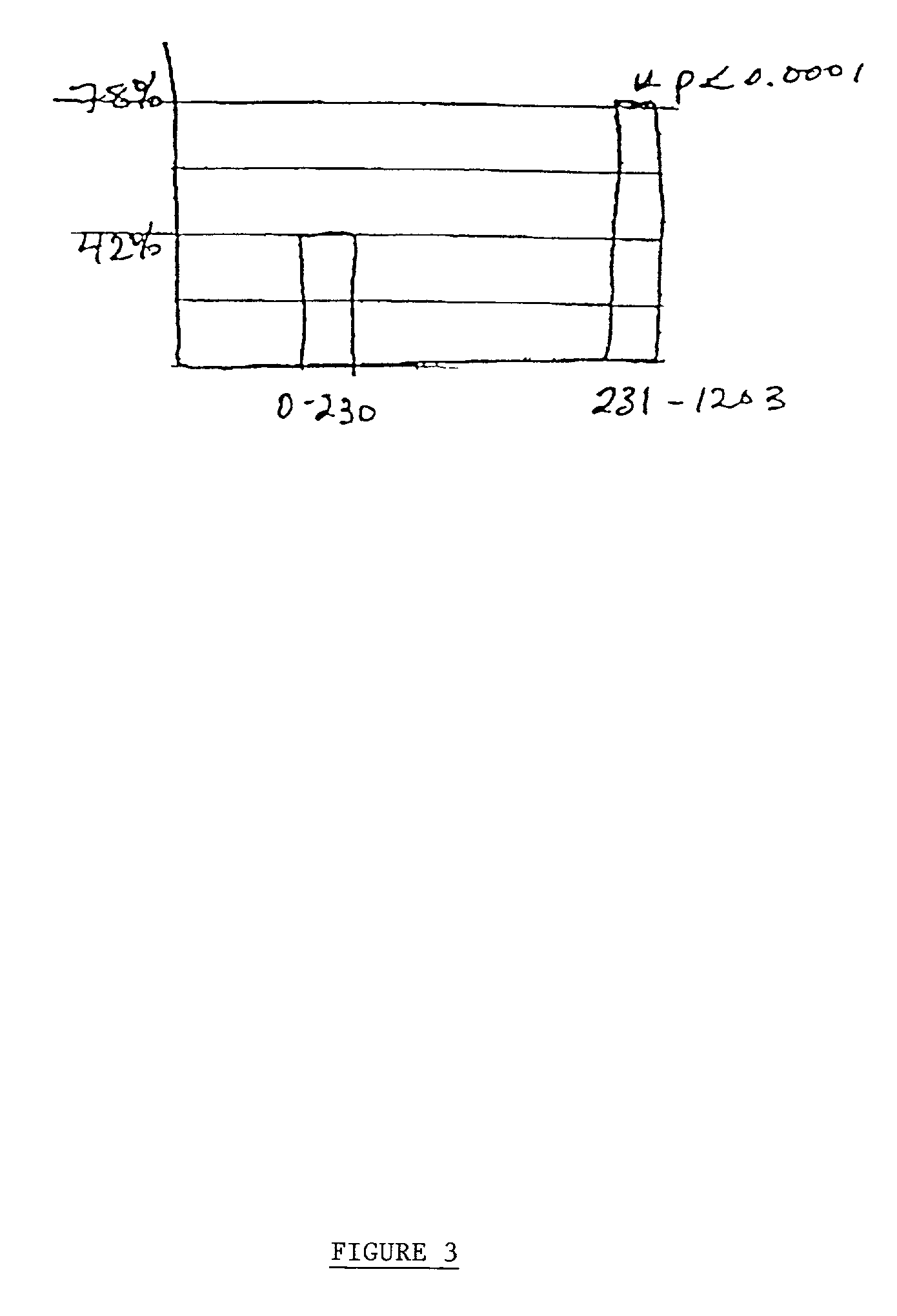
