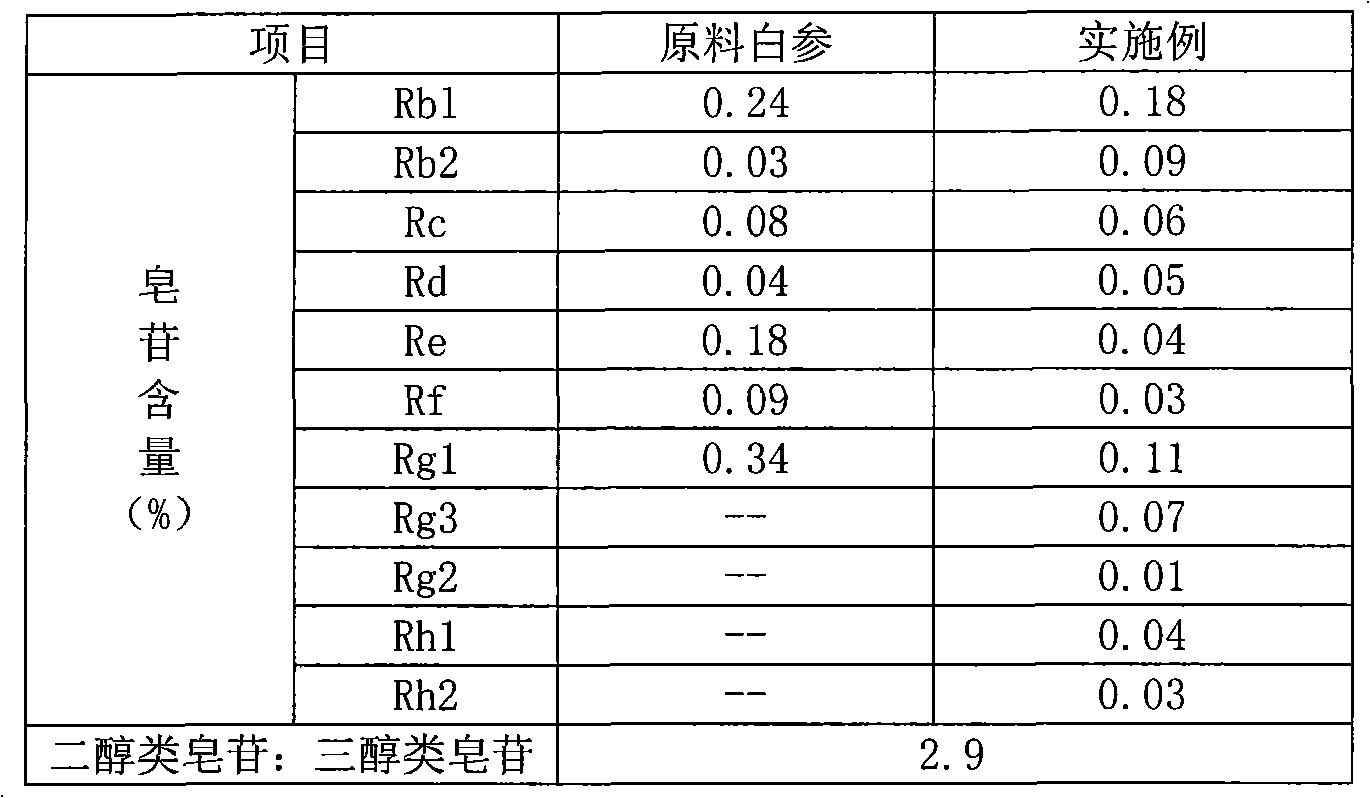
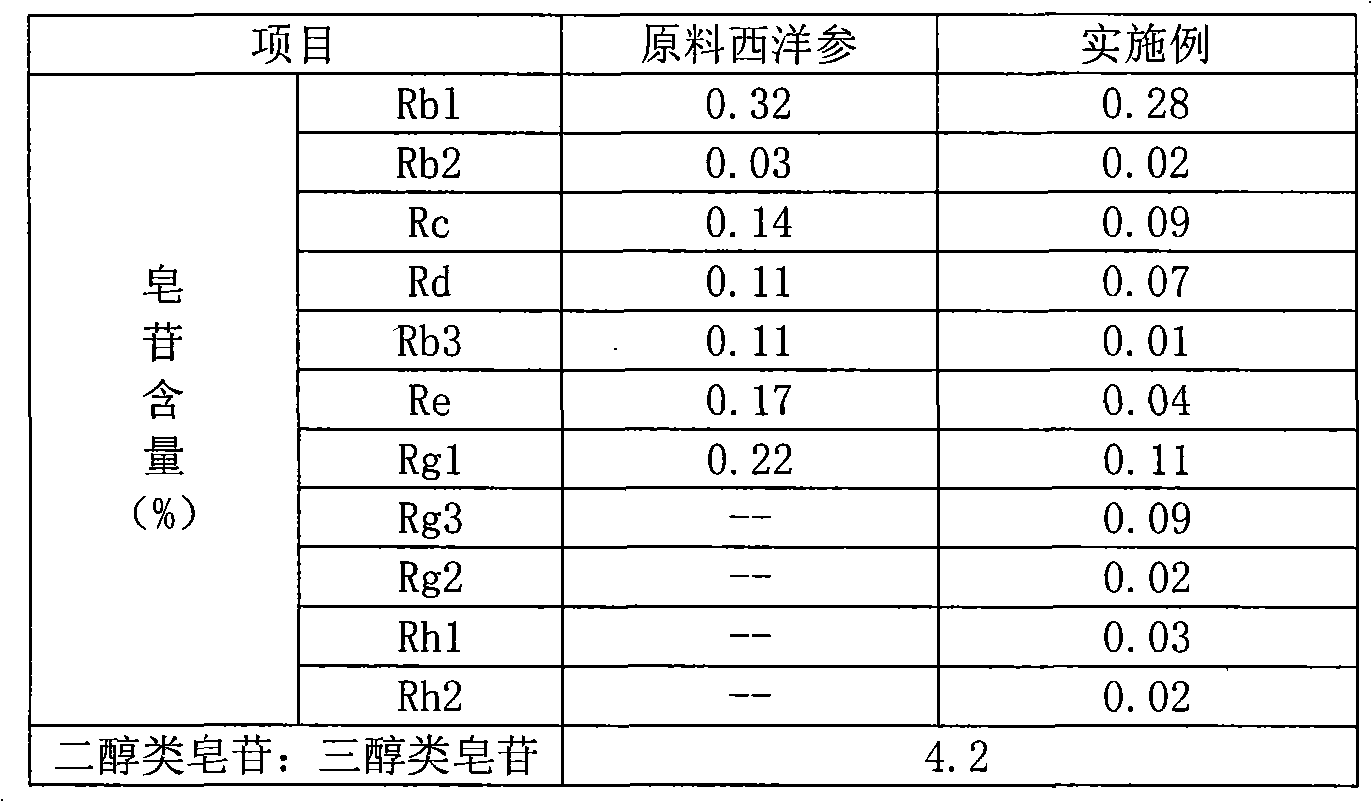
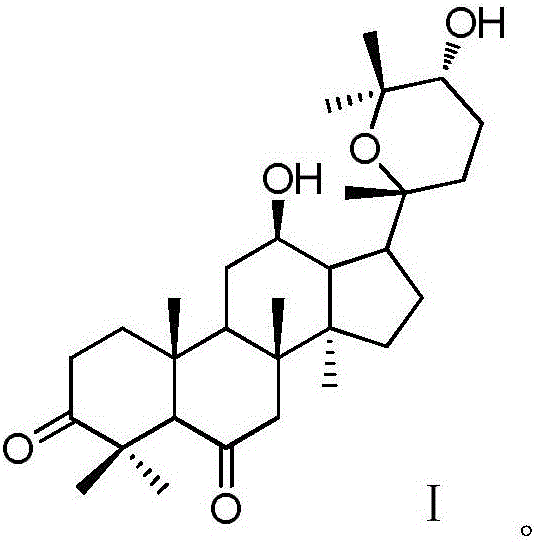
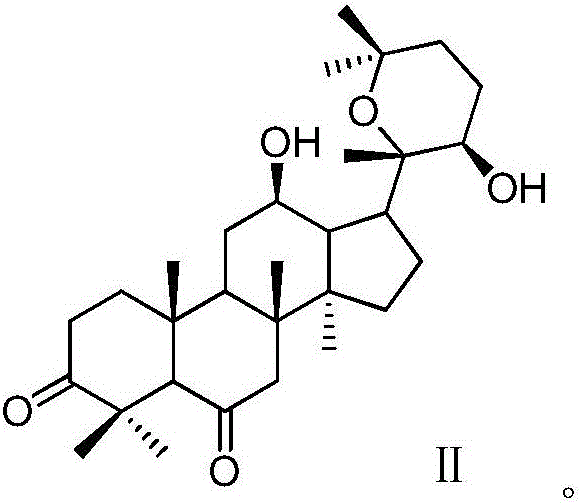
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
82 results about "Panaxatriol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
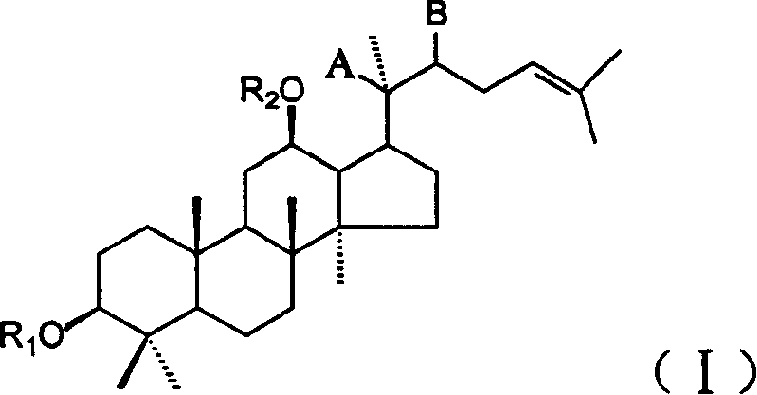
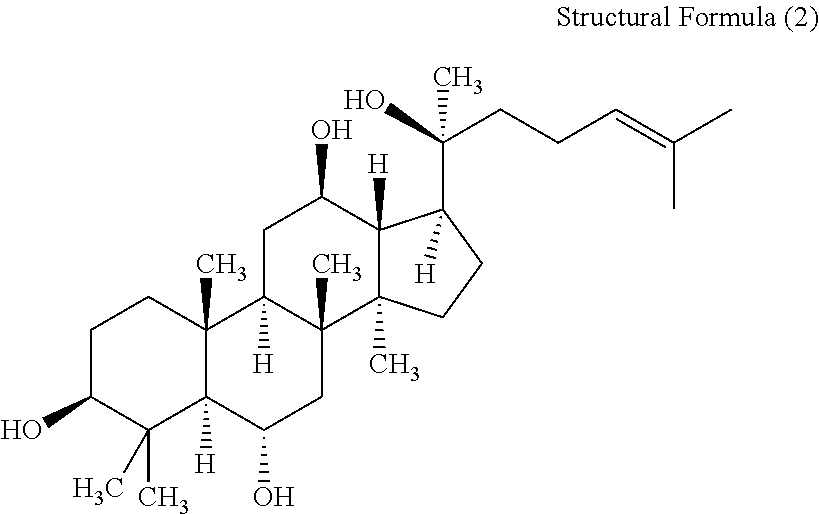
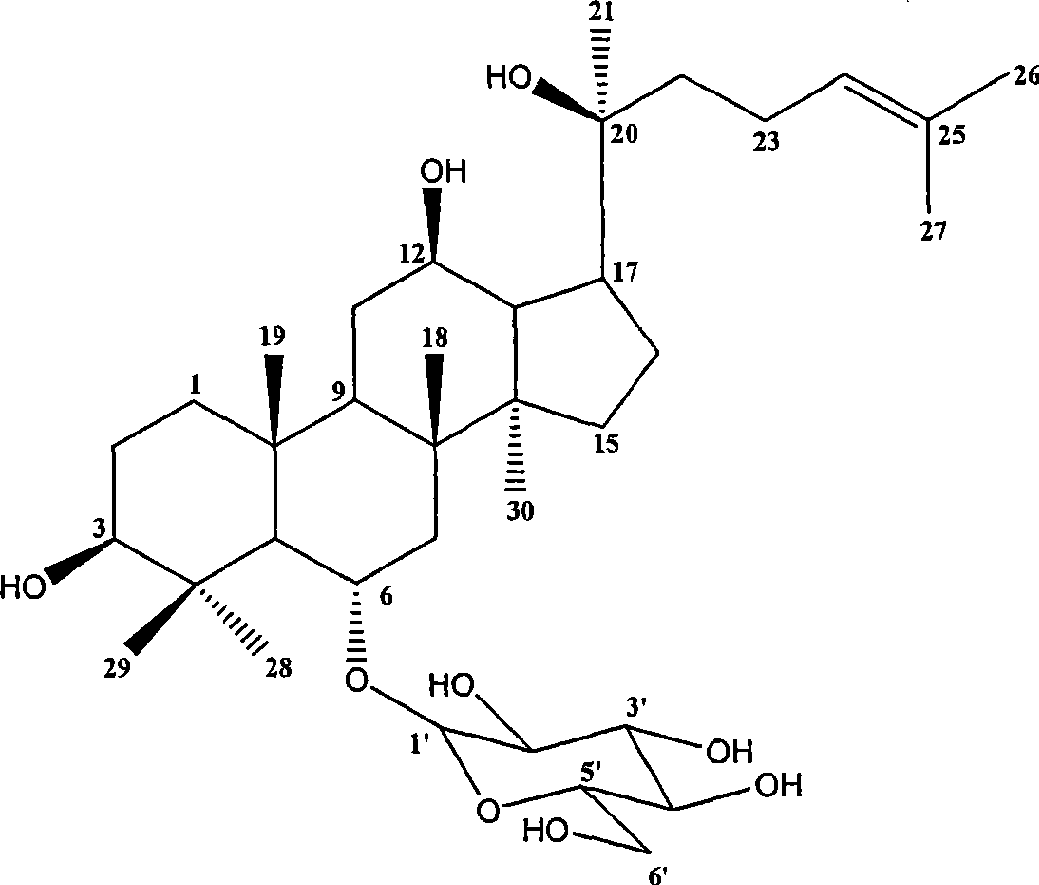
Panaxatriol is an organic compound characterizing a group of ginsenosides. It is a dammarane-type tetracyclic triterpene sapogenin found in ginseng (Panax ginseng) and in notoginseng (Panax pseudoginseng). It is formed by the dehydration of protopanaxatriol.
