A plurality of antineoplastic sapogenin and its injection preparation method
An injection and anti-tumor technology, applied in the direction of anti-tumor drugs, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of low bioavailability, no anti-tumor effect research, and poor utilization of resources, etc. problem, achieve the effect of saving production cost and reducing usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
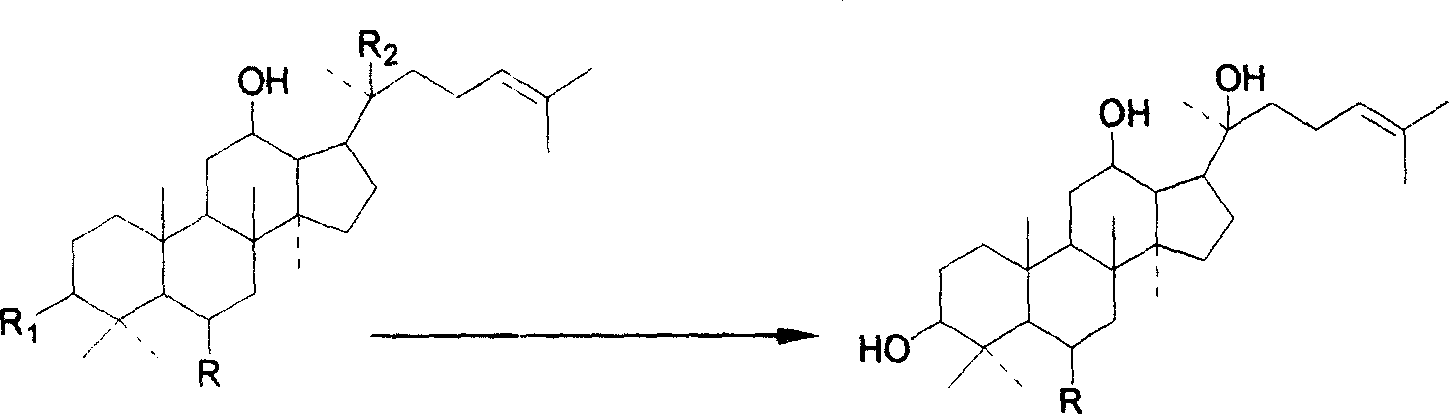
[0041]Dissolve 100g of Panax notoginseng saponins in 1.5L of anhydrous n-butanol, add 40g (1.73mol) of sodium particles while stirring rapidly until there are no bubbles, then continue to feed oxygen, heat to 90°C, and react for 24 hours. , cooled to room temperature, washed three times with 1L of n-butanol saturated water to remove excess alkali and salts generated, then recover n-butanol under reduced pressure, disperse the reactant (about 73g) in 1L of aqueous solution, and use 1L of Ethyl acetate was extracted three times, and ethyl acetate was recovered and dried to obtain 40 g of ethyl acetate extract. The ethyl acetate extract was subjected to silica gel column chromatography, and CHCl 3 - MeOH (100:1 ~ 100:6) gradient elution to obtain 4.6 g of protopanaxadiol and 11.0 g of protopanaxatriol.
[0042] Its NMR data are respectively:
[0043] Protopanaxadiol 1 H-NMR (300MHz, CDCl 3 )δ: 1.70(3H, s), 1.64(3H, s), 1.20(3H, s), 0.99(3H, s), 0.98(3H, s), 0.89(6H, s), 0.78(3...
Embodiment 2
[0049] Dissolve 100g of total ginsenosides in 1.3L of anhydrous n-butanol, add 36g (1.57mol) of sodium particles while stirring rapidly until there are no bubbles, then continue to feed oxygen, heat to 95°C, and react for 18h. After the reaction, Cool to room temperature, wash with 1L of n-butanol-saturated water three times to remove excess alkali and generated salt, then recover n-butanol under reduced pressure, disperse the reactant (about 70 g) in 1L of aqueous solution, and wash with 1L of n-butanol Ethyl acetate was extracted three times, and ethyl acetate was recovered and dried to obtain 43g of ethyl acetate extract. The ethyl acetate extract was subjected to macroporous adsorption column chromatography, and EtOH-H 2 Gradient elution with O (50%-100%) yielded 12 g of protopanaxadiol and 3.8 g of protopanaxatriol.
[0050] Its NMR data is consistent with the literature, and the total TLC of the reference substance is a spot.
Embodiment 3
[0052] Panax ginseng total saponins 100g, dissolved in 1.1L anhydrous n-butanol, add 46g (2mol) of sodium particles while stirring rapidly until there are no bubbles, then continue to feed oxygen, heat to 95°C, react for 15h, after the reaction is completed, cool to room temperature, washed three times with 1L of n-butanol saturated water to remove excess alkali and salt produced, then recover n-butanol under reduced pressure, disperse the reactant (about 80g) in 1L of aqueous solution, wash with 1L of ethyl acetate Ethyl acetate was extracted three times, ethyl acetate was recovered, dried to obtain 47g ethyl acetate extract, ethyl acetate extract was subjected to ODS column chromatography, and EtOH-H 2 Gradient elution with O (50%-100%) yielded 10.7 g of protopanaxadiol and 3.0 g of protopanaxatriol.
[0053] Its NMR data is consistent with the literature, and the total TLC of the reference substance is a spot.
[0054] 2 preparation of injection
[0055] Example 1
[005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com