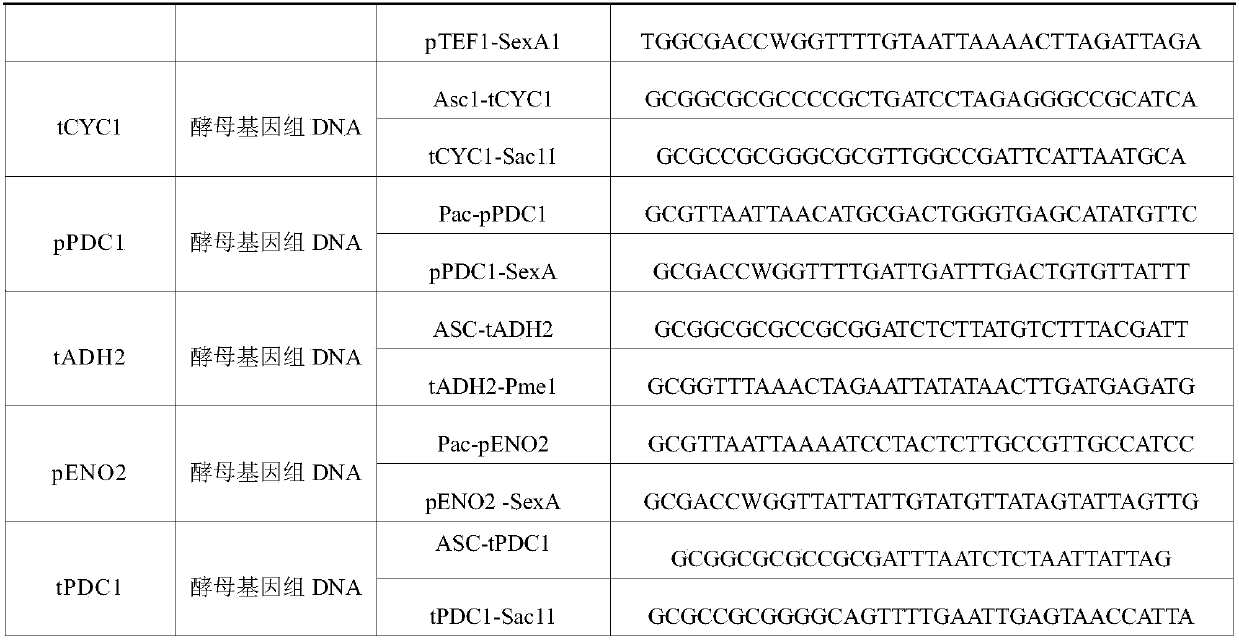
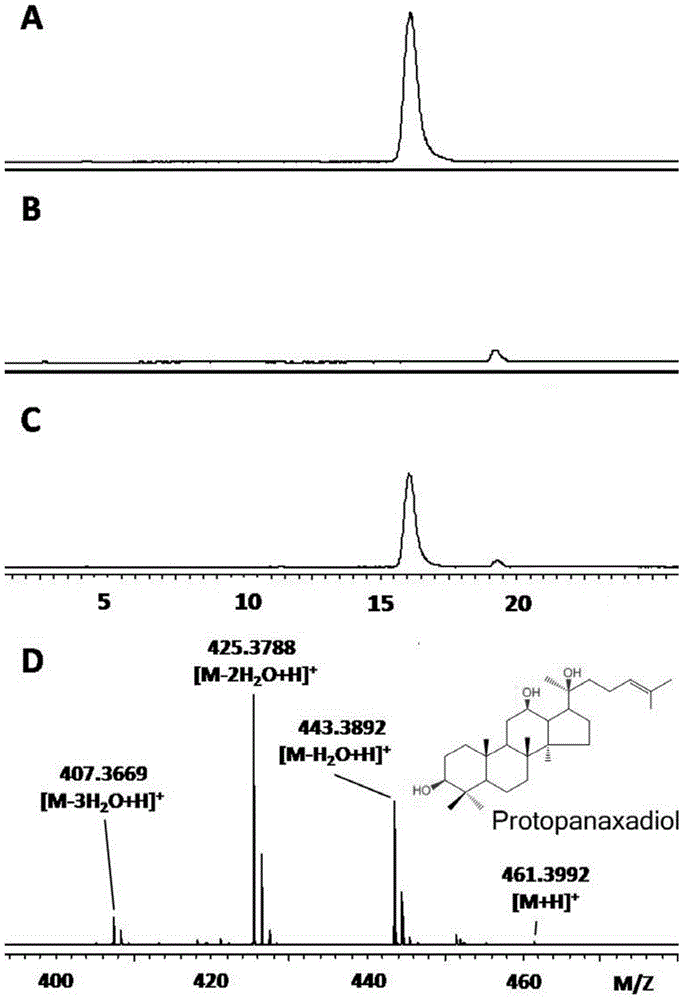
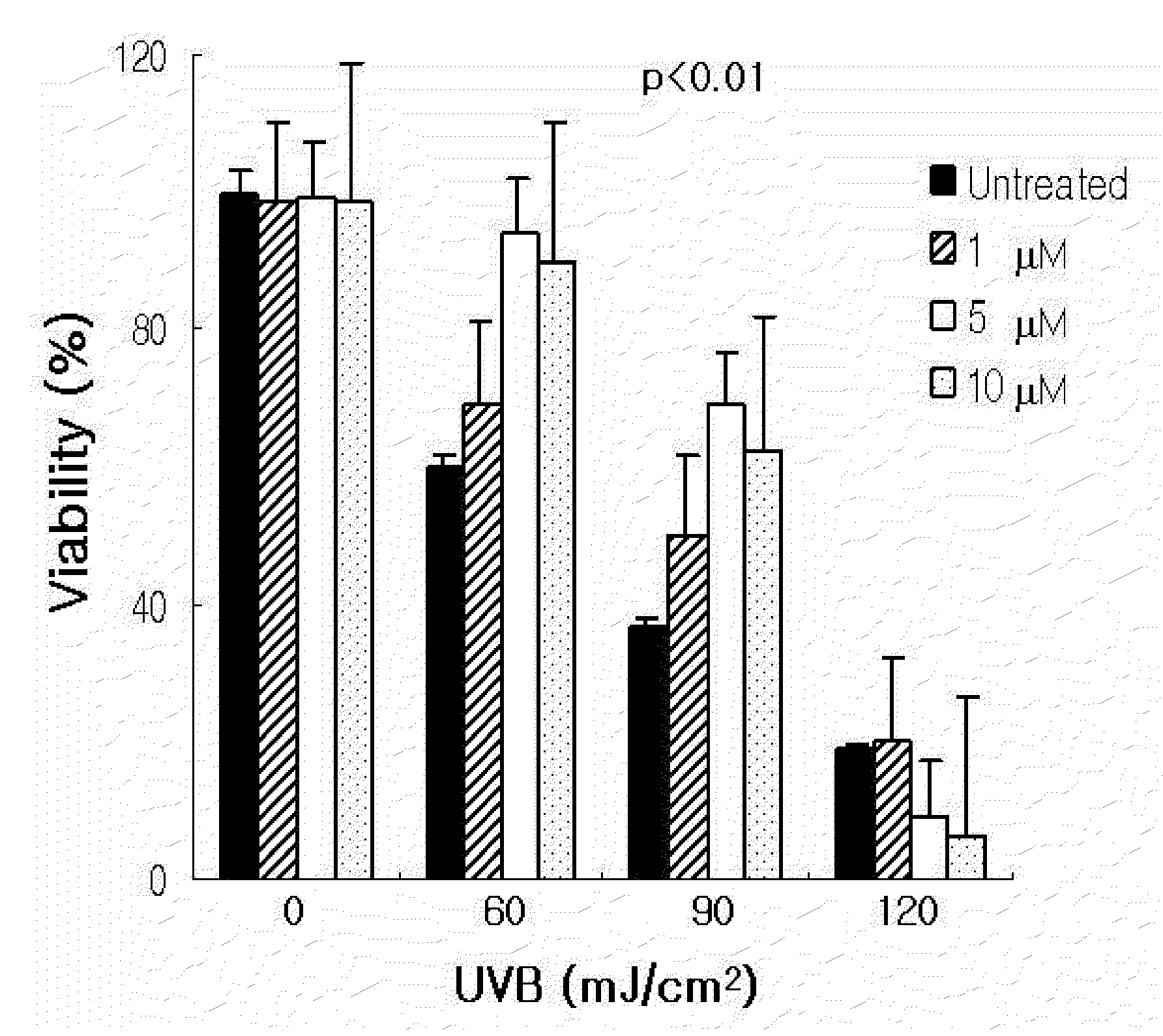
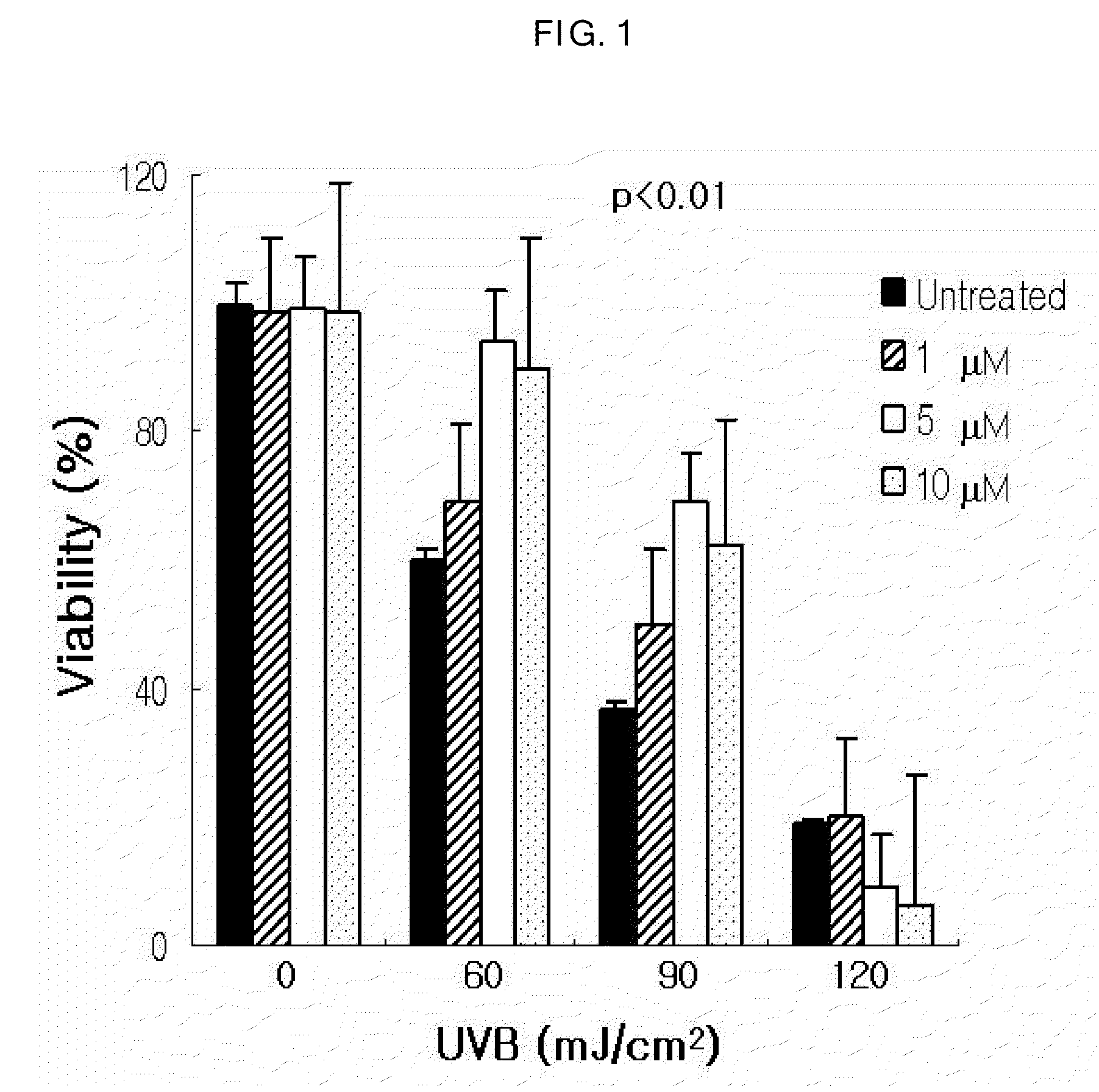
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
88 results about "Protopanaxatriol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
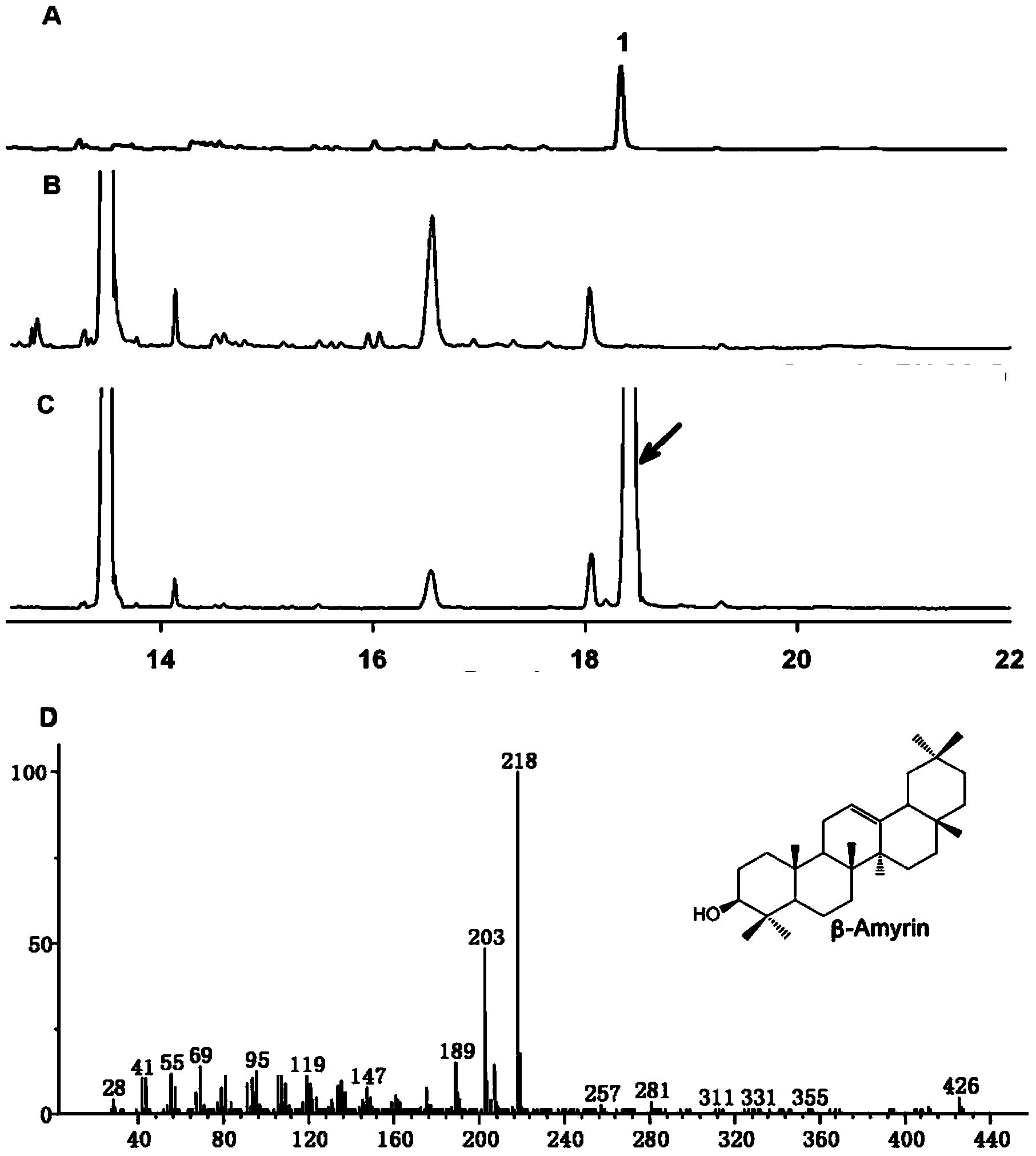
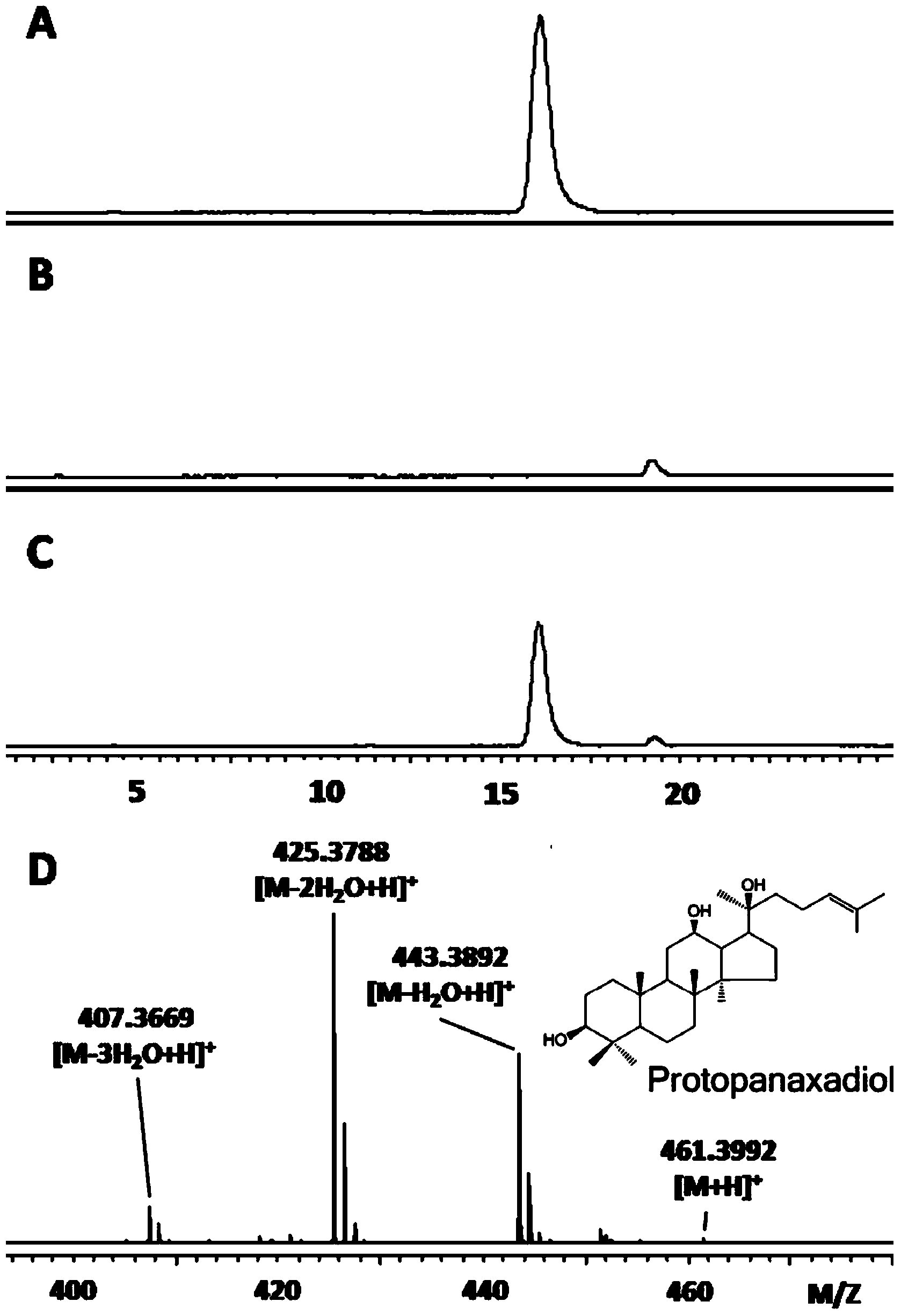