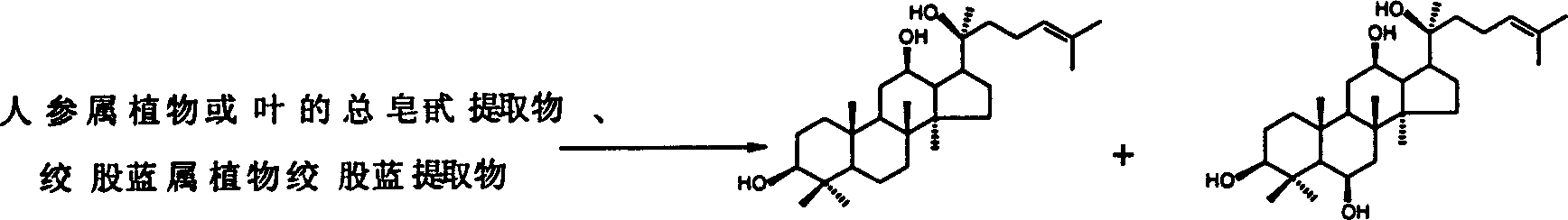
Process for preparing protopanoxadiol and protopanaxatriol
ActiveCN1569882AThere is no problem of instability, easy destruction, and easy cyclizationHigh yieldSteroidsOrganic solventProtopanaxadiol
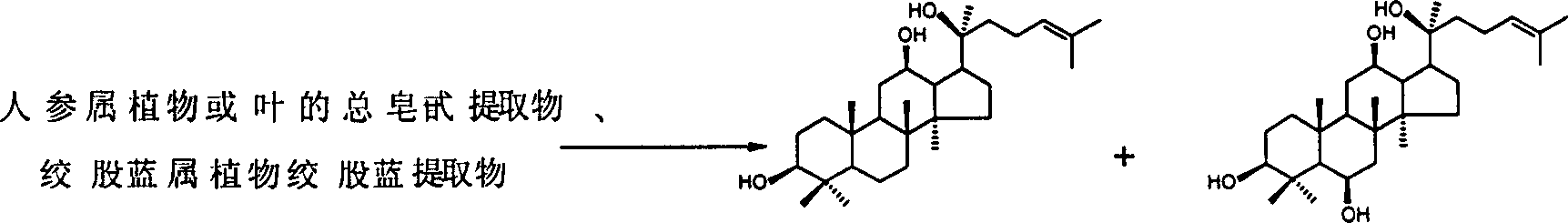
The invention relates to the process for preparing protopanoxadiol and protopanaxatriol by using general saponin extract from panax ginseng plant leaves and gynostemma pentaphylla extractive as raw material for alkaline hydrolysis reaction in organic solvent through column chromatography and purification.
