents, adhesion agents, surface active agents and lubricating agents; the isolating layer consists of film-forming agents, pore-foaming agents and hydrophobic materials; and the enteric-coated layer consists of an enteric-coated material, plasticizers, antisticking agents and light-screening agents. The formula and the preparation technology of the isolating layer are key and core technologies of controlling in-vitro release of drugs. By control on the formula and the preparation technology of the isolating layer and weight increment of the isolating layer, the releasing rate of the proton pump inhibitor enteric-coated tablet in different dissolution media with pH (potential of hydrogen) 1.2, pH 6.0, pH 6.8 and pH 8.0 and water. A prepared proton pump inhibitor enteric-coated tablet product is stable in quality and has a good market prospect.
Owner:珠海润都制药股份有限公司
Prepn of medicine for treating hepatosis
InactiveCN1403134AImprove bioavailabilityProlong the action timeDigestive systemAntiviralsOrganic solventMedicine
The present invention relates to the preparation of medicine for treating hepatosis. Coarse Ganhuangcao powder is extracted with water or organic solvent to obtain extractive and the extractive is mixed with supplementary material to prepare the medicine in different forms, including tablet, capsule, dropping pill, injection, delayed releasing capsule, etc. The medicine is easy to take, high in biological utilization and long in the time for the active component of Ganhuangcao to act in the body.
Owner:江云 +1
Granulating and coating process of esomeprazole magnesium contained in esomeprazole magnesium enteric-coated tablet
ActiveCN103040774AGuaranteed insolublePromote dissolutionOrganic active ingredientsPill deliveryEnteric-coated granulesEsomeprazole Sodium
The invention provides a granulating and coating process of esomeprazole magnesium contained in esomeprazole magnesium enteric-coated tablets. The granulating and coating process comprises the following steps of: firstly preparing esomeprazole magnesium granules; then preparing esomeprazole magnesium enteric-coated granules sequentially through isolating layer coating and enteric-coated layer coating; and finally blending adjuvants with the esomeprazole magnesium enteric-coated granules and tabletting to prepare the esomeprazole magnesium enteric-coated tablets. An appropriate granulating method comprises the following steps of: crushing and grinding the esomeprazole magnesium and the adjuvants into powder, and then uniformly mixing; mixing with an adhesive to obtain a water solution, stirring for 8-10 minutes in a wet type granulator to prepare appropriate granules; drying at 40-45 DEG C, and screening to obtain the granules with grain size being between 40 meshes and 80 meshes. According to the process including granulating and coating of raw materials, the enteric-coated granules are prepared firstly and then blended with the adjuvants and finally tabletting is carried out, pellets are not used, the content uniformity of the prepared esomeprazole magnesium enteric-coated tablet product is greatly enhanced, and the problem of unqualified uniformity of the product content caused by excessive material flowability in an original process is solved.
Owner:SHANGHAI SINE WANXIANG PHARMA
Omeprazole enteric coated tablet and preparation method thereof
ActiveCN101744788ARapid dissolutionGood water solubilityOrganic active ingredientsDigestive systemPlasticizerDissolution
The invention provides an omeprazole enteric coated tablet and a preparation method thereof. The enteric coated tablet is formed by an inner tablet core and an outer enteric coating, wherein the inner tablet core takes omeprazole as active constituent. No protective isolating layer is arranged between the tablet core and the enteric coating. The inner tablet core is made of omeprazole cyclodextrin inclusion compound and other pharmaceutically acceptable auxiliary materials. The enteric coating contains no plasticizer and the dosage of the enteric coating accounts for 5 percent to 15 percent of the weight of the tablet. The invention has the advantages that the stability and the dissolution of omeprazole are improved, the dosage of basic materials and the dosage of all kinds of inert auxiliary materials are greatly reduced, omeprazole can be stably and rapidly released in the intestinal tract and the bioavailability is improved.
