Novel catalytic system for preparing rare ginsenosides and application thereof
A kind of ginsenoside, a rare technology, applied in the field of biotechnology and plant biology, can solve the problems of high price, severe reaction conditions and limitations of chemical hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Example 1. Cloning of glycosyltransferases UGTPg1, UGTPg5, UGTPg29, UGTPg50, BC10 and sucrose synthase SUS1
[0150] (1) Cloning of glycosyltransferases UGTPg1, UGTPg5, UGTPg29, UGTPg50 and BC10
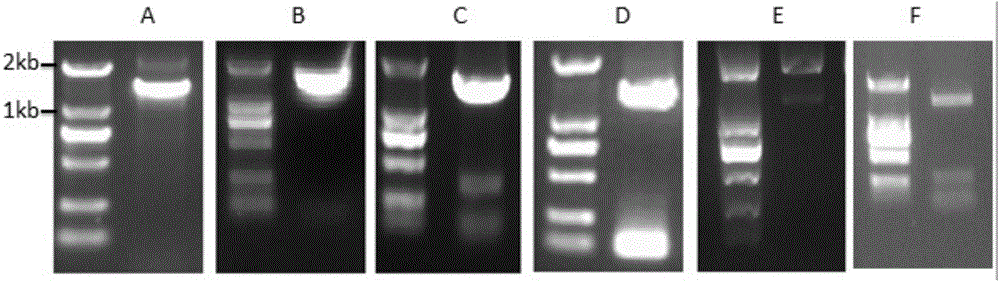
[0151]Two primers respectively having the nucleotide sequences of SEQ ID NO:7 and SEQ ID NO:8 in the sequence listing were synthesized. Using the cDNA obtained by reverse transcription of RNA extracted from ginseng as a template, PCR was performed using primers SEQ ID NO: 7 and SEQ ID NO: 8. As the DNA polymerase, the high-fidelity KOD DNA polymerase from Treasure Bioengineering Co., Ltd. was selected. The PCR amplification program is: 94°C for 2min; 94°C for 15s, 58°C for 30s, 68°C for 2min, a total of 35 cycles; 68°C for 10min, then drop to 10°C. The PCR products were detected by agarose gel electrophoresis, and the results were as follows: figure 1 a. Under UV irradiation, the target DNA band is excised. Then AxygenGelExtraction Kit (AXYGEN Company) was used to recover...
Embodiment 2
[0166] Example 2. Glycosyltransferase and sucrose synthase SUS1 expression
[0167] (1) Expression of UGTPg1, UGTPg5, UGTPg29, UGTPg50 and BC10 in Escherichia coli
[0168] Two primers respectively having the nucleotide sequences of SEQ ID NO: 19 and SEQ ID NO: 20 in the sequence listing were synthesized. Two restriction sites, BamHI and XhoI, were respectively set at both ends of the synthesized primers SEQIDNO:19 and SEQIDNO:20, and PCR was performed using pMDT-UGTPg1 as a template. The PCR amplification procedure is the same as in Example 2. The PCR product was separated and recovered by agarose gel electrophoresis, and after being digested by BamHI and XhoI, it was ligated into the pET28a vector (Novagen Company) that was also digested by BamHI and XhoI using T4 DNA ligase from NEB Company. The obtained recombinant plasmid was named pET28a-UGTPg1. The recombinant plasmid pET28a-UGTPg1 was transformed into Escherichia coli BL21(DE3) (Novagen Company) to construct the rec...
