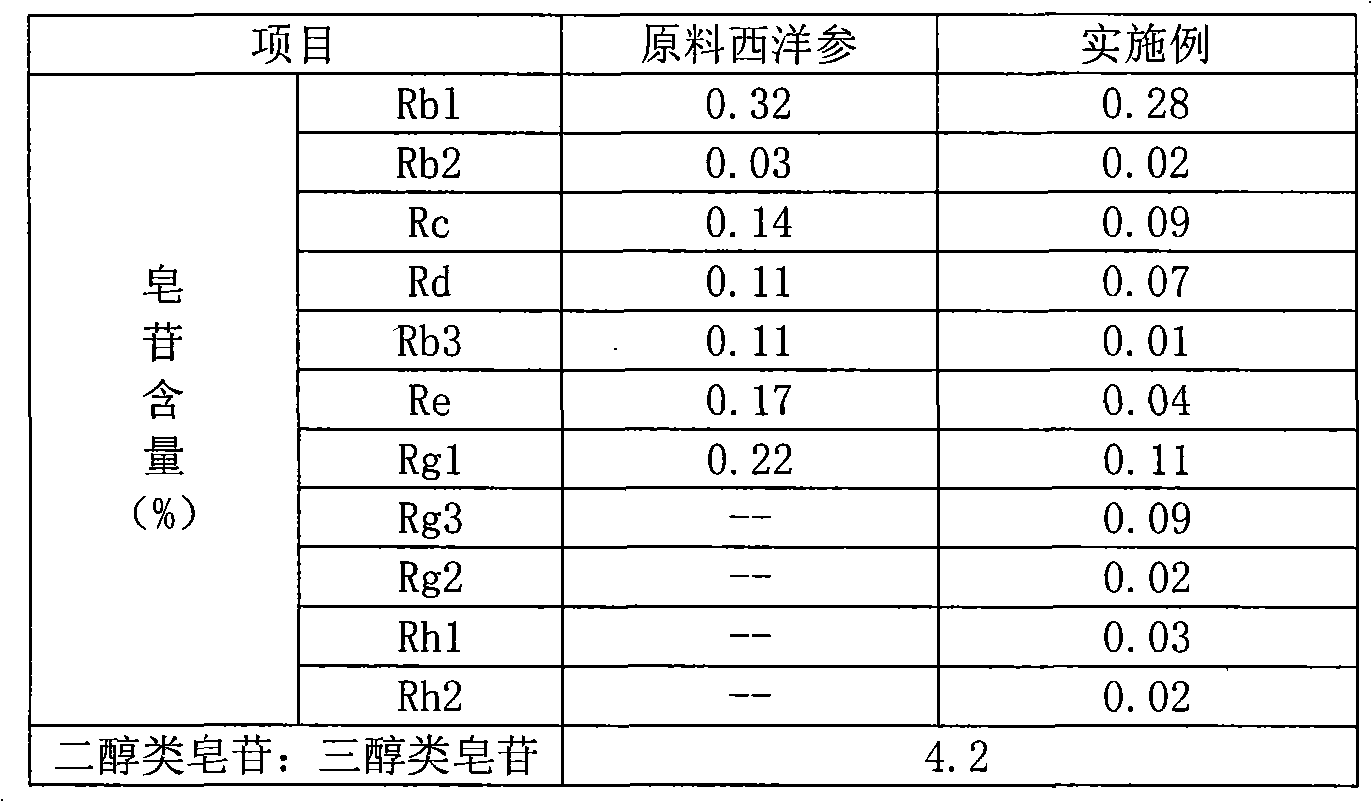
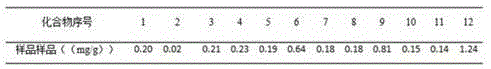
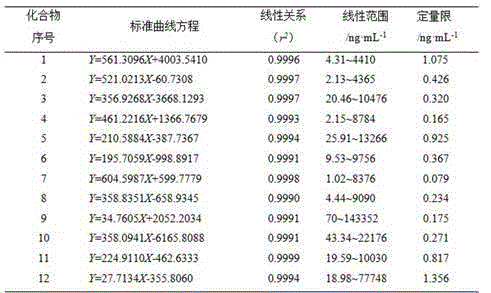
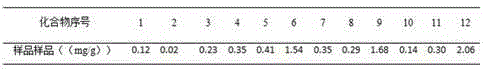
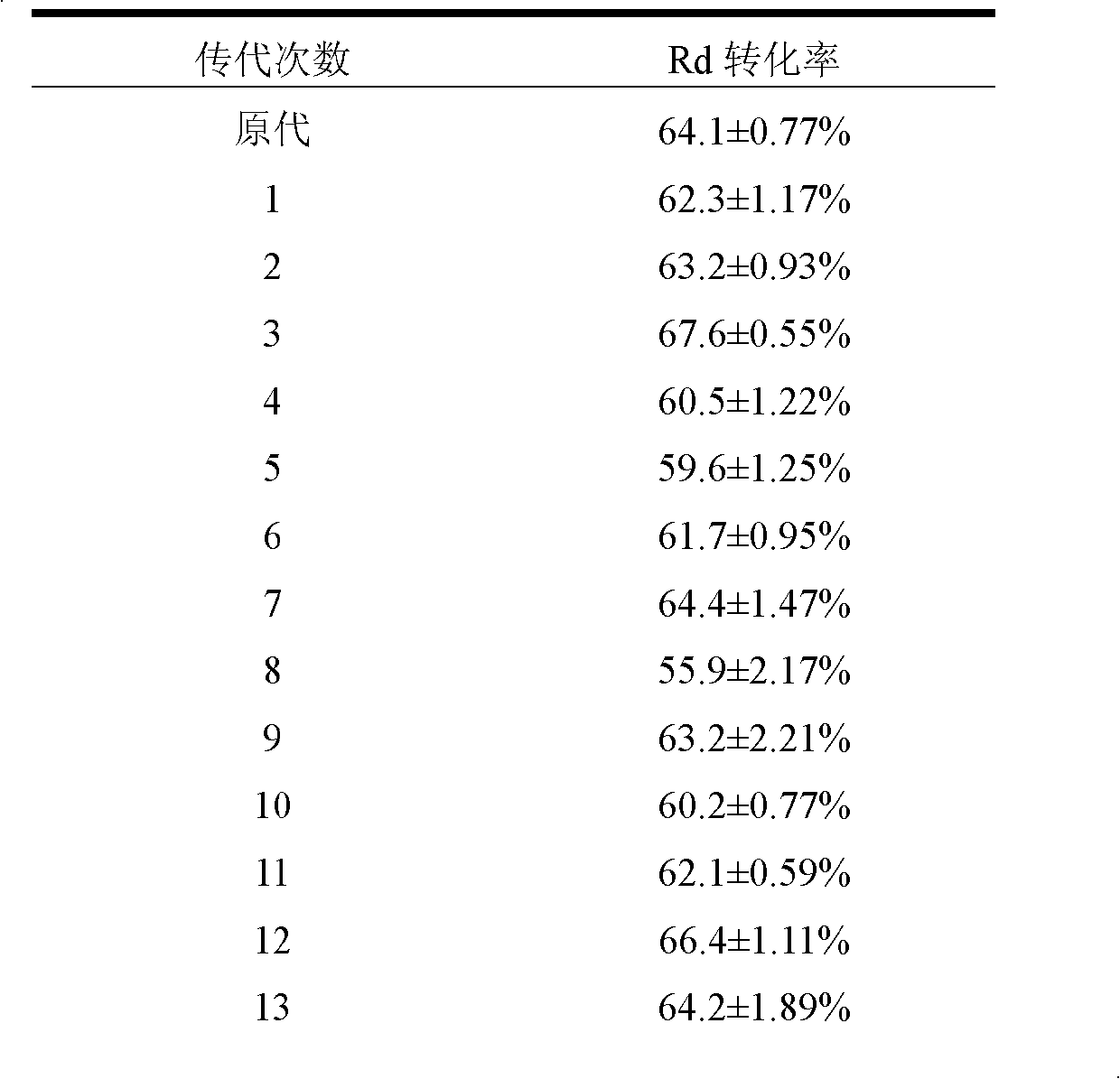
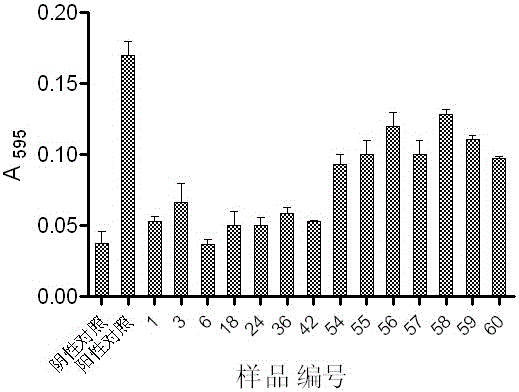
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
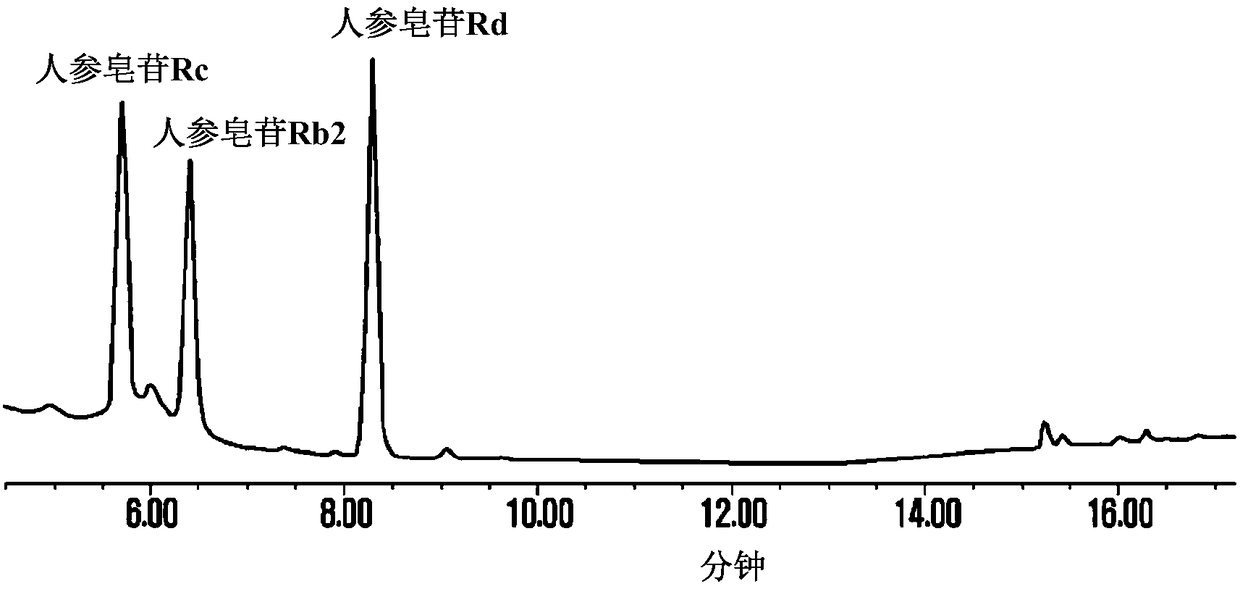
111 results about "Ginsenoside Rd" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Personal care compositions and methods for the beautification of mammalian skin and hair
Personal care composition comprising from about 0.05% to about 5% of at least one aquaporin-stimulating compound selected from the group consisting of xanthine, caffeine; 2-amino-6-methyl-mercaptopurine; 1-methyl xanthine; 2-aminopurine; theophylline; theobromine; adenine; adenosine; kinetin; p-chlorophenoxyacetic acid; 2,4-dichlorophenoxyacetic acid; indole-3-butyric acid; indole-3-acetic acid methyl ester; beta-naphthoxyacetic acid; 2,3,5-triiodobenzoic acid; adenine hemisulfate; n-benzyl-9-(2-tetrahydropyranyl)adenine; 1,3-diphenylurea; 1-phenyl-3-(1,2,3-thiadiazol-5-yl)urea; zeatin; indole-3-acetic acid; 6-benzylaminopurine; alpha-napthaleneacetic acid; 6-2-furoylaminopurine; green tea extract; white tea extract; menthol; tea tree oil; ginsenoside-RB1; ginsenoside-RB3; ginsenoside-RC; ginsenoside-RD; ginsenoside-RE; ginsenoside-RG1; ginseng root extract; ginseng flower extract; pomegranate extract, extracts from Ajuga turkestanica; extracts from viola tricolor and combinations thereof; an additional ingredient selected from the group consisting of niacinamide, glycerin and mixtures thereof, and a dermatologically-acceptable carrier.