Owner:怡瑞达(上海)医药科技有限公司
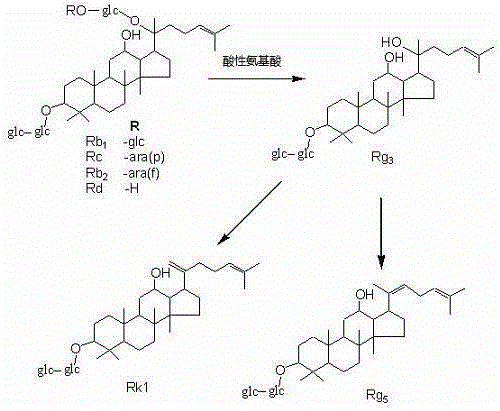
Method for preparing rare ginsenoside by hydrolyzing ginsenoside with acidic amino acid
ActiveCN105273032AOvercoming poor hydrolysis specificityLow overcoming rateSteroidsNeutral Amino AcidsNew medications
The invention discloses a method for preparing rare ginsenoside by hydrolyzing ginsenoside with acidic amino acid. Panoxadiol saponins are converted to generate 20R-Rg3, 20S-Rg3, Rg5 and Rk1, and panaxatriol saponins are converted to generate Rg6, F4, Rk3 and Rh4. Compared with an existing preparation process, the hydrolysis capacity of acidic amino acid is more than 10 times of that of neutral amino acid and that of basic amino acid and can better meet requirements of industrial preparation of rare ginsenoside, the defects that the hydrolysis specifity of ginsenoside is poor, the yield is low, the corrosion is high, environment pollution is caused, multiple byproducts are produced and the like due to the fact that a large amount of strong acid and strong base are used are overcome, ginseng resources are fully used, rare ginsenoside is extracted and enriched to the largest extent, the purpose of simple, quick, environment-friendly and low-cost enrichment of rare ginsenoside is achieved, and the method guarantees industrial production and preparation of new medicines.
Owner:JILIN YATAI PHARM CO LTD
Production method of flavor panax ginseng
The invention relates to a preparation method of flavor ginseng; after ginseng is added with nature fruit juice or organic acid for reaction, the content of the precious ginsenoside in products is enhanced, the efficacies of ginseng is improved, while the specific flavor of ginseng is maintained. The main quality characteristics is that: the flavor ginseng includes at least one of the following ingredients: ginsenoside Rg3, ginsenoside Rh1 and ginsenoside 20R-Rh2; wherein, the ratio of the total amount of precious ginsenoside group, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rd and ginsenoside Rc to the total amount of ginsenoside Re, ginsenoside Rg1 and ginsenoside Rf is more than 2.5; the preparation method can convert part of the component causing internal heat, namely, panaxatriol ginsenoside, into precious ginsenoside.
Owner:JILIN HONGJIU BIO TECH
Method for preparing protopanoxadiol and protopanaxatriol by using synergistic oxidation and alkaline bydrolysis of oxide and hyperoxide
This invention relates to a method for preparing protopanaxadiol and protopanaxatriol by co-oxidizing with oxygen and peroxide and alkali-hydrolyzing total notoginsenoside. The method comprises: (1) oxidizing and alkali-hydrolyzing Panax ginseng total saponin extract and Gynostemma pentaphyllum total saponin extract by applying cooperative effect of oxygen and peroxide, and alkali-hydrolyzing using alkoxide in situ formed in alcohol at 80-95 swg..C for 8-24 h, where the Panax ginseng total saponin extract and Gynostemma pentaphyllum total saponin extract / alcohol ratio is 30-150 g / L; (2) purifying by column chromatography to obtain protopanaxadiol and protopanaxatriol. The method adopts cooperative effect of oxygen and peroxide, thus can obtain high-purity protopanaxadiol and protopanaxatriol with high yields at low reaction temperatures and short reaction time. This invention provides a simple, convenient and low-cost method for preparing protopanaxadiol and protopanaxatriol in large amounts, which can satisfy the needs of drugs for treating cardiovascular and cerebrovascular diseases and tumor.
Owner:中山以诺生物科技有限公司
Brand discoloration wet-process polyurethane resin with good hydrolysis resistance
The invention relates to a brand discoloration wet-process polyurethane resin with good hydrolysis resistance and a preparation method of the brand discoloration wet-process polyurethane resin. The polyurethane resin comprises the following components in percentage by weight: 8-15% of diphenylmethane-4, 4'-diisocyanate, 12-20% of poly(polyol adipate), 1-4% of 1, 4-butanediol, 0.05-1% of ethylene glycol, 0.3-2% of hydroxyl fluorine-silicone oil, 60-77% of N, N-dimethylformamide and 1-4% of polyoxypropylene propylene-panaxatriol. The polyurethane resin has the characteristics of good surface smoothness and good hydrolysis resistance; after the polyurethane resin is processed by using a branding process, the discoloring effects of grains on the surface of the polyurethane resin are remarkable, and the polyurethane resin has a strong stereoscopic impression.
Owner:SHANGHAI HUIDE TECH CO LTD
Preparation process for ginsenoside Rg1
The invention relates to a preparation process for panaxoside monomer comprising the steps of, (1) dissolving total notoginseng saponin with alcohol, (2) passing through pretreated big hole resin columns, washing panaxatriol saponin with alcohol, rinsing the resin columns, (3) reclaiming alcohol through decompression, spray drying, obtaining panaxadiol saponin, (4) adsorbing on chromatographic silica gel, drying, (5) proceeding low pressure chromatography, (6) reclaiming solvent, vacuum drying to obtain the panaxoside Rg1 crude product, (7) proceeding step 4, 5 and 6 to obtain refined Rg1.
Owner:云南特安呐制药股份有限公司
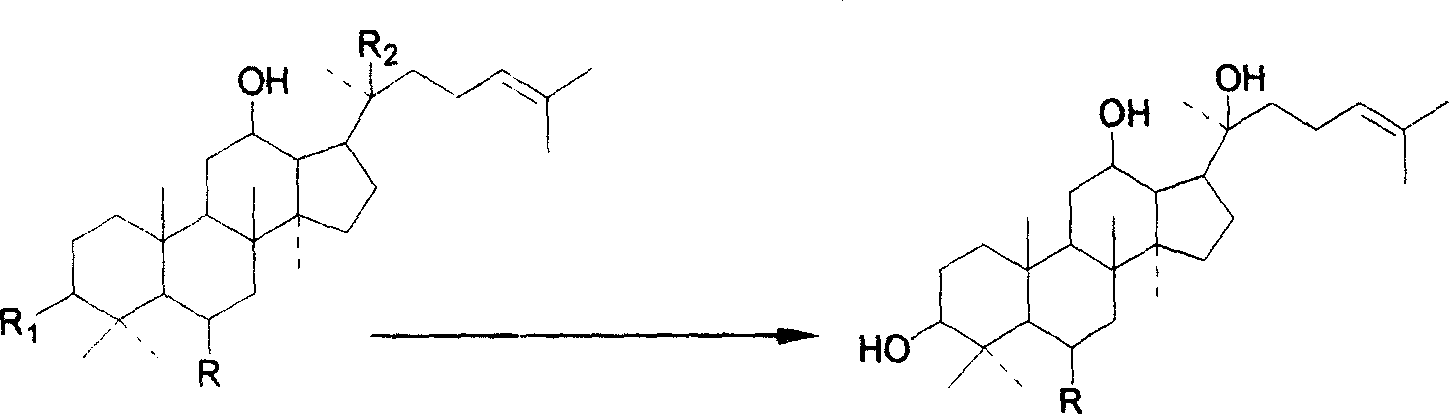
Preparation method and application of 20 (R)-panaxatriol derivative
ActiveCN105949265AGood antitumor activityWide range of usesOrganic active ingredientsMicroorganism based processesMicrobial transformationStructural formula
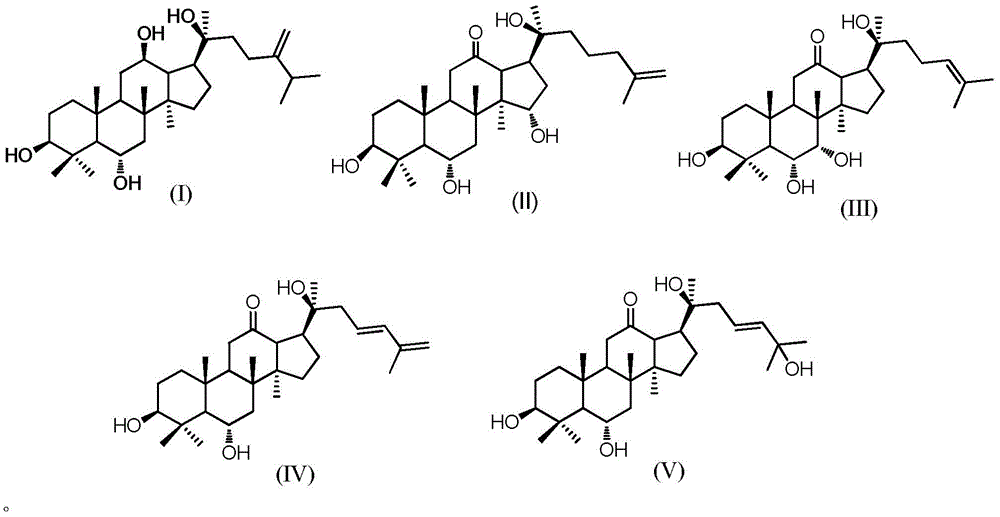
The invention discloses a 20 (R)-panaxatriol derivative and a preparation method and application thereof. Structural formulas of the 20 (R)-panaxatriol derivative are formulas I, II, III, IV, V, VI, VII, VIII, IX, X, XI and XII. Microbial conversion technology is utilized, 20 (R)-panaxatriol is structurally modified successfully, and various novel compounds are obtained; in-vitro anti-tumor cell experiments verify that the compounds have high anti-tumor activity, can serve as active ingredients of anti-tumor drugs and have wide application range.