Embodiment 3
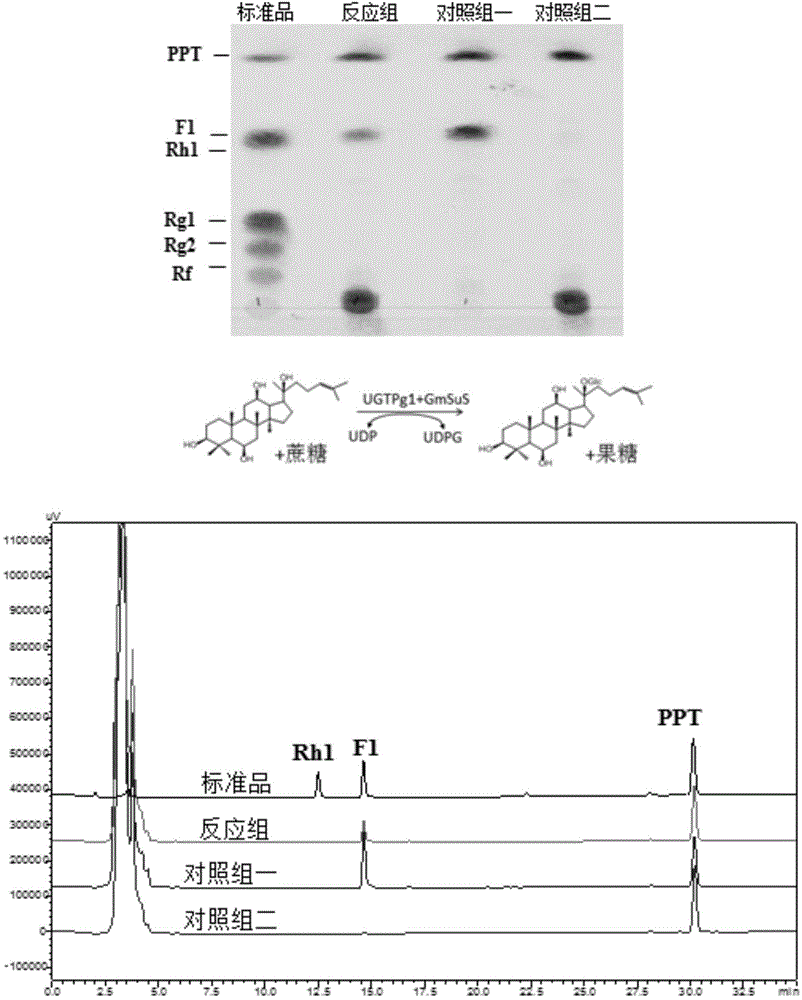
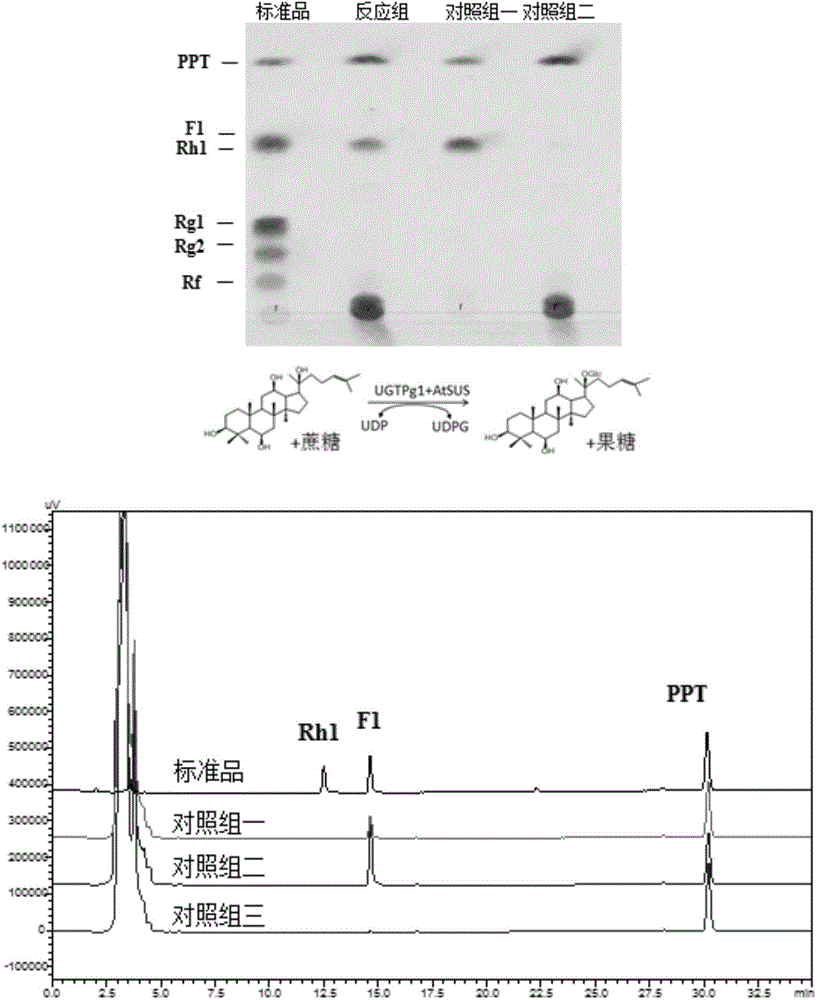
[0178] Example 3. Synthesis of rare ginsenosides from ginsenogenin catalyzed by sucrose synthase and glycosyltransferase
[0179] The reaction of glycosyltransferase catalyzing the synthesis of ginsenoside from ginsenogenin needs to add UDP-glucose as the glycosyl donor, and the current commercial UDP-glucose price is about 4000 RMB per gram (Sigma Company). In the reaction system, UDP-glucose needs to be added in excess (about 10 mM UDP-glucose needs to be added to the reaction system for converting 1 mM ginsenogenin) to ensure that the reaction proceeds. Moreover, UDP-glucose is in a state of continuous consumption during the reaction process and cannot be recycled. These comprehensive factors make it extremely expensive to directly use UDP-glucose as a glycosyl donor for glycosylation to prepare glycosides. In the present invention, by using plant-derived sucrose synthase, by adding UDP and sucrose to the reaction system, sucrose synthase catalyzes UDP and sucrose to synthe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com