Owner:THE PROCTER & GAMBLE COMPANY
Extracting purified ginsenoside from leaves of Panax quinquefolium and ginseng at the same time and the preparing method thereof
InactiveCN101032535ASimple processLow costOrganic active ingredientsSteroidsSide effectGinsenoside Rc
The present invention relates to Chinese medicine and its extracting and processing technology, and is especially the effective part extracted from American ginseng leaf and ginseng leaf and its preparation process. The extracted effective part contains six kinds of ginsenoside substances, including ginsenoside Re, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rb3, ginsenoside Rc and ginsenoside Rd. Its preparation process includes water extracting American ginseng leaf and ginseng leaf in the weight ratio of 1 to 0.3, macroporous resin adsorption of the water extract liquid, water eluting to eliminate impurity, further eluting with two kinds of elutents, collecting the eluted liquid, and refining the total solid matter to reach purity up to 63.45 %. The extracted effective part has less side effects, and may be used widely in compound medicine preparation and functional food and for separating ginsenoside substance.
Owner:吉林人参研究院
Radix notoginseng extract and preparation thereof
ActiveCN101732378AHigh purity of ingredientsIncrease concentrationCardiovascular disorderPlant ingredientsGinsenoside RcPanax notoginseng extract
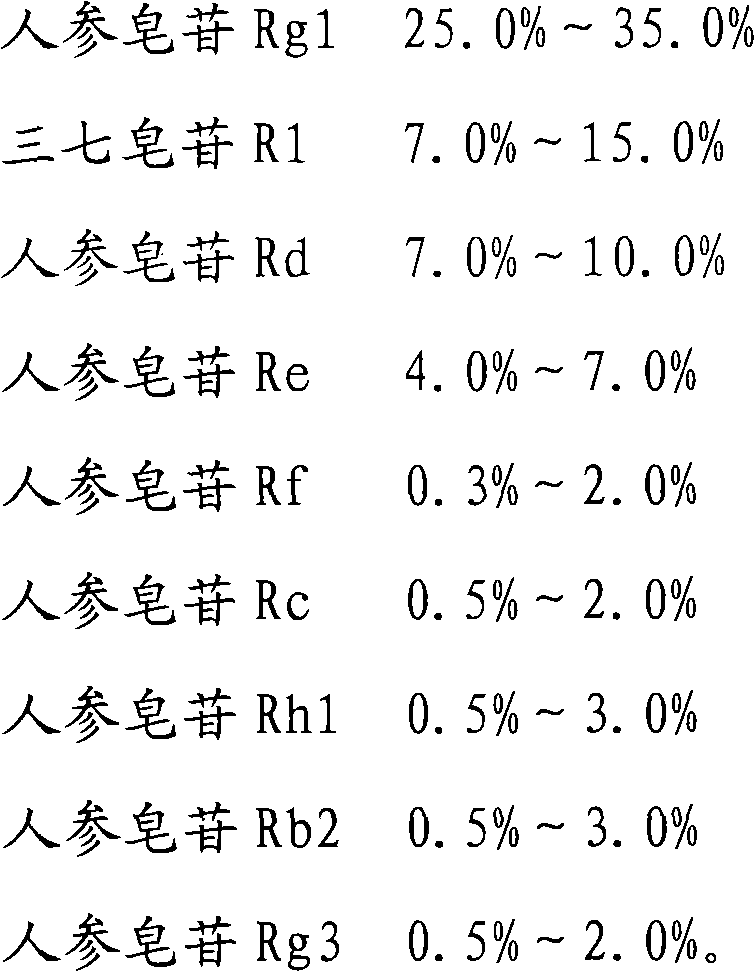
The invention provides a radix notoginseng extract which contains 5-10% of notoginsenoside R1, 25-36% of ginsenoside Rg1, 2.5-5% of ginsenoside Re, 30-39% of ginsenoside Rb1, 5-10% of ginsenoside Rd and at least 2% of ginsenoside Rf, ginsenoside Rh1, ginsenoside Rc, ginsenoside Rb2 and ginsenoside Rg3, wherein the ginsenoside R1, the ginsenoside Rg1, the ginsenoside Re, the ginsenoside Rb1 and the ginsenoside Rd account for 75-95% of the total weight. The invention also provides a preparation method of the radix notoginseng extract. The radix notoginseng extract prepared by the method has little impurities, the purity of the component of total saponin is higher, and especially, the components of the ginsenoside Rf, the ginsenoside Rh1, the ginsenoside Rc, the ginsenoside Rb2, the ginsenoside Rg3 and the like with very low content are purified. The medicinal preparation prepared by the radix notoginseng extract has better curative effect and higher safety.
Owner:HARBIN ZHENBAO PHARMA
Notoginseng medicine composition for treating cardiac and cerebral vascular diseases
The present invention relates to a kind of total arasaponin composition, which has the active component of total arasaponin comprising arasaponin R1, ginsenoside Rg1, ginsenoside Re and ginsenoside Rb1 not less than 55.0 wt% and ginsenoside Rd not more than 2.5 wt%. The total arasaponin composition is prepared into injection, powder for injection, enteric coated tablet, bolus, medicine powder and other preparation forms. The medicine of the present invention has the functions of promoting blood circulation to disperse blood clots and activating collateral flow, and is used in treating blood stasis to block collateral channels, apoplexy, hemiplegia and other cardiac and cerebral vascular diseases.
Owner:GUANGXI WUZHOU PHARMA GRP
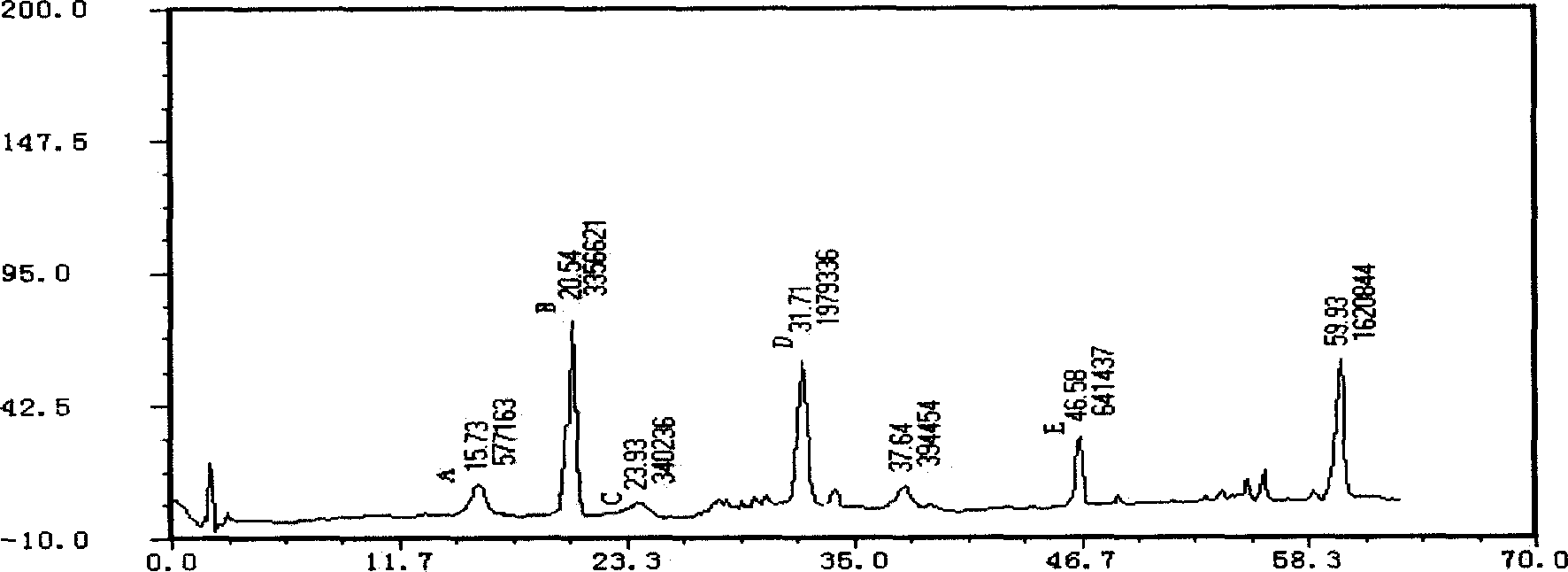