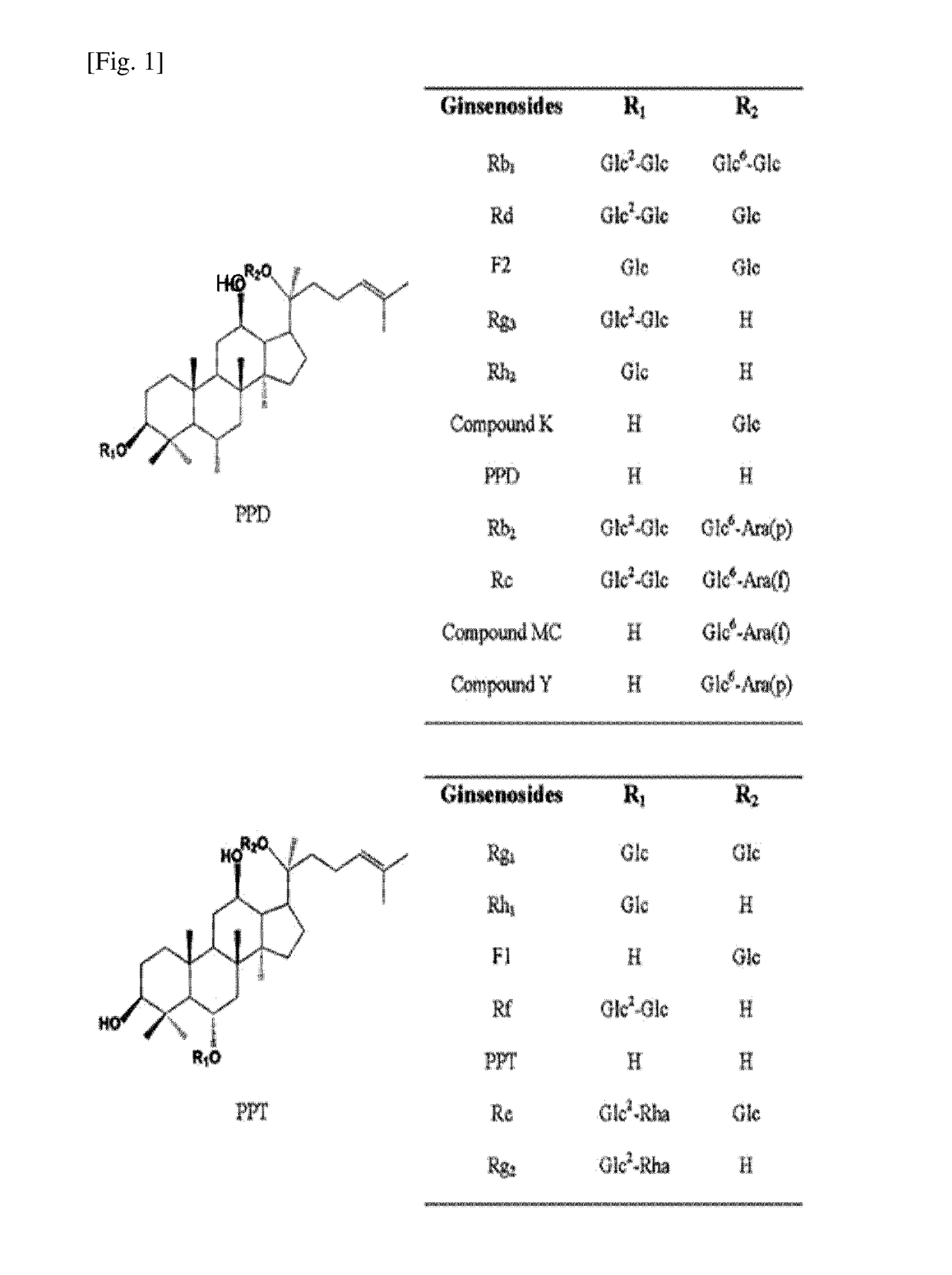
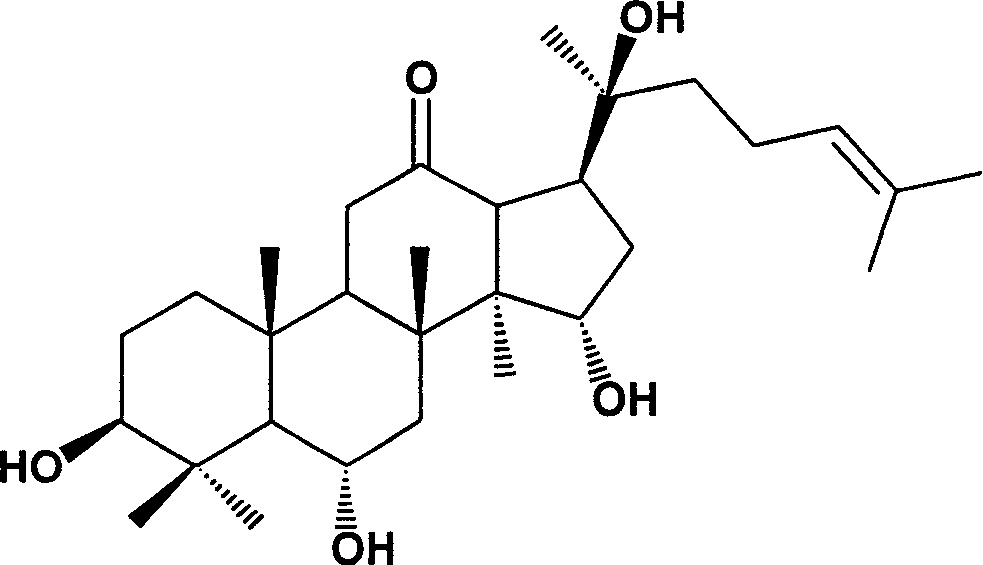
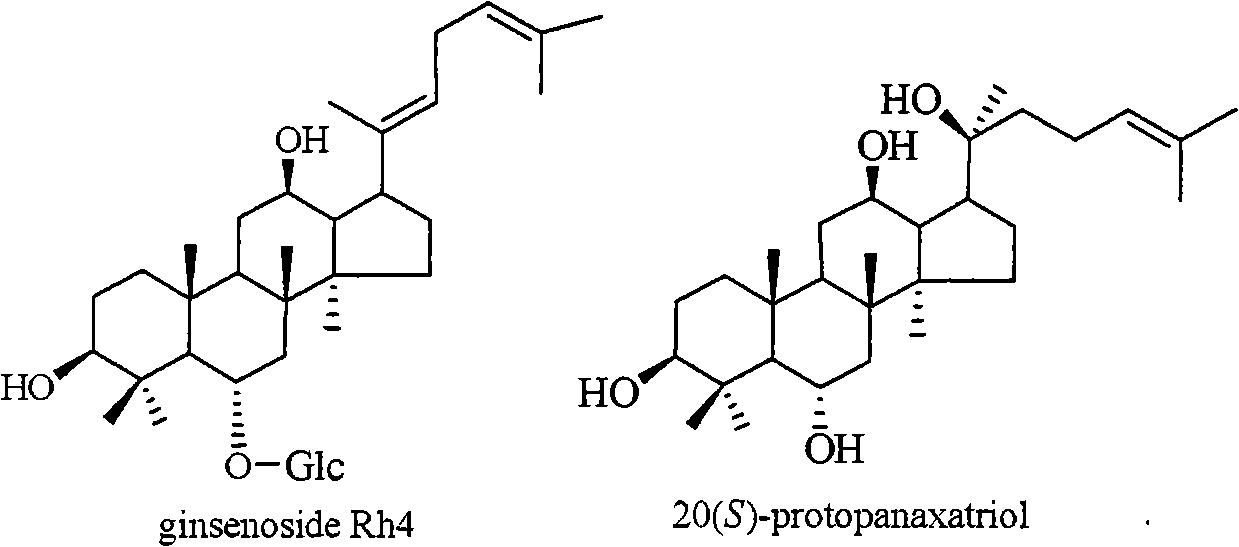
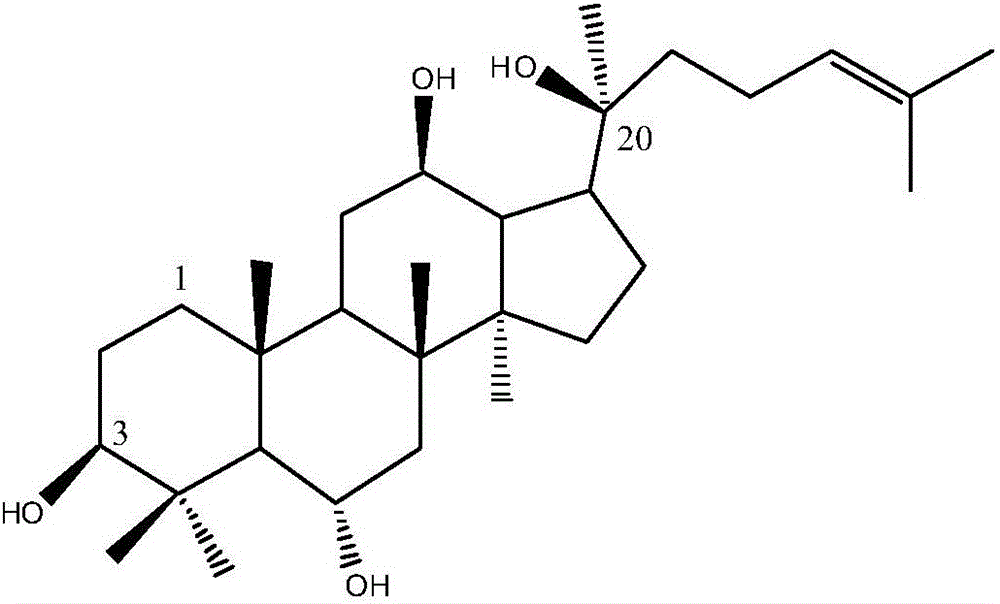
Protopanaxatriol (PPT) is an organic compound characterizing a group of ginsenosides. It is a dammarane-type tetracyclic triterpene sapogenins found in ginseng (Panax ginseng) and in notoginseng (Panax pseudoginseng).
