Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
55 results about "Antiepileptic Agents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
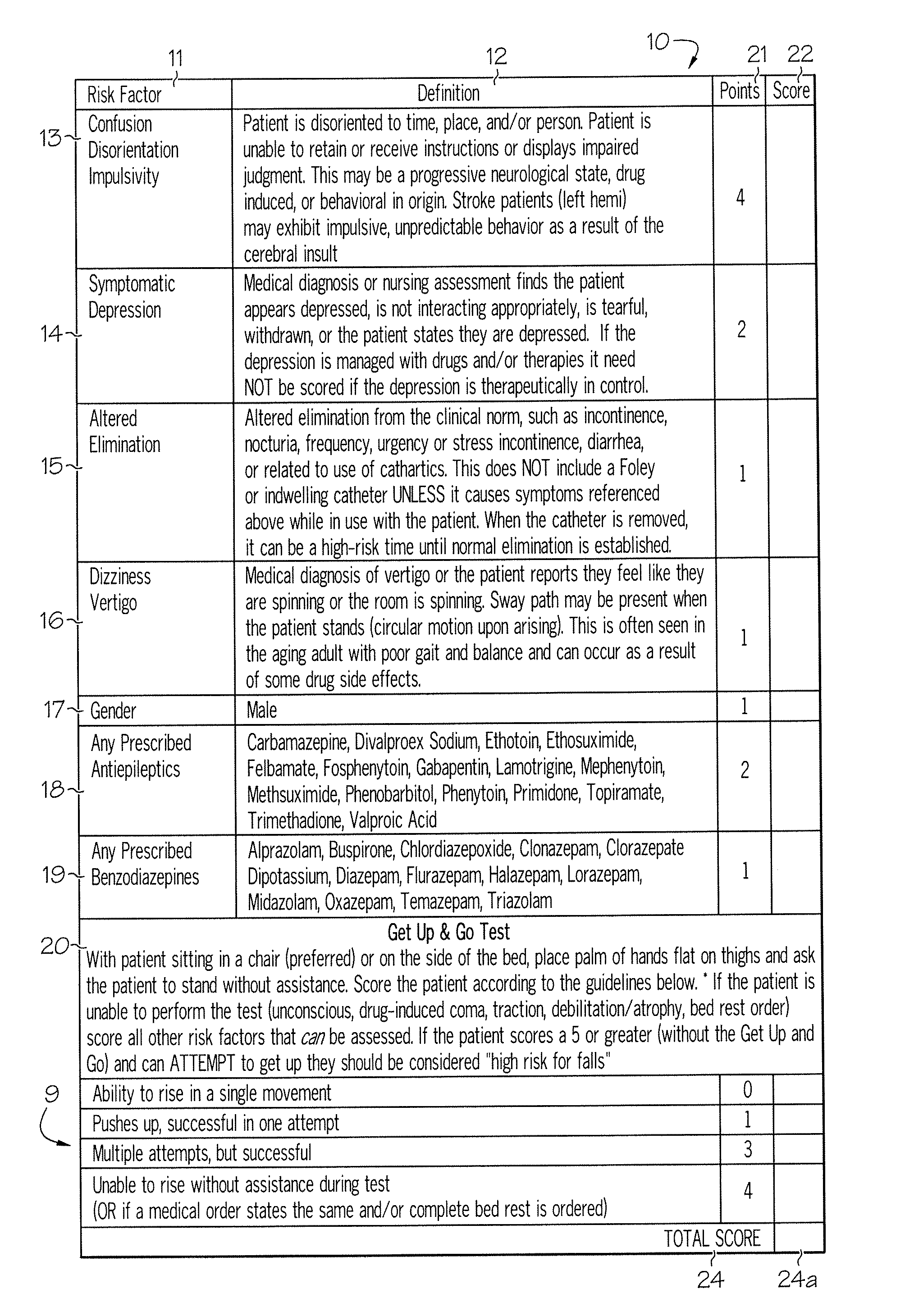
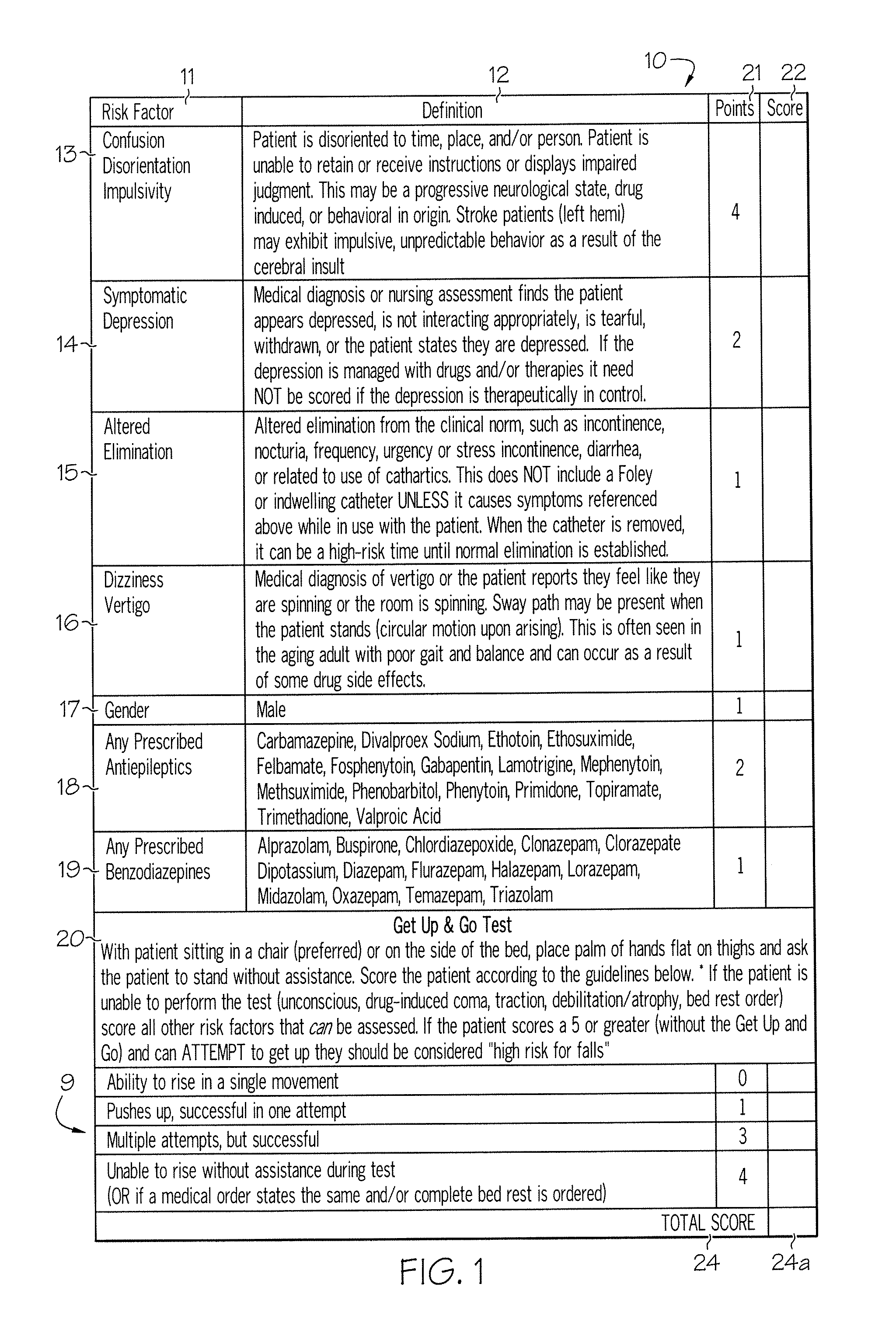
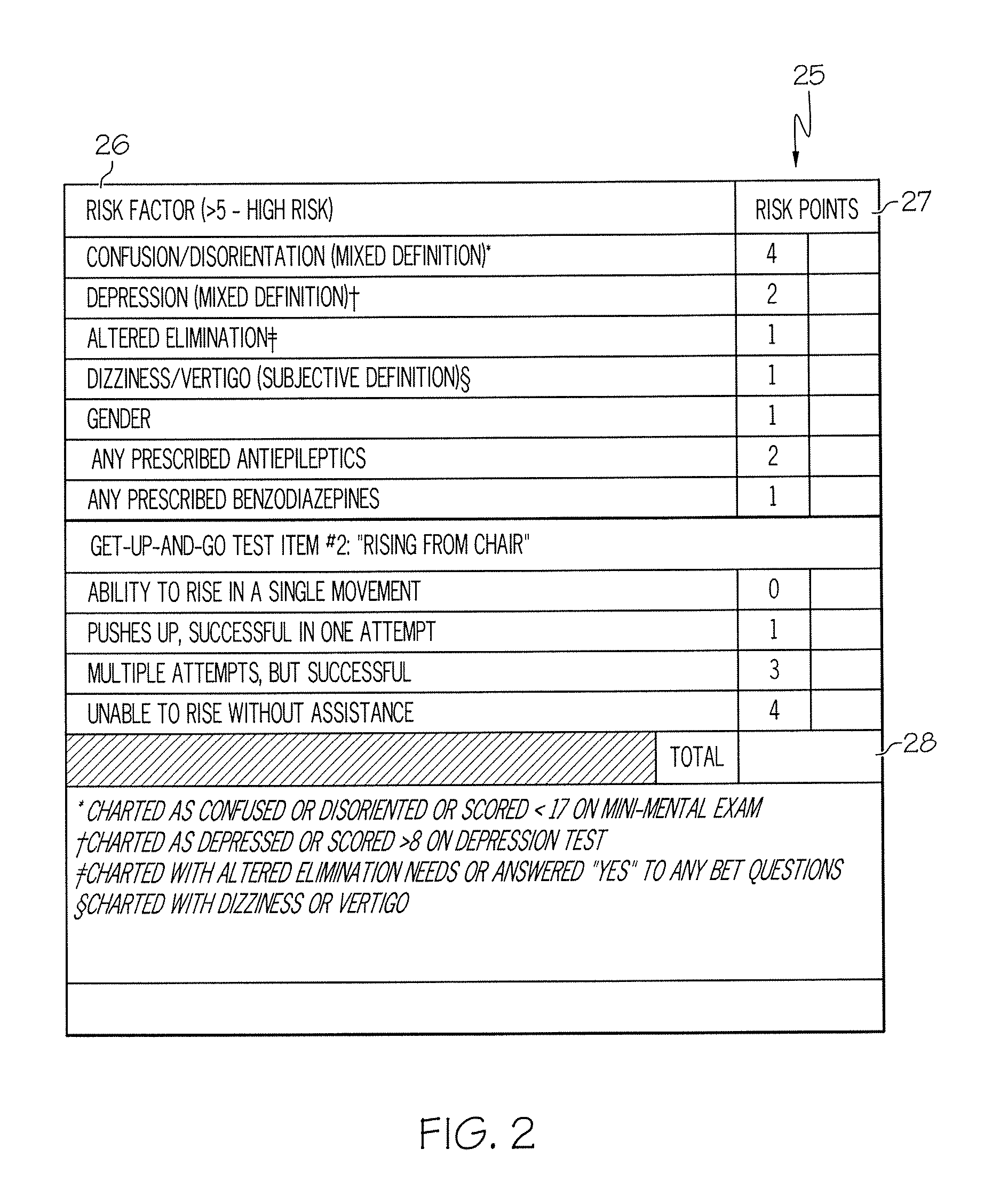
Method and system for assessing fall risk
ActiveUS20080009686A1Easy to addAccurate predictionData processing applicationsTherapiesBenzodiazepineTotal risk
A method and system for determining the fall risk of a patient is provided. The method includes the evaluation of a patient to determine whether the patient exhibits one or more intrinsic fall risk factors selected from a group consisting of confusion, depression, altered elimination, dizziness, male gender, antiepileptic / anticonvulsant prescriptions and benzodiazepine prescriptions. A specific point value is assigned to each of the intrinsic risk factors found to be exhibited by the patient. A mobility test is also performed on the patient to evaluate the patient's ability to rise from a seated position, and a specific mobility test point value is assigned to the patient based upon the patient's performance of the mobility test. Each intrinsic risk factor's specific point value is then summed together with the specific mobility test point value to achieve a total risk score, and the patient's fall risk is determined based on the total risk score. An intervention process may be developed for the patient based on the patient's fall risk.