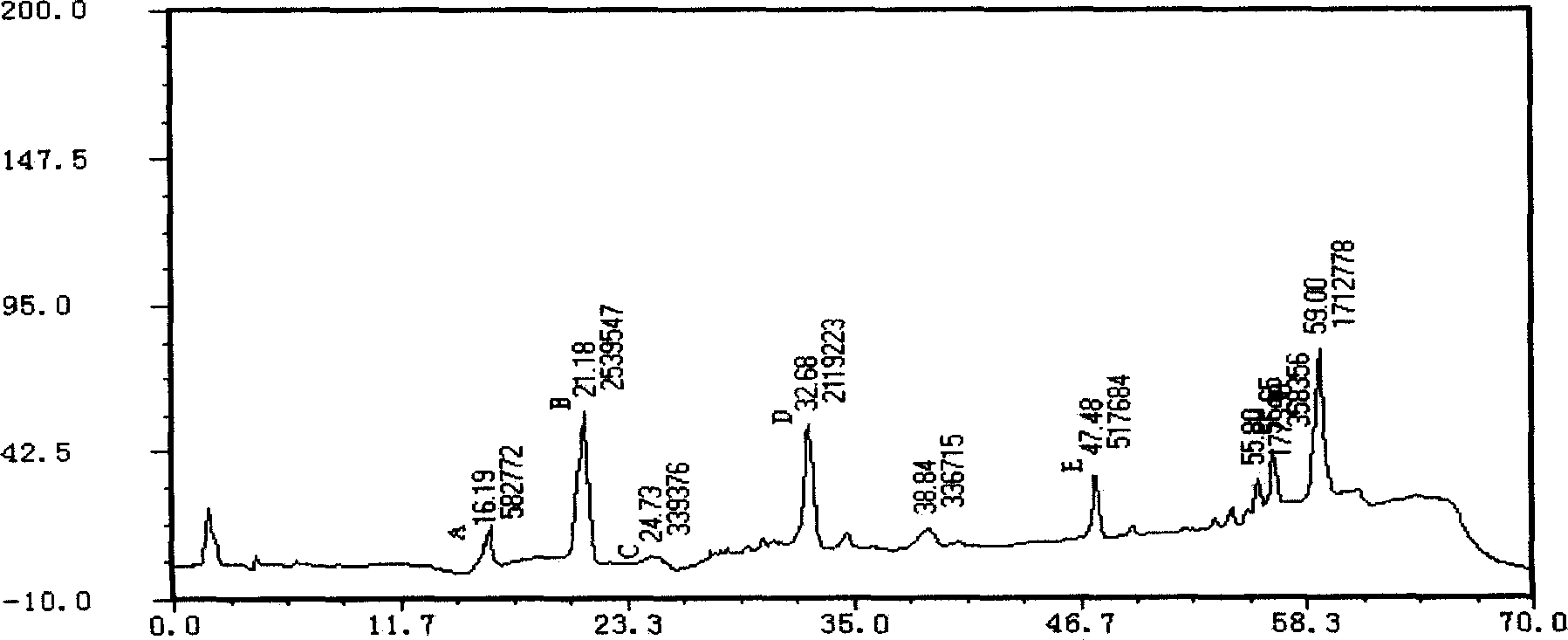
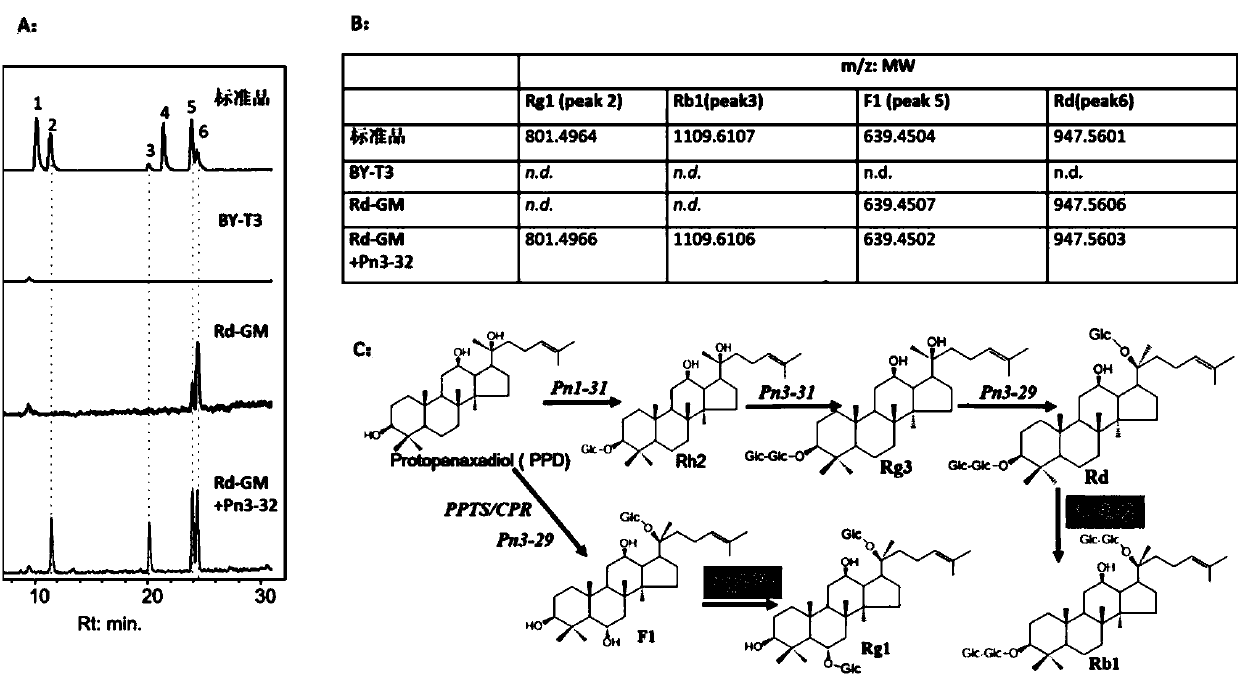
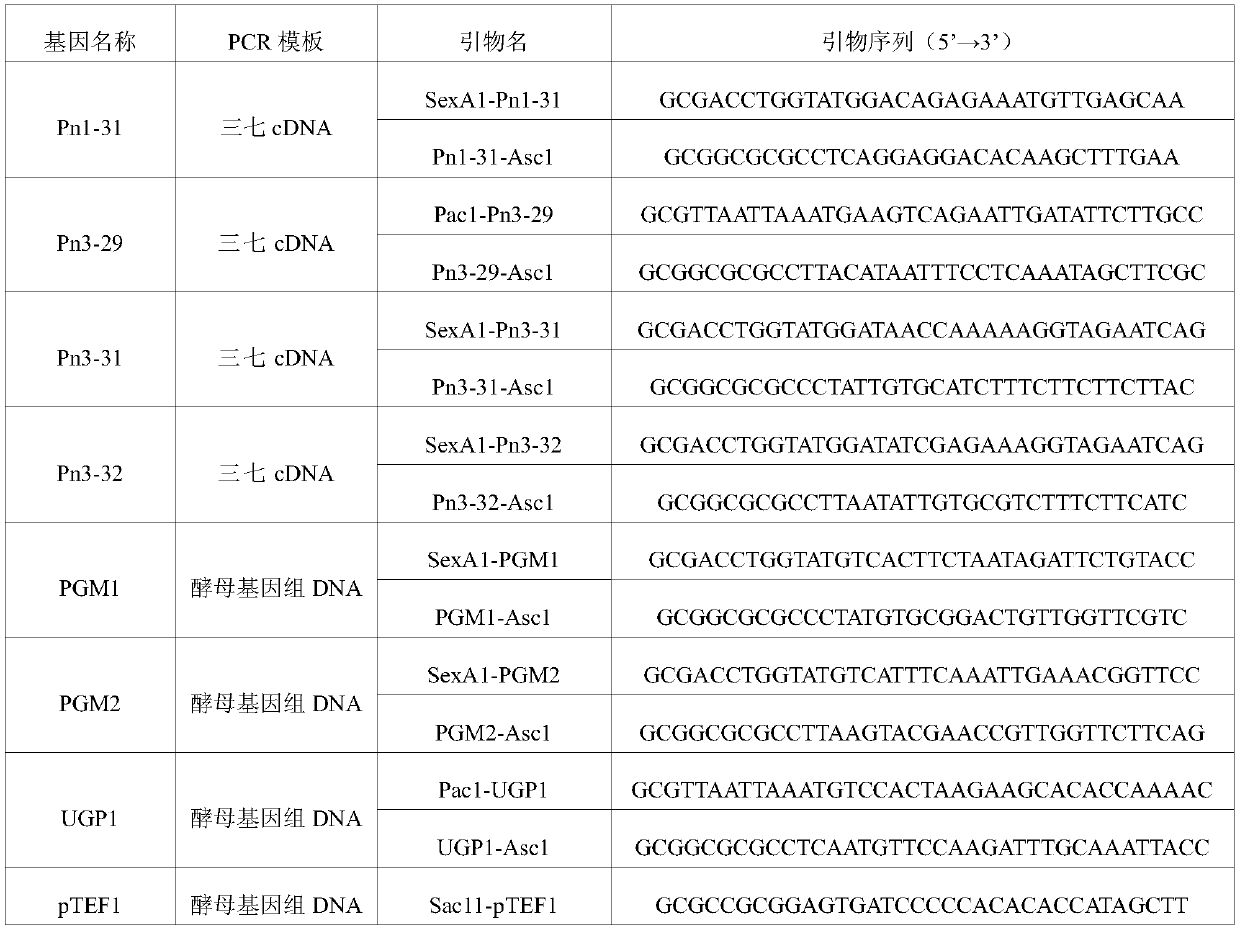
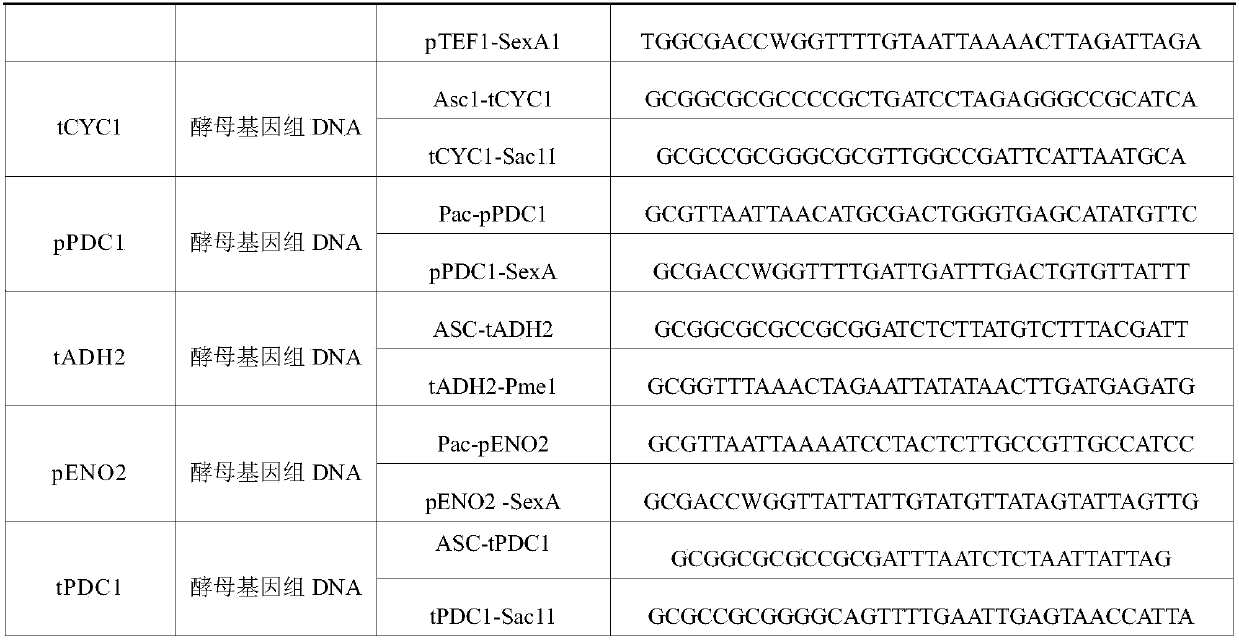
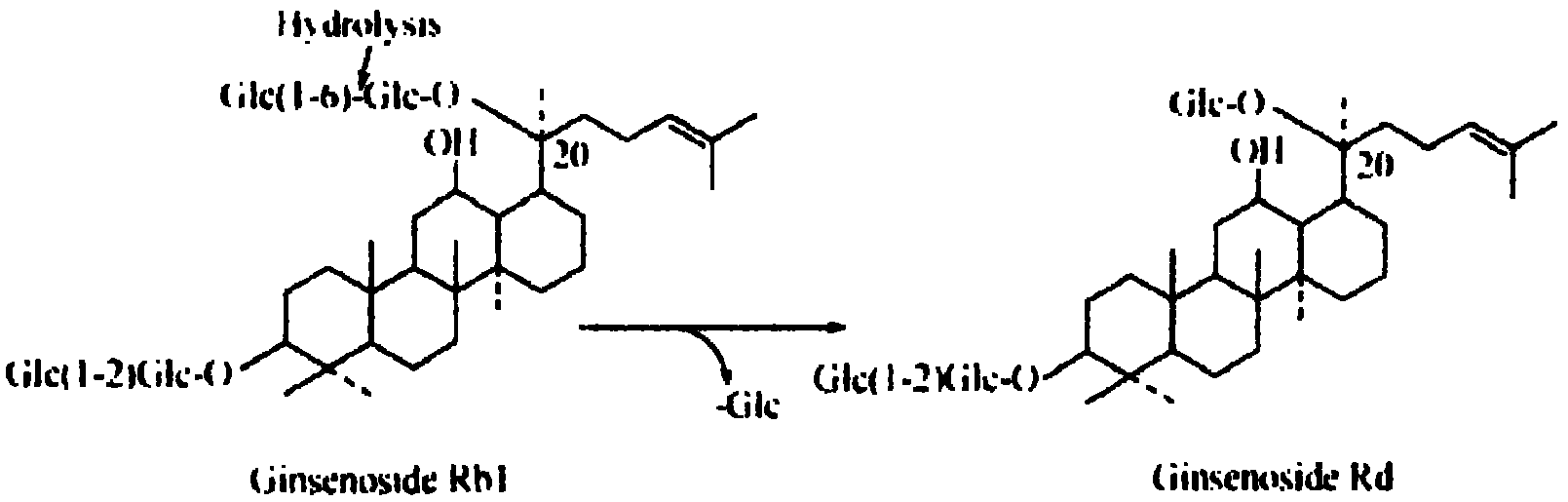
Applications of glycosyltransferase and related materials thereof in construction of engineering bacteria for producing ginsenoside Rb1 and Rg1
The invention discloses applications of glycosyltransferase and related materials thereof in the construction of engineering bacteria for producing ginsenoside Rb1 and Rg1. A glycosyltransferase genePn3-32 which can catalyze ginsenoside Rd to generate ginsenoside Rb1 can be successfully identified through a synthetic biological method; and the gene can simultaneously catalyze ginsenoside F1 to generate ginsenoside Rg1 and construct recombinant yeast producing the ginsenoside Rb1 and the ginsenoside Rg1. Through experiments, the constructed recombinant yeast producing the ginsenoside Rb1 and the ginsenoside Rg1 can simultaneously generate the ginsenoside Rb1 and the ginsenoside Rg1. Pn1-31, Pn-3-29, Pn3-31 and Pn3-32 glycosyltransferase genes in medicinal plant radix notoginseng are firstly utilized to continuously catalyze protopanaxadiol and protopanaxatriol to synthetize the ginsenoside Rb1, the ginsenoside Rg1 and corresponding intermediates, so that novel cases can be provided formicrobial strains to produce natural products.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Medicinal composition and preparation method thereof
ActiveCN102028700AIncrease the maximum tolerated doseRaise the median lethal doseOrganic active ingredientsPeptide/protein ingredientsHemolysisAcute toxicity testing
The invention provides a medicinal composition, which comprises the following components: ginsenoside Rb1, ginsenoside Rg1, notoginsenoside R1, ginsenoside Rd, ginsenoside Re, ginsenoside Rf, ginsenoside Rc, ginsenoside Rh1, ginsenoside Rb2 and ginsenoside Rg3. The invention also provides a preparation method for the medicinal composition. The medicinal composition has definite components; compared with the total notoginsenoside in the prior art, the medicinal composition has stable quality and good controllability; and results of experiments of acute toxicity, undue toxicity, hemolysis and the like show that the medicinal composition has higher safety and wide clinical application prospect.
Owner:KPC PHARM INC
Method for extracting and separating total saponins of panax ginseng from american ginseng
ActiveCN102772462AHigh purityHigh yieldNervous disorderAntinoxious agentsAMERICAN GINSENG ROOTGinsenoside Rc
The invention discloses a method for extracting and separating total saponins of panax ginseng from american ginseng. The method employs a series of high-efficiency extraction, separation and purification technical measures of alcohol-backflow extracting, water precipitating, purifying by a macroporous adsorption resin, decolorizing with an ion exchange resin, etc. The obtained total saponins of panax ginseng are white powder and comprise ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, ginsenoside Rc and ginsenoside Rd, wherein the total purity of ginsenoside Rg1, ginsenoside Re and ginsenoside Rb1 is more than 74.0%. The production process is simple and practical, has no pollution, and is suitable for large-scale production.
