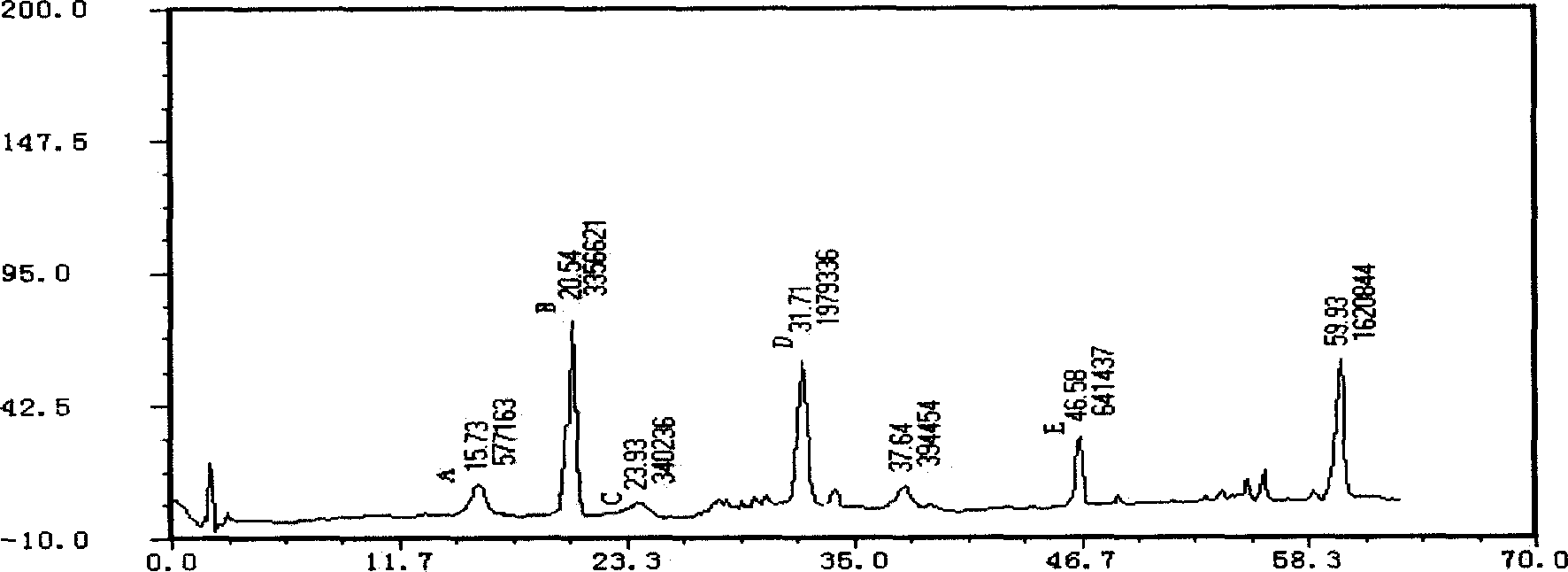
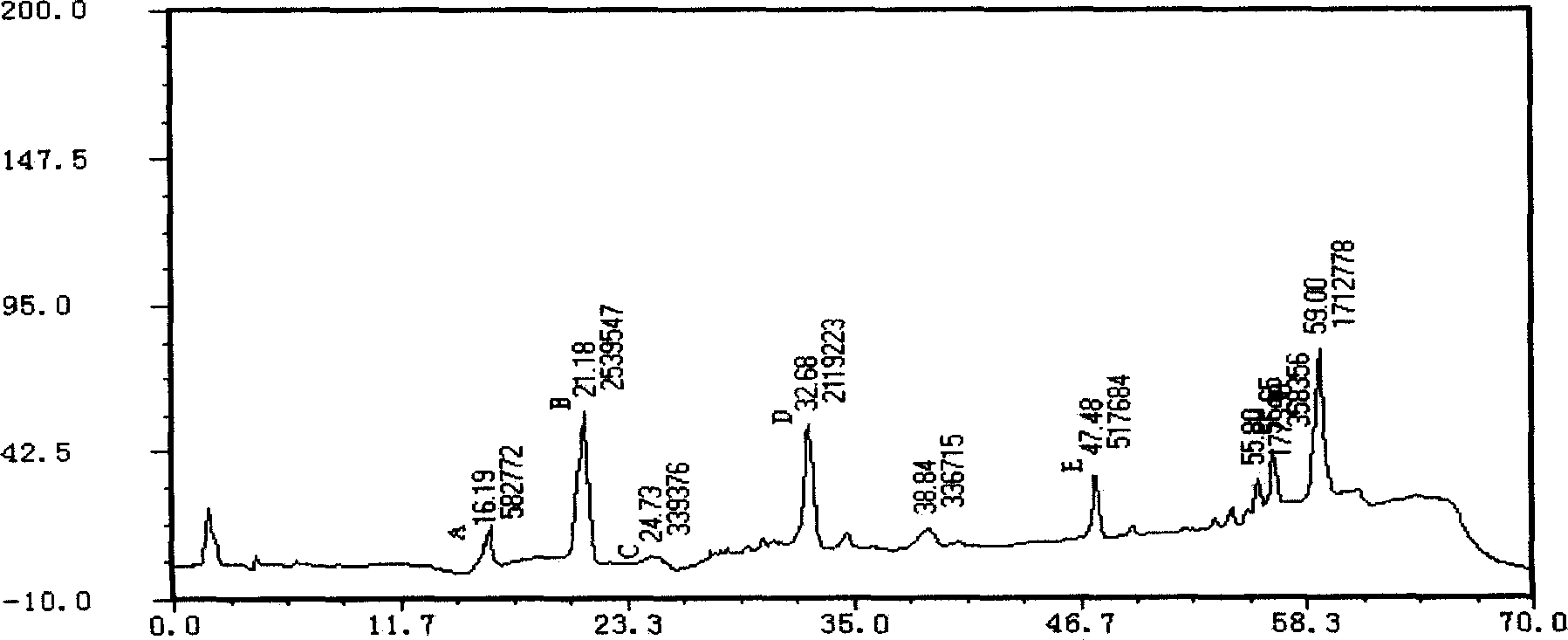
Notoginseng medicine composition for treating cardiac and cerebral vascular diseases
A technology for cardiovascular and cerebrovascular diseases and a composition is applied in the field of active ingredients of Panax notoginseng saponins, and can solve problems such as adverse reactions, severe allergic reactions, complex ingredients and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Embodiment 1: the preparation of pharmaceutical composition of the present invention
[0102] Take notoginseng and grind it into 1000g of coarse powder, add 8000ml of 60% ethanol to extract for 7 hours, collect the extract, filter, take the filtrate to recover the ethanol until it has no alcohol smell, add water to make a solution containing 0.5g of crude drug per 1ml, and use a macroporous resin column (Class D macroporous resin, resin volume 3000ml, resin bed height to diameter ratio is about 8) and alumina adsorption, gradient elution with different concentrations of ethanol, the ethanol concentration is adjusted from 5% to 95% within 6 hours , flow rate 4.5BV / h, discard the eluate containing ethanol concentration other than 30% to 70%, collect the eluate containing ethanol concentration above 30% to below 70%, recover the solvent under reduced pressure below 65°C and concentrate To a clear paste with a relative density of 1.13 (60°C) to obtain the active ingredient ...
Embodiment 2
[0104] Embodiment 2: the preparation of pharmaceutical composition of the present invention