Owner:SHENZHEN SUN BIO SCI & TECH CO LTD
20(SO)ortho ginseng diol derivative, medicinal composition containing them and its application
ActiveCN1651451AStrong anti-virusReduced activity of anticancer drugsOrganic active ingredientsSuppositories deliveryDrugToxicity
Owner:HAINAN ASIA PHARM CO LTD
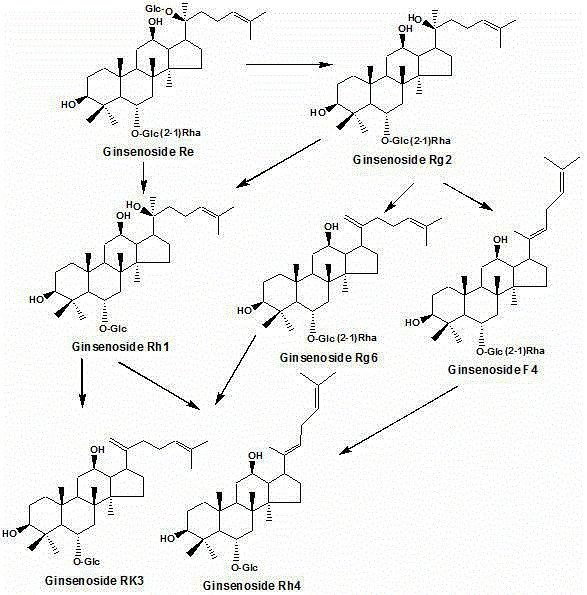
Preparation method of 20th carbon hydroxyl dehydrated ginseng rare saponin and aglycone
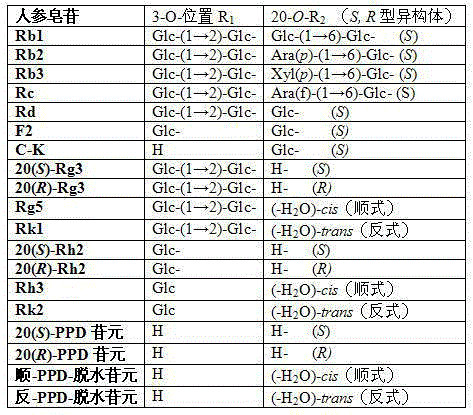
The present invention discloses a preparation method of 20th carbon hydroxyl dehydrated ginseng rare saponin and aglycone. The method is characterized in that metal inorganic salt catalyzes decomposition of decomposition of ginsenoside 20th carbon glycosyl in an organic solvent for preparing 20-O-hydroxy dehydrated Rg5 and Rk1 of Rg3, 20-O-hydroxy dehydrated Rg2 and Rg6 of Rg4, 20-O-hydroxy dehydrated Rh3 and Rk2 of Rh2, 20-O-hydroxy dehydrated Rh4 and Rk3 of Rh1, panoxadiol saponin aglycone 20-O-hydroxy dehydrated cis-PPD (-H2O) aglycone and trans-PPD (-H2O) aglycone panoxadiol saponin aglycone 20-O-hydroxy dehydrated cis-PPT (-H2O) aglycone and trans-PPT (-H2O) aglycone. The method has the advantages of simple operation, low cost, high yield and high purity (both greater than 90%), and is applicable to mass production; and the obtained product can be used in drug development, ginseng products, health products and cosmetics.
Owner:金凤燮
Preparation process for ginsenoside Rb1
The invention relates to a preparation process for ginsenoside monomer comprising the steps of, (1) dissolving total notoginseng saponin with alcohol, (2) passing through pretreated big hole resin columns, washing panaxatriol saponin with alcohol, rinsing the resin columns, (3) reclaiming alcohol through decompression to obtain panaxadiol saponin, (4) adsorbing on chromatographic silica gel, drying, (5) proceeding low pressure chromatography, (6) reclaiming solvent, vacuum drying to obtain the panaxoside Rb1 crude product, (7) repeating step 4-6 to obtain refined ginsenoside Rb1.
Owner:云南特安呐制药股份有限公司
Preparation method of pseudo-ginseng protopanoxadiol saponin and its uasage
InactiveCN101156882AEfficient removalIncrease contentAntineoplastic agentsPlant ingredientsProtopanaxadiolGradient elution
The invention provides a preparation method for extractive originating from an araliaceae plant, notoginseng. The extractive of the category mainly contains extractive of dammarane type, tetracyclic triterpene category, protopanaxadiol type and saponin category, wherein the content of ginsenoside Rb1 and Rd is not lower than 60 percent, and the content of ginsenoside Re, Rg1, and notoginseng saponin R1 is not higher than 5 percent. The notoginseng is extracted by using alcohol containing water as the solvent, chromatography to the extracting solution through a chromatographic column is performed to obtain elutriant, reversed phase and liquid phase gradient elution is performed, the elutriant is collected, and then the target extractive is obtained through concentration. The extraction process of the invention can effectively remove impurities of sugar, protein, amino acid, and the saponin of the protopanaxadiol type of the notoginseng, etc., and improve the content of effective components. The effective components are definite, the process is simple, the quality is controllable, and the invention is suitable for industrialized production, thereby being applied for preparing medicines treating malignant solid tumors.