Owner:HEBEI YILING MEDICINE INST
Production method of flavor panax ginseng
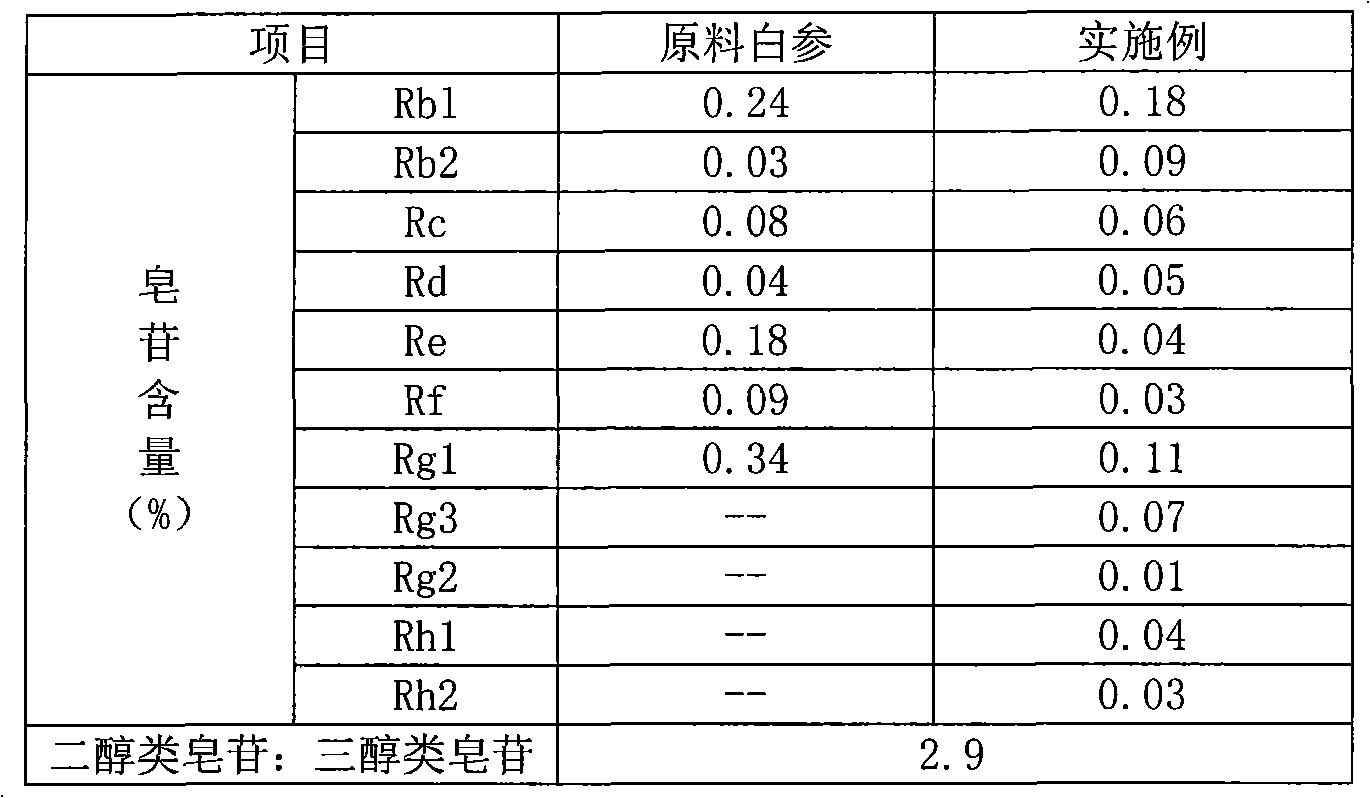
The invention relates to a preparation method of flavor ginseng; after ginseng is added with nature fruit juice or organic acid for reaction, the content of the precious ginsenoside in products is enhanced, the efficacies of ginseng is improved, while the specific flavor of ginseng is maintained. The main quality characteristics is that: the flavor ginseng includes at least one of the following ingredients: ginsenoside Rg3, ginsenoside Rh1 and ginsenoside 20R-Rh2; wherein, the ratio of the total amount of precious ginsenoside group, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rd and ginsenoside Rc to the total amount of ginsenoside Re, ginsenoside Rg1 and ginsenoside Rf is more than 2.5; the preparation method can convert part of the component causing internal heat, namely, panaxatriol ginsenoside, into precious ginsenoside.
Owner:JILIN HONGJIU BIO TECH
Determining method for contents of twelve components in traditional Chinese medicine composition preparation
ActiveCN104914199AQuality improvementGood repeatabilityComponent separationBiotechnologyAstragaloside
The present invention discloses a UPLC-MS quantitative method for the contents of twelve components in a traditional Chinese medicine composition preparation, specifically determination of the contents of calycosin-7-O-beta-D-glucoside (1), isoquercitrin (2), narirutin (3), hesperidin (4), ginsenoside Re (5), ginsenoside Rg1(6), periplocoside (7), ginsenoside Rf (8), ginsenoside Rb1 (9), astragaloside (10), ginsenoside Rd (11) and periplocin H1 (12) in the traditional Chinese medicine composition, and belongs to the field of traditional Chinese medicine composition preparation component detection. The determining method of the present invention has characteristics of short period, good reproducibility and high sensitivity.
Owner:HEBEI YILING MEDICINE INST
Extract of panax notoginseng saponins and preparation method thereof
InactiveCN101829170AIncrease cerebral blood flowHigh trafficBlood disorderCardiovascular disorderPANAX NOTOGINSENG ROOTAdjuvant
The invention relates to an extract of panax notoginseng saponins, a preparation method thereof, a preparation prepared from the total saponins and a method for preparing the preparation. The extract comprises panaxtriol saponins (PTS) and panaxadiol saponins (PDS), wherein the PTS mainly comprises notoginsenoside R1 and ginsenoside Rg1; the PDS mainly comprises the ginsenoside Rb1 and the ginsenoside Rd; the extract is characterized in that the weight ratio of the PTS to the PDS is 1:0.5-2; and the total content of the notoginsenoside R1, the ginsenoside Rg1, the ginsenoside Rb1 and the ginsenoside Rd accounts for over 80 weight percent of the extract of the panax notoginseng saponins. The preparation of the extract of the panax notoginseng saponins comprises the therapeutically effective amount of the panax notoginseng saponins and pharmaceutically acceptable adjuvant.
Owner:北京中海康医药科技发展有限公司
Application of panaxadiol saponins fraction in preparing medicine for preventing dermatitis and scar
ActiveCN102743402AHigh purityStable efficacyOrganic active ingredientsCosmetic preparationsMedicinal herbsGlucocorticoid
The invention provides application of a panaxadiol saponins fraction in preparing a medicine for preventing dermatitis and scar and a health-care cosmetic. The panaxadiol saponins fraction comprises the following main constituents: ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rb3, ginsenoside Rc and ginsenoside Rd. The medicine or the cosmetic is prepared from active constituents in the single panaxadiol saponins fraction or / and other medicines together and a pharmaceutically acceptable or cosmetic acceptable carrier. The medicine or the cosmetic is prepared by utilizing raw materials: ginseng rhizome medicinal materials, American ginseng rhizome medicinal materials, ginseng stem leaf medicinal materials, American ginseng leaf medicinal materials and total extractives or total saponins of the ginseng rhizome medicinal materials, the American ginseng rhizome medicinal materials, the ginseng stem leaf medicinal materials and American ginseng leaf medicinal materials through a chromatographic separation and purification method combining macroporous resin column chromatography and octadecylsilane chemically bonded silica column chromatography. The scar can be prevented from forming while the tissue regeneration and repair are promoted by the medicine or the cosmetic. Compared with glucocorticoids, cellular immunity is integrally regulated, the drug action is stable after the medicine is suspended, and the drug action advantage is obvious. The medicine or the health-care cosmetic is highly safe. The structural formula of panaxadiol saponins is shown in the specification.
Owner:ZHEJIANG UNIV
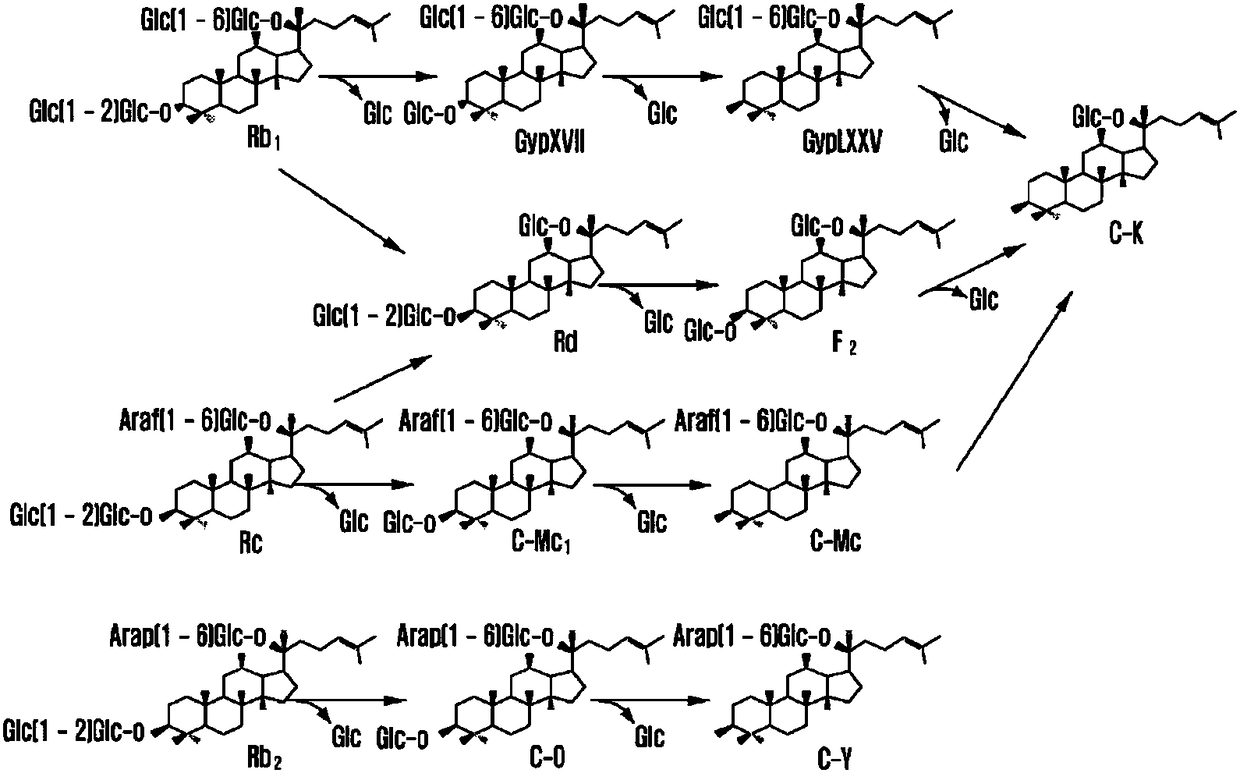
Method for preparing ginsenoside Rd through biotransformation of panax notoginseng saponins
ActiveCN105331668AIncrease contentImprove conversion efficiencyMicroorganism based processesFermentationMicroorganismPANAX NOTOGINSENG ROOT
The invention discloses a method for preparing ginsenoside Rd through biotransformation of panax notoginseng saponins. According to the method, coniochaetasp.EA-9 or crude enzyme secreted by the coniochaetasp.EA-9 is adopted to conduct biotransformation on panax notoginseng saponins, and the ginsenoside Rd and C-K are obtained. The method is easy to operate, high in conversion specificity, mild in reaction condition, little in microbial secondary metabolite, simple in extraction and fermentation product, high in conversion rate and capable of being used for large-scale preparation of the ginsenoside Rd and achieving industrialization of the ginsenoside Rd.
Owner:KUNMING UNIV OF SCI & TECH
Ginseng endogenesis zygorhynchus moelleri mildew as well as method for preparing ginsenoside Rd by utilizing same
The invention relates to ginseng endogenesis zygorhynchus moelleri mildew (CGMCC (China General Microbiological Culture Collection): No.4315). The mildew has the capability for preparing Rd by converting a substrate ginsenoside Rb1 and has higher substrate unicity and product unicity, and the preparation of the ginsenoside Rd by utilizing the mildew can adopt in-site conversion. A method for preparing the ginsenoside Rd comprising the following steps of: dibbling the mildew in a PDA (Potato Dextrose Agar) culture medium containing the ginsenoside Rb1, standing still at 25 DEG C and culturing for 5-7 days or vaccinating the mildew on an enzyme production culture medium by adopting a microorganism enzymic method and culturing at 28 DEG C for 5-7 days, collecting enzyme liquid, mixing with the ginsenoside Rb1 and reacting at 40 DEG C for 24h. The ginsenoside Rd produced by adopting the technical scheme of the invention has the advantages of strong specificity, simplicity, convenience, safety, reliability and low cost without side products, the purity of the fermentation product Rd is higher than 90 percent, and the conversion rate can be higher than 60 percent.