Owner:AHI OF INDIANA
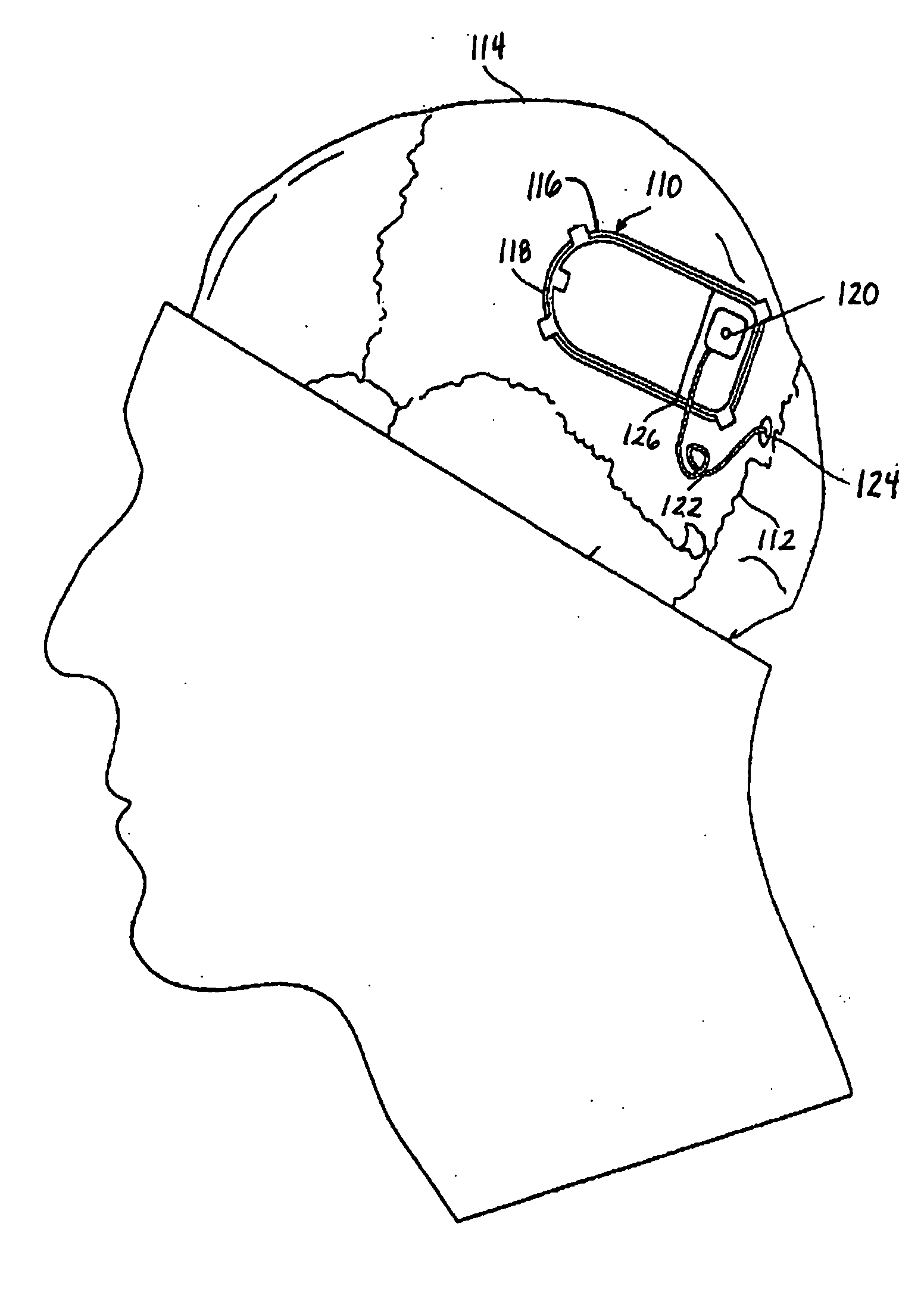
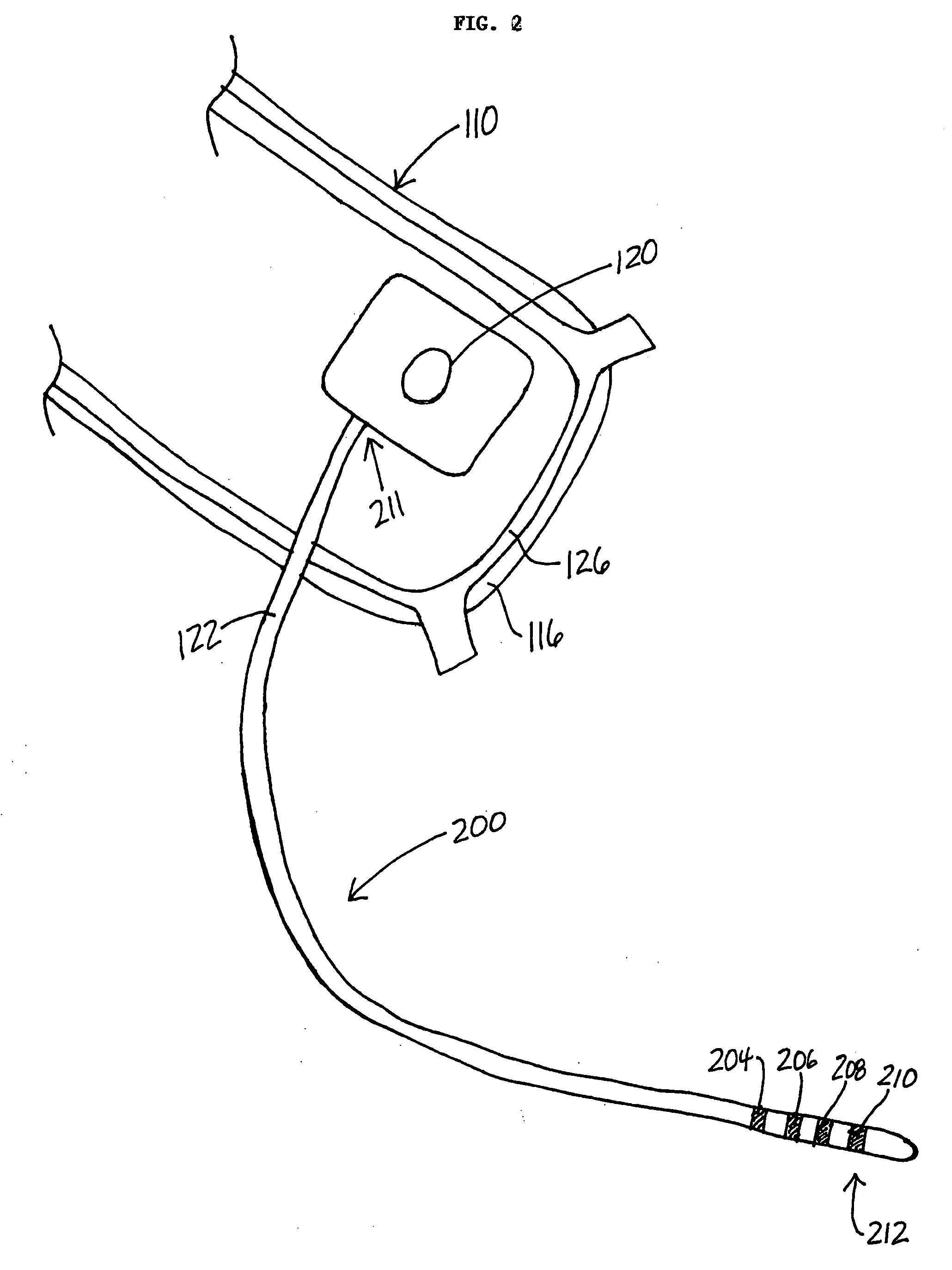
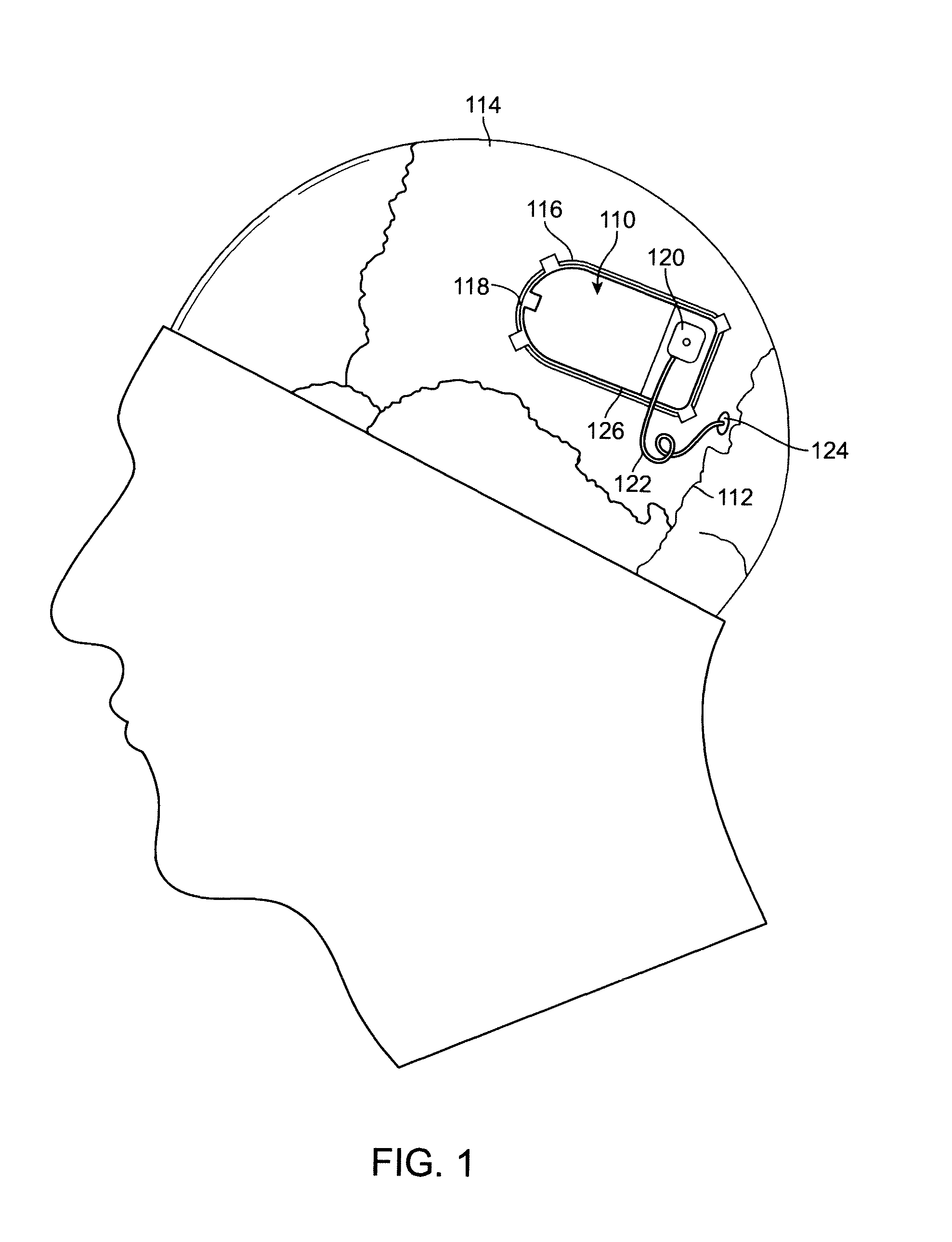
Drug eluting lead systems
Medical electrical lead systems and related methods are described. The lead systems may be configured to be at least partially implanted in neural tissue of a subject, such as a brain of a subject. Some variations of the lead systems may comprise a lead body, an electrode connected to the lead body, and a bioactive agent. The electrode and / or lead body may comprise a substrate, and the bioactive agent may be supported by the substrate (e.g., by a substantial portion of the area of the substrate). Examples of bioactive agents that may be used in the lead system include antiproliferative agents, bactericidal agents, bacteriostatic agents, antiepileptic agents, and / or antifungal agents. Methods described herein may comprise coating a lead body and / or an electrode of a medical electrical lead system with at least one bioactive agent, where the lead body and the electrode are connected to each other.