Owner:ZHEJIANG UNIV
Panaxatriol saponin composition and uses in preparing medicament for immunity enhancement
InactiveCN101401813AImprove immune boosting activityGood curative effectOrganic active ingredientsImmunological disordersSide effectActive component
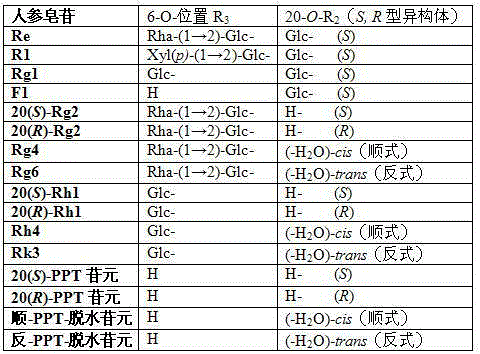
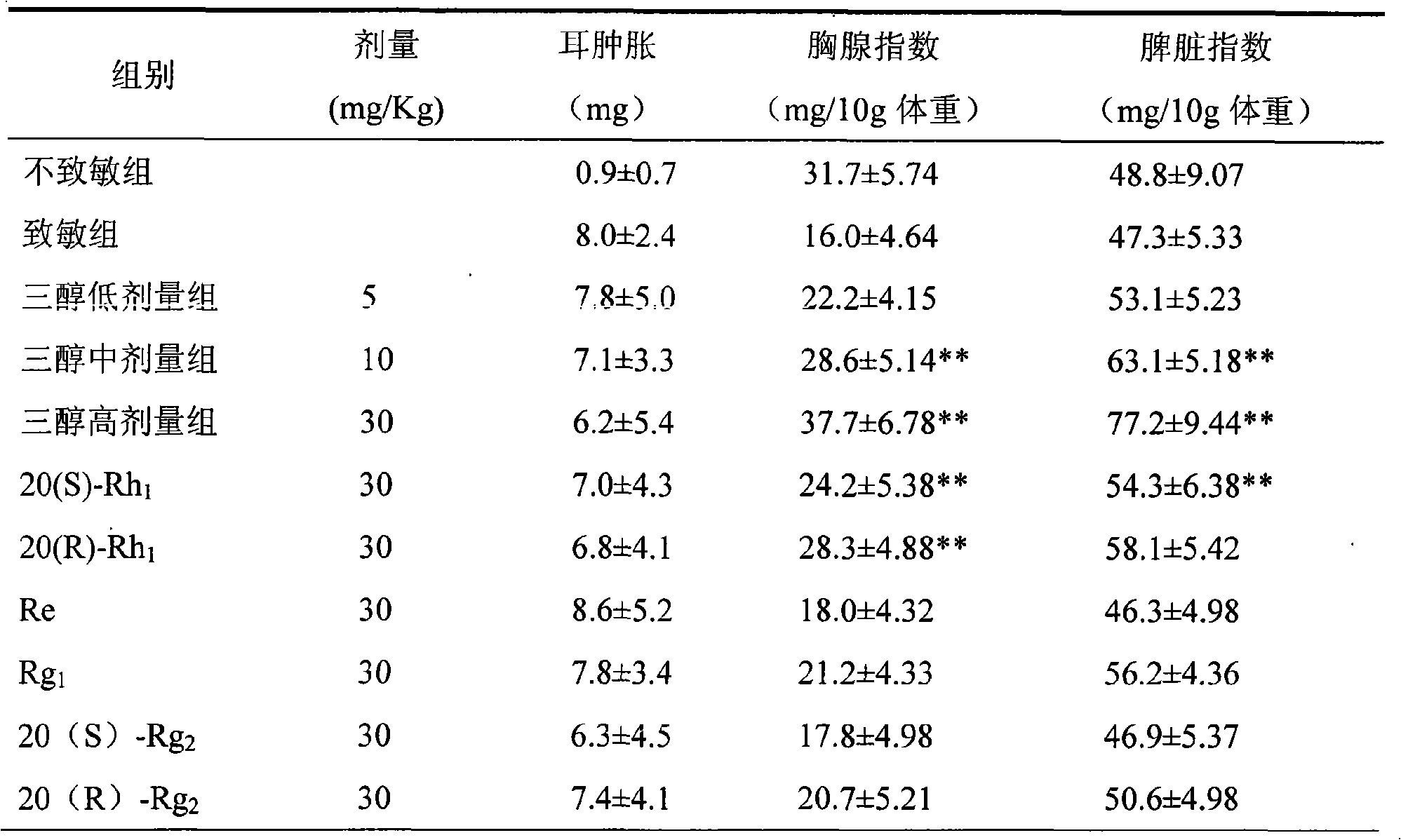
The invention relates to a panaxatriol saponin composition, wherein active components with immune-enhancing function in the composition mainly comprise ginsenoside-Re, ginsenoside-Rg1, ginsenoside-Rg2 and ginsenoside-Rh1, and the components in percentage by weight are: 25 to 50 percent of the ginsenoside-Re, 15 to 50 percent of the ginsenoside-Rg1, 0 to 30 percent of the ginsenoside-Rg2, and 0 to 30 percent of the ginsenoside-Rh1. The panaxatriol saponin composition of the proportions has the immune-enhancing activity far larger than that of a ginseng monomer saponin, improves the curative effect of the immune-enhancing function, and reduces the toxic side effects. In addition, the preparation method is simple, and the production cost is low.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
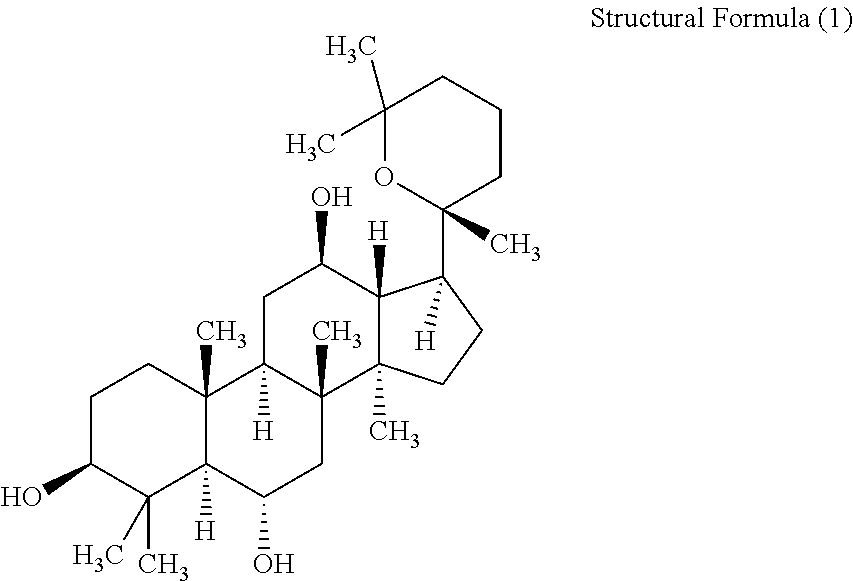
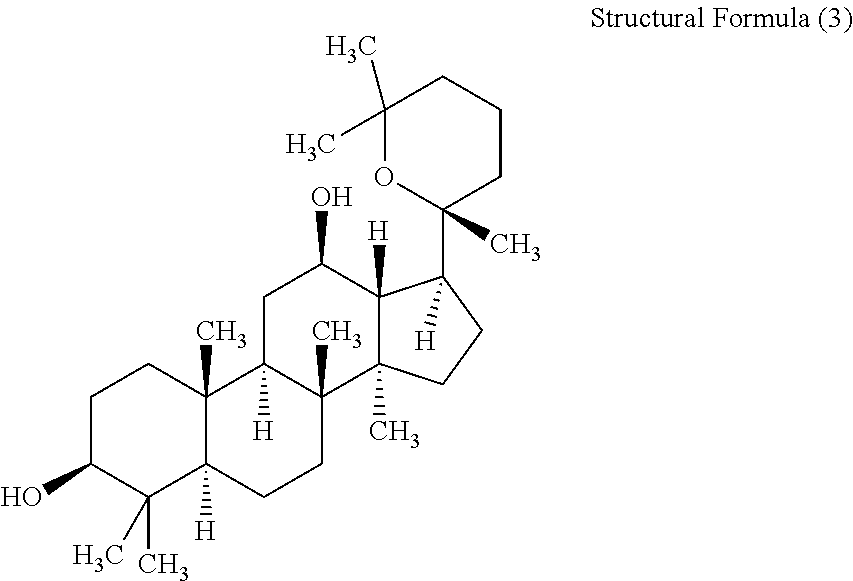
Composition containing protopanaxatriol and protopanaxadiol
InactiveUS20120252768A1Improve securityImprove stabilityOrganic active ingredientsBiocideProtopanaxadiolPhysical chemistry
A composition including: at least one mixture selected from the group consisting of a mixture of (A) panaxatriol and (B) protopanaxatriol and a mixture of (C) panaxadiol and (D) protopanaxadiol, wherein a ratio (A) / (B) of a mass of the (A) panaxatriol to a mass of the (B) protopanaxatriol is 1 or greater, and a ratio (C) / (D) of a mass of the (C) panaxadiol to a mass of the (D) protopanaxadiol is 1 or greater.
Owner:LION CORP
Compound panoxatriol saponin with physiological activity and its prepn and use
InactiveCN1608629AFunction increaseImprove oxygen supply and blood supplyOrganic active ingredientsNervous disorderNervous systemCranial nerves
The compound panoxatriol saponin with physiological activity contains panoxatriol saponin, gingko extractive, theanine and supplementary material is prepared into various preparations. The compound panoxatriol saponin can treat cerebral atherosclerosis, senile dementia, etc. by means of the functions of strengthening the activity of cerebral cell and cerebral nerve cell, improving brain's oxygen and blood supplying state, etc.
Owner:玉溪维和香格喜玛生物技术有限责任公司
Rabies vaccine stabilizer and application thereof
InactiveCN104383550AGuaranteed stabilityCompatibility is scientific and reasonableAntiviralsMacromolecular non-active ingredientsBovine serum albuminMannitol
The invention discloses a rabies vaccine stabilizer. The rabies vaccine stabilizer is prepared from the following components by weight: 1-1.2 percent (W / V) of bovine serum albumin, 2.1-2.7 percent (W / V) of amino acid composition, 0.2-0.8 percent (W / V) of sorbitol or mannitol, 20-50mmol / L of buffer solution, 1.2-1.5 percent (W / V) of proto-panaxatriol and the balance of water, wherein a pH value of the buffer solution is 7.0-7.2. An application method of the rabies vaccine stabilizer comprises the following steps: (1) BSR cell passage, culture and extension; (2) virus inoculation, culture and harvest; (3) virus inactivation; and (4) vaccine preparation: after virus inactivation passes inspection, adding a virus in the rabies vaccine stabilizer. Without containing animal serum, the rabies vaccine stabilizer can not have harmful effects in an immune animal body and can ensure the stability of a rabies vaccine at low, normal and high temperatures.