Process for preparing protopanoxadiol and protopanaxatriol
ActiveCN1569882AThere is no problem of instability, easy destruction, and easy cyclizationHigh yieldSteroidsOrganic solventProtopanaxadiol
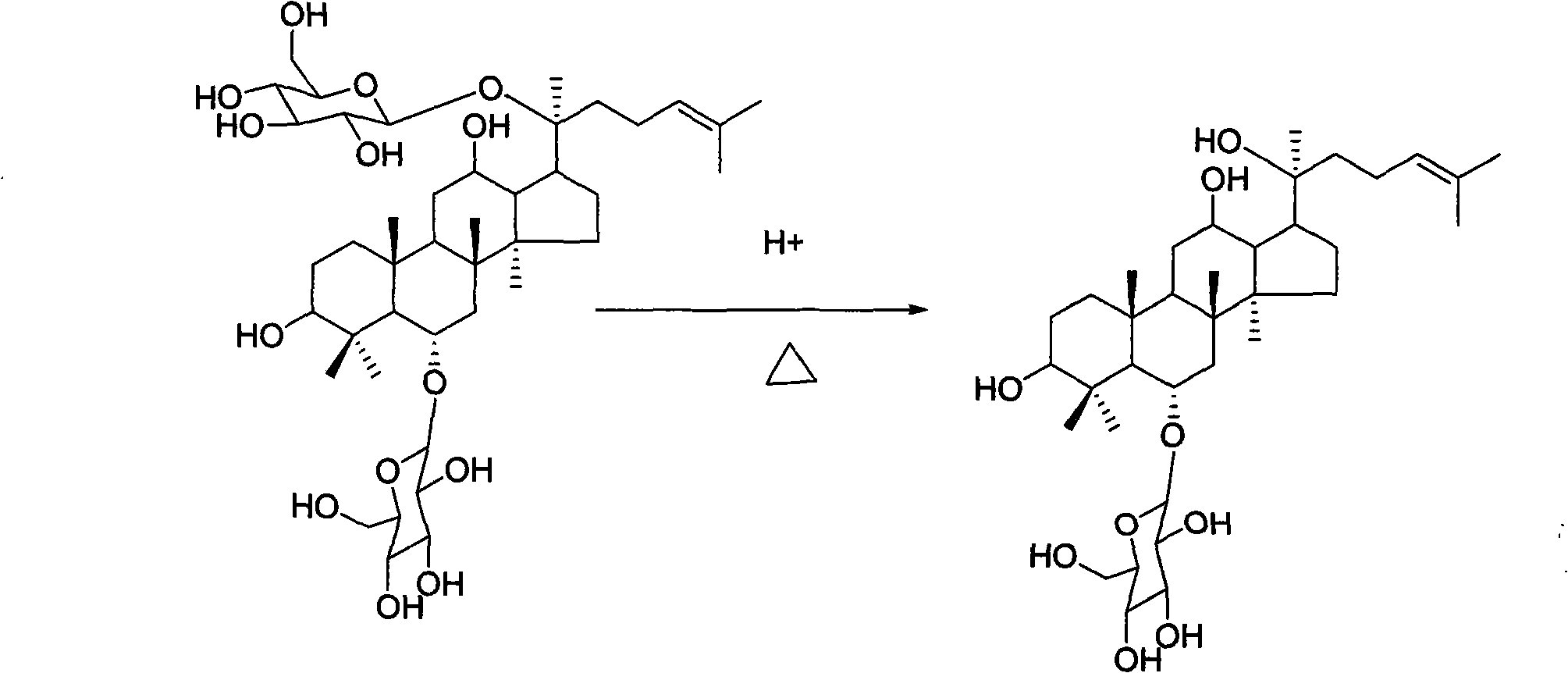
The invention relates to the process for preparing protopanoxadiol and protopanaxatriol by using general saponin extract from panax ginseng plant leaves and gynostemma pentaphylla extractive as raw material for alkaline hydrolysis reaction in organic solvent through column chromatography and purification.
Owner:怡瑞达(上海)医药科技有限公司
Medical application of protopanaxatriol and protopanaxadiol in nervous system diseases
The invention relates to medical application of protopanaxa-triol (PPT), protopanaxadiol (PPD) and mixtures of the PPT and the PPD in any proportion in nervous system diseases, which are preferably used for treating and / or preventing epilepsy, cerebral ischemic diseases, memory disorder and parkinsonism syndrome.
Owner:王泽君
Recombinant saccharymyces cerevisiae for producing ginsengenins as well as construction method and application of same
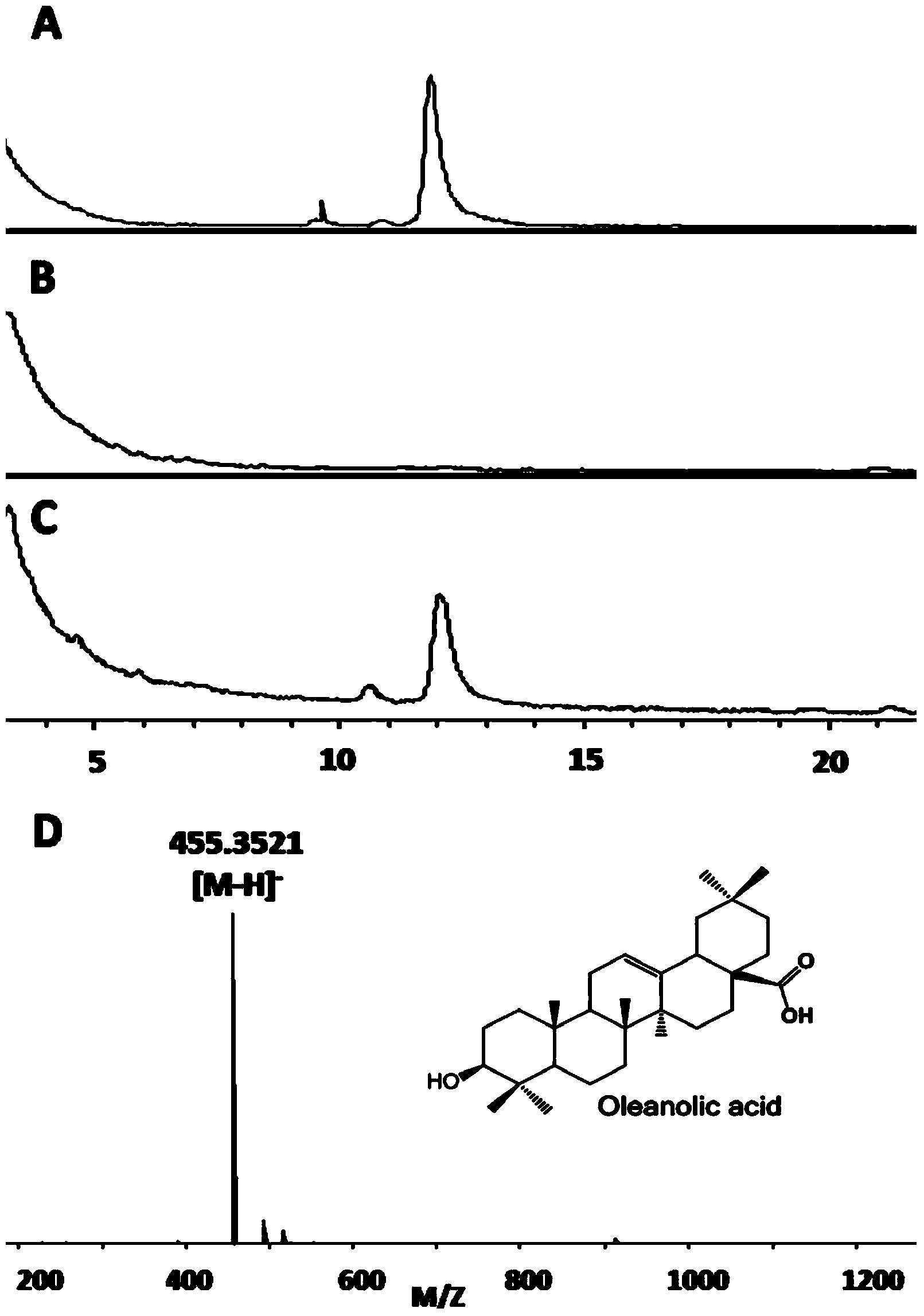
The invention discloses a recombinant saccharymyces cerevisiae for producing ginsengenins as well as a construction method and an application of the same. The recombinant saccharymyces cerevisiae capable of producing three ginsengenins of oleanolic acid, protopanoxadiol and protopanaxatriol simultaneously is Saccharymyces cerevisiae GY-1, with a collection number of CGMCC No. 7725 in the China General Microbiological Culture Collection Centre. The saccharymyces cerevisiae GY-1 is capable of producing three ginsengenins of oleanolic acid, protopanoxadiol and protopanaxatriol simultaneously, wherein the yields achieve 21.4 mg / L in fermentation broth, 17.2 mg / L in fermentation broth and 15.9 mg / L in fermentation broth respectively, the proportions of which in the total ginsengenin content are 39.3%, 31.5% and 29.2% respectively.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Novel catalytic system for preparing rare ginsenosides and application thereof
ActiveCN105087739AHigh catalytic efficiencyReduce use costFungiBacteriaSucrose synthetaseProtopanaxadiol
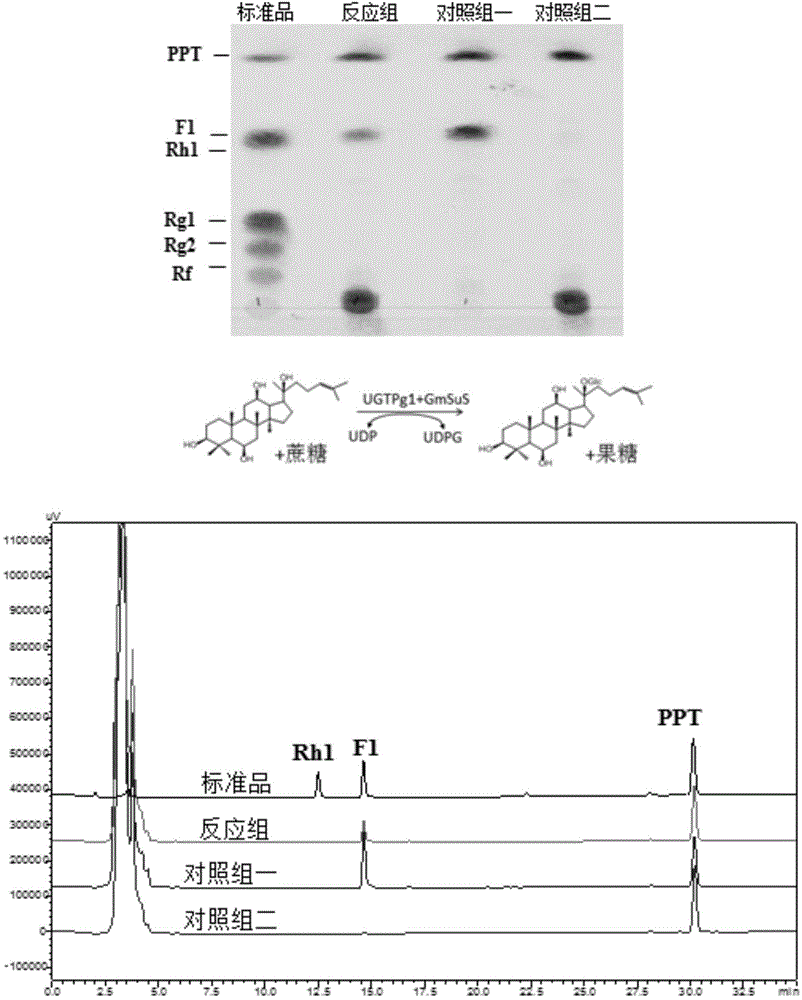
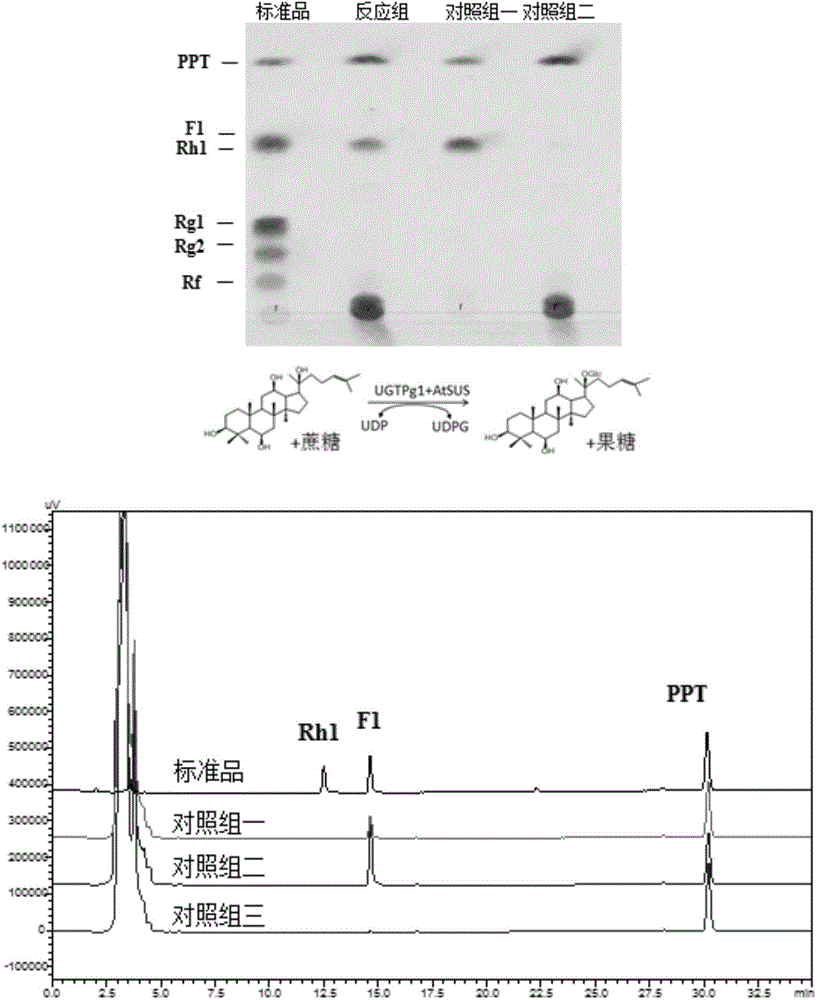
The invention discloses a co-catalytic reaction system for preparing rare ginsenosides. The co-catalytic reaction system comprises glycosyltransferase GT (a); sucrose synthase SUS (b); uridine diphosphate UDP (c); and saccharose (d). Experiments show that in the presence of the saccharose and little UDP, an enzyme combination composed of the glycosyltransferase and the saccharose is capable of replacing an in-vitro reaction system to synthesize UDP sugar, one of expensive materials for the rare ginsenosides, and efficiently and economically converting substrates such as protopanoxadiol or protopanaxatriol into the rare ginsenosides, wherein the UDP is about 1 / 4 of the UDP sugar in price and is 1% of its original dosage; thus, preparation cost of the rare ginsenosides is greatly saved, and large-scale commercial preparation of the ginsenosides is better facilitated.