Owner:NEUROPACE
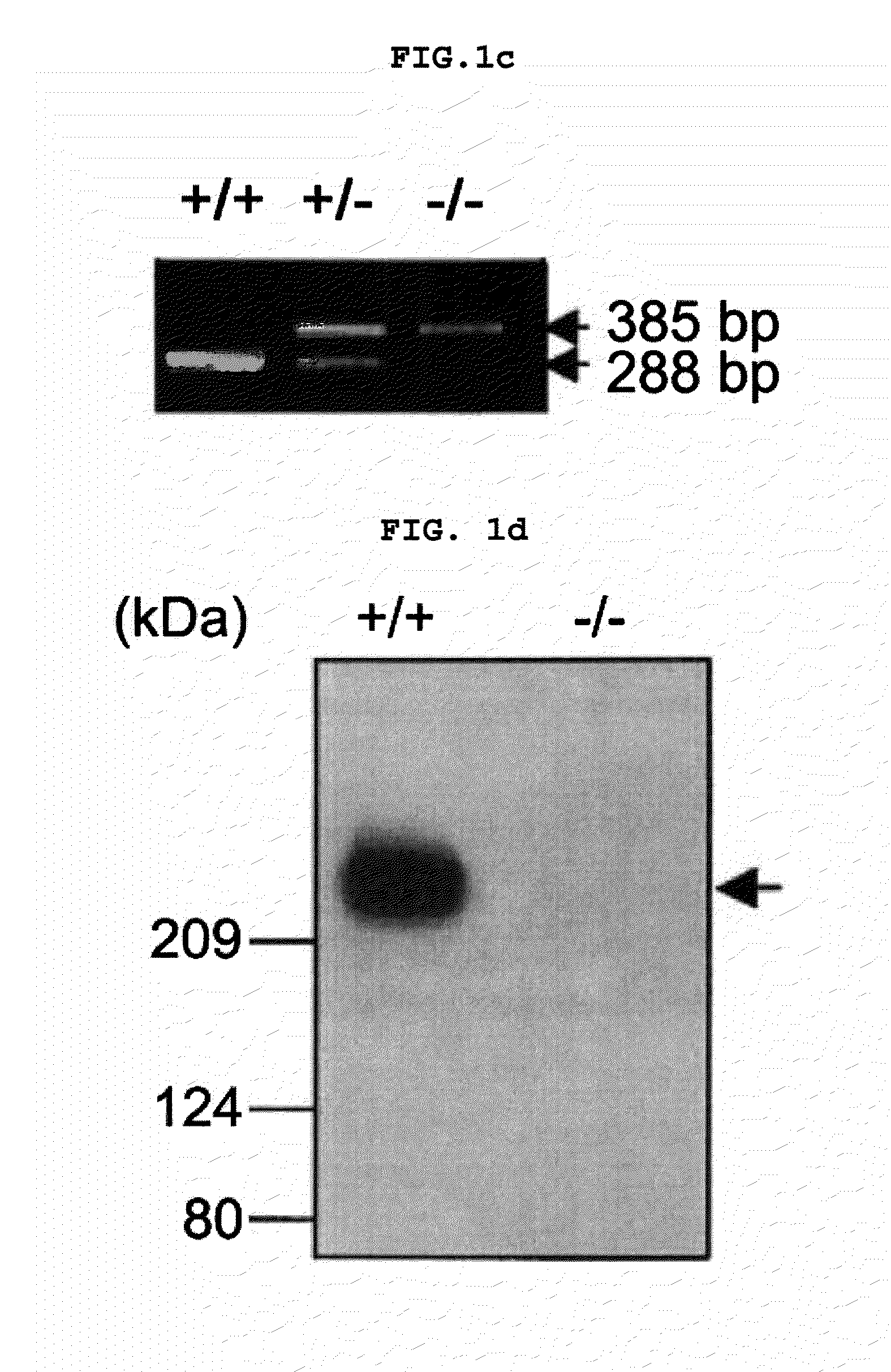
Transgenic mouse whose genome comprises a homozygous disruption of its α1G gene, a method of preparing the same and use thereof
InactiveUS7626076B2Compounds screening/testingCell receptors/surface-antigens/surface-determinantsNervous systemKnockout animal
The disclosure concerns a method for resistance of epilepsy by suppressing the function of alpha 1G protein of T-type calcium channels, use of suppressor of alpha 1G protein for prevention or treatment for epilepsy, knockout mice resisting epilepsy by disrupting alpha 1G subunit of T-type calcium channel, and preparation method thereof. The α1G-knockout transgenic mouse can be used for investigating the relationship between diseases particularly neuropathy or psychopathy and the function of α1G T-type calcium channel via various behavioral tests since α1G subunit is mainly expression in central nervous system (CNS) and pheripheral nervous system (PNS). Further, the α1G-knockout transgenic mouse can be used for screening antileptic agents.
Owner:ORIENTBIO
Drug eluting lead systems
Medical electrical lead systems and related methods are described. The lead systems may be configured to be at least partially implanted in neural tissue of a subject, such as a brain of a subject. Some variations of the lead systems may comprise a lead body, an electrode connected to the lead body, and a bioactive agent. The electrode and / or lead body may comprise a substrate, and the bioactive agent may be supported by the substrate (e.g., by a substantial portion of the area of the substrate). Examples of bioactive agents that may be used in the lead system include antiproliferative agents, bactericidal agents, bacteriostatic agents, antiepileptic agents, and / or antifungal agents. Methods described herein may comprise coating a lead body and / or an electrode of a medical electrical lead system with at least one bioactive agent, where the lead body and the electrode are connected to each other.
Owner:NEUROPACE
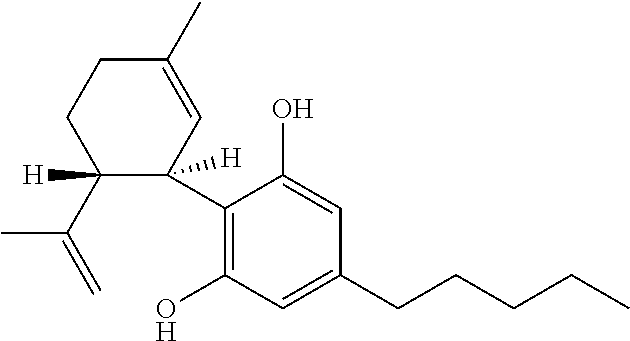
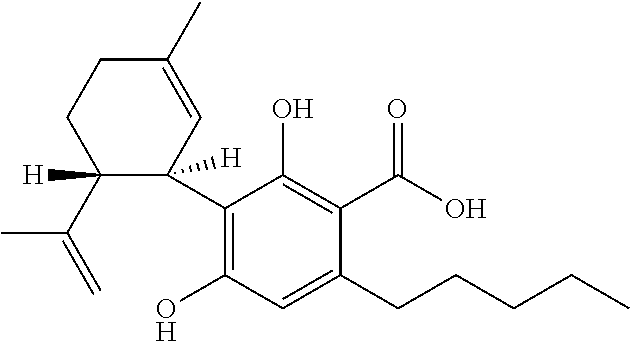
Use of cannabinoids in the treatment of epilepsy
InactiveUS20200352878A1Reduce seizuresPoor response rateNervous disorderHydroxy compound active ingredientsAntiepileptic AgentsStiripentol
The present invention relates to the use of cannabidiol (CBD) in the treatment of patients with childhood-onset epilepsy who are concurrently taking one or more antiepileptic drugs that works via GABA receptor agonism. Preferably the AED is stiripentol. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD.
Owner:GW RES LTD
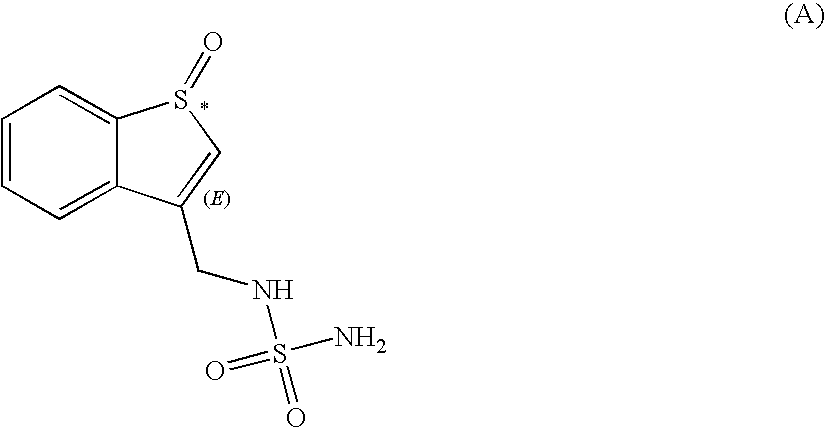
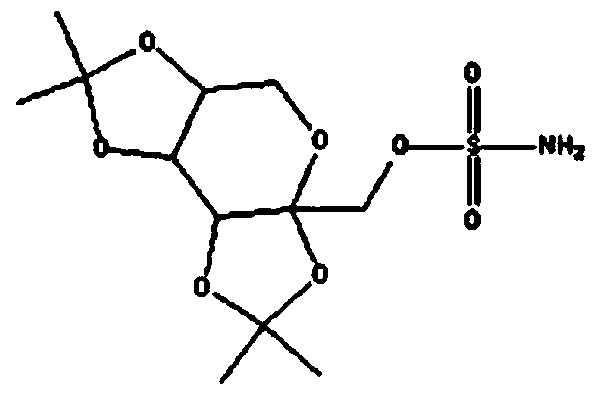
Sulfamide derivative useful for the treatment of epilepsy
The present invention is directed to novel sulfamide derivatives, pharmaceutical compositions comprising said compounds and methods for the treatment of epilepsy and related disorders comprising administering to a subject in need thereof, said compounds, either alone or as co-therapy with one or more anticonvulsant and / or anti-epileptic agents.
Owner:JANSSEN PHARMA NV
Application of total flavonoid extract of tagetes patula in antiepileptic drug
The invention relates to an application of a total flavonoid extract of tagetes patula in antiepileptic drug. A method comprises the following steps: crushing whole herb of the tagetes patula, conducting reflux extraction by virtue of ethanol, and implementing degreasing by virtue of petroleum ether; and then, implementing macroporous adsorption resin purification, so that the total flavonoid of tagetes patula is obtained, wherein the content of the total flavonoid of tagetes patula is greater than 30%; and the total flavonoid of tagetes patula, together with medicinal adjuvants, can be prepared into granules, tablets or capsules as drugs for therapy or adjuvant therapy of epilepsy. Pharmacological tests prove that the total flavonoid is relatively good in pharmacological effect on a pentrazole-induced mouse acute epilepsy model, and the total flavonoid can obviously prolong an incubation period of pentrazole-induced mouse epileptic seizure, reduce the occurrence rate of tonic convulsion in experimental epileptic mice induced by pentrazole and prolong the death time of the pentrazole-induced epileptic mice.