Owner:DALIAN NATIONALITIES UNIVERSITY
Method for preparing ginsenoside Rd by utilizing microbial conversion
InactiveCN102888437ARealize industrial preparationExpand resource sourcesMicroorganism based processesFermentationMicrobial transformationGinsenoside Rd
The invention belongs to the technical field of biological medicine, relates to a method for preparing ginsenoside Rd by utilizing microbial conversion and in particular relates to a microbial conversion method for preparing the ginsenoside Rd by fermenting and converting aspergillus niger into various glycol-type ginsenosides. In the method disclosed by the invention, a high-conversion industrial aspergillus niger strain with culture preservation number being CICC40426 is adopted, various glycol-type ginsenosides are obtained by fermentation and conversion in a full-automatic fermentation tank, and high-purity ginsenoside Rd is prepared through purification and crystallization by virtue of macroporous resin. The method disclosed by the invention is simple in process, low in cost and high in yield and is environment-friendly; and with adoption of the method disclosed by the invention, industrial preparation of the ginsenoside Rd can be realized, source of the ginsenoside Rd drug resource is expanded, and wide medical care market demand is met, so that the method disclosed by the invention has great value.
Owner:FUDAN UNIV
Panax notoginseng saponin composition and preparation method and application thereof
ActiveCN105816471AClear validityClear contentOrganic active ingredientsBlood disorderGinsenoside RdLow toxicity
The invention provides a panax notoginseng saponin composition and a preparation method and application thereof.The composition is prepared from, by weight, 30-50% of ginsenoside Rg1, 25-40% of ginsenoside Rb1, 7-16% of notoginsenoside R1, 2.7-8% of ginsenoside Re, 0.5-7.0% of ginsenoside Rd, 0.5-2% of ginsenoside Rf, 0.3-2.0% of ginsenoside Rh2, 1-3% of ginsenoside Rc, 0.3-2.5% of ginsenoside Rb3 and 0.5-2.5% of ginsenoside Rg3, wherein ginsenoside Rg1, ginsenoside Rb1 and notoginsenoside R1 account for 70-95% of the total weight of the active components, and the sum of the contents of ginsenoside Rb1 and notoginsenoside R1 is not smaller than 8 times of the content of ginsenoside Rd.Compared with commercially available Xueshuantong injections and Xueshuantong preparations, the panax notoginseng saponin composition has a more obvious arterial and venous thrombosis resistant effect, low toxicity and high safety.
Owner:HARBIN ZHENBAO PHARMA
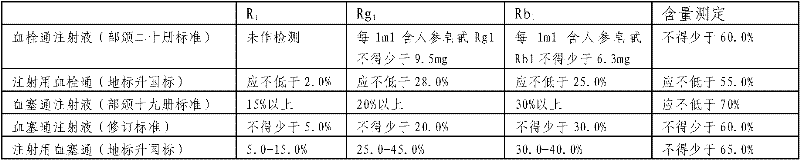
Method for measuring content of Shenqi blood sugar reducing preparation and application thereof in overall quality control
The invention provides a method for measuring the content of a Shenqi blood sugar reducing preparation. The method is used for measuring the content of 10 active ingredients in the Shenqi blood sugar reducing preparation: ginsenoside Rb1, ginsenoside Rc, ginsenoside Rd, ginsenoside Rg1, ginsenoside Re, astragaloside, deoxyschizandrin, schisandrol A, schisandrol B. In the invention, with the guidance of blood sugar reducing activity of a compound, multiple active substances in the compound are detected, and the quality level of the whole compound preparation can be reflected better; meanwhile, a Q-MS ion monitoring mode is selected for content measurement, and the mass spectrum quantification has high accuracy, specificity and sensitivity and is superior to traditional HPLC analysis method; and moreover, fingerprint is established according to the liquid phase diagrams of different batches of Shenqi blood sugar reducing preparations obtained by the method, the similarity is evaluated, the inherent quality of the Shenqi blood sugar reducing preparation product is evaluated more scientifically and comprehensively, and a basis is provided for establishing a quality standard of higher level.
Owner:ZHEJIANG UNIV OF TECH
Extraction and transformation coupling technique for preparing ginsenoside composition
InactiveCN107383144ASynchronous fetchSimultaneous conversionGlycoside steroidsHydrolysateGinsenoside Rd
The invention relates to a preparation method for a traditional Chinese medicinal ingredient composition, and in particular to an extraction and transformation coupling technique for extracting, preparing and separating a ginsenoside composition from ginseng and American ginseng materials. The method includes the following steps: fine ginseng and American ginseng material powder (screened by 40 meshes) is chosen and added with an enzyme solution which is 12 to 24 times in amount, pH is regulated to acid by diluted hydrochloric acid, and ultrasonic extraction is carried out under a certain temperature. The pH of hydrolysate is regulated to 6.5 to 7.0 by ammonia, flow velocity is controlled, the supernate is added onto a pretreated macroporous resin column, 0 to 30 percent of ethanol is used for washing, 50 to 80 percent of ethanol is used for elution, the eluent is concentrated and dried, the obtained dried material contains 3 to 5 percent of ginsenoside Rg1, 20 to 40 percent of ginsenoside Re, 0 to 10 percent of ginsenoside Rb1 and 0 to 70 percent of ginsenoside Rd4, and the composition of the ginsenoside composition is different from the existing products, such as total ginsenoside, panax notoginseng saponins and notoginseng leaf triterpenes. The preparation method disclosed by the invention is simple and convenient, extraction and transformation are synchronized, the cost is low, yield is high, and the preparation method is suitable for the mass preparation of the ginsenoside composition, particularly high-content ginsenoside Rd composition.
Owner:QILU UNIV OF TECH
Traditional Chinese medicine composition for treating cerebral thrombosis and preparation method therefor
InactiveCN104887747ASolve the root cause of the diseaseGood treatment effectAnthropod material medical ingredientsBlood disorderAngelica Sinensis RootToxic material
The invention discloses a traditional Chinese medicine composition for treating cerebral thrombosis and belongs to the technical field of traditional Chinese medicines. The traditional Chinese medicine composition is prepared from the following raw materials: moringa oleifera leaves, angelica sinensis, ground beetle, ginsenoside Rb1, ginsenoside Rg1, ginsenoside Rd, ginsenoside Re, panax pseudo-ginseng, safflower, spatholobus stem, chuanxiong rhizome, herba artemisiae, earthworms and glycyrrhiza uralensis. The traditional Chinese medicine composition has the effects of nourishing Yin and supplementing kidney, replenishing essence and enriching blood, removing and dispelling blood stasis, soothing nerves and benefiting intelligence, invigorating spleen and replenishing Qi and clearing away heat and toxic materials and further has the advantages that the preparation process is simple, the treatment effect is good, the side effect is low, the taking is convenient, popularization and application are easy, and the like.