Owner:SYNBIOTECH (SUZHOU) CO LTD
Applications of glycosyltransferase and related materials thereof in construction of engineering bacteria for producing ginsenoside Rb1 and Rg1
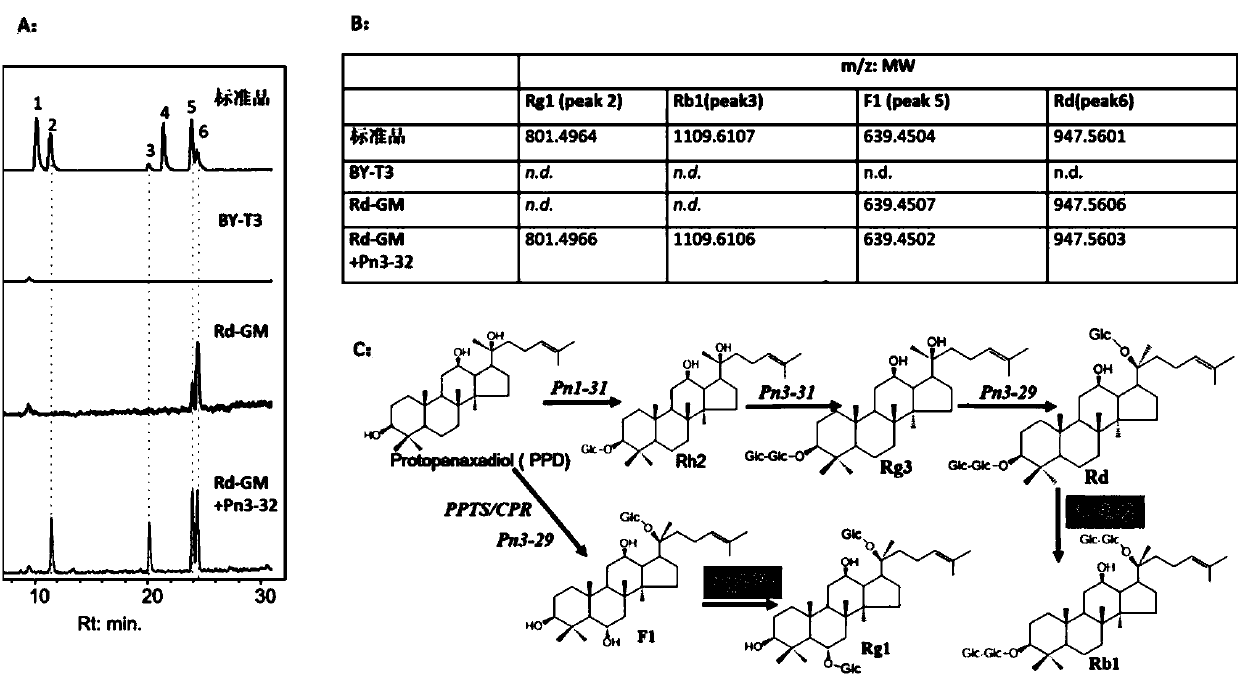
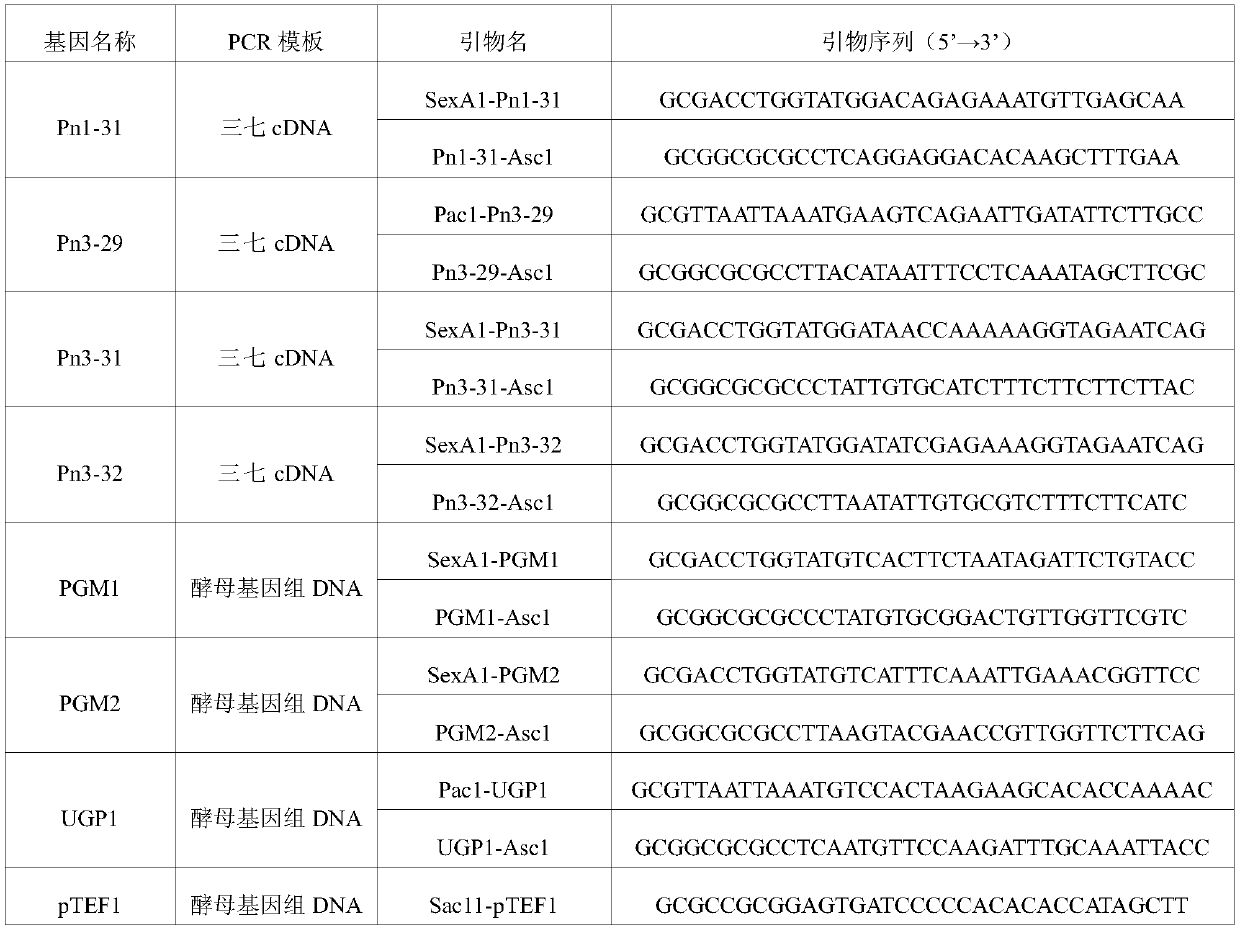
The invention discloses applications of glycosyltransferase and related materials thereof in the construction of engineering bacteria for producing ginsenoside Rb1 and Rg1. A glycosyltransferase genePn3-32 which can catalyze ginsenoside Rd to generate ginsenoside Rb1 can be successfully identified through a synthetic biological method; and the gene can simultaneously catalyze ginsenoside F1 to generate ginsenoside Rg1 and construct recombinant yeast producing the ginsenoside Rb1 and the ginsenoside Rg1. Through experiments, the constructed recombinant yeast producing the ginsenoside Rb1 and the ginsenoside Rg1 can simultaneously generate the ginsenoside Rb1 and the ginsenoside Rg1. Pn1-31, Pn-3-29, Pn3-31 and Pn3-32 glycosyltransferase genes in medicinal plant radix notoginseng are firstly utilized to continuously catalyze protopanaxadiol and protopanaxatriol to synthetize the ginsenoside Rb1, the ginsenoside Rg1 and corresponding intermediates, so that novel cases can be provided formicrobial strains to produce natural products.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Method for preparing protopanoxadiol and protopanaxatriol by using synergistic oxidation and alkaline bydrolysis of oxide and hyperoxide
This invention relates to a method for preparing protopanaxadiol and protopanaxatriol by co-oxidizing with oxygen and peroxide and alkali-hydrolyzing total notoginsenoside. The method comprises: (1) oxidizing and alkali-hydrolyzing Panax ginseng total saponin extract and Gynostemma pentaphyllum total saponin extract by applying cooperative effect of oxygen and peroxide, and alkali-hydrolyzing using alkoxide in situ formed in alcohol at 80-95 swg..C for 8-24 h, where the Panax ginseng total saponin extract and Gynostemma pentaphyllum total saponin extract / alcohol ratio is 30-150 g / L; (2) purifying by column chromatography to obtain protopanaxadiol and protopanaxatriol. The method adopts cooperative effect of oxygen and peroxide, thus can obtain high-purity protopanaxadiol and protopanaxatriol with high yields at low reaction temperatures and short reaction time. This invention provides a simple, convenient and low-cost method for preparing protopanaxadiol and protopanaxatriol in large amounts, which can satisfy the needs of drugs for treating cardiovascular and cerebrovascular diseases and tumor.
Owner:中山以诺生物科技有限公司
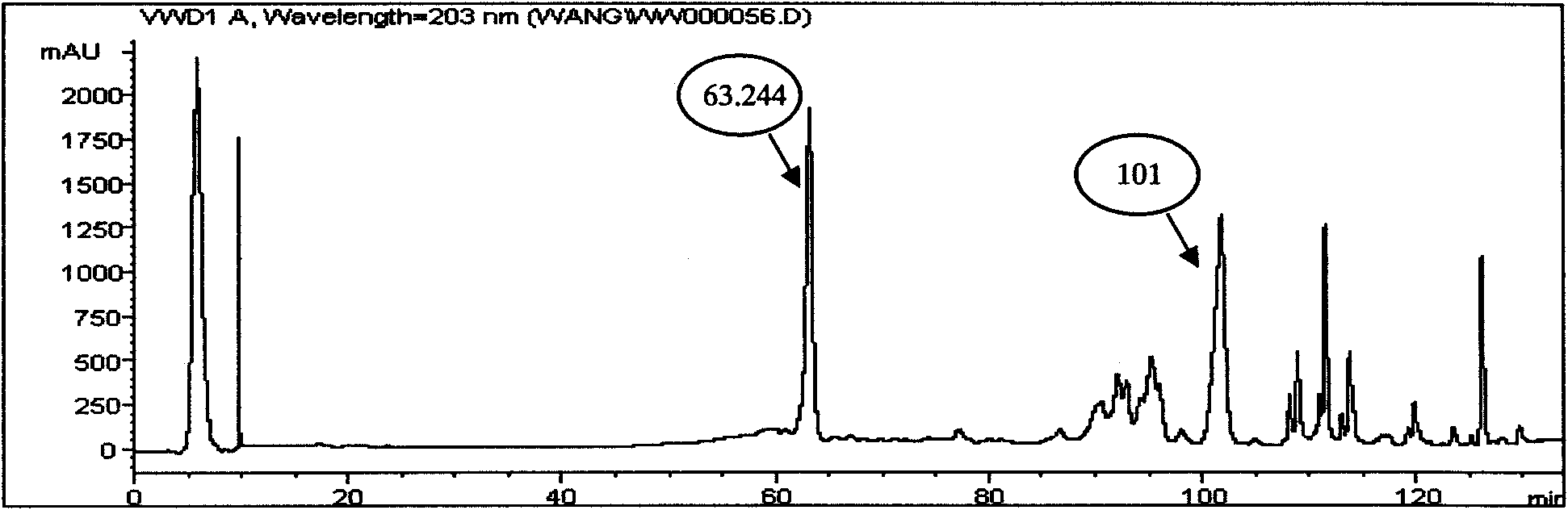
High performance liquid chromatography (HPLC) method for simultaneously determining 16 ginseng saponin monomers
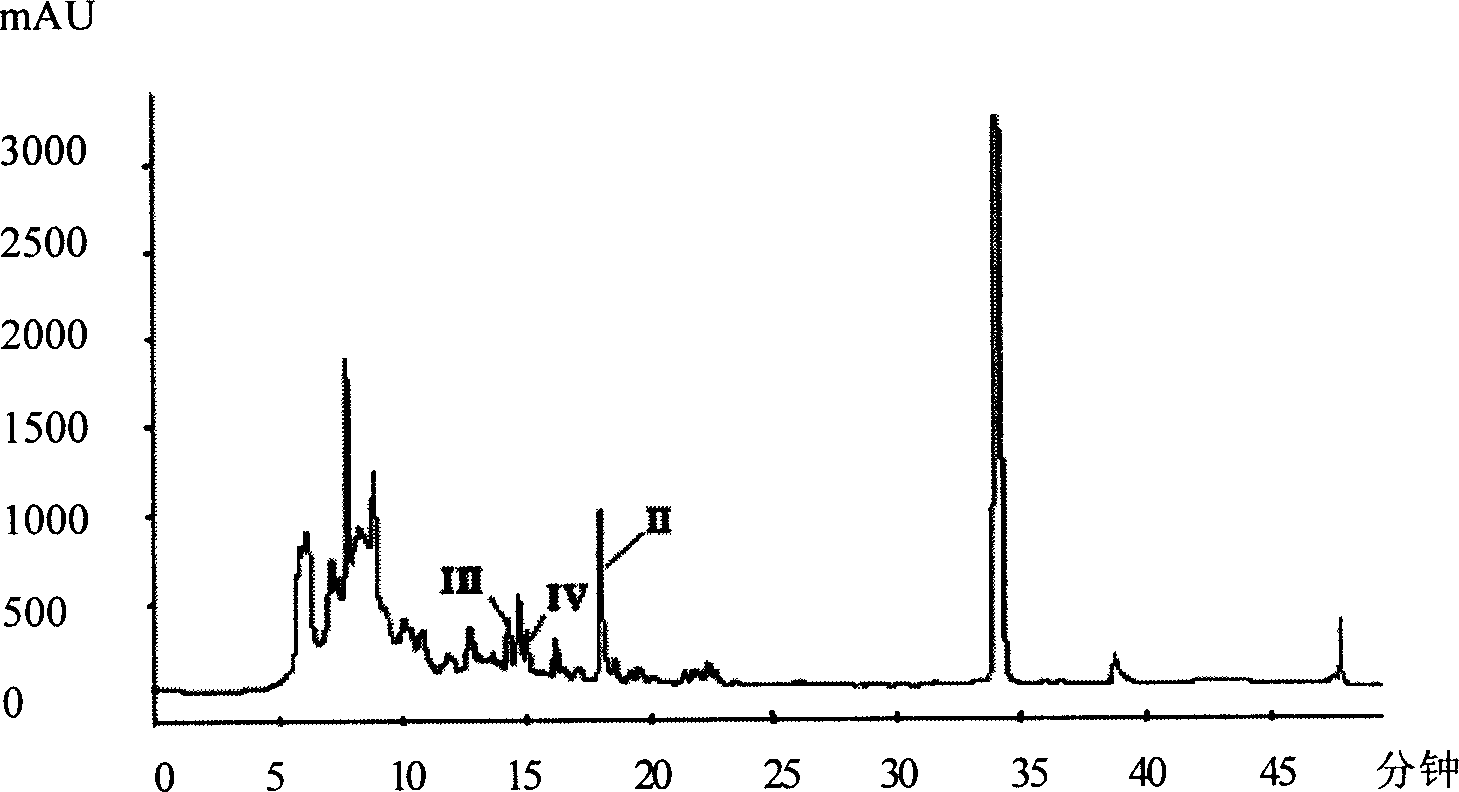
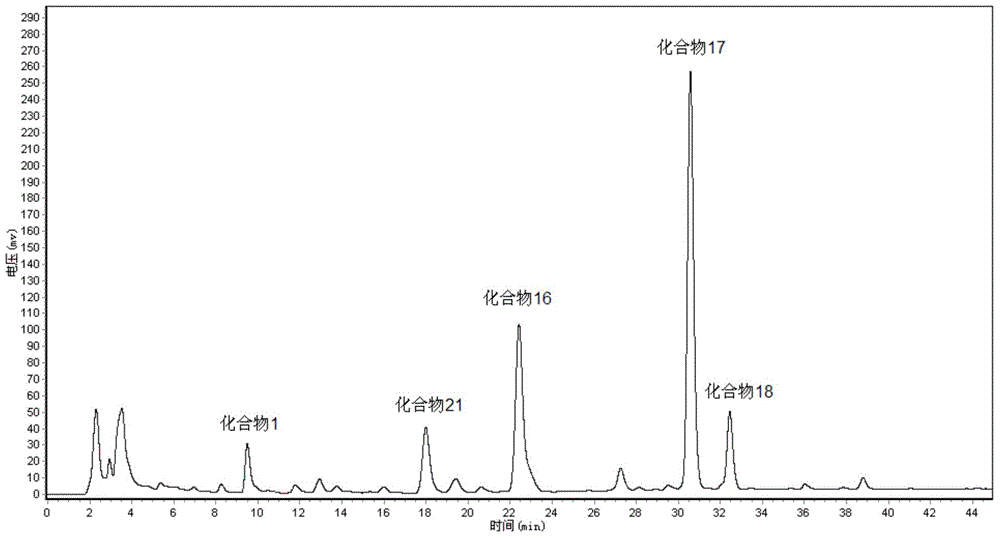
The invention provides a high-performance liquid chromatography (HPLC) method for simultaneously determining 16 ginseng saponin monomers, such as Rg1, Re, Rf, Rb1, Rg2, Rc, Rb2, Rb3, F1, Rd, F2, Rg3, protopanaxatriol, Compound K, Rh2 and protopanaxadiol. The determination range comprises medicinal plants such as ginseng and American ginseng containing more than one saponin monomer, and preparations thereof. Compared with the prior art, the HPLC method has the advantages that the variety of the ginseng saponin monomers simultaneously determined by the method can be up to 16, and the efficiency is improved. Only are acetonitrile and water used as mobile phases, so that the problems that phosphate is large in loss on a chromatographic column, the system is troublesome to clean and the like are solved. The built HPLC method is wide in linearity range, accurate in method, good in reproducibility, fast and convenient, stable and reliable. A theoretical basis is provided for quality control of the medicinal plants, such as ginseng and American ginseng, and the preparations thereof.