Owner:常州同泰生物药业科技股份有限公司
Process of preparing 20(S)-ginsenoside Rh1 with streptomycete fermentation of pseudo-ginseng saponin
A Streptomyces fradiae NTGA-344 strain with the preservation number of CGMCC No.2074 and a method for preparing 20(S)-ginsenoside Rh1 (6-O-belta-D-Glucopyranoside-20(S)-Protopanaxatriol by using the strain to ferment and convert panaxatriol saponins in various notoginsenosides in a fermentation cylinder are provided. 1(GA), ISP2, humic acid (HSG culture medium) and asparagines culture medium are selected as the culture media. In the process of fermentation, the inventory rating proportion of various notoginsenosides is 0.1% to 10 % (w / v), the fermentation temperature is 18 to 50 DEG C, the pH value of 0.1% to 35% (w / v) ammonia is dynamically adjusted to be between 2 and 8, the ventilation ratio is 0.1 to 10 (v / v), the stir speed is 10 to 900r / min, the fermentation time is 5h to 200h, and the macroporous absorbent resin is 0.1% to 30% (w / v). The invention provides the streptomyces yunnanensis with stable property, simple nutritional requirement and good growth and a practical and convenient fermentation cylinder fermentation and separation extraction process for large-scale fermentation and conversion of various notoginsenoside and preparation of 20(S)-ginsenoside Rh1. The 20(S)-ginsenoside Rh1 is prepared by the strain and the technology, thereby having low manufacturing cost, less impurity and high product yield.
Owner:KUNMING NOVOGINSENG BIOENG
Preparation method of panaxadiol saponin and panaxadiol type saponin monomer
ActiveCN107188910AImprove adsorption capacityNot easy to adsorbSugar derivativesSugar derivatives preparationEthyl acetateTriol
The invention discloses a preparation method of a panaxadiol saponin and a panaxadiol type saponin monomer. The preparation method comprises the following steps: taking radix notoginseng, ginseng or American ginseng as raw materials, extracting and concentrating the raw materials through low alcohols and filtering through diatomite; then selectively absorbing panaxatriol saponin with macroporous resin with an aperture of 50-70 [mu]m, collecting effluent liquid and drying to obtain a panaxadiol saponin coarse product; dissolving the panaxadiol saponin coarse product through methanol, ethanol, methanol water or ethanol water, adding acetonitrile or ethyl acetate to precipitate, collecting a precipitate to dissolve, putting a panaxadiol saponin aqueous solution on a chromatographic resin column, firstly resolving triol impurities with an ethanol solution, and dissolving and resolving by adopting different concentrations of ethanol or methanol solutions to obtain the panaxadiol saponin or the panaxadiol type saponin monomer. The preparation method disclosed by the invention overcomes the defects of low yield, high production cost, low purity, long production period and the like in the prior art, obtains the high-purity panaxadiol saponin or the panaxadiol type saponin monomer and is simple and easy to operate, low in cost, suitable for industrial production, environment-friendly and free of pollution.
Owner:云南特安呐制药股份有限公司
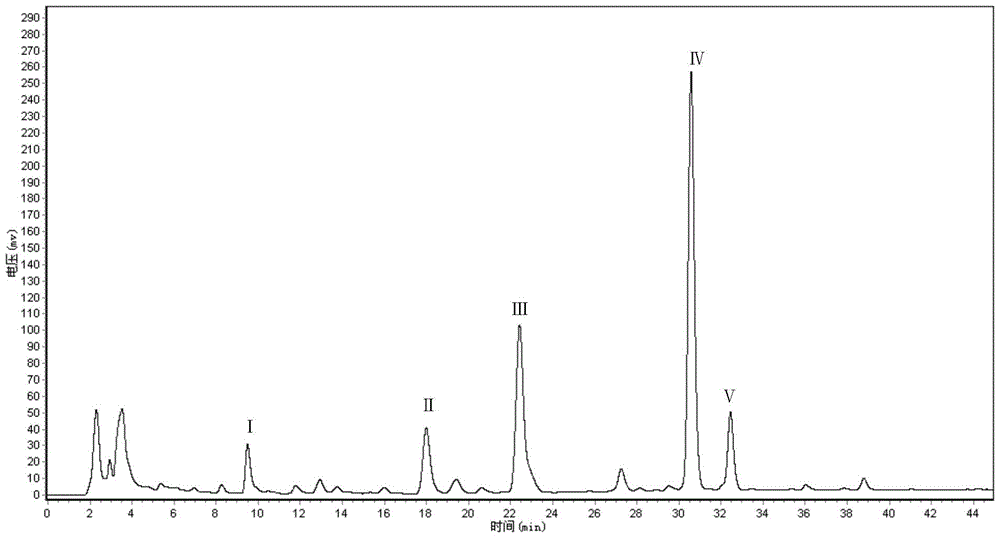
Conversion action of black rhizopus on ginsenosides
The invention discloses a rhizopus stolonifer capable of biologically converting ginsenosides. A fermentation product is analyzed by an HPLC (High Performance Liquid Chromatography) system to prepare a component with a reduced color spectrum peak value in an original panaxatriol saponins and a component with an increased peak value in the panaxatriol saponins fermentation product thereof. By isolation and identification, a converted substance in the panaxatriol saponins is confirmed to be ginsenosides Re, a compound (1) in the panaxatriol saponins fermentation product is confirmed to be ginsenosides Rh1, a compound (2) is confirmed to be ginsenosides Rg3, and a compound (3) is confirmed to be ginsenosides Rg2. By speculation, the ginsenosides Re in the panaxatriol saponins is converted into the ginsenosides Rh1 by the black rhizopus or the ginsenosides Re is firstly converted into the ginsenosides Rg2 and then converted into ginsenosides Rh1, and the detailed conversion path is to be further researched.
Owner:包海鹰
A plurality of antineoplastic sapogenin and its injection preparation method
InactiveCN1962681AReduce usageReduce manufacturing costOrganic active ingredientsPowder deliveryProtopanaxadiolOxygen
The invention discloses a saponin compound to prevent tumour preparing method of its agent, which comprises the following steps: adopting panaxoside, American ginseng saponin, Notoginsen triterpenes and gynostemma pentaphylla saponin as raw material; dissolving in the high-boiling point organic solvent; aerating oxygen to do oxide alkaline degradation; adsorbing product through column chromatography to obtain protopanaxadiol, protopanaxatriol and Octoid.