Owner:苗怡文
Traditional Chinese medicine composition treating diabetic nephropathy and preparing method thereof
InactiveCN104983759AGood curative effectImprove efficiencyOrganic active ingredientsMetabolism disorderGinsenoside RdGlucoside
The invention belongs to the technical field of traditional Chinese medicine, and relates to a traditional Chinese medicine composition treating diabetic nephropathy and a preparing method thereof. The traditional Chinese medicine composition comprises total flavones of moringa oleifera leaf, stibene glucoside, ginsenoside Rb 1, ginsenoside Rg1, ginsenoside Rd, ginsenoside Re, bilobalide, kaempferol and quercetin. The medicine components are reasonable, the synergism effect is high, the traditional Chinese medicine composition serves as the medicine compatibility for treating the diabetic nephropathy, and the clinic verifies that very obvious effects are achieved.
Owner:QINGDAO LANSHENGYANG PHARMA & BIOTECH CO LTD
Application of panaxadiol saponins fraction in preparing medicine for preventing epilepsia
ActiveCN102743401AOvercome curative effectOvercome securityOrganic active ingredientsNervous disorderMedicinal herbsAntiepileptic Agents
The invention provides application of a panaxadiol saponins fraction in preparing a medicine for preventing epilepsia. The component comprises the following main constituents: ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rb3, ginsenoside Rc and ginsenoside Rd, and the medicine is prepared from active constituents in the single panaxadiol saponins fraction or from the active constituents in the single panaxadiol saponins fraction and other medicines. By taking one of five panaxadiol saponins: ginseng, American ginseng rhizome medicinal materials, ginseng stems and leaves, American ginseng stem and leaf medicinal materials and total extractives or total saponins of the ginseng, the American ginseng rhizome medicinal materials, the ginseng stems and leaves and American ginseng stem and leaf medicinal materials as a starting raw material independently or taking a mixture which consists of two or more than two different raw materials as the starting raw material comprehensively, the medicine is prepared by establishing the chromatographic separation and purification technology. According to the medicine prepared from the panaxadiol saponins fraction provided by the invention, an anti-epileptic effect can be greatly improved, and moreover, the toxic and side effects, especially dermatitis and intelligence toxicity, of traditional anti-epileptic medicines can be resisted, therefore, long-standing defects that the anti-epileptic medicines are not enough in curative effect and low in safety are overcome. The structural formula of the panaxadiol saponin is shown in specification.
Owner:ZHEJIANG UNIV
Detection method of ginseng formula granules
InactiveCN106290629AComprehensive qualitative testingStrong specificityComponent separationTheoretical plateGinsenoside Rd
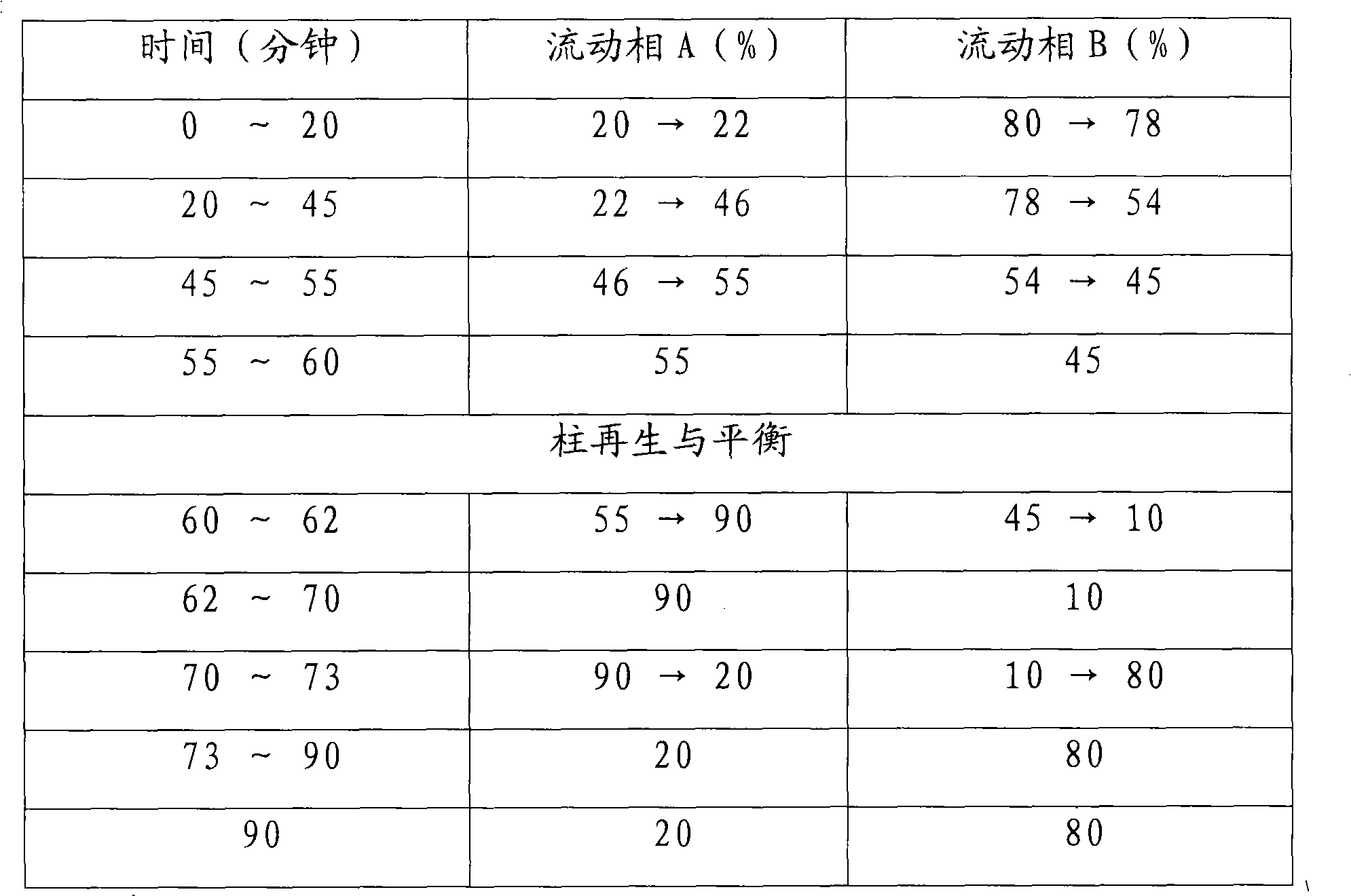
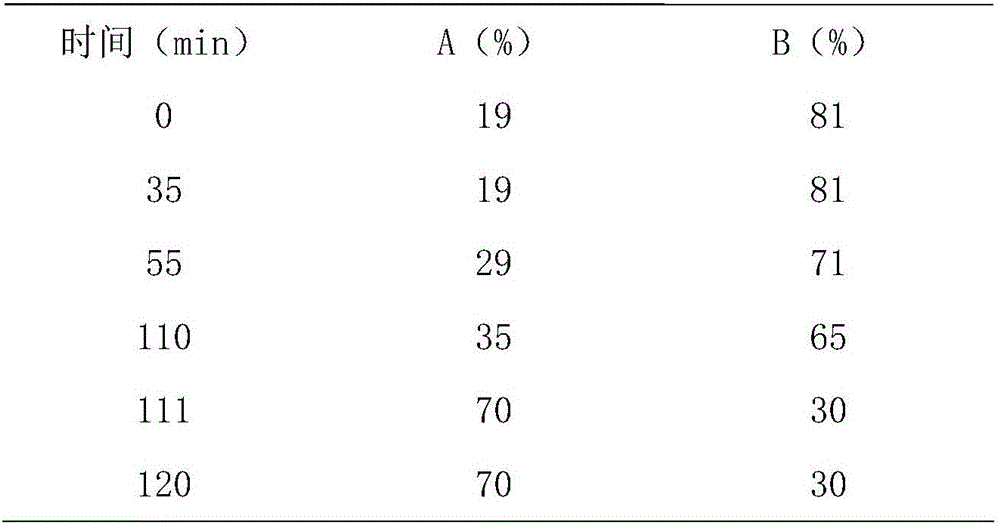
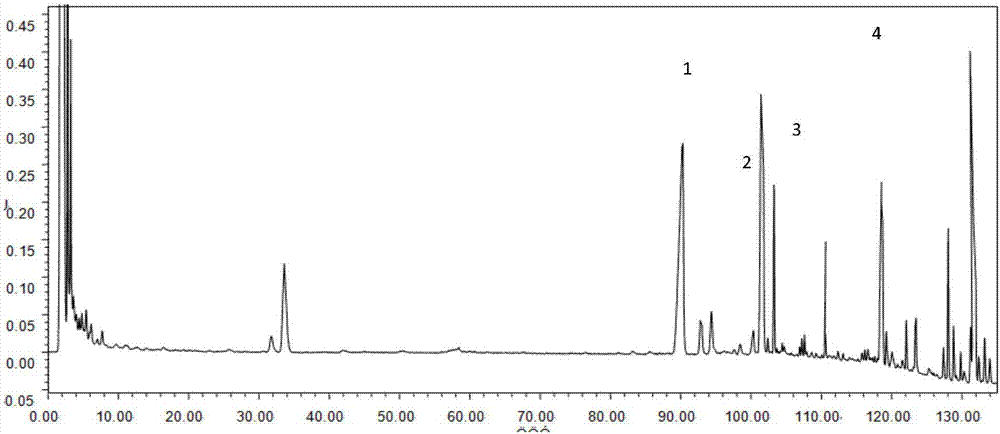
The invention relates to a detection method of ginseng formula granules, and belongs to the field of modernization of Chinese traditional medicine. Octadecylsilane chemically bonded silica is used as a filler; the column is CAPCELL PAK MG, 4.6*250 mm, and 5 microns; acetonitrile is used as a mobile phase A, water is used as a mobile phase B, and gradient eluting is carried out; the detection wavelength is 203 nm, the number of theoretical plates is not lower than 6000 in terms of ginsenoside Re peaks and is not lower than 200000 in terms of ginsenoside Rd peaks; in the preparation of a reference substance solution and a test solution, 5 microliters of the reference substance solution and 5 microliters of the test solution are adsorbed precisely respectively and then are injected into a liquid chromatograph, collection lasts for 120 minutes, and a chromatogram map is recorded. According to the detection method, the ginseng formula granules are qualitatively detected relatively comprehensively, and the method is a qualitative ginseng formula granule detection method with relatively high specificity.
Owner:JILIN AODONG GRP LIYUAN PHARM CO LTD
Pharmaceutical composition for treating cardiovascular and cerebrovascular diseases
InactiveCN102309496AReduce infarct sizeAlleviate behavioral disturbances arising from necrosisOrganic active ingredientsNervous disorderDiseaseCoronary heart disease
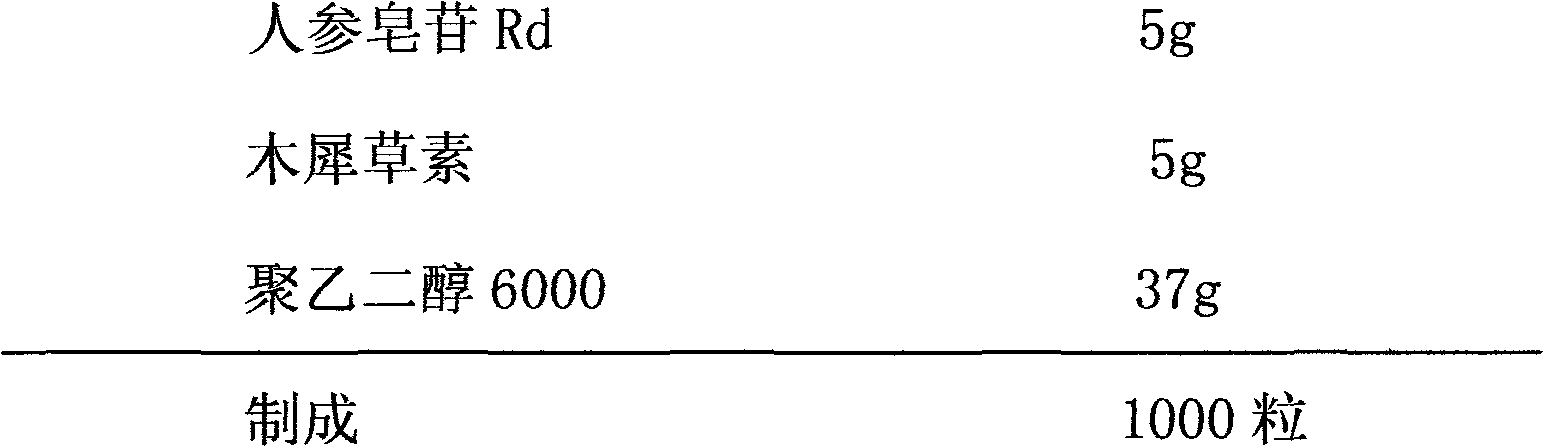
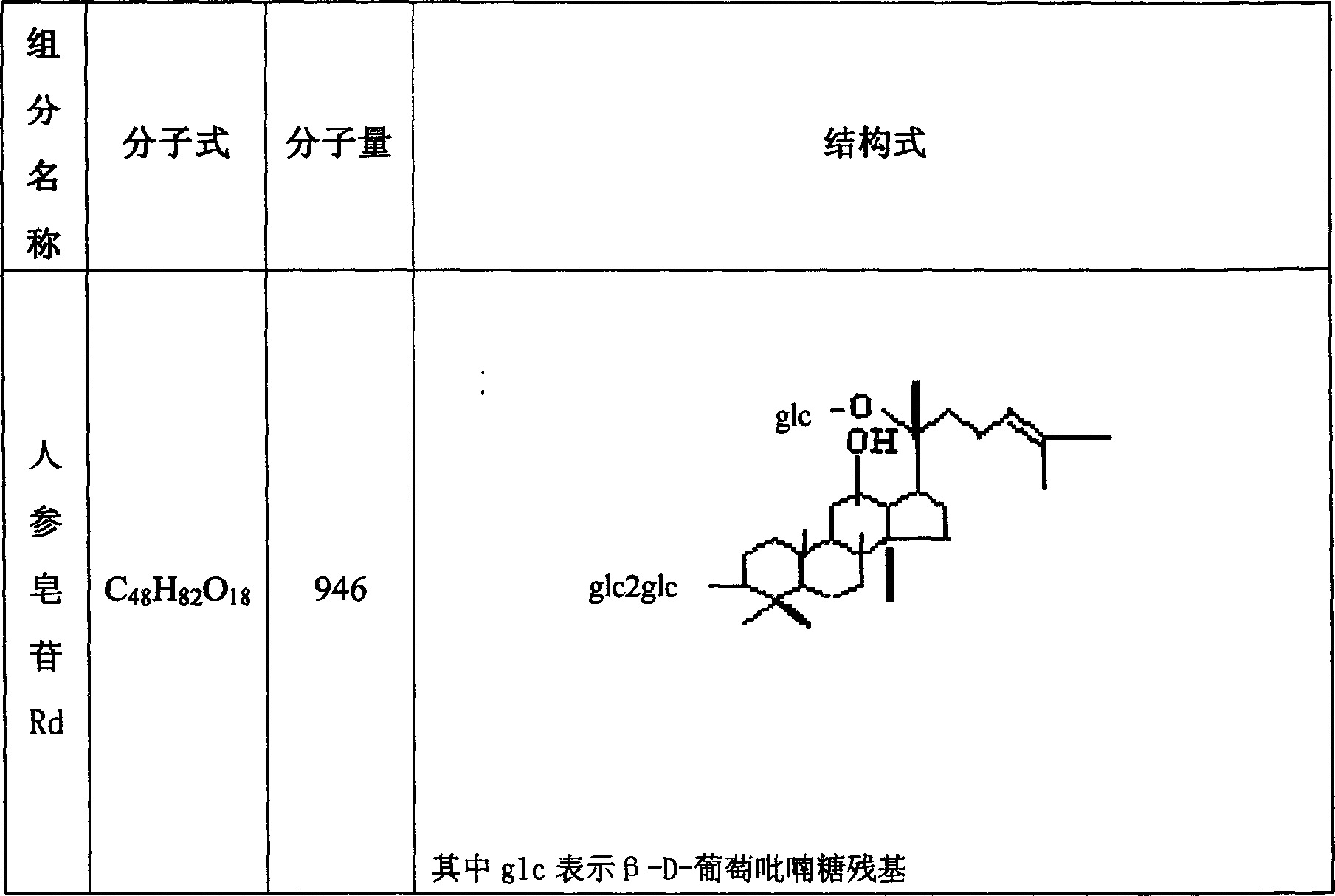
The invention relates to a pharmaceutical composition for treating cardiovascular and cerebrovascular diseases and an application. The pharmaceutical composition takes ginsenoside Rd and luteolin as main components, is capable of obviously minimizing an infarction area of brain tissue and obviously mitigating the behavior obstacle due to necrotic brain tissue, and achieves the purpose of treating insufficiency of cerebral blood supply, cerebral thrombus, brain embolism, cerebrovascular spasm, brain dysfunction, Alzheimer disease, parkinsons syndrome, cerebral apoplexy, hypertension, hyperlipoidemia, arteriosclerosis, coronary heart disease, angina pectoris and miocardial infarction.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Notoginseng glycol-saponin composition and its prepn and use
ActiveCN1771977AGood anti-inflammatory and analgesic effectSmall toxicityOrganic active ingredientsAntipyreticGinsenoside RdTraditional medicine
The present invention belongs to the field of Chinese medicine, and is especially the purified product of notoginseng as one kind of Chinese medicinal materials and its preparation process and pharmaceutical use. The notoginseng glycol-saponin composition obtained through purifying notoginseng consists of ginsenoside Rb1 and ginsenoside Rd as main components, and contains ginsenoside Rb1 30-70 wt% and ginsenoside Rd 4-18 wt%, with the total content of ginsenoside Rb1 and ginsenoside Rd being up to 80 wt%. The notoginseng glycol-saponin composition has obvious antiflogistic and analgetic effect, low toxicity, and the features of nonsteroidal antiphlogistic. The composition may be used in preparing medicine for treating rheumatic and rheumatoid arthritis.
Owner:成都中医大华神药业有限责任公司
Method for selectively producing ginsenoside rd from saponins of ginseng through enzymatic method
The present invention relates to a method for selectively producing ginsenoside Rd, which is originally present in a trace amount in ginseng, from panaxadiol-based saponins in ginseng and, more specifically, to a method capable of obtaining a desired target compound, that is, ginsenoside Rd, in high yield, by treating panaxadiol-based saponins, obtained from ginseng, with particular enzymes to structurally convert the saponins.