Owner:JILIN AGRICULTURAL UNIV
A novel glycosyltransferase derived from dolwoe and use thereof
Provided are a novel UDP-glycosyltransferase (uridine diphosphate glycosyltransferase) protein from Dolwoe (Gynostemma pentaphyllum) having glycosyltransfer activity for glucose linked by a glycosidic bond at the C-20 position of PPD (protopanaxadiol)-type or PPT (protopanaxatriol)-type ginsenoside, and use thereof.
Owner:KOREA ADVANCED INST OF SCI & TECH +1
Nanoemulsion comprising metabolites of ginseng saponin as an active component and a method for preparing the same, and a skin-care composition for anti-aging containing the same
ActiveUS20060216261A1Good skin permeabilityPromote collagen-biosynthesisCosmetic preparationsBiocideMetaboliteProtopanaxadiol
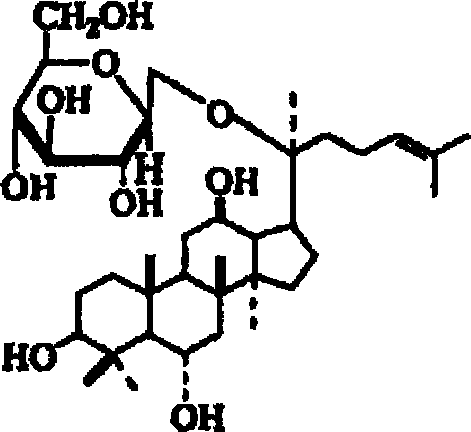
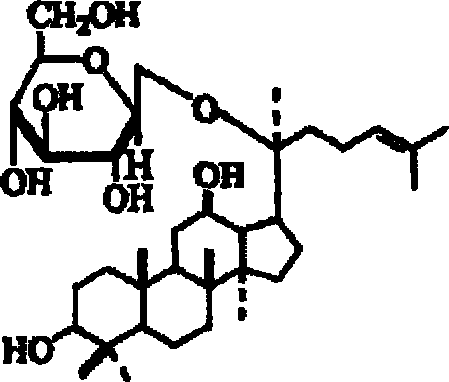
Disclosed herein is nanoemulsion prepared by emulsifying main metabolites of ginseng saponin obtained by conversion of glucose, i.e. compound K (20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol), ginsenoside F1 (20-O-β-D-glucopyranosyl-20(S)-protopanaxatriol) and compound Y (20-O-[α-L-arabinopyranosyl(1→6)-β-D-glucopyranosyl]-20(S)-protopanaxadiol); and admixture-thereof, in fine emulsion or liposome with dermotropic emulsifier by nano-emulsification; and having enhanced skin penetration, so to be effective in promoting proliferation of fibroblast and biosynthesis of collagen.
Owner:AMOREPACIFIC CORP
Method for preparing ginsenoside Rh1
ActiveCN101671384ALow hydrolysis temperatureEase of industrial productionSteroidsAcetic acidLymphatic Spread
The invention discloses a method for preparing ginsenoside Rh1. Although the acid-hydrolysis method is applied to the preparation of total saponins and preparation thereof from ginsenoside, the complex procedure is needed for deacidification after acid-hydrolysis and no reports indicate that the special method for preparing high-purity Rh1 exists. The invention provides a method for preparing ginsenoside Rh1, which is characterized by comprising the steps of adding glacial acetic acid in protopanaxtriolsaponins for hydrolysis, obtaining Icariside part enriched with ginsenoside Rh1 by chromatography deacidification of macroporous resin column, carrying out purification by reversed-phase medium-pressure column chromatography, and finally obtaining the ginsenoside Rh1 with the purity of morethan 90%. The method adopts the macroporous resin column for chromatography deacidification, ensures high metastasis rate of the total saponins, has moderate hydrolysis condition and short reaction period, effectively saves cost and energy resource, is easy for industrial production, thus ensuring the high yield of Rh1 and improving purity.
Owner:YUXI WINHEY BIO TECH
A novel method for glycosylation of ginsenoside using a glycosyltransferase derived from panax ginseng
The present invention relates to a uridine diphosphate (UDP)-glycosyltransferase protein which has glycosylation activity for a hydroxyl group at the C-20 position of a protopanaxadiol (PPD)- or protopanaxatriol (PPT)-type ginsenoside, and a method for glycosylation of UDP using the same.
Owner:KOREA ADVANCED INST OF SCI & TECH
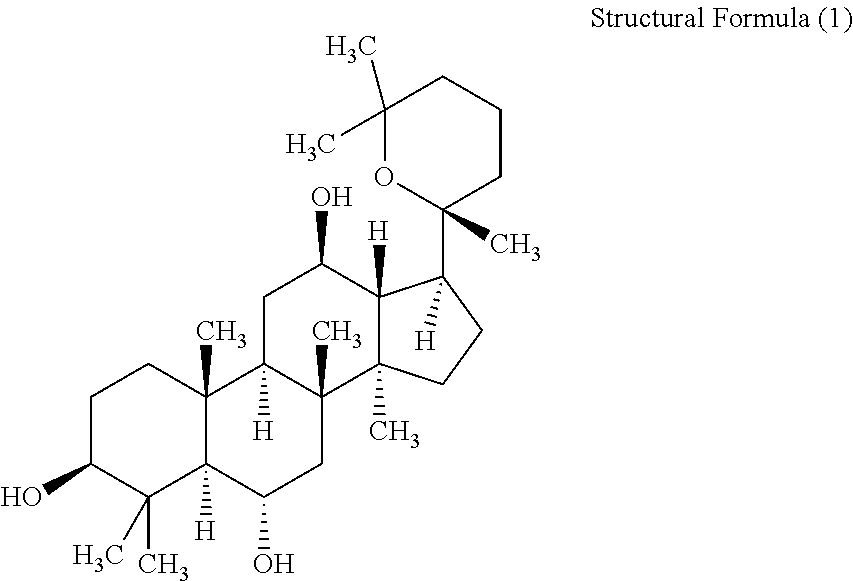
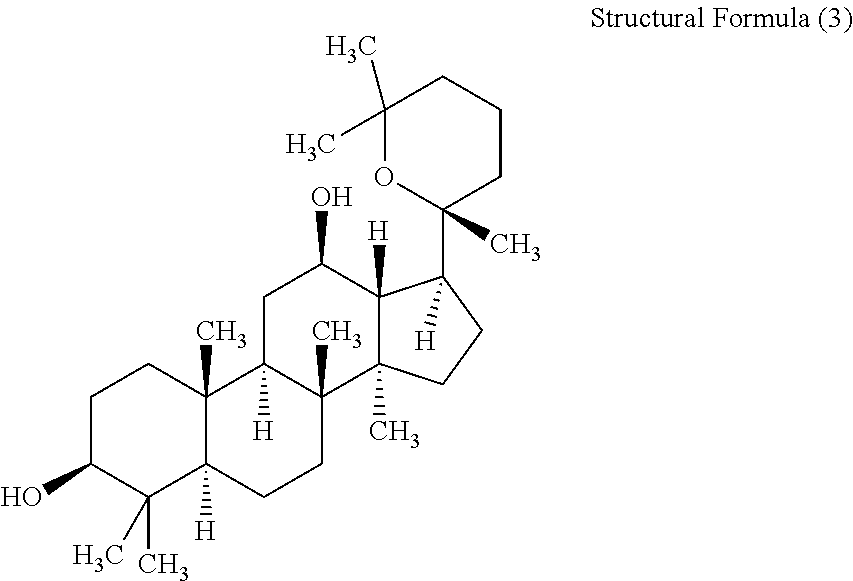
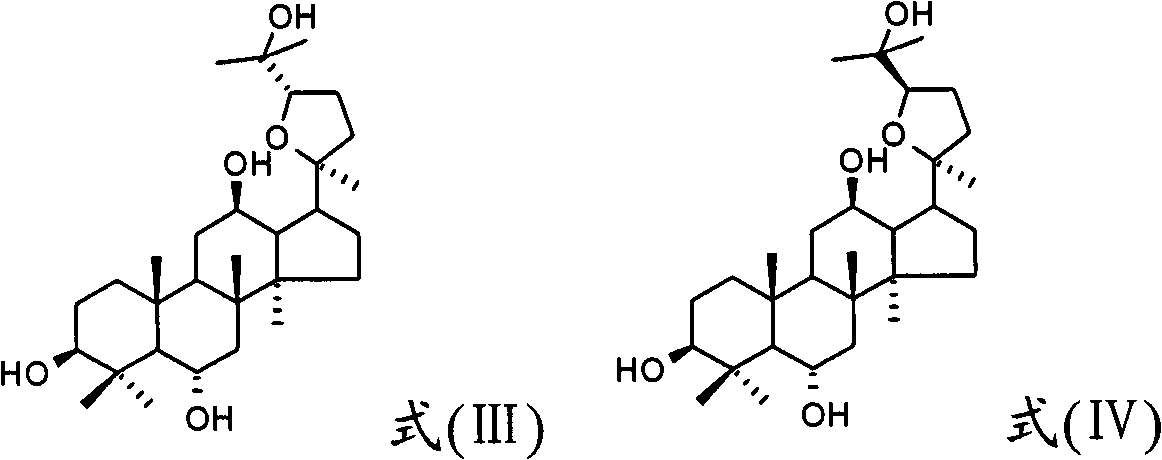
Derivative of protopanoxatriol, prepn. method and application thereof
InactiveCN1830994AGood antitumor activityWide range of usesOrganic active ingredientsSteroidsMicroorganismMicrobial transformation
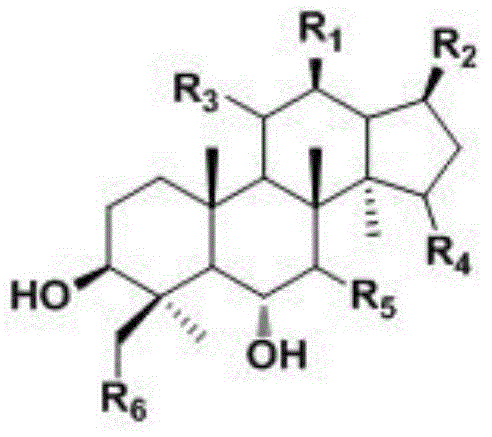
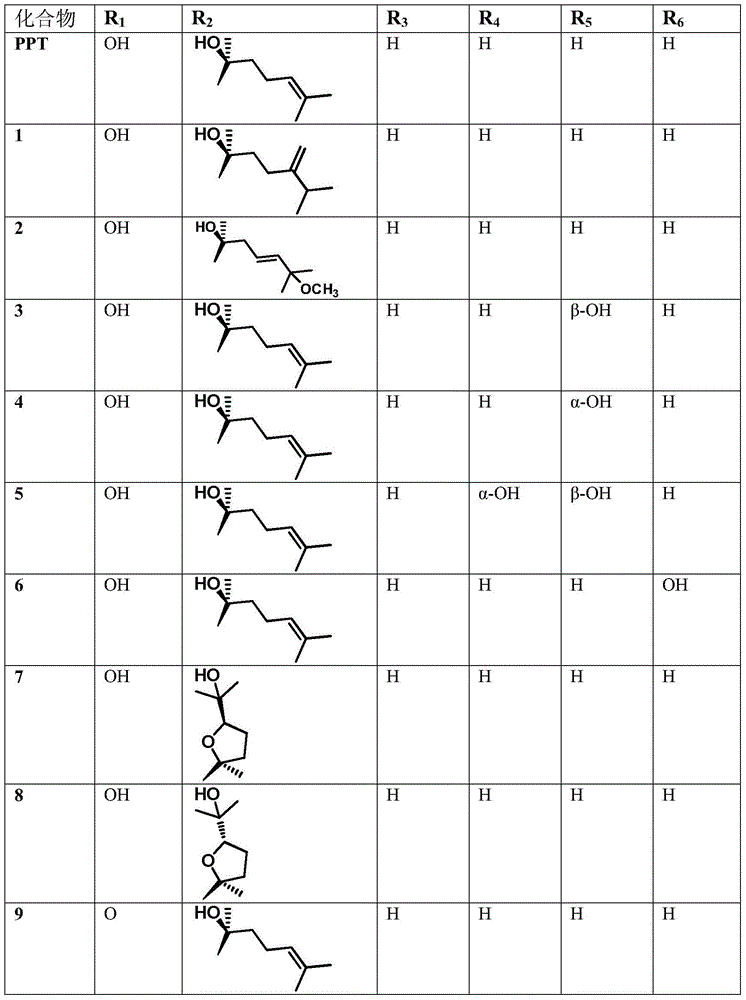
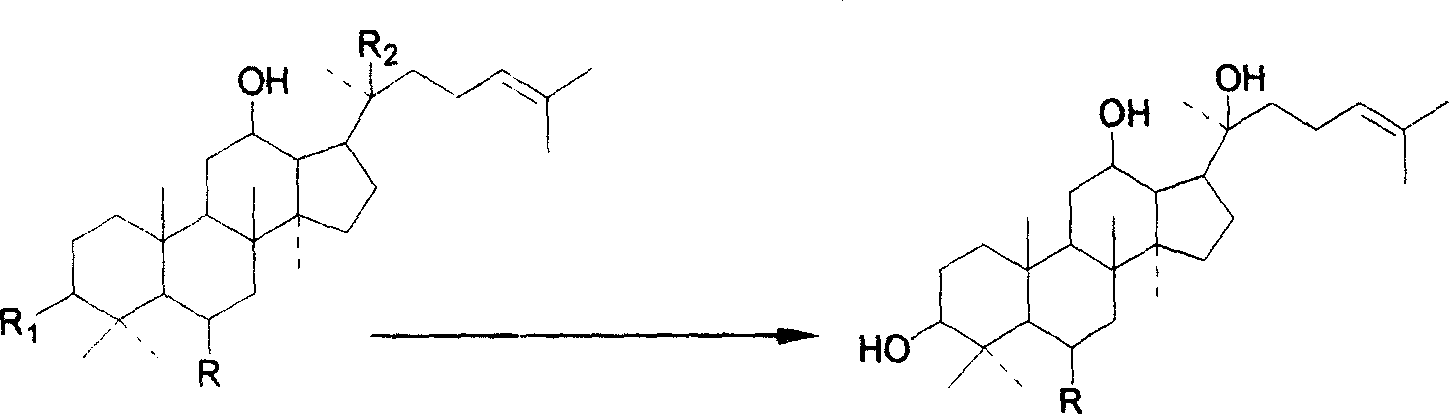
The invention discloses the ramification of the protopanaxatriol and a method of the production and the appliance. The formula of the ramification of the protopanaxatriol is the formula I, thereinto, R1 is -OH or H; R2 is -CH3 or -CH20H; R3 is -CH3 or -CH20H. The invention adopts the technique of the microbial conversion to the structure qualification of the protopanaxatriol, Then a great variety of new compounds are gained. With the verification of the vitro resisting tomour cell test, the compound has the better function of the anti-tumor activity and can be used for the active constitution of the antineoplastic. The compound has the broad purpose.