Owner:蔡德成 +1
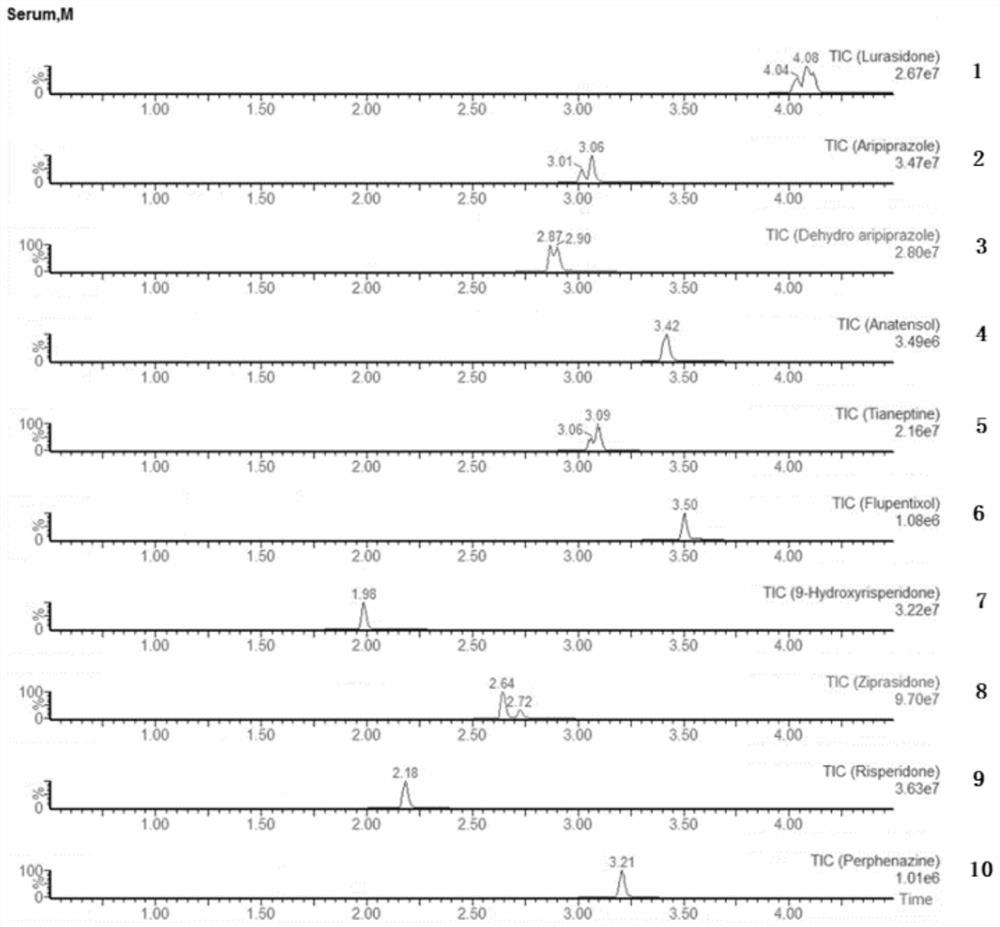
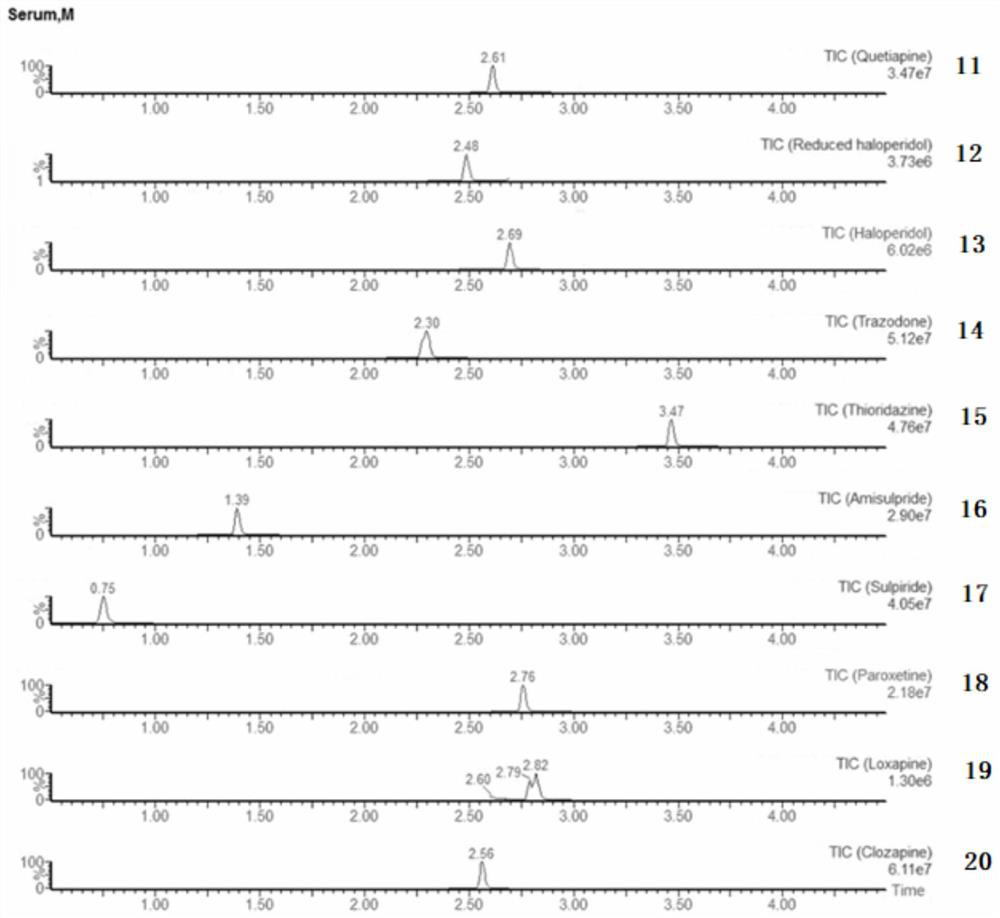
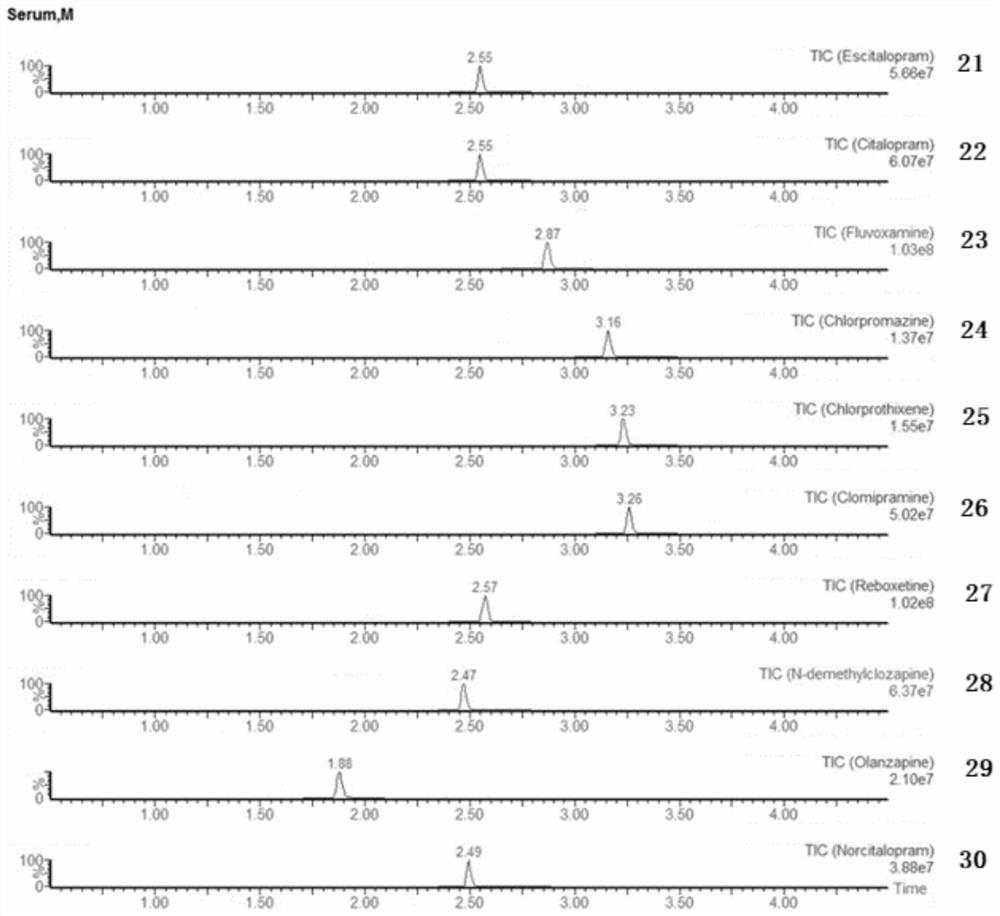
Detection kit for antiepileptic drugs in serum and application thereof
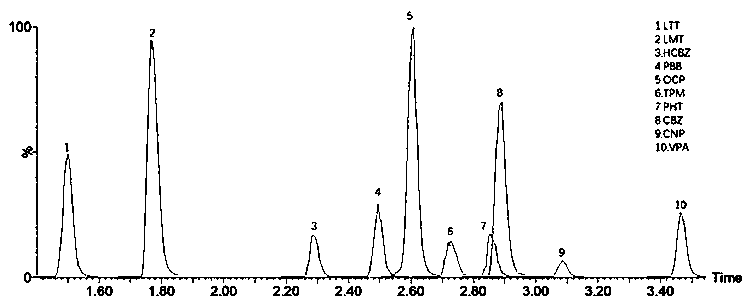
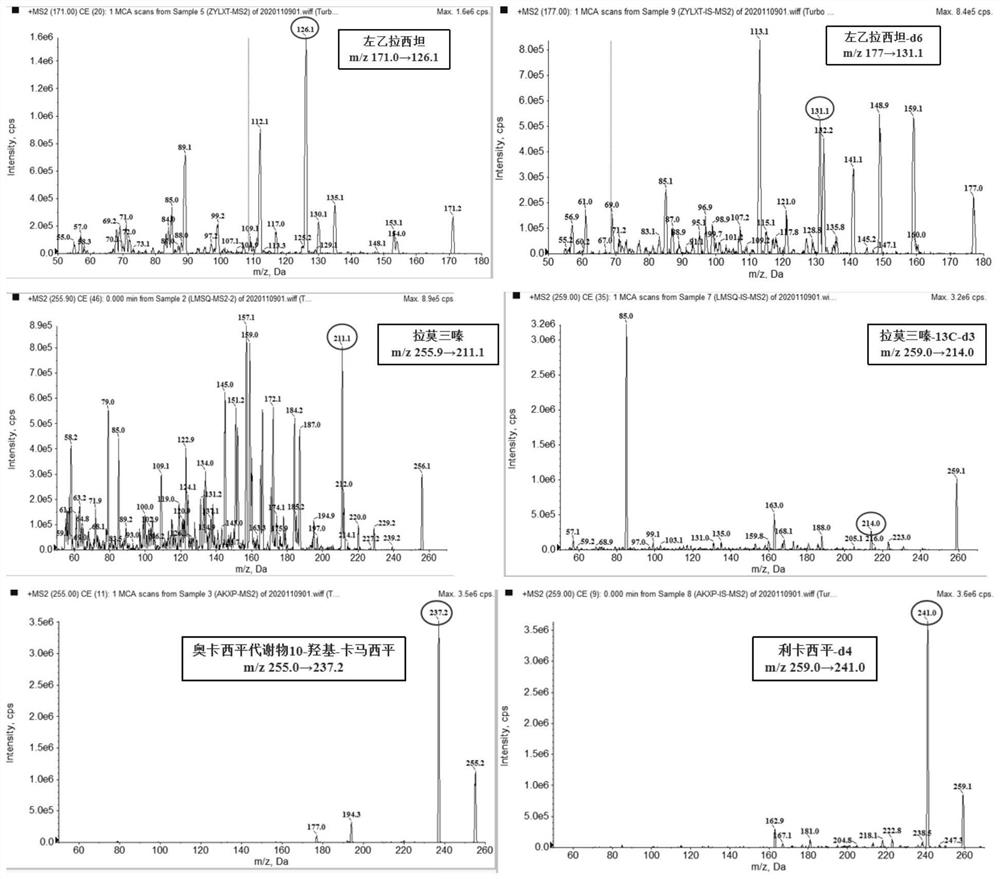
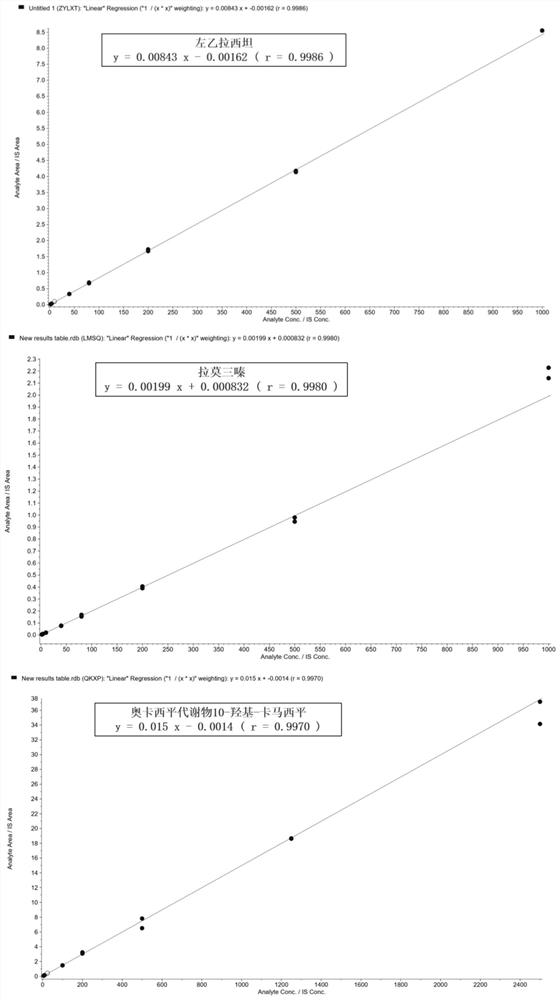
The invention discloses a detection kit for antiepileptic drugs in serum and application thereof, and belongs to the technical field of drug analysis. The kit disclosed by the invention adopts an LC-MS / MS method; ten common antiepileptic drugs can be detected at one time; the kit is simple in pretreatment process, low in cost, high in sensitivity and high in specificity; separation and detection of the antiepileptic drugs are completed within 6 min, the accuracy and precision basically meet the requirements, the kit can be used for quantitative analysis of the clinical antiepileptic drugs, anda reliable detection method is provided for monitoring the treatment concentration of the clinical antiepileptic drugs.