[0105]Take notoginseng and grind it into 1000g of coarse powder, add 10000ml of 50% ethanol to extract for 6 hours, collect the extract, filter, take the filtrate to recover the ethanol until it has no alcohol smell, add water to make a solution containing 0.4g of crude drug per 1ml, and use a macroporous resin column (Class D macroporous resin, resin volume 3000ml, resin bed height to diameter ratio is about 8) and alumina adsorption, carry out gradient elution with different concentrations of ethanol (the ethanol concentration is adjusted from 5% to 95% within 5.5 hours ), the flow rate is 5.0BV / h, discard the eluate containing ethanol concentration other than 30% to 70%, collect the eluate containing ethanol concentration above 30% to below 70%, recover the solvent under reduced pressure below 65°C and Concentrate to a clear paste with a relative density of 1.15 (60°C), dry to obta...
Embodiment 3
[0107] Embodiment 3: the preparation of pharmaceutical composition of the present invention
[0108] Take notoginseng and grind it into coarse powder 1000g, add 75% ethanol 7500ml to extract for 8 hours, collect the extract, filter, take the filtrate to recover ethanol until it has no alcohol smell, add water to make a solution containing 0.6g crude drug per 1ml, and use a macroporous resin column (Class D macroporous resin, resin volume 3000ml, resin bed height to diameter ratio is about 8) and alumina adsorption, carry out gradient elution with different concentrations of ethanol (the ethanol concentration is adjusted from 5% to 95% within 6.5 hours ), the flow rate is 4.0BV / h, discard the eluate containing ethanol concentration other than 30% to 70%, collect the eluate containing ethanol concentration above 30% to below 70%, recover the solvent under reduced pressure below 65°C and Concentrate to a clear paste with a relative density of 1.16 (60°C) to obtain the active ingr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com