Owner:PEKING UNIV
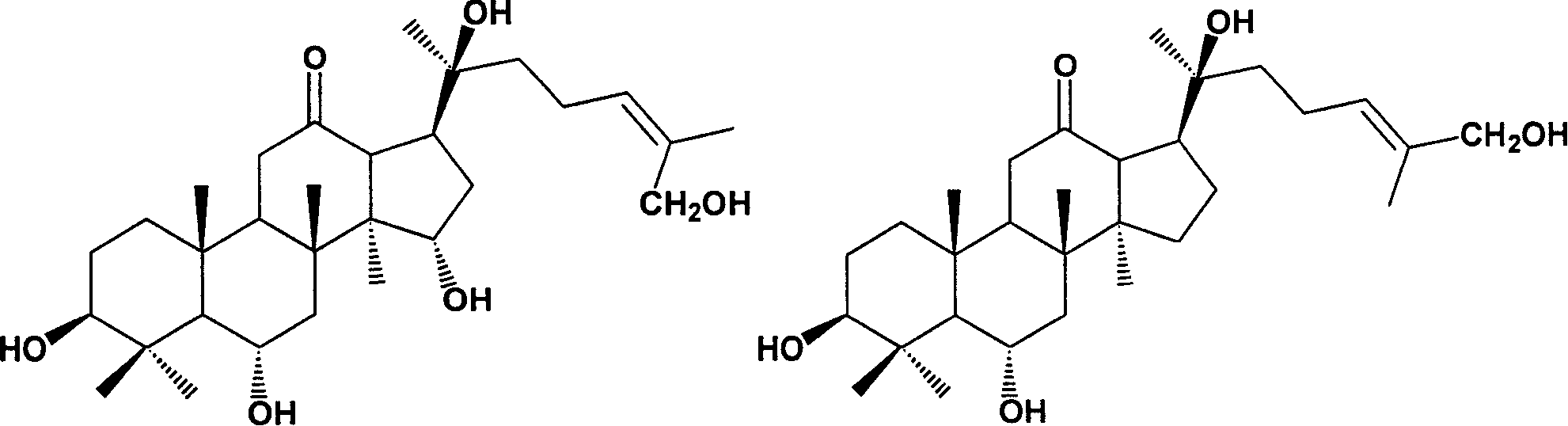
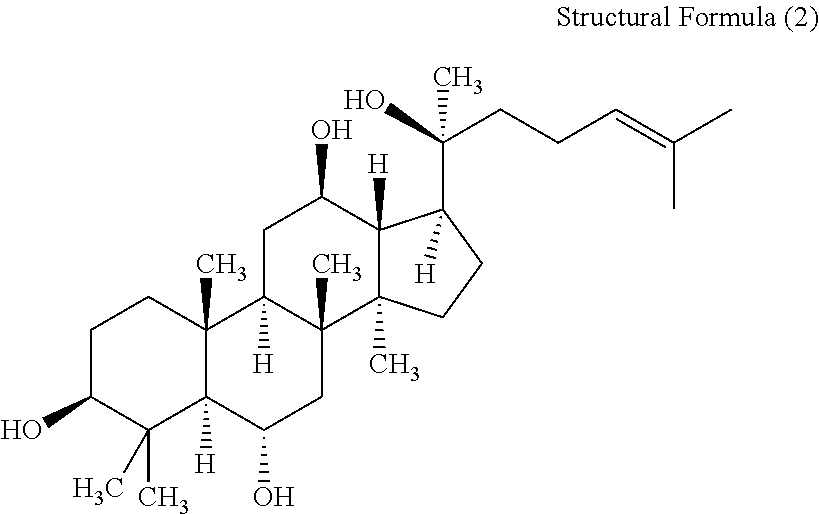
Composition containing protopanaxatriol and protopanaxadiol
InactiveUS20120252768A1Improve securityImprove stabilityOrganic active ingredientsBiocideProtopanaxadiolPhysical chemistry
A composition including: at least one mixture selected from the group consisting of a mixture of (A) panaxatriol and (B) protopanaxatriol and a mixture of (C) panaxadiol and (D) protopanaxadiol, wherein a ratio (A) / (B) of a mass of the (A) panaxatriol to a mass of the (B) protopanaxatriol is 1 or greater, and a ratio (C) / (D) of a mass of the (C) panaxadiol to a mass of the (D) protopanaxadiol is 1 or greater.
Owner:LION CORP
Process of preparing 20(S)-ginsenoside Rh1 with streptomycete fermentation of pseudo-ginseng saponin
A Streptomyces fradiae NTGA-344 strain with the preservation number of CGMCC No.2074 and a method for preparing 20(S)-ginsenoside Rh1 (6-O-belta-D-Glucopyranoside-20(S)-Protopanaxatriol by using the strain to ferment and convert panaxatriol saponins in various notoginsenosides in a fermentation cylinder are provided. 1(GA), ISP2, humic acid (HSG culture medium) and asparagines culture medium are selected as the culture media. In the process of fermentation, the inventory rating proportion of various notoginsenosides is 0.1% to 10 % (w / v), the fermentation temperature is 18 to 50 DEG C, the pH value of 0.1% to 35% (w / v) ammonia is dynamically adjusted to be between 2 and 8, the ventilation ratio is 0.1 to 10 (v / v), the stir speed is 10 to 900r / min, the fermentation time is 5h to 200h, and the macroporous absorbent resin is 0.1% to 30% (w / v). The invention provides the streptomyces yunnanensis with stable property, simple nutritional requirement and good growth and a practical and convenient fermentation cylinder fermentation and separation extraction process for large-scale fermentation and conversion of various notoginsenoside and preparation of 20(S)-ginsenoside Rh1. The 20(S)-ginsenoside Rh1 is prepared by the strain and the technology, thereby having low manufacturing cost, less impurity and high product yield.
Owner:KUNMING NOVOGINSENG BIOENG
Applications of protopanaxatriol and derivatives thereof in preparation of medicines for treating hepatic disease
ActiveCN104352505AGood anti-hepatic fibrosis activityWide range of usesOrganic active ingredientsDigestive systemHepatic lipaseHepatic Diseases
The invention discloses applications of protopanaxatriol derivatives or pharmaceutically formable salts thereof and protopanaxatriol or pharmaceutically formable salts of the protopanaxatriol in preparation of medicines for treating a hepatic disease. An in-vitro anti-liver fibrosis cell test proves that these compounds have relatively good anti-liver fibrosis activity, can be used as active components of anti-liver fibrosis medicines, and can be applied widely.
Owner:SHENZHEN SUN BIO SCI & TECH CO LTD
A composition containing ginsenoside F1 or compound K for skin external application
The present invention relates to an inhibitor for the biosynthesis of gelatinase comprising ginsenoside F1 (20-O-beta-D-glucopyranosyl-20(S)-protopanaxatriol) or compound K (20-0-beta-D-glucopyranosyl-20(S)-protopanaxadiol), which is a chief metabolite of ginseng saponin, as an active ingredient; and a cosmetic / medical composition for the prevention of skin-aging comprising the same which is superior in inhibiting the decomposition of epidermal-dermal junction and also in accelerating the generation thereof.
Owner:AMOREPACIFIC CORP
Conversion action of black rhizopus on ginsenosides
The invention discloses a rhizopus stolonifer capable of biologically converting ginsenosides. A fermentation product is analyzed by an HPLC (High Performance Liquid Chromatography) system to prepare a component with a reduced color spectrum peak value in an original panaxatriol saponins and a component with an increased peak value in the panaxatriol saponins fermentation product thereof. By isolation and identification, a converted substance in the panaxatriol saponins is confirmed to be ginsenosides Re, a compound (1) in the panaxatriol saponins fermentation product is confirmed to be ginsenosides Rh1, a compound (2) is confirmed to be ginsenosides Rg3, and a compound (3) is confirmed to be ginsenosides Rg2. By speculation, the ginsenosides Re in the panaxatriol saponins is converted into the ginsenosides Rh1 by the black rhizopus or the ginsenosides Re is firstly converted into the ginsenosides Rg2 and then converted into ginsenosides Rh1, and the detailed conversion path is to be further researched.