Owner:NANJING PINSHENG MEDICAL TECH CO LTD
Kit and detection method for accurately determining blood concentration of multiple antiepileptic drugs in human serum
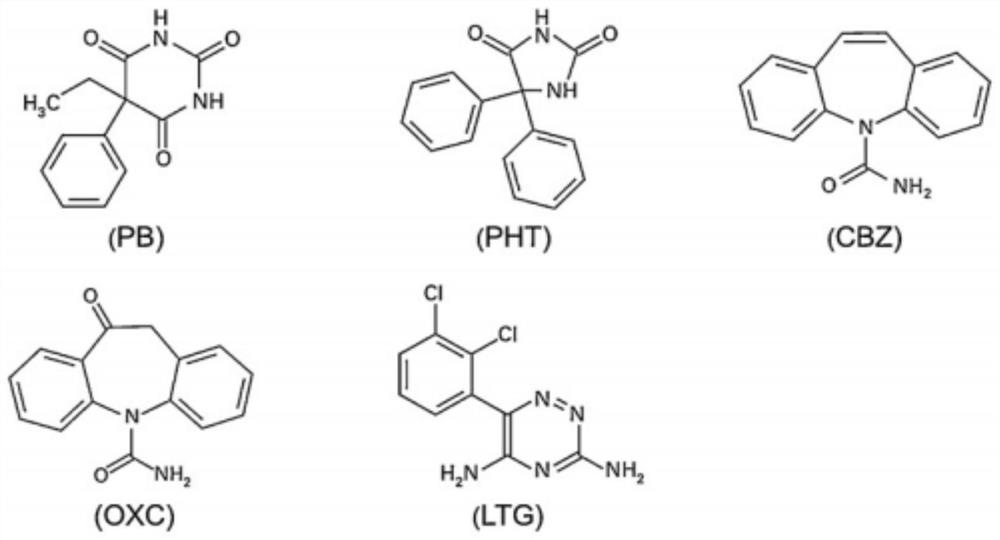
The invention discloses a kit and a detection method for accurately determining the blood concentration of multiple antiepileptic drugs in human serum. The kit mainly comprises a calibration product mother liquor, methanol or acetonitrile is used as a diluent to prepare the calibration product mother liquor comprising six concentration points, the calibration product mother liquor includes lamotrigine, phenobarbital, oxcarbazepine, carbamazepine and phenytoin in order; the kit also comprises an internal standard, a quality control material, a matrix correction solution and an extract, whereinthe extract is a tert-butyl methyl ether solution, a reconstitution solution and a mobile phase. Complex purification steps are not needed, and the required analysis time is short; and the detection speed is increased.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Application of panaxadiol saponins fraction in preparing medicine for preventing epilepsia
ActiveCN102743401AOvercome curative effectOvercome securityOrganic active ingredientsNervous disorderMedicinal herbsAntiepileptic Agents
The invention provides application of a panaxadiol saponins fraction in preparing a medicine for preventing epilepsia. The component comprises the following main constituents: ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rb3, ginsenoside Rc and ginsenoside Rd, and the medicine is prepared from active constituents in the single panaxadiol saponins fraction or from the active constituents in the single panaxadiol saponins fraction and other medicines. By taking one of five panaxadiol saponins: ginseng, American ginseng rhizome medicinal materials, ginseng stems and leaves, American ginseng stem and leaf medicinal materials and total extractives or total saponins of the ginseng, the American ginseng rhizome medicinal materials, the ginseng stems and leaves and American ginseng stem and leaf medicinal materials as a starting raw material independently or taking a mixture which consists of two or more than two different raw materials as the starting raw material comprehensively, the medicine is prepared by establishing the chromatographic separation and purification technology. According to the medicine prepared from the panaxadiol saponins fraction provided by the invention, an anti-epileptic effect can be greatly improved, and moreover, the toxic and side effects, especially dermatitis and intelligence toxicity, of traditional anti-epileptic medicines can be resisted, therefore, long-standing defects that the anti-epileptic medicines are not enough in curative effect and low in safety are overcome. The structural formula of the panaxadiol saponin is shown in specification.
Owner:ZHEJIANG UNIV
Bi-path detecting card capable of simultaneously detecting carbamazepine and sodium valproate and detecting method of same
InactiveCN102331499AEasy to manufactureLow detection costMaterial analysisAntiepileptic AgentsEngineering
The invention provides a bi-path detecting card capable of simultaneously detecting carbamazepine and sodium valproate and a detecting method of the same, and belongs to the technical field of the monitoring on the blood concentration of antiepileptic drug. In the invention, a detecting window and a sample adding hole are arranged on the surface of a shell of the detecting card; a testing bar is arranged in the shell; a nitrocellulose film is adhered to the middle part of a backing board of the testing bar; a water absorbent film is adhered to one end of the backing board; a sample pad is adhered to the other end of the backing board; two segments of colloidal gold films are arranged between the water absorbent film and the sample pad in an adhesive manner; the colloidal gold films are respectively glass fiber films containing colloidal gold markers of monoclonal antibodies resistant to the carbamazepine and the sodium valproate; three separated imprint display strips are transversely arranged on the nitrocellulose film, and include a detecting strip containing carbamazepine protein conjugate, an another detecting strip containing sodium valproate protein conjugate and a quality control strip containing antibodies resistant to a rabbit or a rat; the sample pad is just opposite to the sample adding hole; and the nitrocellulose film is just opposite to the detecting window. The detecting card can be used for simultaneously detecting components of two anticonvulsants in a serum sample, and has the advantages of saved cost for detection, convenience for use, quickness in detection, high sensitivity and accuracy in result.
Owner:无锡安迪生物工程有限公司
Solid Forms Of An N-(Phenylmethyl)Propanamide Derivative And Processes Of Preparation
ActiveUS20110034731A1Carboxylic acid amides optical isomer preparationCarboxylic acid amide separation/purificationEnantiomerMedicinal chemistry
The invention relates to solid forms of the anti-epileptic agent lacosamide (I). The invention also relates to mixtures of solid forms of lacosamide. The invention further relates to mixtures of lacosamide enantiomers crystallized in a conglomerate Form and the use thereof in providing enantiomerically enriched lacosamide, preferably lacosamide enriched with the (R)-enantiomer of lacosamide.
Owner:MEDICHEM
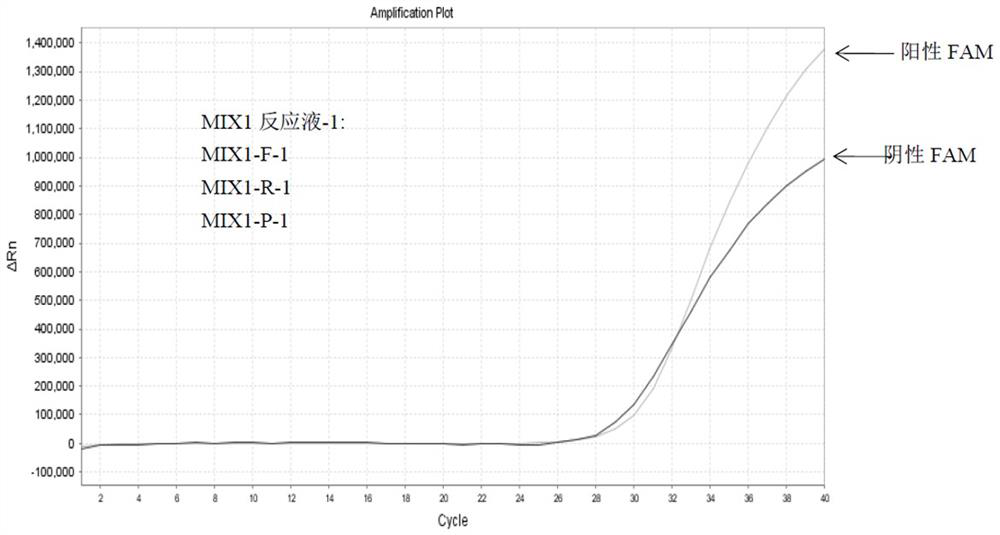
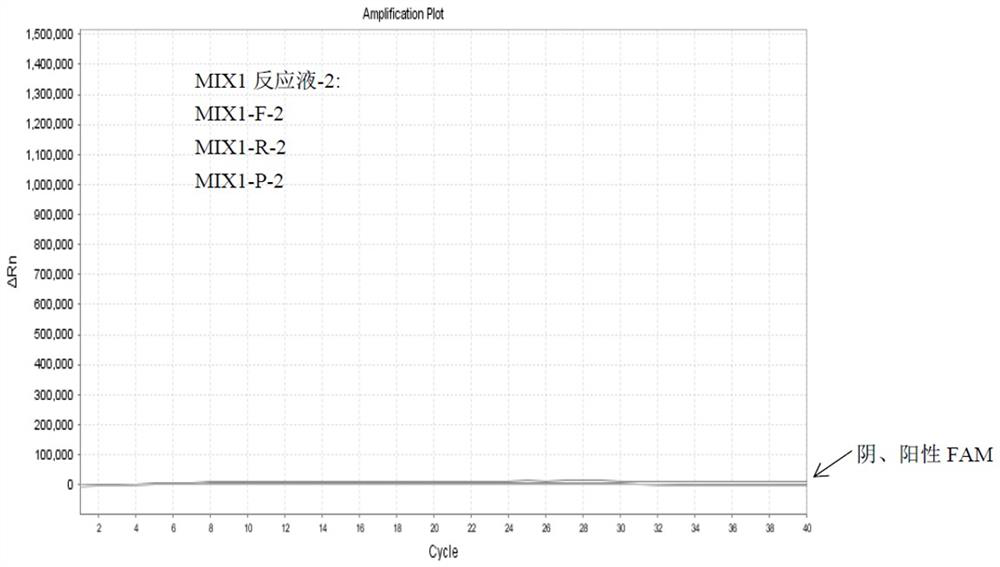
Detection method of HLA-B*1502 gene, detection kit and application thereof
The invention belongs to the technical field of biology, and provides a reagent for detecting human leukocyte antigen B site 1502 genotype (HLA-B*1502), application of the reagent in preparation of akit for guiding administration of antiepileptic drugs such as carbamazepine, oxcarbazepine, phenytoin and lamotrigine, a corresponding kit and a detection method of the kit. According to the HLA-B*1502 genotyping, three groups of MIX reaction solutions are detected by adopting a PCR-fluorescent probe method, and target nucleic acid molecules are circularly amplified through an amplification reaction, so that a fluorescence generation group is indirectly combined with an amplified target nucleotide sequence; the amount of fluorescence generated by the fluorescence generation group is determined, and the existence of the target nucleotide is determined. The detection reagent comprises a nucleic acid amplification system of three groups of MIX reaction solutions, wherein the nucleic acid amplification system comprises an upstream primer 1 and a downstream primer 1 which can be combined with target nucleotide, and an upstream primer 2 and a downstream primer 2 which can be combined with the target nucleotide; and three groups of probes 1 and probes 4 of a fluorescence detection system matched with the nucleic acid amplification system.
Owner:上海恩元生物科技有限公司
Method for simultaneously determining contents of various antiepileptic drugs in blood and application of method
ActiveCN112946101AObvious superiorityHigh sensitivityComponent separationAntiepileptic AgentsDosing regimen
The invention provides a method for simultaneously determining the content of various antiepileptic drugs in blood and application of the method, and belongs to the technical field of medicine detection. According to the method of the invention, a protein precipitation method is combined with HPLC-MS / MS to determine the content of the nine antiepileptic drugs in the plasma. The determination method of the nine antiepileptic drugs in the plasma is fully verified from the aspects of specificity, linearity, sensitivity, accuracy, precision, matrix effect, recovery rate, stability and the like; finally, the method is applied to animal pharmacokinetic research of oral medication, can calculate pharmacokinetic parameters through a non-atrioventricular model, can give the real level of the real concentration of the medicine for treating epilepsy in the body of a patient and can guide doctors to reasonably formulate a drug administration scheme, and therefore, the patient can reasonably take the medicine, side effects can be reduced; and the method has important significance on achieving the optimal curative effect of the patient.