Owner:SHANDONG NEWTIME PHARMA
Pantoprazole sodium enteric-coated tablet and preparation method thereof
ActiveCN103006613AImprove stabilityReduced stabilityOrganic active ingredientsDigestive systemAdhesivePantoprazole Sodium
The invention provides a pantoprazole sodium enteric-coated tablet and a preparation method thereof. The pantoprazole sodium enteric-coated tablet comprises a pantoprazole sodium tablet, an isolated layer and an enteric-coated layer, wherein the pantoprazole sodium tablet comprises main drug pantoprazole sodium and auxiliaries; and the auxiliaries include a filler, a disintegrant, a lubricating agent, an adhesive, a pH regulating agent, and the like. The pantoprazole sodium enteric-coated tablet accelerates the disintegration time of the pantoprazole sodium enteric-coated tablet and solves the problem of drug instability during a storage period.
Owner:SHIJIAZHUANG HUAXIN PHARMA
Kallidinogenase enteric coated tablet and preparation method thereof
InactiveCN102813637AImprove adhesionPrevent titer lossPeptide/protein ingredientsInorganic non-active ingredientsAdjuvantAdhesive
The invention discloses a kallidinogenase enteric coated tablet which contains kallidinogenase which is used as an active ingredient, and a tablet core contains sodium alga acid or inorganic salt which is taken as a stabilizer. The invention further discloses a preparation method of the kallidinogenase enteric coated tablet, wherein adjuvants are taken as blank particles at first, kallidinogenaseserving as the main active ingredient is then added and is uniformly mixed, the tablet is produced according to a conventional method, and finally, enteric coating is used to coat a premixing agent into enteric film coating. The added stabilizer has the activity of being combined with the kallidinogenase into activating enzyme, so the kallidinogenase is prevented from titer loss during the production process, and the active ingredient input is in accordance with finished product yield. An adhesive has strong adhesive force, large particle adhesive power, and is easily mixed with the kallidinogenase uniformly. The formula has the characteristics of little unit titer loss during the production process and high finished product titer recovery rate; all adjuvants have no interference with thefinished product inspection, the finished product is high in quality, safe and effective.
Owner:武陟维尔康生化制药有限公司
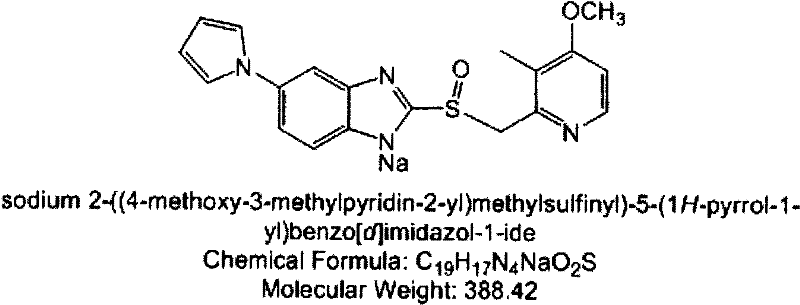
Combination medicament of ilaprazole sodium and preparation process thereof
InactiveCN102058593AEliminate adverse reactionsGood regeneration performanceOrganic active ingredientsDigestive systemCoated tabletsFreeze-drying
The invention provides a combination medicament of ilaprazole sodium. The medicament is characterized by comprising the following raw materials in percentage by weight: 5-10 percent of ilaprazole sodium, 25-35 percent of reduction composition containing glutathione and tiopronin with a weight ratio of 1:10 and 45-55 percent of diammonium glycyrrhizinate. The invention also provides a preparation process of the medicament. According to a pharmaceutically acceptable dosage of the ilaprazole sodium, the combination medicament can be respectively prepared into medical preparations of the dosage forms of injections of the combination medicament of the ilaprazole sodium, freeze-drying injections of combination medicament of the ilaprazole sodium, coated tablets of the combination medicament of the ilaprazole sodium, enteric capsules, sprays, and the like and is used for treating gastric ulcer.
Owner:吴赣英
Pantoprazole sodium enteric-coated tablet and preparation method thereof
ActiveCN104382875ACreativeMeet the requirements of consistency evaluationOrganic active ingredientsDigestive systemCoated tabletsOrganic chemistry
The invention provides a pantoprazole sodium enteric-coated tablet. The pantoprazole sodium enteric-coated tablet is prepared by coating a pantoprazole sodium tablet with an isolation layer and an enteric-coated layer, wherein the pantoprazole sodium tablet comprises the following components in percentage by weight: 35-45% of pantoprazole sodium, 30-59% of a filling agent, 0.2-3% of a binder, 8.1-18% of a disintegrant, 5-15% of a stabilizer and 0.2-1.5% of a lubricant. According to the pantoprazole sodium enteric-coated tablet and the preparation method thereof provided by the invention, a dissolution curve of the pantoprazole sodium enteric-coated tablet in each of four dissolution media is consistent with that of an originally developed preparation, thereby being in line with the requirements of consistency evaluation; and furthermore, the stability of the pantoprazole sodium enteric-coated tablet is better than that of the originally developed preparation.