Owner:AMOREPACIFIC CORP
Green platycodon grandiflorus and ginseng fermented yogurt and production method thereof
InactiveCN106900856AImprove sleepingImprove symptoms of chest tightness and shortness of breathMilk preparationGinsenoside RdSolvent
The invention relates to a green platycodon grandiflorus and ginseng fermented yogurt and a production method thereof and belongs to the technical field of food processing. The method comprises extracting effective constituents of platycodon grandiflorus and ginseng through supercritical fluid extraction; fermenting the ginseng through beta-glucosaccharase to significantly increase the content of rare ginsenoside Rd, PPD, Rg3 and Rh2; performing fermentation through streptococcus thermophilus and lactobacillus bulgaricus to obtain the purely vegetative platycodon grandiflorus and ginseng fermented yogurt. The green platycodon grandiflorus and ginseng fermented yogurt contains no such additive as preservative, sweetening agent, thickener or colorants, thereby being particularly applicable to middle aged and elderly people. The green platycodon grandiflorus and ginseng fermented yogurt can achieve the effects of sleep improvement, cardio-pulmonary function enhancement, chest distress release, breath shortness avoidance, phlegm expelling and cough arresting as well as significantly enhance body immune functions. By means of the green chemical technology of supercritical fluid extraction, the preparation method of the green platycodon grandiflorus and ginseng fermented yogurt is efficient, clean, simple in process, high in extracting rate, safe and easy-recycled in solvent and green and pollution-free in product. The green platycodon grandiflorus and ginseng fermented yogurt is a novel purely vegetative and environmentally friendly nutritious food.
Owner:JILIN UNIV
Efficient panax notoginseng saponins injection and preparation method thereof
InactiveCN102335214ARaise the ratioGood effectOrganic active ingredientsPowder deliveryDiseasePANAX NOTOGINSENG ROOT
The invention discloses an efficient panax notoginseng saponins injection and a preparation method thereof. The injection comprises active ingredients in percentage by weight: 9.5-15.0% of notoginsenoside R1, 37.0-56.0% of ginsenoside Rg1, 5.0-8.0% of ginsenoside Re, 21.0-32.0% of ginsenoside Rb1 and no less than 0.45% of ginsenoside Rd; and a weight ratio of panaxtriol saponins to panaxadiol saponins is 1.5-3.5:1. By verifying through a pharmacodynamic experiment, the efficient panax notoginseng saponins injection, according with the ratio of the panaxtriol saponins to the panaxadiol saponins in the invention, has good effects in the treatment of cardio-cerebrovascular diseases, such as obstruction of collaterals by blood stasis, apoplectic hemiplegia, chest stuffiness and pains, central retinal vein occlusion and the like.
Owner:GUANGXI WUZHOU PHARMA GRP
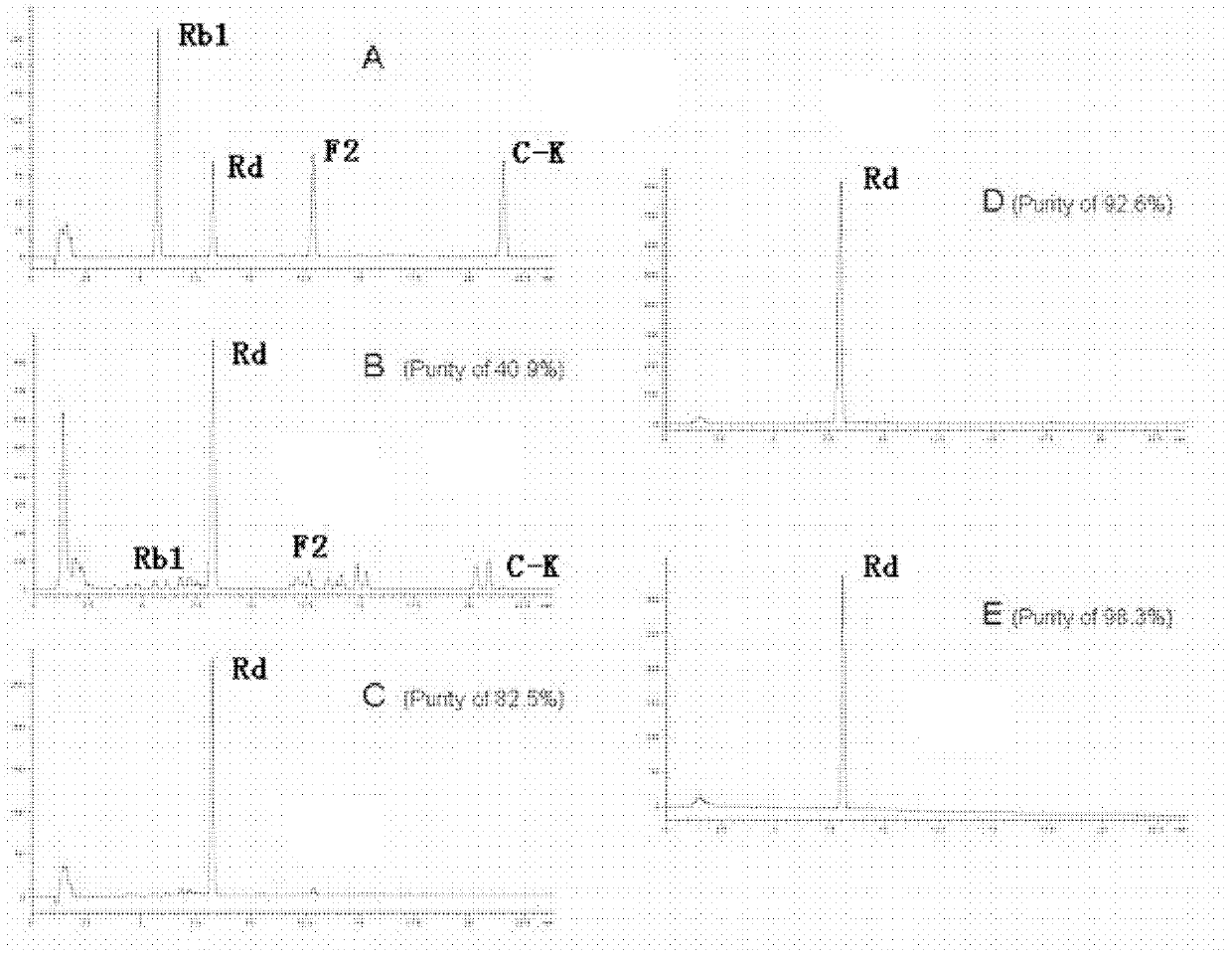
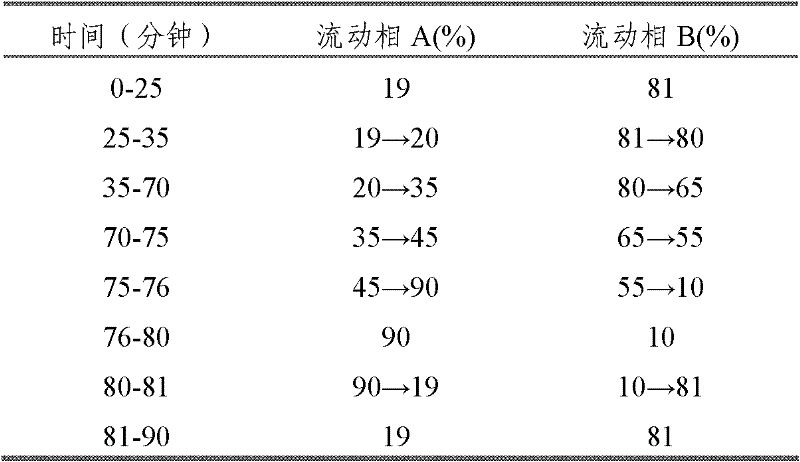
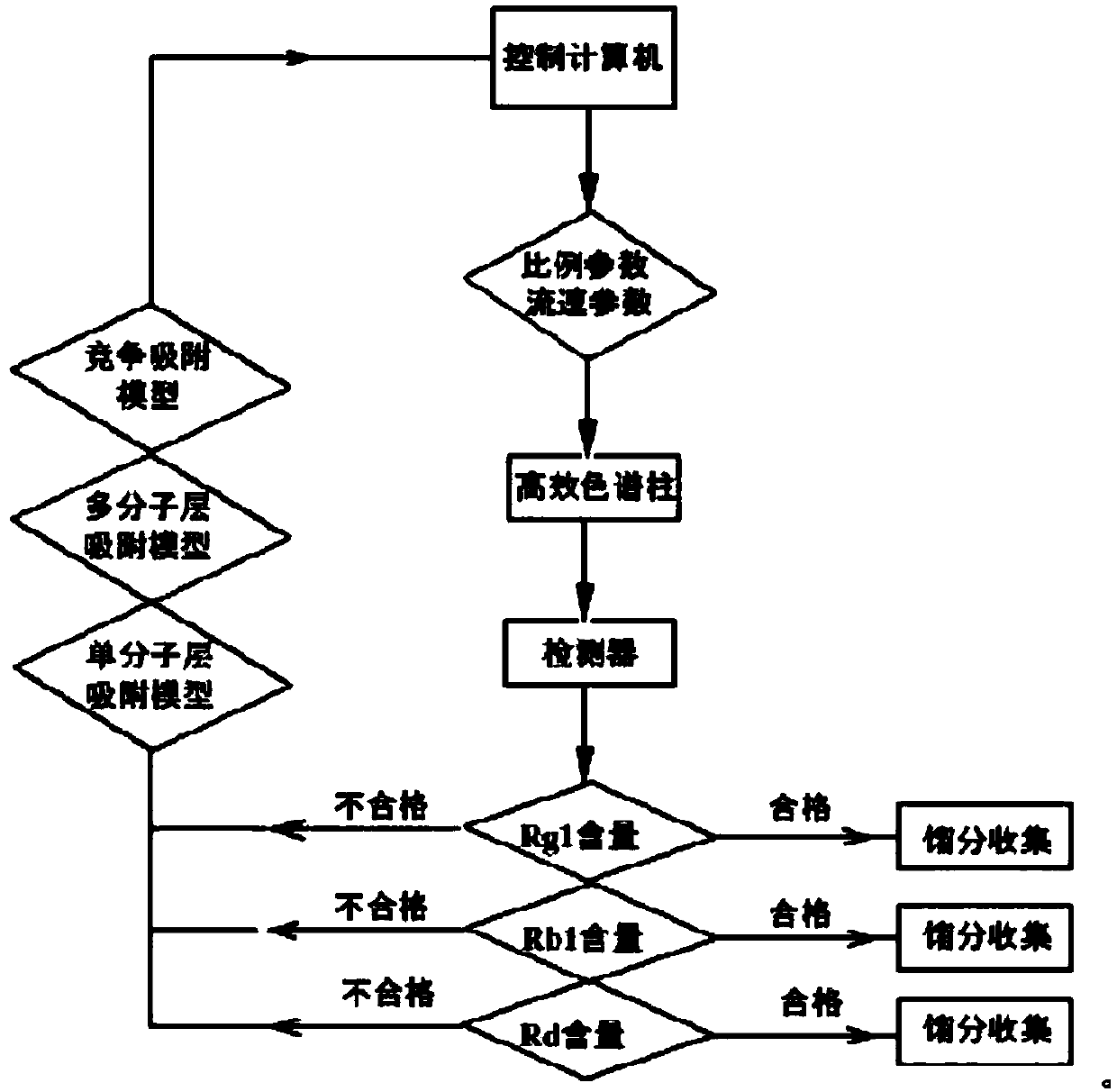
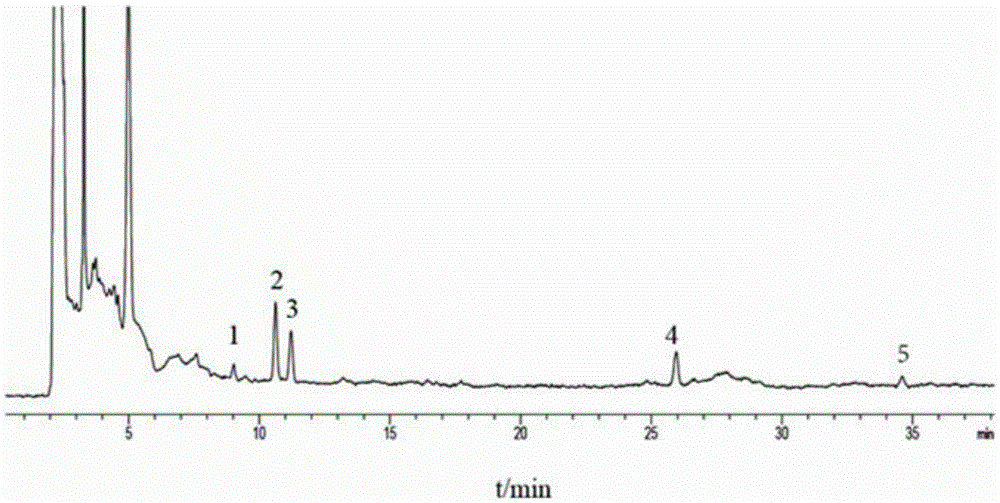
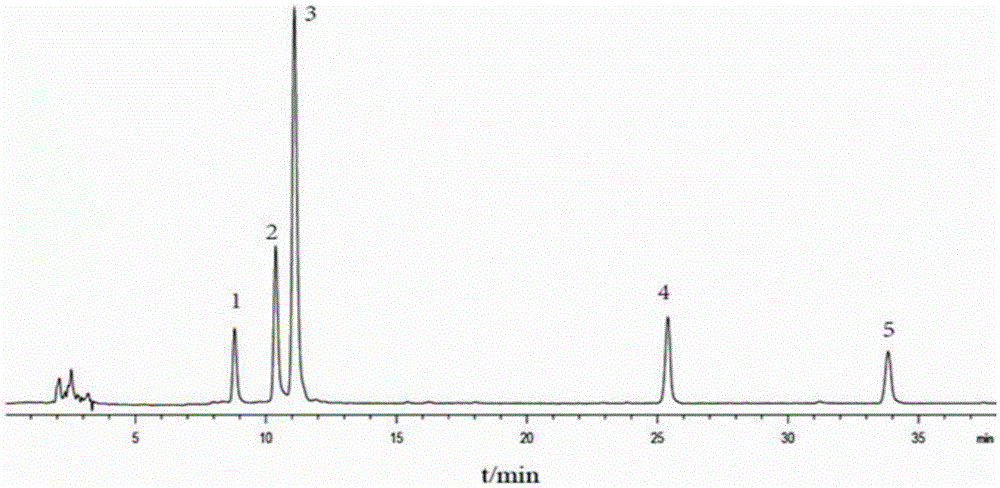
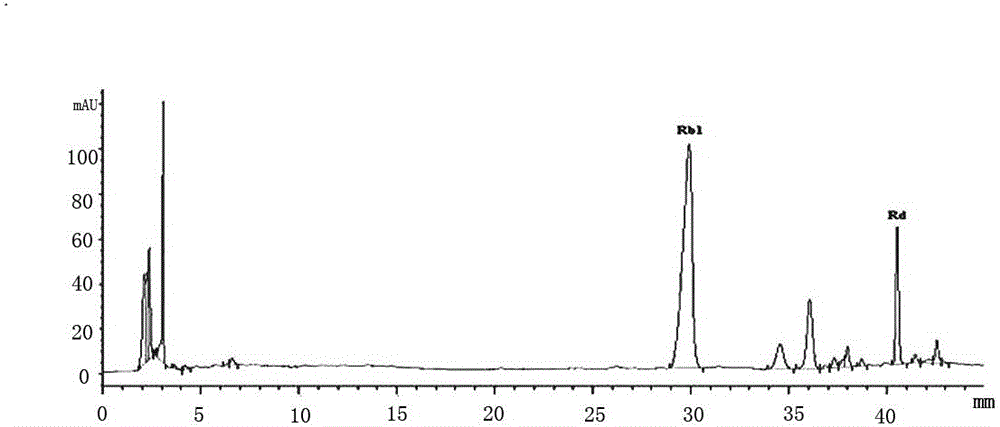
Automatic control method for separation and purification of ginsenoside Rd
ActiveCN110156863ARealize automatic controlCollection Precise ControlGlycoside steroidsSolid sorbent liquid separationAutomatic controlElution
The invention relates to an automatic control method for separation and purification of ginsenoside Rd. The method adopts a reversed-phase high performance chromatographic column to separate and purify ginsenoside extract. An elution solvent is formed by mixing of ethanol and water, and the elution mode is constant flow velocity or variable flow velocity stable pressure elution, also the content of ginsenoside in the eluent is detected and an adsorption model is combined to adjust the elution parameters to complete elution separation, thus obtaining high purity ginsenoside Rg1, Rb1 and Rd, especially high purity ginsenoside Rd. The automatic control method for separation and purification of ginsenoside Rd provided by the invention has the characteristics of high ginsenoside separation purity, high separation efficiency, solvent saving, energy saving and consumption reduction, safety and environmental protection, improves the economic value of the product, and is conducive to large-scale production.