Owner:JINAN YING SHENG BIOTECH
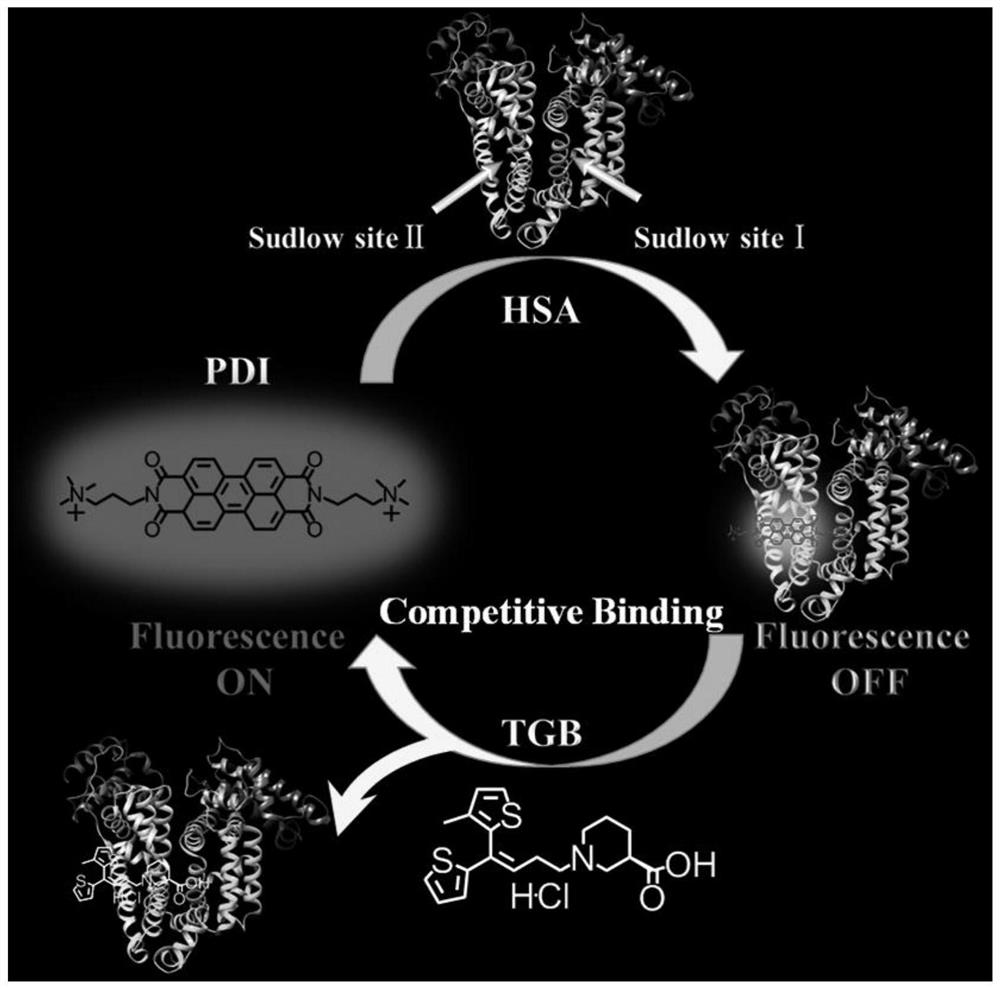
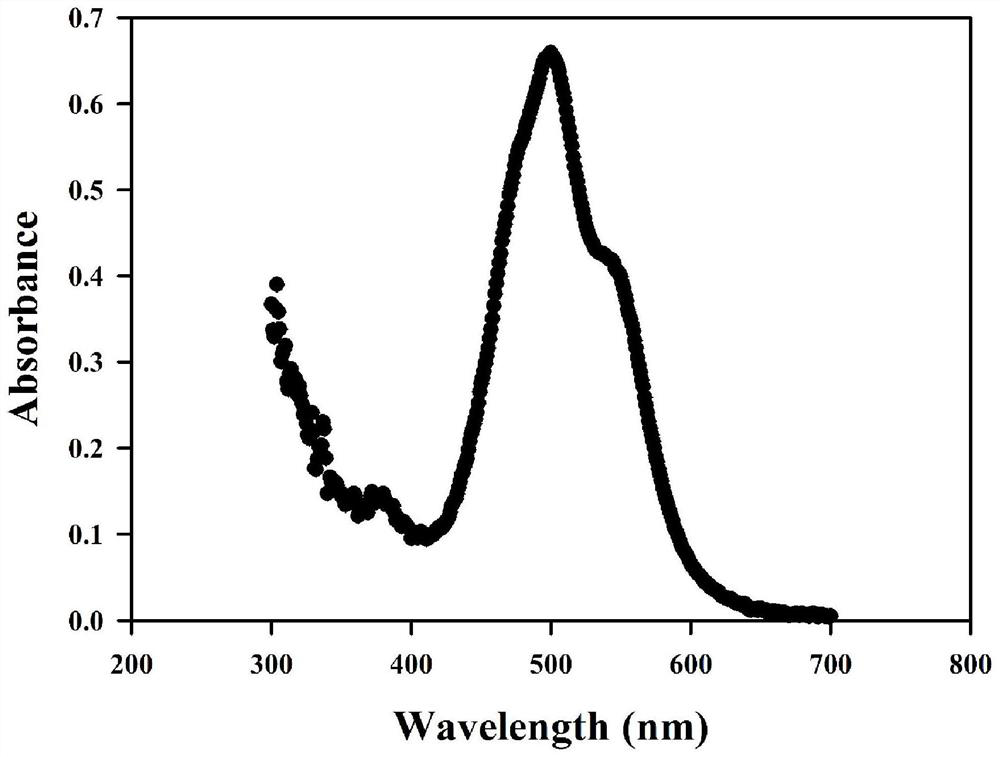
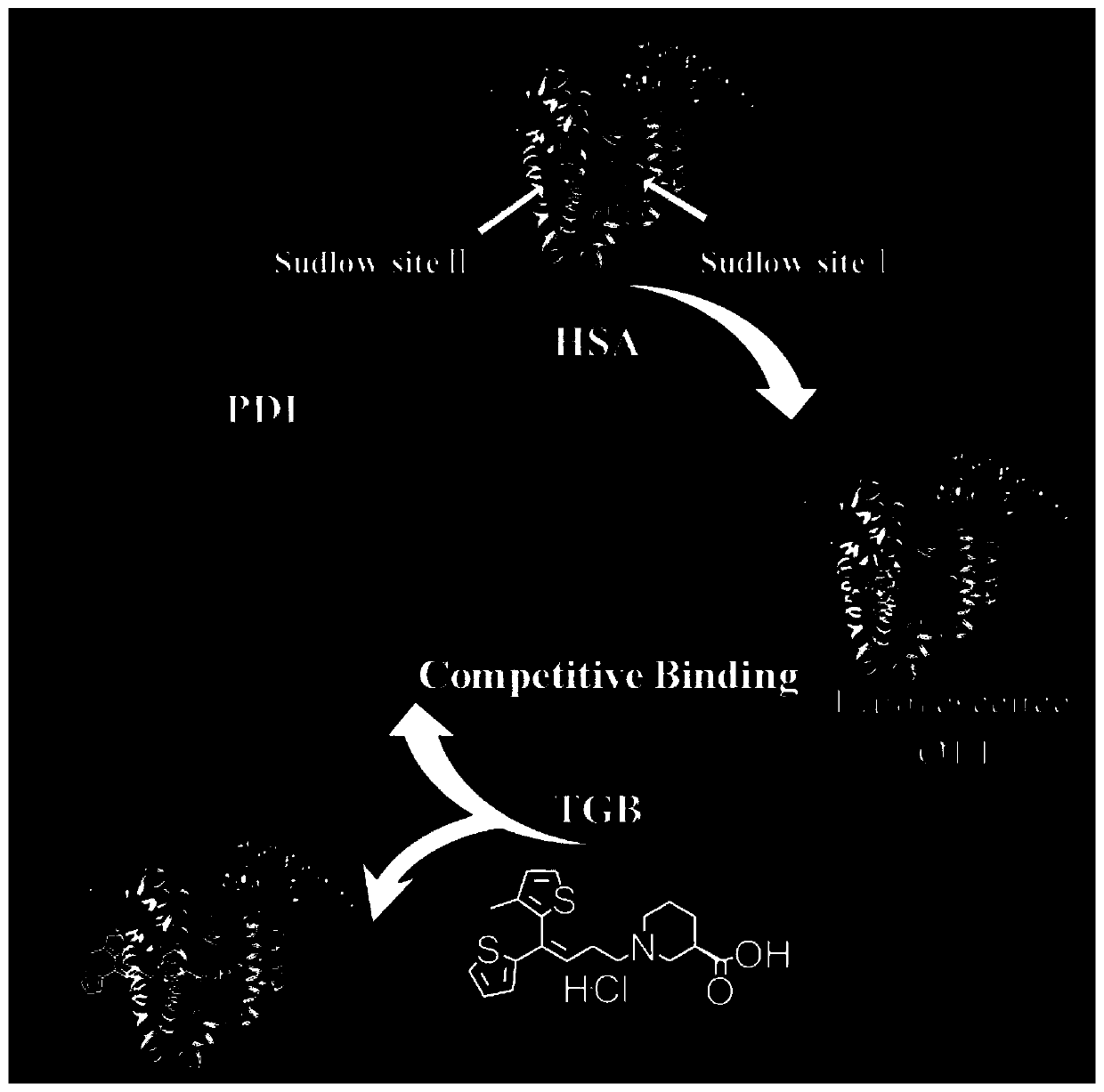
Fluorescence detection method for antiepileptic drug-tiagabine hydrochloride (TGB)
ActiveCN110308121AEasy to detectHigh sensitivityFluorescence/phosphorescenceAntiepileptic AgentsPharmacology
The invention discloses a fluorescence detection method for an antiepileptic drug-tiagabine hydrochloride (TGB). Through study on interaction among dye perylene diimide derivative (PDI), human serum albumin (HSA) and the tiagabine hydrochloride (TGB), the fluorescence of the PDI is found to be quenched by the HSA, and the study indicates that the PDI is bound at a site II of the HSA, the TGB is able to compete for being bound at the site II of the HSA, the PDI is beaten at the site II of the HSA, and fluorescence recovery is thus realized. On the basis, the invention provides a TGB fluorescence detection method. With the PDI-HSA as a probe, under effects of the TGB, fluorescence recovery is realized, and the TGB is thus detected. The method has the advantages of original innovation, simpledetection process, mild experimental condition, high sensitivity, good selectivity, fast detection time and low detection limit and the like, and has good social value and application prospects.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
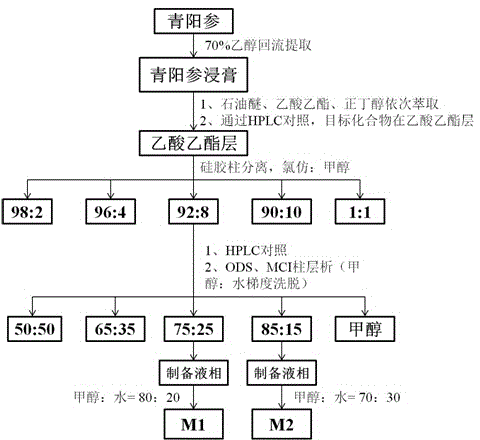
Cynanchum otophyllum saponin composition and application thereof
ActiveCN104644663AGood oral absorption propertiesClear compositionOrganic active ingredientsNervous disorderAntiepileptic AgentsPharmaceutical drug
The invention discloses a cynanchum otophyllum saponin composition. The cynanchum otophyllum saponin composition comprises cynanchum otophyllum saponin M1 and cynanchum otophyllum saponin M2, and the cynanchum otophyllum saponin M1 and the cynanchum otophyllum saponin M2 are combined at a mass ratio of 1:1. Tests show that the cynanchum otophyllum saponin composition can be taken orally and absorbed, can be used together with phenobarbital sodium, can be used for enhancing the antiepileptic effect of phenobarbital sodium, is superior to the single cynanchum otophyllum saponin M1 and the single cynanchum otophyllum saponin M2 in effect and can be prepared into an antiepileptic medicine.
Owner:KUNMING UNIV OF SCI & TECH
Pharmaceutical composition comprising racetam and carnitine and process for its preparation
InactiveUS20110052556A1Avoid side effectsMinimize adverse effectsBiocideNervous disorderAntiepileptic AgentsMedicine
The present invention refers to a pharmaceutical composition comprising racetam and carnitine. Said pharmaceutical composition may further comprise coenzyme Q10 and / or agent associated with mitochondrial and / or metabolic disturbance, such as hypocholesterolemic agent of the statins group, a hypoglycemic agent, or an antiepileptic agent. The invention also includes a method of treatment for mitochondrial disturbances comprising administration of the pharmaceutical composition of the invention.