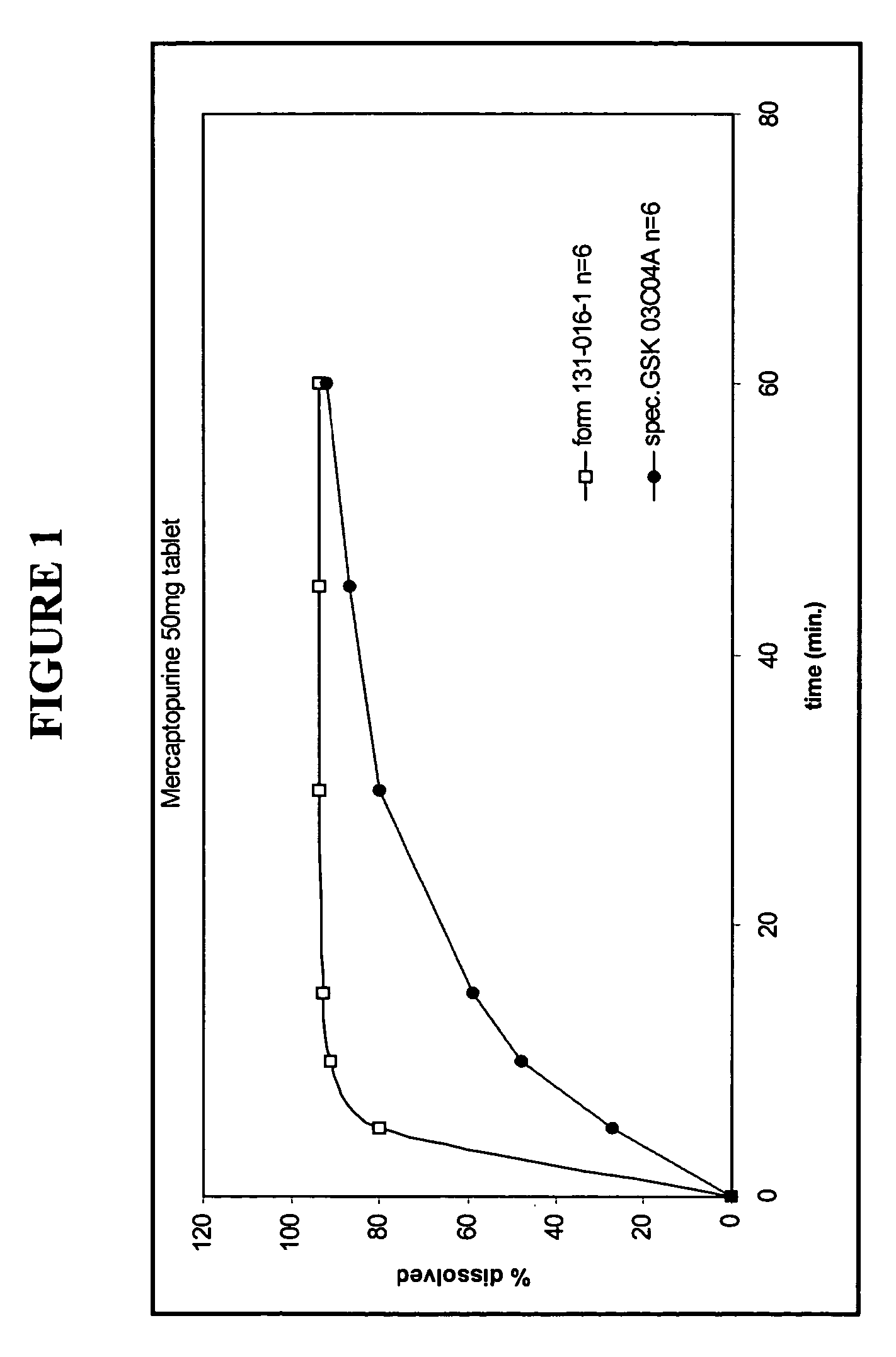