Owner:包海鹰
Anti-coxsackie medicament composition and medicine use thereof
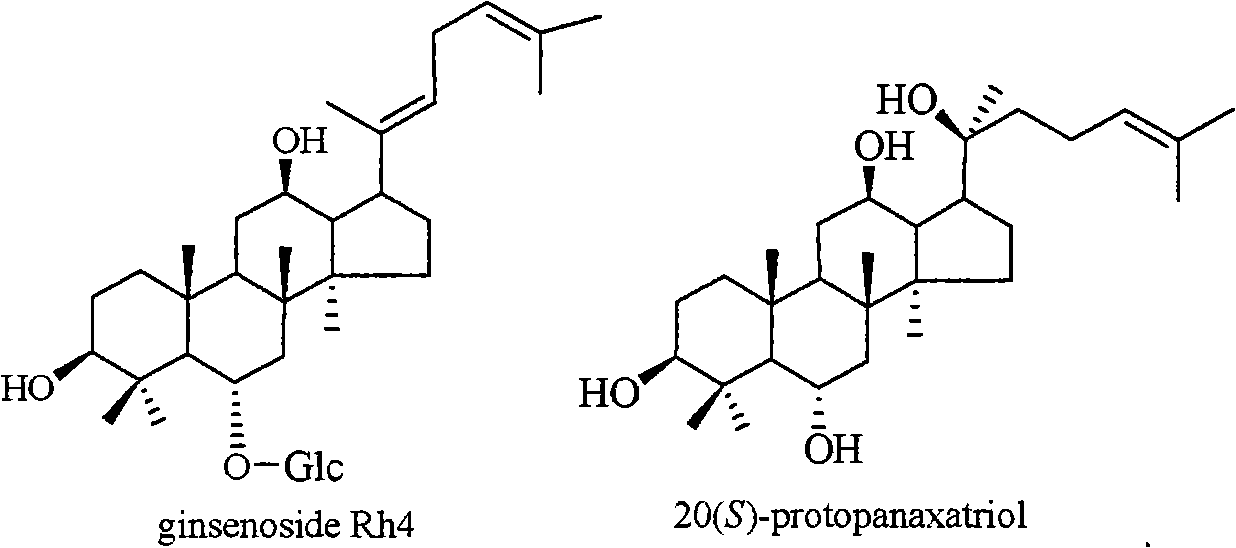
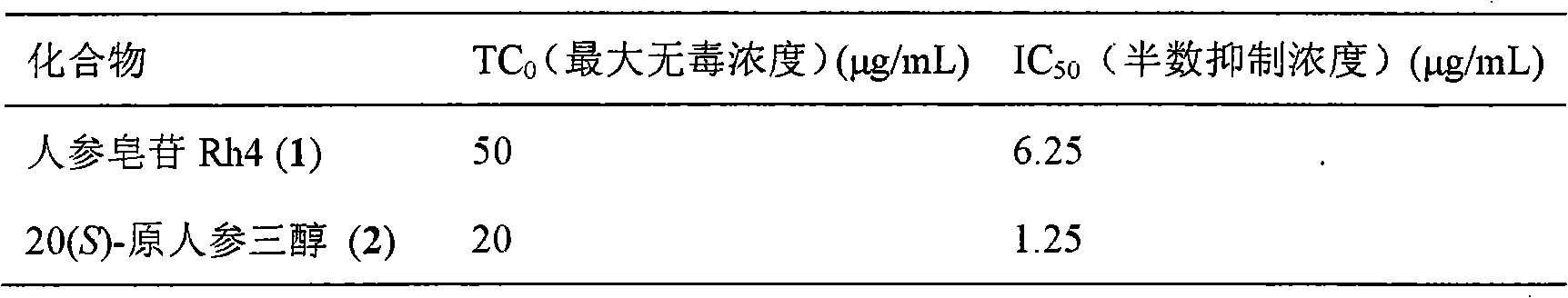
The invention relates to a pharmaceutical composition with dammarane-type tetracyclic triterpene compound ginsenoside Rh4 as shown in the following structural formula and 20(S)-protopanaxatriol as the active components and the application thereof to the pharmaceutical field. The in vitro pharmacological experiments show that the pharmaceutical composition has good in vitro inhibitory activity for coxsackie B3 virus and can be used for treating viral myocarditis caused by coxsackie B3 virus.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Cultivation method of novel dammarane-type ginsenoside-containing paddy rice variety
InactiveCN103333914AVector-based foreign material introductionAngiosperms/flowering plantsBiotechnologyEnzyme Gene
The invention discloses a cultivation method of a novel dammarane-type ginsenoside-containing paddy rice variety and belongs to the field of genetic engineering. The breeding method is characterized in that a full-length cDNA of a gene of a dammarenediol synthetase as a key enzyme of dammarane-type ginsenoside biosynthesis is constructed to double-T-DNA plant expression vectors; the constructed product is subjected to genetic transformation in paddy rice; and through screening cultivation, the novel dammarane-type ginsenoside-containing paddy rice variety is obtained. A test proves that the novel dammarane-type ginsenoside-containing paddy rice variety comprises 0.020% of 20(S)-protopanoxadiol (PPD) and 0.173% of 20(S)-protopanaxatriol (PPT). The cultivation method can be used for cultivation of high-quality ginseng rice varieties containing dammarane-type ginsenoside, can improve a health care value of rice as a main food of a human body, can widen a ginsenoside source, can alleviate the scarcity of a ginsenoside drug source, and has a good application prospect.
Owner:FUJIAN CHAODA GROUP
A plurality of antineoplastic sapogenin and its injection preparation method
InactiveCN1962681AReduce usageReduce manufacturing costOrganic active ingredientsPowder deliveryProtopanaxadiolOxygen
The invention discloses a saponin compound to prevent tumour preparing method of its agent, which comprises the following steps: adopting panaxoside, American ginseng saponin, Notoginsen triterpenes and gynostemma pentaphylla saponin as raw material; dissolving in the high-boiling point organic solvent; aerating oxygen to do oxide alkaline degradation; adsorbing product through column chromatography to obtain protopanaxadiol, protopanaxatriol and Octoid.
Owner:王丹
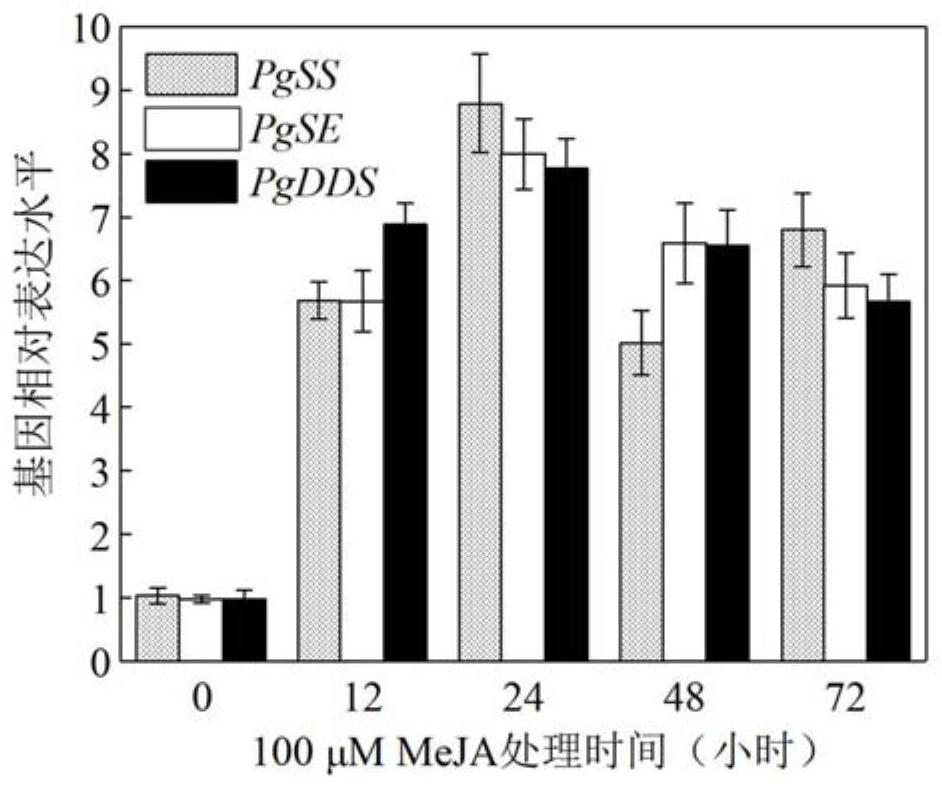
Ginseng PgJAZ1 gene, method for improving protopanaxatriol saponin based on gene and application
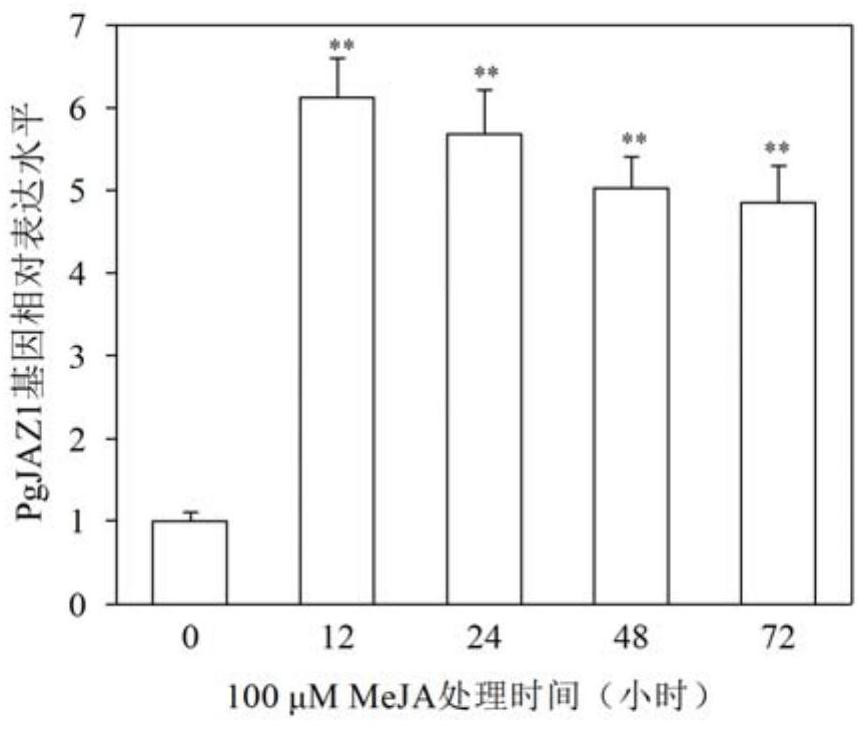
The invention discloses a ginseng PgJAZ1 gene, a method for improving protopanaxatriol saponin based on the gene and application of the ginseng PgJAZ1 gene. The gene is derived from Panax ginseng C.A.Meyer and is named as PgJAZ1, and a protein coded by the gene has a conservative TIFY structural domain and a jas structural domain which are specific to the JAZ family. The constructed PgJAZ1 gene RNA interference vector is used for transforming a ginseng root, and a PgJAZ1 gene silenced ginseng hairy root is obtained. Compared with a control ginseng hairy root, the content of protopanaxatriol saponin and the content of total saponin in the PgJAZ1 gene silenced ginseng hairy root are remarkably increased. Researches show that the PgJAZ1 gene is utilized to regulate the content of endogenous jasmonic acid methyl ester in ginseng hairy roots so as to regulate the biosynthesis of protopanaxatriol type saponins and total saponins. The invention has important application value in the aspects of increasing the yield of the protopanaxatriol saponin and improving the quality of the ginseng by utilizing the PgJAZ1 gene in the ginseng.
Owner:HUNAN INSTITUTE OF ENGINEERING
Preparation method and application of high-activity anti-aging component with natural source
InactiveCN107326059AHigh activityIncrease production costCosmetic preparationsToilet preparationsNatural sourceProtopanaxadiol
The invention discloses a preparation method of a high-activity anti-aging component with a natural source. The preparation method comprises the following steps of screening out a proper aerobic single strain; using the plant tissue of a ginseng plant as a main raw material, adding at least one of wheat bran, wheat straw, grain flour and a rice husk, water and other inductors, sufficiently agitating, and sterilizing; inoculating the strain, and fermenting, wherein a fermentation product is a ginseng product which is enriched in F1 and has high anti-aging activity; adding lower alcohol to extract a ginsenoside mixture; extracting a hydrolytic enzyme from an extracted filter residue by using water or a buffer solution; converting ginsenoside by utilizing the hydrolytic enzyme, so that the high-activity anti-aging component with the natural source can be obtained. By using the preparation method, the total ginsenoside does not need to be divided into diol saponin PPD (Protopanaxadiol) and triol saponin PPT (Protopanaxatriol); the ginsenoside glycosidase also does not need to be specially prepared; an anti-aging component F1 is directly prepared through a fermentation method.
Owner:肖永坤
Use of melanin biosynthesis inhibitors from korean ginseng and the cosmetic composition containing thereof for skin whitening
ActiveUS20100310485A1Inhibit melanin productionImproves pigmentationCosmetic preparationsHair cosmeticsGINSENG EXTRACTBULK ACTIVE INGREDIENT
Disclosed herein is a whitening cosmetic composition containing plant-derived ginsenoside Fl (20-O-β-D-glucopyranosyl-20(S)-protopanaxatriol) as an active ingredient. More specifically, the ginsenoside Fl is obtained from particularly a ginseng extract using an acid, a base, an enzyme or microorganism, and a whitening cosmetic composition containing the ginsenoside Fl has an excellent effect of inhibiting melanin biosynthesis, and thus provides an excellent skin whitening effect.
Owner:AMOREPACIFIC CORP
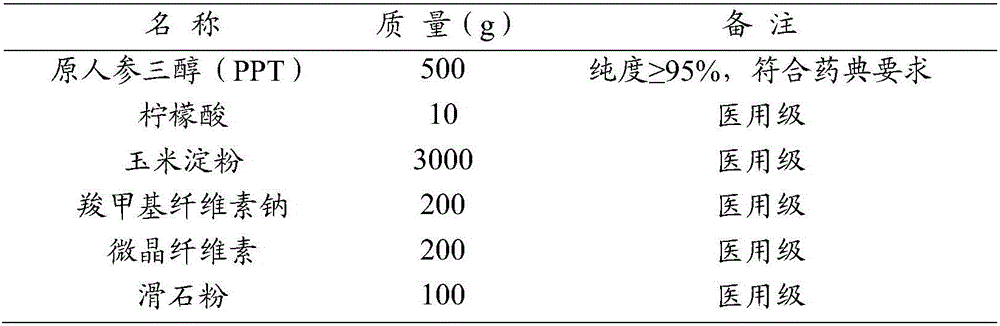
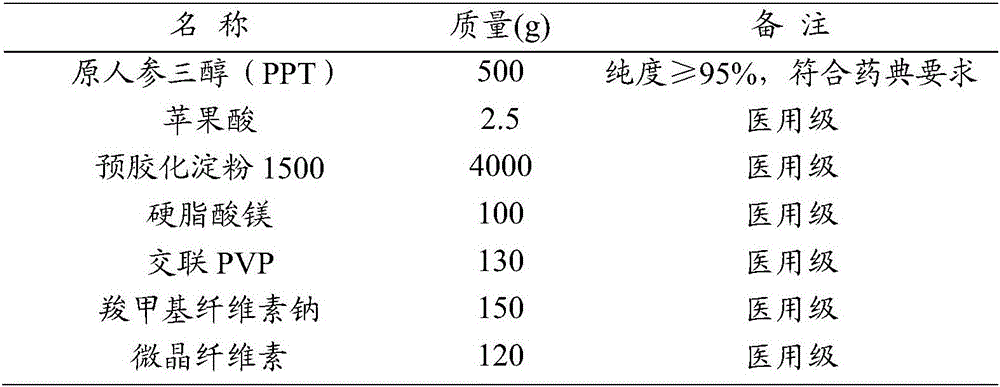
Rare ginsenoside composition including rare protopanaxatriol (PPT)
InactiveCN106236757AHigh efficiency and low toxicity anti-tumor effectGood anti-tumor effectOrganic active ingredientsAntineoplastic agentsProtopanaxatriolTricarboxylic acid
The invention provides a rare ginsenoside composition including rare PPT. The rare ginsenoside composition comprises rare PPT and edible C2-6 organic dicarboxylic acid or tricarboxylic acid, wherein the edible C2-6 organic dicarboxylic acid or tricarboxylic acid accounts for 0.05 to 5 parts by weight on the basis of 100 parts by weight of PPT. The rare ginsenoside composition has substantial antineoplastic activity and has high efficiency and low toxicity.
Owner:SHAANXI GIANT BIOTECHNOLOGY CO LTD
Application of protopanaxadiol derivative and protopanaxatriol derivative in preparation of drugs
ActiveCN103961358AOptimize spaceGood orientationOrganic active ingredientsNervous disorderProtopanaxadiolPressure reduction
The invention provides an application of a protopanaxadiol derivative and a protopanaxatriol derivative in the preparation of drugs for treating or preventing neurodegenerative diseases. Pharmacological experiment results show that the above derivatives have a stronger anti-oxidation protection effect than 20(S)-protopanaxadiol and 20(S)-protopanaxatriol in H2O2 damage models of PC12 cells and N2a cells. Two vascular dementia models comprising a repeated occlusion rat bilateral vascular carotid cooperation sodium nitroprusside pressure reduction process stimulated dementia model and a bilateral hippocampus injection Abeta1-42 duplicated senile dementia model can improve the rat space exploration and orientation capability.