Owner:BIOLAB SANUS FARMACEUTICA LTD
Quality control product and/or calibration product for simultaneously monitoring 78 neuropsychiatric drugs
ActiveCN114739774AReduce matrix effectImprove accuracyComponent separationPreparing sample for investigationAntiepileptic AgentsButylated hydroxytoluene
The invention provides a quality control material and / or a calibration material for simultaneously monitoring 78 neuropsychiatric drugs as well as a preparation method and application of the quality control material and / or the calibration material. The quality control material and / or the calibration material comprises the 78 neuropsychiatric drugs, a matrix and an antioxidant, the antioxidant is a combination of ascorbic acid, butylated hydroxytoluene and ethylenediamine tetraacetic acid; the mass content of the antioxidant in the quality control product and / or the calibration product is 0.2-0.5%; the 78 kinds of neuropsychiatric drugs comprise 15 kinds of antiepileptic drugs, 23 kinds of antipsychotic drugs and 40 kinds of antidepressant drugs. The quality control product and / or the calibration product prepared by adding the antioxidant in a specific combination have high stability, effectively eliminate the matrix effect generated in the process of mass spectrometric detection of the neuropsychiatric drugs of the serum sample, and significantly improve the accuracy and indoor precision of mass spectrometric detection; the technical bottleneck is solved for the industrialization and wide application of the technology for detecting the psychotherapy drugs by mass spectrometry.
Owner:SHANGHAI MENTAL HEALTH CENT (SHANGHAI PSYCHOLOGICAL COUNSELLING TRAINING CENT) +1
Topiramate sustained-release capsule and preparation method thereof
PendingCN110638791AEffective and stable blood drug concentrationOrganic active ingredientsNervous disorderAntiepileptic AgentsSustained Release Capsule
The invention relates to the field of antiepileptic drug preparations, in particular to a topiramate sustained-release capsule and a preparation method thereof. The topiramate sustained-release capsule comprises a drug-containing sustained-release pill core, an enteric coating layer and an immediate-release layer. The weight ratio of pharmaceutical active ingredient topiramate to the drug-containing sustained-release pill core is 40%-80%, and the weight ratio of the topiramate to the immediate-release layer is 20%-60%. According to the opiramate sustained-release capsule, a part of the pharmaceutical active ingredient topiramate is distributed in the drug immediate-release layer, when the topiramate sustained-release capsule enters the gastrointestinal tract, the drug immediate-release layer begins to disintegrate, the topiramate is dissolved, and the effective blood concentration can be quickly reached, at the moment, the sustained-release part of the drug is not dissolved under the protection of enteric coating, when the drug enters the intestine, the topiramate is released in a sustained mode under the action of the sustained-release coating, and effective and stable blood drugconcentration can be continuously maintained.
Owner:ZHEJIANG POLY PHARMA +1
Combination treatment comprising administration of 2-amino-3,5,5-trifluoro-3,4,5,6-tetrahydropyridines
The present invention is directed to the combined use of BACE1 inhibitor of Formula Iand a compound useful in active or passive Tau immunotherapy, a compound useful in active or passive Aβ peptide immunotherapy, an NMDA receptor antagonists, an acetylcholine esterase inhibitor, an antiepileptic, an anti-inflammatory drug, a Tau aggregation inhibitor or an SSRI for the treatment of neurodegenerative or cognitive disorders.
Owner:H LUNDBECK AS
Method for detecting antiepileptic drugs and metabolites thereof in hair by liquid chromatography-mass spectrometry
PendingCN113092615AEfficient extractionEfficient separationComponent separationAntiepileptic AgentsDrug utilisation
The invention belongs to the technical field of detection of drugs and metabolites thereof and particularly relates to a method for detecting antiepileptic drugs and metabolites thereof in hair by liquid chromatography-mass spectrometry. The invention provides a system which is used for judging whether the dosage of an anti-epileptic drug is excessive or insufficient by detecting or inputting the content of the anti-epileptic drug or metabolites thereof in hair. The detection method comprises the following steps of (1) pretreating a hair sample to obtain a solution to be detected; and (2) detecting the concentration of the anti-epileptic drug in the hair by adopting a liquid chromatography-mass spectrometry method. The detection method is simple, convenient and sensitive, the long-term medication condition of a subject can be reflected by detecting the anti-epileptic medicine in the hair, the hair sample is simple and convenient to take, the storage requirement is low, and the method is suitable for clinical large-scale popularization.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
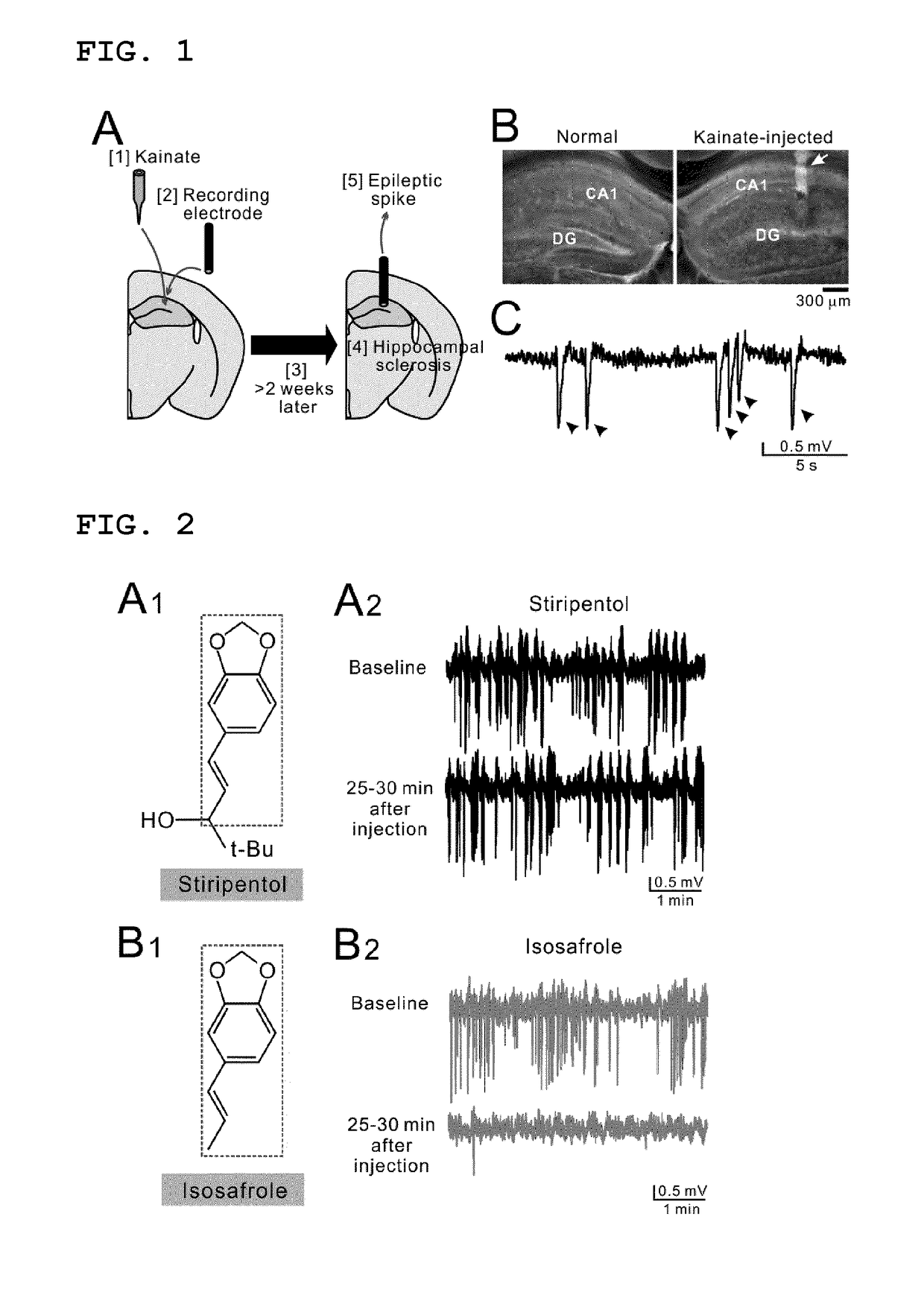
Lactate dehydrogenase inhibitor and antiepileptic drug containing the same
ActiveUS20180015068A1Inhibited refractory epilepsyNervous disorderOrganic chemistryAntiepileptic AgentsDepressant
The invention provides a lactate dehydrogenase inhibitor that makes it possible to suppress refractory epilepsy in which conventional antiepileptic drugs are ineffective, and an antiepileptic drug containing said inhibitor. The lactate dehydrogenase inhibitor of the invention contains a compound represented by formula (III); i.e., isosafrole or a compound having isosafrole as a scaffold, and the antiepileptic drug of the invention has these compounds as an active ingredient.
Owner:UNIV OKAYAMA
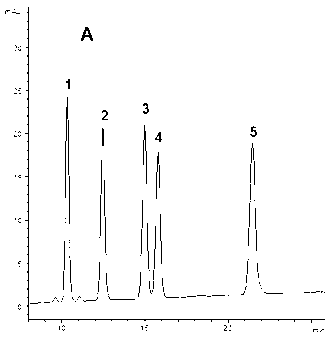
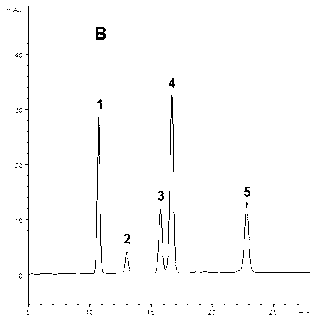
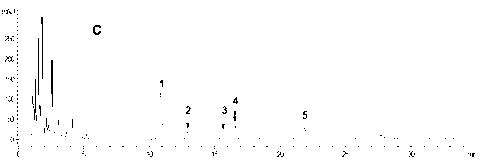
5-substituted phenoxy-1, 2, 4-triazole [4, 3-a] pyridine compound used for antiepileptic medicament, and medical salt thereof
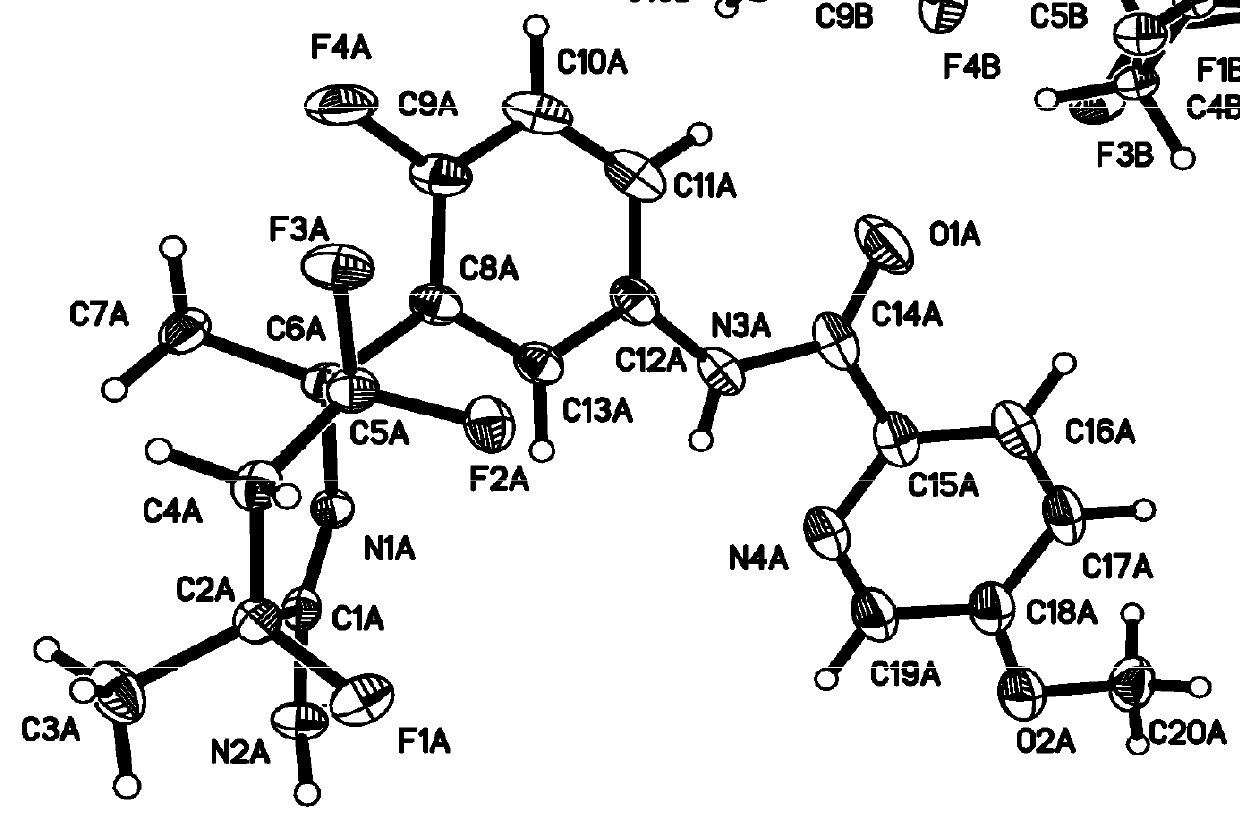
InactiveCN102633794ASmall side effectsOrganic active ingredientsNervous disorderAntiepileptic AgentsSide effect
The invention relates to a 5-substituted phenoxy-1, 2, 4-triazole [4, 3-a] pyridine compound used for antiepileptic medicament, and medical salt of the compound. The invention provides the new 5-substituted phenoxy-1, 2, 4-triazole [4, 3-a] pyridine compound which has comparatively low toxic and side effects and shows the valuable pharmacological properties as anticonvulsant, and addition salt of pharmaceutically acceptable acid of the compound. The formula I shows the compound and the addition salt of the pharmaceutically acceptable acid of the compound, wherein R is selected from all bromoalkanes. The compound is high in anticonvulsion activity, comparatively low in toxic and side effects and high in safety index, thus laying the foundation for the research and application of the antiepileptic medicament.