Owner:吉林紫鑫参工堂生物科技有限公司
High-performance liquid phase detection method for heart-calming granules
ActiveCN105548425AIncrease contentComprehensive quality control indicatorsComponent separationGinsenoside RdGinsenoside Rb1
The invention provides a high-performance liquid phase detection method for heart-calming granules. The method comprises the step of adopting a high-performance liquid phase method for measuring the content of notoginsenoside R1, the content of ginsenoside Rg1 and the content of ginsenoside Rb1, and the step of adopting the high-performance liquid phase method for measuring the content of ginsenoside Rd and the content of lobetyolin. On the basis of utilizing the high-performance liquid phase method for measuring the content of notoginsenoside R1, the content of ginsenoside Rg1 and the content of ginsenoside Rb1, the high-performance liquid phase method is additionally adopted for measuring the content of ginsenoside Rd and the content of lobetyolin, and therefore the quality control index for the heart-calming granules is more comprehensive, and the detection method is good in precision, stability and repeatability.
Owner:SHANDONG BUCHANG PHARMA +1
Method for preparing ginsenoside-Rd through immobilization of cellulase and enzymolysis of ginsenoside-Rb1 by virtue of covalent cross-linking process
The invention belongs to the technical field of immobilization of enzymes and particularly relates to a method for preparing ginsenoside-Rd through immobilization of cellulase and enzymolysis of ginsenoside-Rb1 by virtue of a covalent cross-linking process. An immobilized enzyme is prepared through three steps of polyethyleneimine amination, glutaraldehyde cross-linking and enzyme immobilization. Compared with a manner of simply utilizing a free enzyme, the method has the advantages of recyclability, high selectivity, no pollution and the like. Compared with other enzyme immobilization methods, the method has many advantages that the bonding is firm, the operation is stable and the repeated utilization is realized, and the method is more suitable for industrial production.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Application of ginsenoside Rd in aspect of controlling diamondback moths
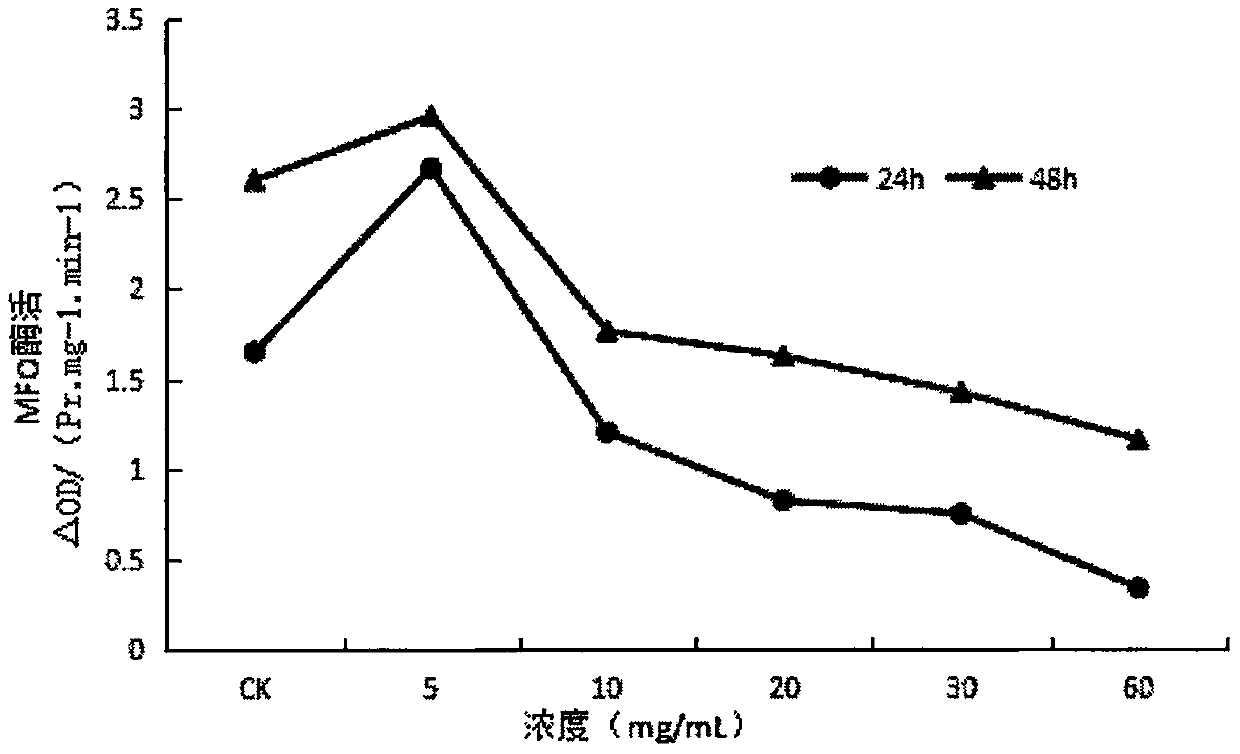
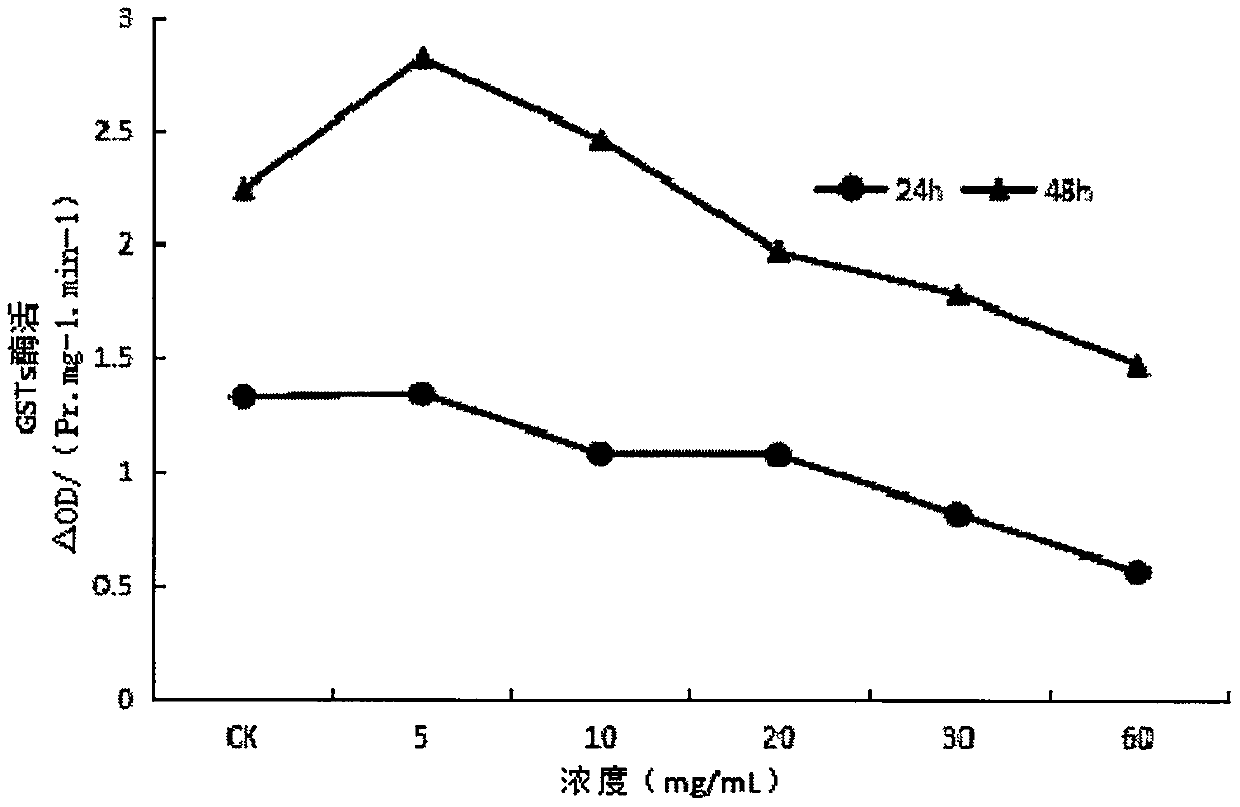
InactiveCN107668052AReduce enzyme activityEnhanced inhibitory effectBiocidePest repellentsNervous systemExperimental methods
The invention provides application of ginsenoside Rd in the aspect of controlling diamondback moths. A large number of experimental methods shows that the ginsenoside Rd has an avoidance function on feeding of second instar diamondback moths and has a certain inhibiting effect on the activity of detoxifying enzymes (mixed-functional oxidase, glutathione transferase and carboxylesterase) and a nervous system enzyme (acetylcholin esterase) in the second instar diamondback moths, and therefore, the ginsenoside Rd can be used for controlling the diamondback moths, and the theoretical foundation isprovided for development of a novel pesticide. When in application, the content of the ginsenoside Rd can be set as 10-60 mg / mL; preferably, when the concentration of the ginsenoside Rd is 60 mg / mL,the activity of a GSTs enzyme reaches the lowest after the diamondback moths are treated for 24 h, and the inhibiting effect is relatively strongest.
Owner:JILIN AGRI SCI & TECH COLLEGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com