Owner:SHANGHAI INNOVATIVE RESEARCH CENTER OF TRADITIONAL CHINESE MEDICINE
Use of 20(S)-protopanoxadiol derivatives and 20(S)-protopanaxatriol derivatives in preparation of antidepressant medicines
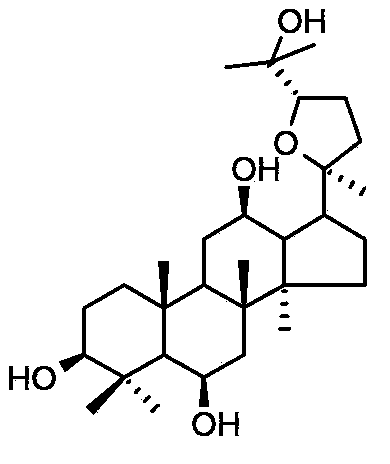
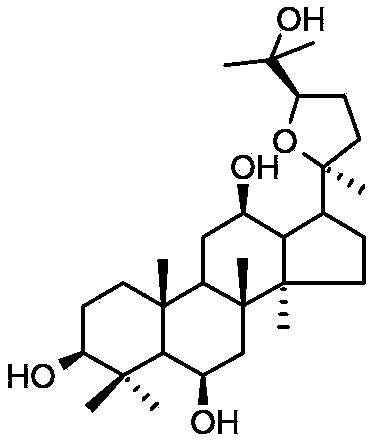
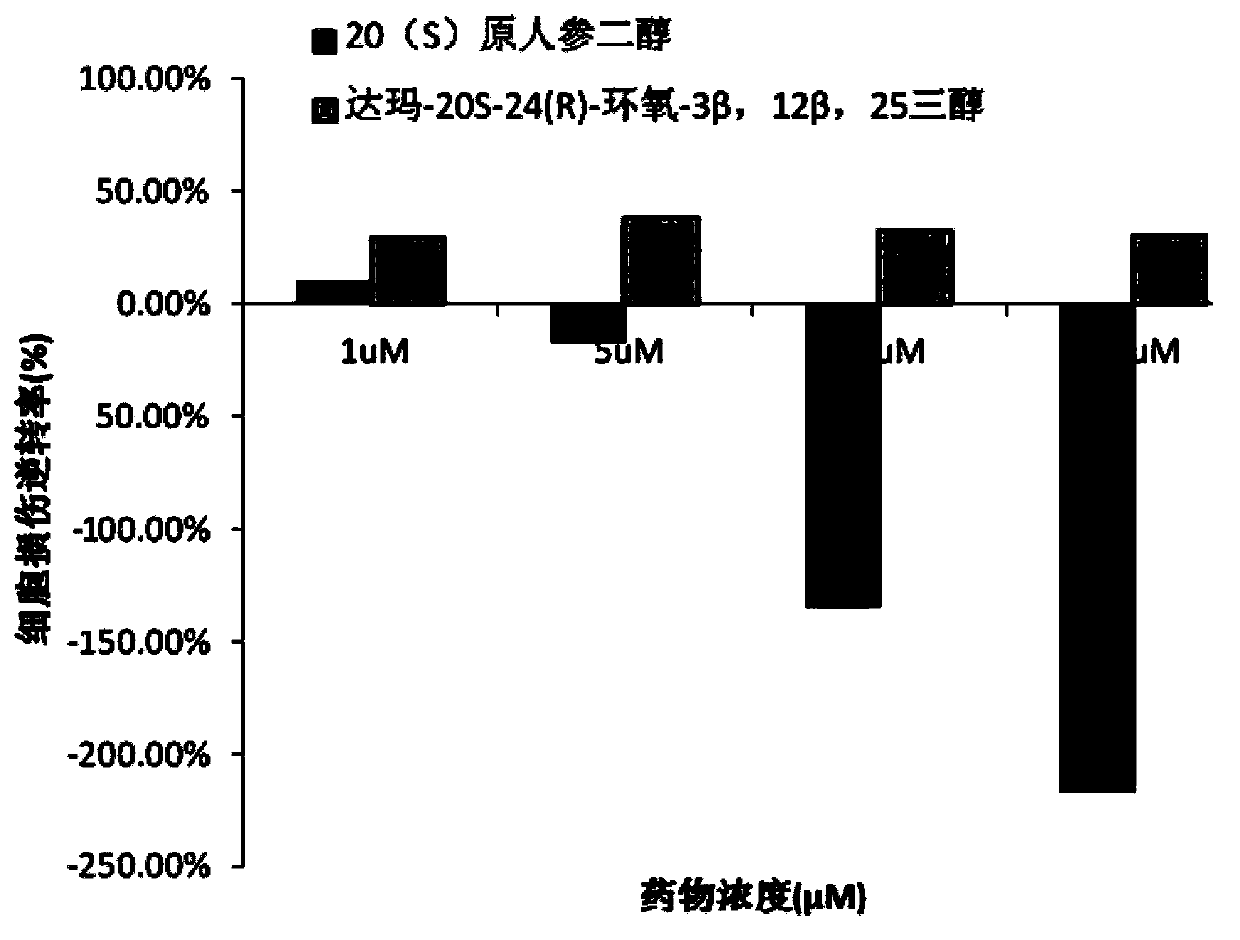
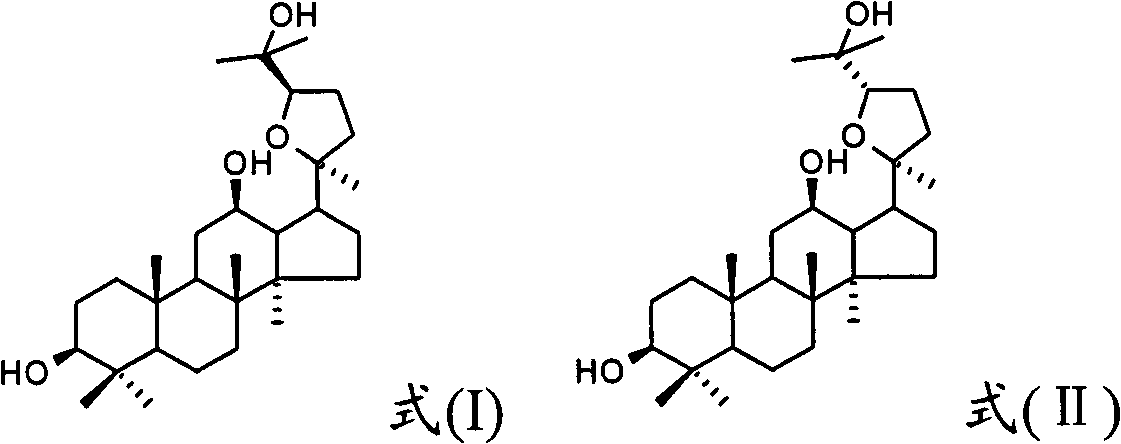
ActiveCN103211823AShorten immobility timeGood antidepressant activityOrganic active ingredientsNervous disorderEpoxyDisease
The invention relates to a new use of 20(S)-protopanoxadiol derivatives and 20(S)-protopanaxatriol derivatives, and mainly relates to a use in the preparation of medicines for treating or preventing depressive mental diseases. 20(S)-protopanoxadiol and 20(S)-protopanaxatriol are used as raw materials, and undergo oxidative cyclization to prepare dammara-20S-24(R)-epoxy-3beta,12beta,25-triol, dammara-20S-24(S)-epoxy-3beta,12beta,25-triol, dammara-20S-24(S)-epoxy-3beta,6beta,12beta,25-tetrol or dammara-20S-24(R)-epoxy-3beta,6beta,12beta,25-tetrol. Researches of pharmacological experiments show that dammara-20S-24(R / S)-epoxy-3beta,12beta,25-triol and dammara-20S-24(R / S)-epoxy-3beta,6beta,12beta,25-tetrol can obviously shorten the tail immobility time of mice in mouse tail suspension tests of a classic depression model, and can obviously shorten the swimming immobility time of the mice in mouse forced swimming model tests, so dammara-20S-24(R / S)-epoxy-3beta,12beta,25-triol and dammara-20S-24(R / S)-epoxy-3beta,6beta,12beta,25-tetraol have potential development values in the preparation of medicines for treating or preventing depressive mental diseases.
Owner:SHANGHAI INNOVATIVE RESEARCH CENTER OF TRADITIONAL CHINESE MEDICINE
Hyperlipemia-ameliorating agent, anemia-ameliorating composition, uric-acid-level-reducing composition, and food or beverage
InactiveUS20120258184A1Good effectImprove securityOrganic active ingredientsBiocideProtopanaxadiolEnvironmental health
A neutral-fat-level-regulating agent, a cholesterol-level-regulating agent, a free-fatty-acid-level-regulating agent or an anti-obesity agent, including: at least one of protopanaxatriol, panaxatriol, protopanaxadiol and panaxadiol which are aglycons of dammarane-type saponins; and a hyperlipemia-ameliorating agent including: at least one of the neutral-fat-level-regulating agent, the cholesterol-level-regulating agent, the free-fatty-acid-level-regulating agent and the anti-obesity agent.
Owner:LION CORP
Recombinant saccharymyces cerevisiae for producing ginsengenins as well as construction method and application of same
The invention discloses a recombinant saccharymyces cerevisiae for producing ginsengenins as well as a construction method and an application of the same. The recombinant saccharymyces cerevisiae capable of producing three ginsengenins of oleanolic acid, protopanoxadiol and protopanaxatriol simultaneously is Saccharymyces cerevisiae GY-1, with a collection number of CGMCC No. 7725 in the China General Microbiological Culture Collection Centre. The saccharymyces cerevisiae GY-1 is capable of producing three ginsengenins of oleanolic acid, protopanoxadiol and protopanaxatriol simultaneously, wherein the yields achieve 21.4 mg / L in fermentation broth, 17.2 mg / L in fermentation broth and 15.9 mg / L in fermentation broth respectively, the proportions of which in the total ginsengenin content are 39.3%, 31.5% and 29.2% respectively.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
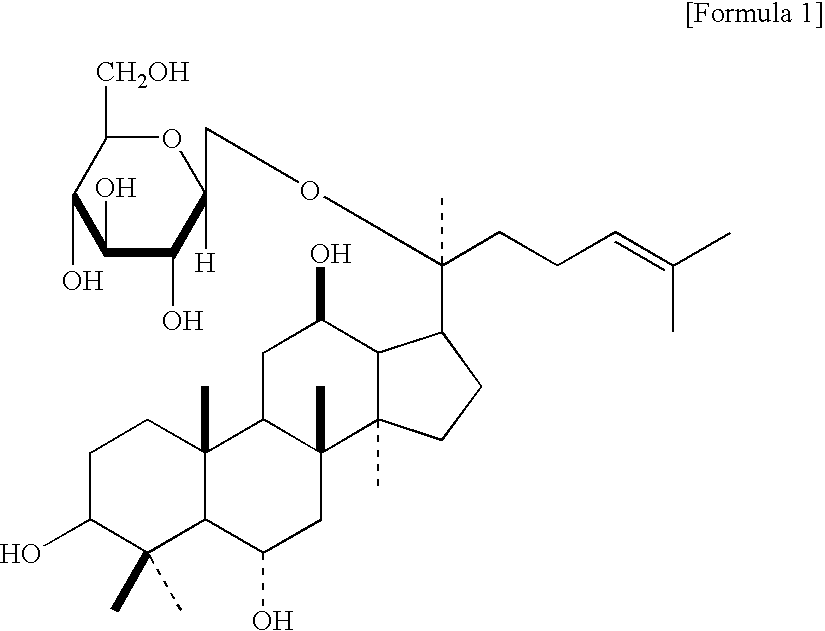
AGENT FOR CONTROLLING Bcl-2 EXPRESSION COMPRISING GINSENOSIDE F1 AS AN ACTIVE COMPONENT
ActiveUS20080261899A1Inhibits downregulationHigh expressionBiocideAntinoxious agentsMedicineActive component
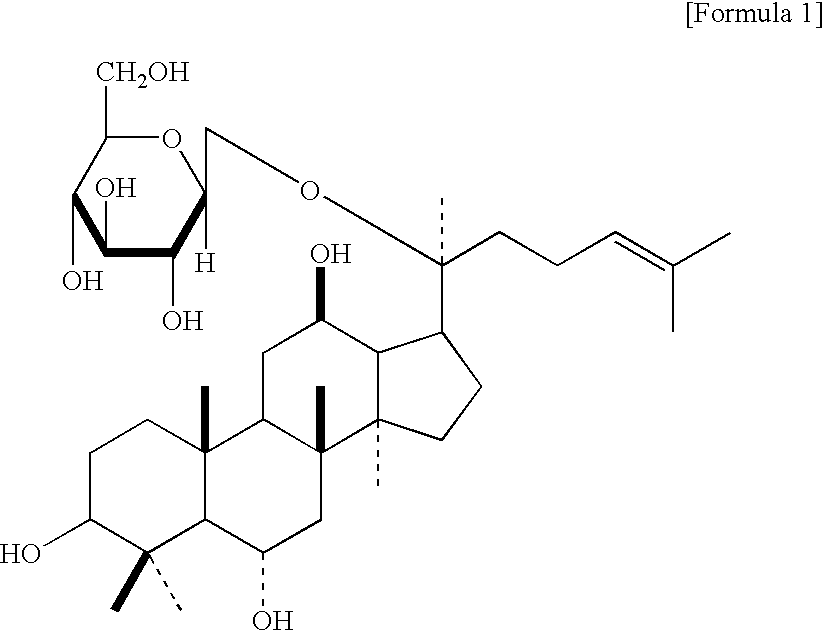
The present invention relates to an agent for controlling Bcl-2 expression comprising ginsenoside F1 (20-O-β-D-glucopyranosyl-20(S)-protopanaxatriol) represented by the following formula 1 as an active component.
Owner:AMOREPACIFIC CORP
Use of melanin biosynthesis inhibitors from korean ginseng and the cosmetic composition containing thereof for skin whitening
ActiveUS8647610B2Inhibit melanin productionImproves pigmentationCosmetic preparationsHair cosmeticsGINSENG EXTRACTBULK ACTIVE INGREDIENT
Disclosed herein is a whitening cosmetic composition containing plant-derived ginsenoside F1 (20-O-β-D-glucopyranosyl-20(S)-protopanaxatriol) as an active ingredient. More specifically, the ginsenoside F1 is obtained from particularly a ginseng extract using an acid, a base, an enzyme or microorganism, and a whitening cosmetic composition containing the ginsenoside F1 has an excellent effect of inhibiting melanin biosynthesis, and thus provides an excellent skin whitening effect.
Owner:AMOREPACIFIC CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com