Owner:王丹
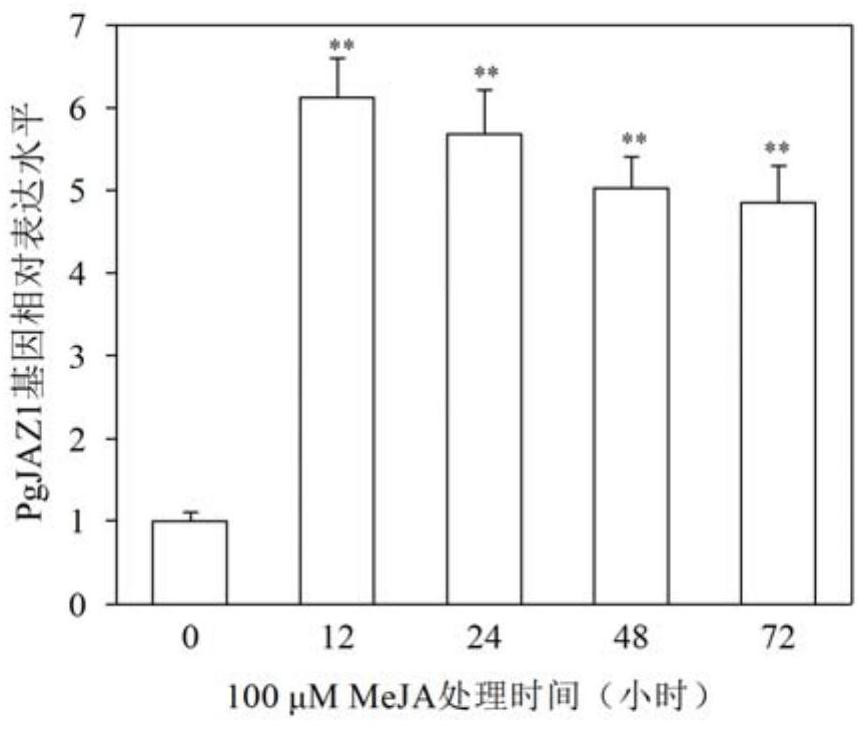
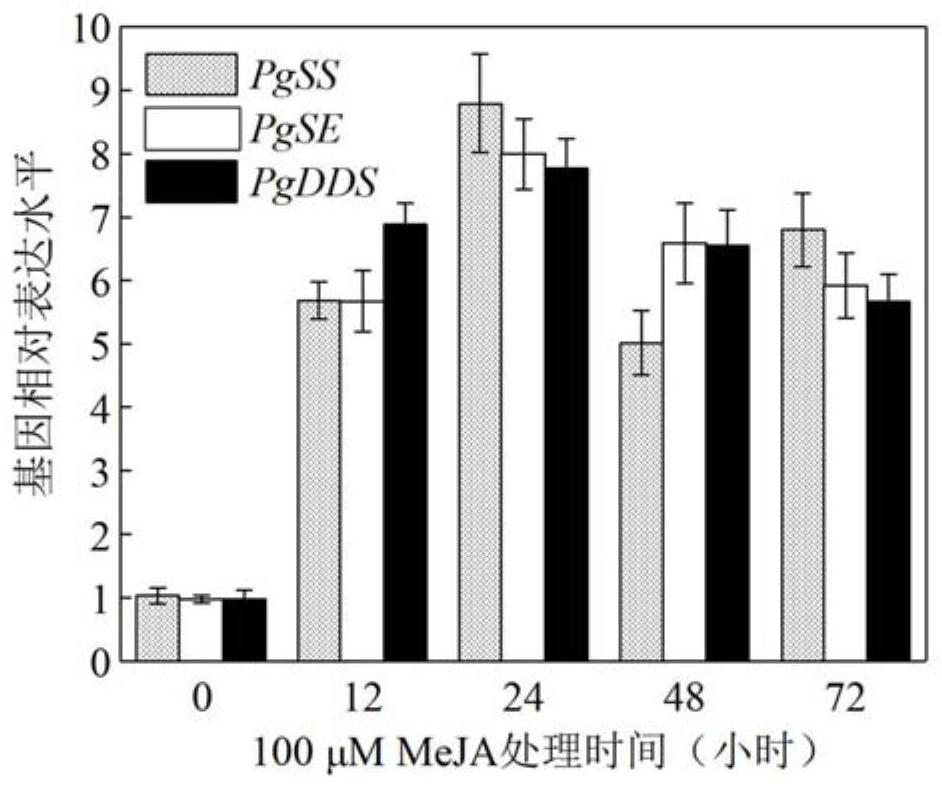
Ginseng PgJAZ1 gene, method for improving protopanaxatriol saponin based on gene and application
The invention discloses a ginseng PgJAZ1 gene, a method for improving protopanaxatriol saponin based on the gene and application of the ginseng PgJAZ1 gene. The gene is derived from Panax ginseng C.A.Meyer and is named as PgJAZ1, and a protein coded by the gene has a conservative TIFY structural domain and a jas structural domain which are specific to the JAZ family. The constructed PgJAZ1 gene RNA interference vector is used for transforming a ginseng root, and a PgJAZ1 gene silenced ginseng hairy root is obtained. Compared with a control ginseng hairy root, the content of protopanaxatriol saponin and the content of total saponin in the PgJAZ1 gene silenced ginseng hairy root are remarkably increased. Researches show that the PgJAZ1 gene is utilized to regulate the content of endogenous jasmonic acid methyl ester in ginseng hairy roots so as to regulate the biosynthesis of protopanaxatriol type saponins and total saponins. The invention has important application value in the aspects of increasing the yield of the protopanaxatriol saponin and improving the quality of the ginseng by utilizing the PgJAZ1 gene in the ginseng.
Owner:HUNAN INSTITUTE OF ENGINEERING
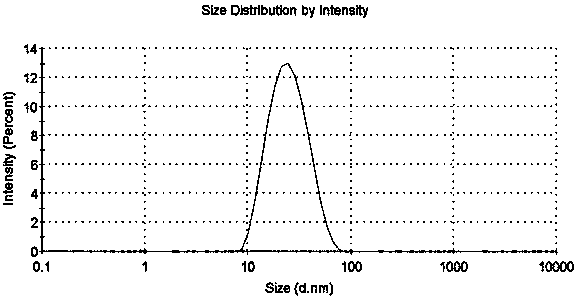
Panaxatriol supersaturated self-microemulsion and preparation method thereof
ActiveCN110623926AImprove bioavailabilityEasy to carryOrganic active ingredientsEmulsion deliveryCelluloseOil phase
The invention belongs to the technical field of medicines, and relates to a panaxatriol supersaturated self-microemulsion and a preparation method thereof. The panaxatriol supersaturated self-microemulsion consists of panaxatriol and a supersaturated self-microemulsion system, wherein the supersaturated self-microemulsion system is composed of an oil phase, an emulsifier, a co-emulsifier and polymers. The panaxatriol supersaturated self-microemulsion specifically consists of the following ingredients in the following percent weight in volume: 0.1-10% of the panaxatriol, 10-50% of the oil phase, 30-70% of the emulsifier, 10-30% of the co-emulsifier, and 0.5-10% of water-soluble cellulose. Compared with conventional self-microemulsions, the panaxatriol supersaturated self-microemulsion is capable of effectively delaying drug precipitation so as to improve emulsion stability; and moreover, the panaxatriol supersaturated self-microemulsion prepared by the preparation method is capable of effectively improving bioavailability of the panaxatriol in vivo. Pharmacokinetic experiments have proven that the bioavailability of the panaxatriol supersaturated self-microemulsion is improved by 2.7 times compared with bulk drugs.
Owner:SHENYANG PHARMA UNIVERSITY
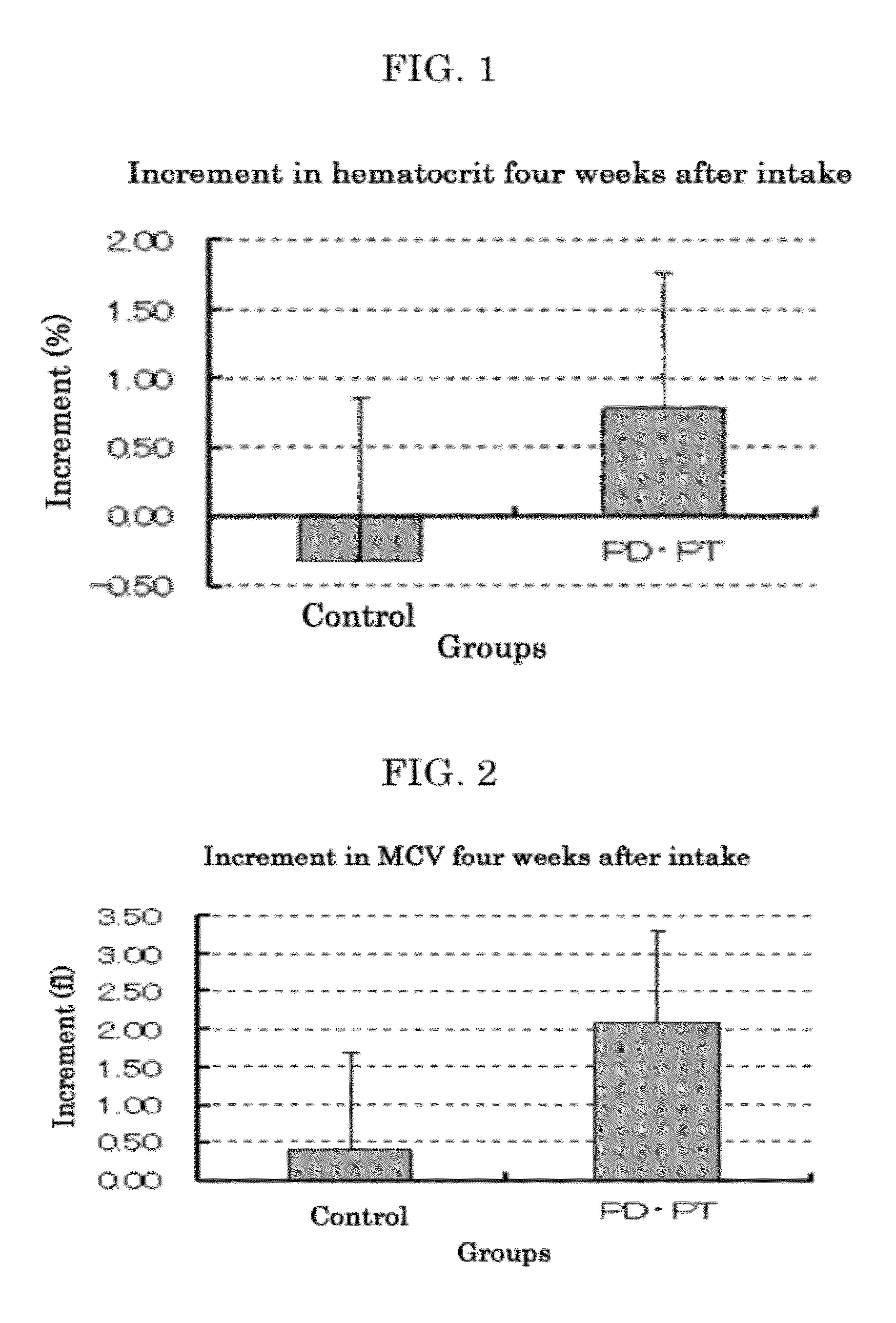
Hyperlipemia-ameliorating agent, anemia-ameliorating composition, uric-acid-level-reducing composition, and food or beverage
InactiveUS20120258184A1Good effectImprove securityOrganic active ingredientsBiocideProtopanaxadiolEnvironmental health
A neutral-fat-level-regulating agent, a cholesterol-level-regulating agent, a free-fatty-acid-level-regulating agent or an anti-obesity agent, including: at least one of protopanaxatriol, panaxatriol, protopanaxadiol and panaxadiol which are aglycons of dammarane-type saponins; and a hyperlipemia-ameliorating agent including: at least one of the neutral-fat-level-regulating agent, the cholesterol-level-regulating agent, the free-fatty-acid-level-regulating agent and the anti-obesity agent.