Owner:HANGZHOU CONBA PHARMA
Content measuring method of metformin hydrochloride enteric coated tablet
InactiveCN101776660AElimination of Assay EffectsImprove accuracyComponent separationMedicineMetformin Hydrochloride
The invention provides a content measuring method of a metformin hydrochloride enteric coated tablet, which comprises the following steps: using metformin hydrochloride as a reference substance and measuring the content of the metformin hydrochloride in the enteric coated tablet by using a high performance liquid chromatography. Compared with the prior art, the invention uses the high performance liquid chromatography to measure the content of the metformin hydrochloride enteric coated tablet, improves the accuracy compared with the prior ultraviolet spectrophotometric method, and provides better standards for the quality control of the metformin hydrochloride enteric coated tablet.
Owner:贵州天安药业股份有限公司
Ilaprazole enteric coated tablet and preparation method thereof
InactiveCN102552190AImprove acid resistanceWidely distributedOrganic active ingredientsDigestive systemOrganic solventIlaprazole
The invention provides an ilaprazole enteric coated tablet and a preparation method thereof. The ilaprazole enteric coated tablet comprises enteric coated pellets and a pharmaceutically-acceptable tablet auxiliary material, wherein each enteric coated pellet comprises a pellet core, an isolating layer and an enteric coated layer; and the pellet core comprises ilaprazole or a pharmaceutically-acceptable salt thereof and a stabilizing agent. The enteric coated pellet tablet made from ilaprazole has high acid resistance; in a prescription, barrier substances such as an acid resisting agent, a surfactant, an organic solvent, a hydrophobic substance and the like are not contained, so that the health and safety of a human body are better facilitated; in the preparation method, an organic solvent is not used, so that operation is easy, and active substances are released quickly and stably; and moreover, the pellets in the tablet can be widely and uniformly distributed into an intestinal tract after administration, a dosage is poured out in a scatter way, and the distribution area of a medicament on the surface of the intestinal tract is increased, so that the stimulation of the medicament on the intestinal tract can be reduced or eliminated, and the bioavailability of the medicament is enhanced.
Owner:LIVZON PHARM GRP INC
Esomeprazole enteric-coated tablets and preparation method thereof
The invention discloses esomeprazole enteric-coated tablets and a preparation method thereof. The formula of the tables comprises esomeprazole magnesium, and an inert pellet core, a binder, a dispersant and a disintegrant which are used for tablet preparation. The preparation method comprises spraying and coating esomeprazole magnesium on the blank pellet core, coating an isolating layer and an enteric coated layer, making into enteric-coated pellets, and making into the required enteric-coated preparation by using the specific tablet adjuvants and tabletting method. The esomeprazole enteric-coated tablets and the preparation method thereof disclosed by the invention have the characteristics that by adopting specific tablet preparation method, the shortcoming that the enteric coated layers of the enteric-coated pellets are easy to be broken during tabletting process to affect the acid resistance of esomeprazole enteric-coated tablets is effectively overcome.
Owner:北京华禧联合科技发展有限公司
Oral delivery of proteins and peptides
InactiveUS20100303901A1Improve bioavailabilityRapid drug releasePeptide/protein ingredientsCalcitoninsPeptide drugFast release
Enteric coated capsules or tablets for oral delivery of a protein, polypeptide or peptide drug, in particular for oral delivery of insulin, are provided, comprising microparticles of the protein, polypeptide or peptide drug, microparticles of a protease inhibitor and, optionally, microparticles of an absorption enhancer. The protease inhibitor and the absorption enhancer may be together in the same microparticles. The microparticles of each component are embedded in an enteric polymer matrix. The enteric coated tablet or capsule of the invention enables fast release of the protein, polypeptide or peptide drug at different times at desired loci in the gastrointestinal tract
Owner:TECHNION RES & DEV FOUND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com