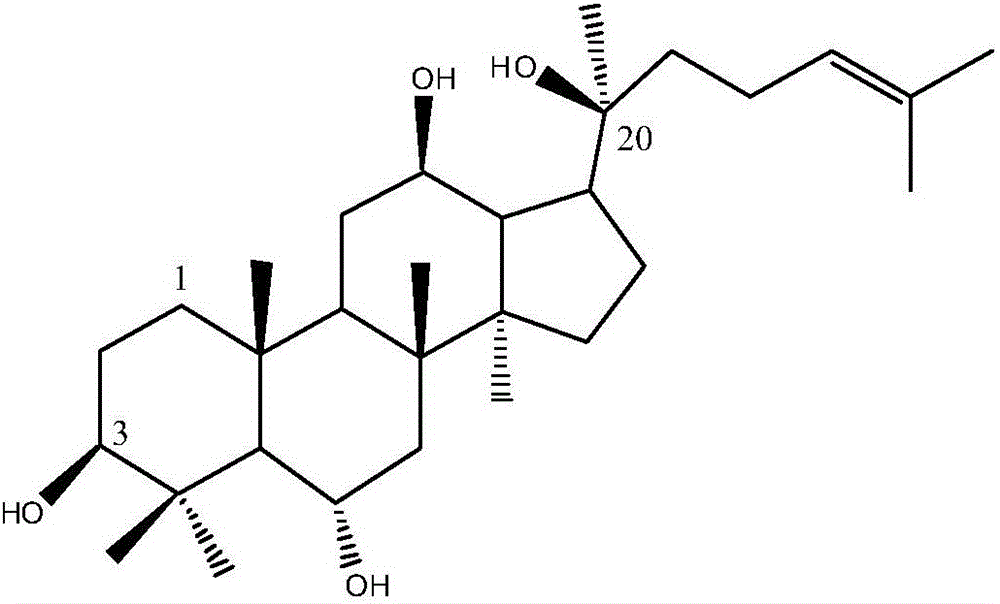
Rare ginsenoside composition including rare protopanaxatriol (PPT)
A technology of protopanaxatriol and ginsenoside, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
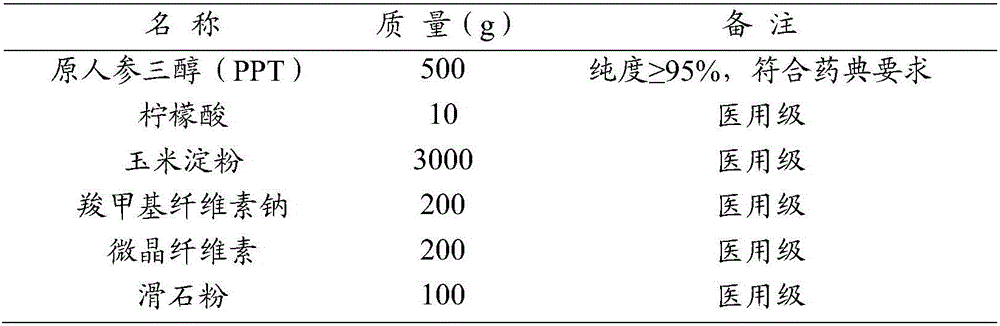
[0049] Example 1 Ginsenoside Gum PPT Capsules
[0050] Make tablets according to the following ratio (10000 tablets, PPT content: 50mg / tablet)
[0051]
[0052] The main process flow is as follows: Pour the weighed cornstarch, protopanaxatriol (PPT), citric acid, sodium carboxymethyl cellulose, microcrystalline cellulose, and talc into the high-speed mixing granulator in sequence, and the operator Operate in accordance with the equipment operating procedures and post operating procedures, rotate at 550 rpm, stir for 10 minutes to make it evenly mixed, then add an appropriate amount of 90% ethanol, continue to stir for 5 minutes until evenly mixed, and granulate to obtain wet granules. Pour the granules into the container and evenly spread them on the plate with a thickness of 3-5cm. Control it within the range of 50°C to 55°C, open the steam drain valve, drain the condensed water in the steam pipe first, then close it, open the steam outlet valve, open the steam inlet valv...
Embodiment 2
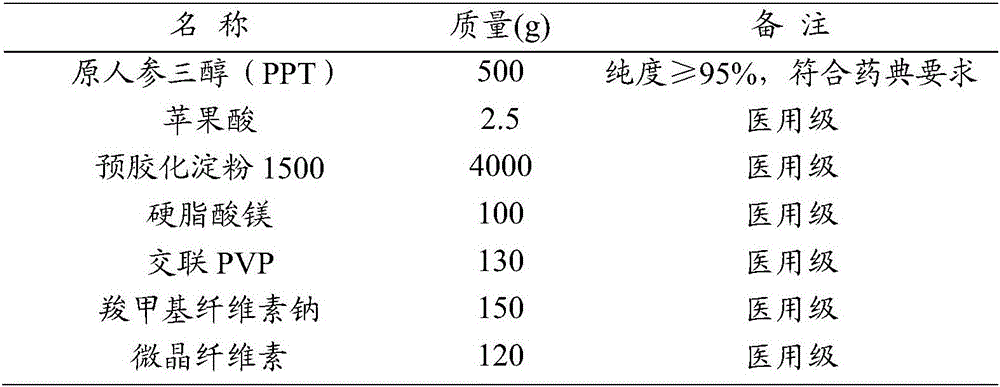
[0053] Embodiment 2 protopanaxatriol (PPT) tablet
[0054] Make tablets according to the following ratio (10000 tablets, PPT content: 50mg / tablet)
[0055]
[0056] The main process flow is as follows: Pour the weighed pregelatinized starch, protopanaxatriol (PPT), malic acid, magnesium stearate, cross-linked PVP, sodium carboxymethyl cellulose and microcrystalline cellulose into the high-speed In the mixing granulator, the operator operates in accordance with the equipment operating procedures and post operating procedures, the speed is 450rpm, stirring for 10 minutes to make it evenly mixed, then add an appropriate amount of 85% ethanol, continue to stir for 5 minutes until the mixing is even, and granulate to obtain wet granules . Pour the granules into the container and evenly spread them on the plate with a thickness of 3-5cm. Control it within the range of 45°C to 50°C, open the steam drain valve, drain the condensed water in the steam pipe first, then close it, ope...
Embodiment 3
[0057] Embodiment 3 protopanaxatriol (PPT) dropping pill
[0058] Make tablets according to the following ratio (10000 pills, PPT content: 5mg / pill)
[0059]
[0060] The main process flow is as follows: keep the room temperature in the operation room at 18°C-25°C, and the relative humidity at 20%-40%. The temperature of the melting tank is 145°C-150°C. After the polyethylene glycol-6000 is melted into a clear liquid, pour protopanaxatriol (PPT) and oxalic acid into it, stir until they are completely dissolved, and put the material into the storage tank. The temperature of the material storage tank is 100°C-110°C, and the temperature of the internal liquid medicine is kept at 90°C-100°C, and the material is discharged into the drop material tank. The temperature of the dripping tank is 100°C-110°C, the temperature of the cooling oil is 5°C-10°C, and the weight of the pills is controlled by the machine to be 22-24mg. After the dripped vegetarian pills are deoiled, the pill...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com