Owner:LION CORP
Method for large-scale production of rare ginsenoside from enzyme-catalysis panaxatriol ginsenoside
The invention discloses a novel process for producing rare ginsenoside from panaxatriol ginsenoside in ionic liquid through enzyme-catalysis directional transformation and for separating and purifying the rare ginsenoside. Specifically, the method comprises the following steps: dissolving panaxatriol ginsenoside in the ionic liquid and adding the ionic liquid dissolved with the panaxatriol ginsenoside to a reaction kettle, and then adding a complex enzyme preparation in a quantitative mode; conducting the directional transformation under corresponding conditions, so that a great amount of rare ginsenoside is obtained; and collecting a finished product and purifying the product, so that various varieties of rare ginsenoside which are higher than 95% in purity are obtained. The process is high in yield of the rare ginsenoside, environment-friendly, relatively simple in downstream purifying process and conducive to large-scale production.
Owner:NORTHWEST UNIV
Protopanaxatriol composition and application thereof to preparation of Parkinson resisting drugs
InactiveCN102631388AGood curative effectSmall toxicityOrganic active ingredientsNervous disorderProtopanaxatriolPharmaceutical drug
The invention discloses a panaxatriol saponins composition and application thereof to preparation of drugs for strengthening immunity. The panaxatriol saponins composition at least comprises 5-60% of ginsenoside-Re and 5-60% of ginsenoside-Rg1 by weight percent. The panaxatriol saponins composition has stronger activity than the single ginsenoside in resisting Parkinson's diseases and has high bioavailability, simple preparation method and low price.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
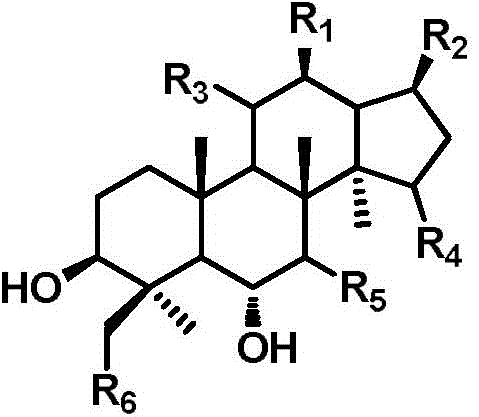
Protopanaxatriol derivatives and their preparation methods and applications
The invention discloses a protopanaxatriol derivative as well as a preparation method and application thereof. The structural formula of the protopanaxatriol derivative includes a formula I, a formula II, a formula III, a formula IV and a formula V. According to the invention, protopanaxatriol is successfully subjected to structure modification by utilizing a microbial transformation technology so as to obtain multiple novel compounds, and an in-vitro antitumor cell test proves that the compounds have preferable antitumor activity, can serve as active components of an antitumor drug and have wide application.
Owner:山东亚泰医疗器械有限公司
Method for preparing protopanoxadiol and protopanaxatriol by using synergistic oxidation and alkaline bydrolysis of oxide and hyperoxide
This invention relates to a method for preparing protopanaxadiol and protopanaxatriol by co-oxidizing with oxygen and peroxide and alkali-hydrolyzing total notoginsenoside. The method comprises: (1) oxidizing and alkali-hydrolyzing Panax ginseng total saponin extract and Gynostemma pentaphyllum total saponin extract by applying cooperative effect of oxygen and peroxide, and alkali-hydrolyzing using alkoxide in situ formed in alcohol at 80-95 swg..C for 8-24 h, where the Panax ginseng total saponin extract and Gynostemma pentaphyllum total saponin extract / alcohol ratio is 30-150 g / L; (2) purifying by column chromatography to obtain protopanaxadiol and protopanaxatriol. The method adopts cooperative effect of oxygen and peroxide, thus can obtain high-purity protopanaxadiol and protopanaxatriol with high yields at low reaction temperatures and short reaction time. This invention provides a simple, convenient and low-cost method for preparing protopanaxadiol and protopanaxatriol in large amounts, which can satisfy the needs of drugs for treating cardiovascular and cerebrovascular diseases and tumor.
Owner:中山以诺生物科技有限公司
Pseudo-ginseng triol saponin enteric pellet and capsule and preparation method thereof
ActiveCN102379885BImprove playbackEvenly wrappedOrganic active ingredientsPharmaceutical product form changeMedicineSilica gel
Owner:CHENGDU HUASUN GRP INC LTD
Process for preparing protopanoxadiol and protopanaxatriol
Owner:怡瑞达(上海)医药科技有限公司
Method for synthesizing panaxytriol
ActiveCN102391074AFew reaction stepsMild reaction conditionsOxygen-containing compound preparationOrganic compound preparationAlcoholPanaxytriol
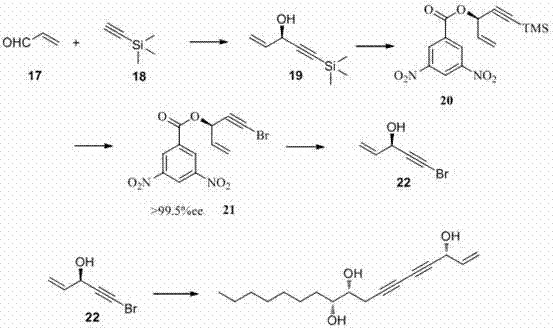
The invention relates to a stereoselectivity complete synthesis method of panaxytriol. In the method, 5-bromine-1-pentene-4-alkyne-3-alcohol is taken as an intermediate, and a Cadiot-Chodkiewcz alkyne-alkyne coupling reaction is carried out on the intermediate and (4R, 5R)-1-dodecyne-4,5-glycol to synthesize the target product panaxytriol. The method provided by the invention has the advantages of simple and available reaction raw materials, short synthesis route, mild reaction conditions and high reaction overall yield.
Owner:PAPANNA BEIJING TECH
Panaxoside composition as well as preparation method and application thereof
InactiveCN104116746AAvoid toxicity of intolerable high-dose chemotherapy drugsAvoid therapeuticOrganic active ingredientsAntineoplastic agentsEpoxyClinical efficacy
The invention discloses a panaxoside composition as well as a preparation method and application thereof. The panaxoside composition comprises the following components in percentage by weight: 10-80 percent of 20(S)-protopanoxadiol, 1-20 percent of 25-alkene-20(S)-protopanoxadiol, 5-50 percent of 20(S) and 24(R)-panaxatriol epoxy compounds, 5-50 percent of 20(S)-panaxatriol, 1-20 percent of 20(S)-24-methyl-23-alkene-24-carbonyl-protopanoxadiol and 1-20 percent of 25-hydroxy-23-alkene-20(S)-protopanoxadiol. The panaxoside composition disclosed by the invention has the beneficial effects that the panaxoside composition has remarkable treatment effects of directly inhibiting and killing cancers, reversing the multidrug resistance of cancer cells and enhancing the treatment effects of a cancer chemotherapeutic medicine, is definite in clinical effect and capable of preventing the defects that no other treatment methods are available after drug discontinuance and drug-resisting relapse because lots of cancer patients cannot tolerate the toxicity of the chemotherapeutic medicine, and meeting the needs of clinical application.
Owner:祁展楷
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com