Owner:ZHEJIANG OCEAN UNIV
A gene detection kit for individualized medication of antiepileptic drugs and its application
ActiveCN113136424BSolve the problem of high incidence of adverse reactionsAccurate and reliable genetic informationMicrobiological testing/measurementDNA/RNA fragmentationAntiepileptic AgentsMultiplex
The invention belongs to the field of gene detection, and in particular relates to a gene detection kit for individualized antiepileptic drug administration and its application. The kit provided by the present invention mainly includes library primer mixture, multiplex PCR library amplification buffer, digestion buffer, ligation buffer, ligase, specific linker, HiFi library amplification buffer, positive / negative quality control, Reporting system, instructions. The kit provided by the invention can be used to detect 10 commonly used anti-epileptic clinical drugs, with a total of 44 genes and 103 detection sites; it realizes the related gene detection and report analysis for the prediction of the curative effect and adverse reactions of anti-epileptic drugs. medication assistance.
Owner:广州合一生物科技有限公司
Pharmaceutical composition comprising racetam and carnitine and process for its preparation
The present invention refers to a pharmaceutical composition comprising racetam and carnitine. Said pharmaceutical composition may further comprise coenzyme QlO and / or agent associated with mitochondrial and / or metabolic disturbance,such as hypocholesterolemic agent of the statins group, a hypoglycemic agent, or an antiepileptic agent. The invention also includes a method of treatment for mitochondrial disturbances comprising administration of the pharmaceutical composition of the invention.
Owner:BIOLAB SANUS FARMACEUTICA LTD
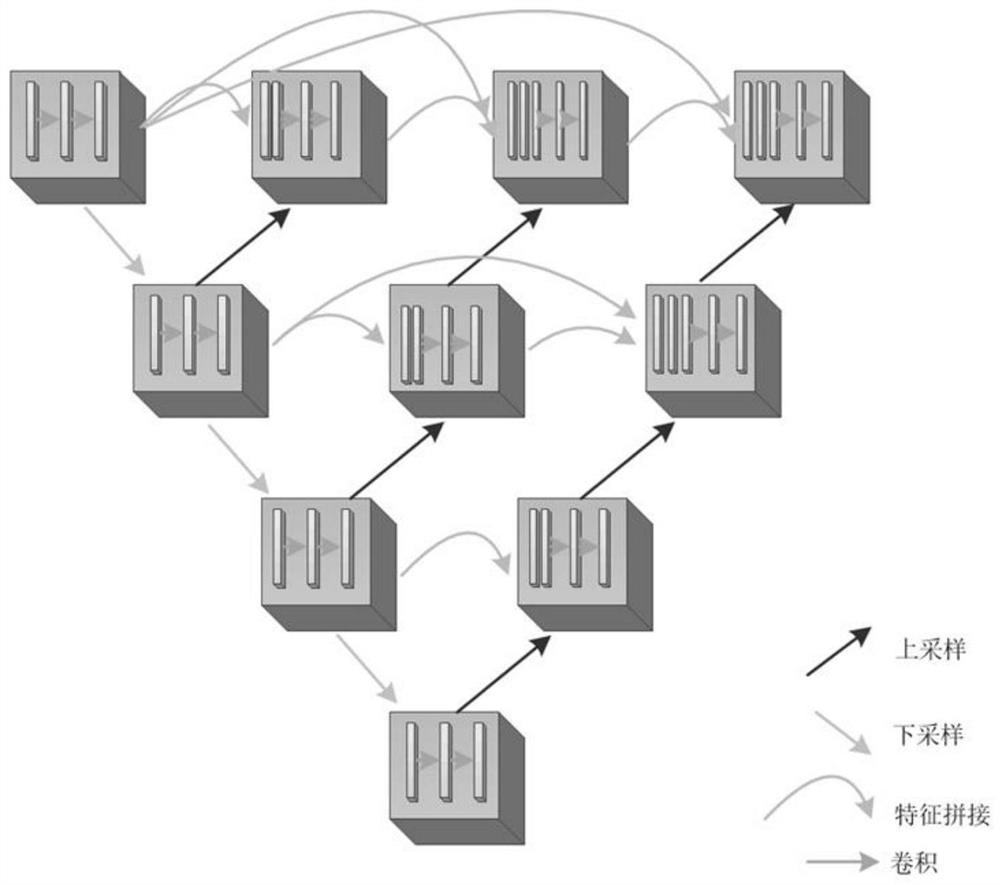
Epilepsy drug treatment outcome prediction method and device based on multi-modal radiomics
PendingCN114711717AReduce labor costsEffective predictionSensorsTelemetric patient monitoringAntiepileptic AgentsAlgorithm
The invention discloses an epilepsy drug treatment outcome prediction method and device. The epilepsy drug treatment outcome prediction method comprises the following steps: acquiring a multi-modal magnetic resonance image of a TSC patient before anti-epilepsy drug treatment; randomly dividing the TSC patients into a training set and a test set according to a proportion; performing region segmentation on each modal magnetic resonance image based on a U-net + + network to obtain a region of interest; performing feature extraction on each region of interest to obtain high-dimensional image omics features; analyzing and screening the high-dimensional image omics features to obtain target image omics features; training a prediction model for the target radiomics characteristics in the training set by using a machine learning algorithm, constructing and obtaining an epilepsy drug treatment outcome prediction model, and verifying the model; and predicting target radiomics characteristics of a patient to be treated by using the constructed epilepsy drug treatment outcome prediction model to obtain a predicted epilepsy drug treatment outcome. According to the method, the drug treatment outcome of the epilepsy patient can be quickly and effectively predicted, and a doctor is assisted in formulating a better treatment scheme.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
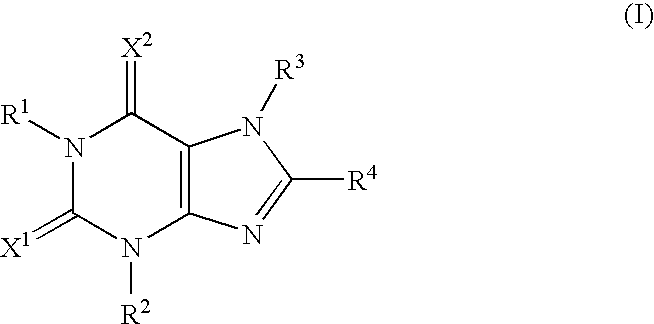
Antiepileptic agent
An antiepileptic agent which contains either a xanthine derivative represented by the formula (I): (I) (wherein R1, R2, and R3 are the same or different and each represents hydrogen, lower alkyl, lower alkenyl, or lower alkynyl; R4 represents cycloalkyl, —(CH2)n.R5, or the formula (II); (II) and X1 and X2 are the same or different and each represents oxygen or sulfur) or a pharmacologically acceptable salt thereof as an active ingredient.
Owner:KYOWA HAKKO KOGYO CO LTD
Biomarker, detection method thereof and application of biomarker in epilepsy pathology research and anti-epilepsy drug screening
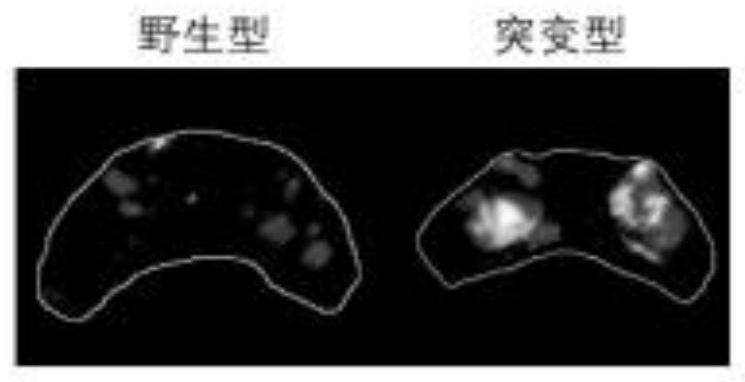
The invention provides a biomarker, a detection method thereof and application of the biomarker in epilepsy pathology research and anti-epilepsy drug screening, the biomarker is related to zebra fish epilepsy pathology characteristics, and the biomarker is a nerve excitation cluster; the nerve excitation cluster is a set of adjacent nerve excitation clusters in time and space obtained when MATLAB analysis is performed on image data obtained by performing GCaMP fluorescent dynamic imaging on zebra fish; and the nerve excitation cluster is a set of at least three connected excitation state pixel points in a single-frame image. According to the invention, a GCaMP dynamic imaging technology and a zebra fish hereditary epilepsy model are combined, data are subjected to visualization and quantitative analysis by adopting an image data processing pipeline based on MATLAB, a local nerve excitation cluster is used as an epilepsy biomarker, efficient judgment of the epilepsy pathology condition of a nervous system is realized, and the biomarker has the advantages of rapidness, objectivity, accuracy and the like and can be used for screening high-throughput antiepileptic drugs.
Owner:浙江赛微思生物科技有限公司
Methods of Treating Epilepsy via Phosphodiesterase 4 (PDE4) Inhibition
PendingUS20220098257A1Efficacy rates for seizure relief have not significantly changedIncreased drug resistanceCompound screeningNervous disorderAntiepileptic AgentsAssay
Provided are methods of treating epilepsy. The methods include administering to an individual having epilepsy a therapeutically effective amount of a phosphodiesterase 4 (PDE4) inhibitor. Also provided are methods of identifying an anti-epileptic agent. Such methods include contacting a PDE4 polypeptide with a candidate agent in a PDE4 activity assay, where inhibition of activity of the PDE4 polypeptide by the candidate agent identifies the candidate agent as an anti-epileptic agent.
Owner:PATH THERAPEUTICS INC
Fluorescence detection method of antiepileptic drug --- tiagabine hydrochloride (tgb)
ActiveCN110308121BEasy to detectHigh sensitivityFluorescence/phosphorescenceAntiepileptic AgentsPharmacology
The invention discloses a fluorescence detection method for antiepileptic drug tiagabine hydrochloride (TGB). Through the study of the interaction of dye perylene diimide derivatives (PDI), human serum albumin (HSA) and tiagabine hydrochloride (TGB), we found that the fluorescence of PDI can be quenched by HSA, and our research It shows that PDI binds at site II of HSA, and TGB can compete for binding at site II of HSA, so that PDI is competed from site II of HSA, thereby realizing fluorescence recovery. Based on this, the present invention proposes a fluorescence detection method for TGB, which uses PDI‑HSA as a probe to realize fluorescence recovery under the action of TGB, so as to detect TGB. The method of the invention has the advantages of original innovation, simple detection process, mild experimental conditions, high sensitivity, good selectivity, fast detection time, low detection limit, etc., and has good social value and application prospect.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
































![5-substituted phenoxy-1, 2, 4-triazole [4, 3-a] pyridine compound used for antiepileptic medicament, and medical salt thereof 5-substituted phenoxy-1, 2, 4-triazole [4, 3-a] pyridine compound used for antiepileptic medicament, and medical salt thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e37c5318-30f7-4267-aa04-624fe612b4f0/DEST_PATH_HSB00000811522000011.PNG)
![5-substituted phenoxy-1, 2, 4-triazole [4, 3-a] pyridine compound used for antiepileptic medicament, and medical salt thereof 5-substituted phenoxy-1, 2, 4-triazole [4, 3-a] pyridine compound used for antiepileptic medicament, and medical salt thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e37c5318-30f7-4267-aa04-624fe612b4f0/BSA00000655123500011.PNG)
![5-substituted phenoxy-1, 2, 4-triazole [4, 3-a] pyridine compound used for antiepileptic medicament, and medical salt thereof 5-substituted phenoxy-1, 2, 4-triazole [4, 3-a] pyridine compound used for antiepileptic medicament, and medical salt thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e37c5318-30f7-4267-aa04-624fe612b4f0/BSA00000655123500012.PNG)