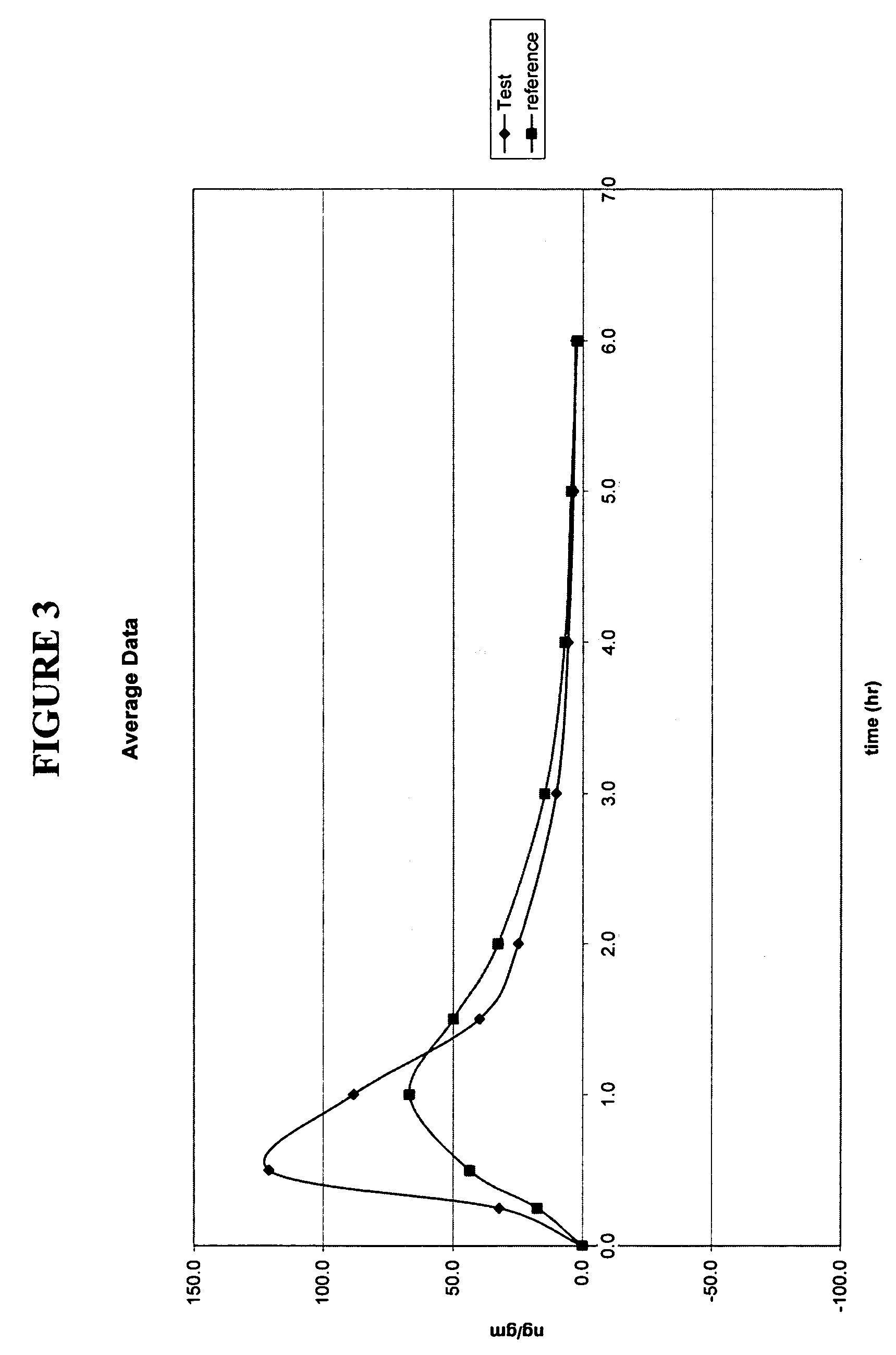
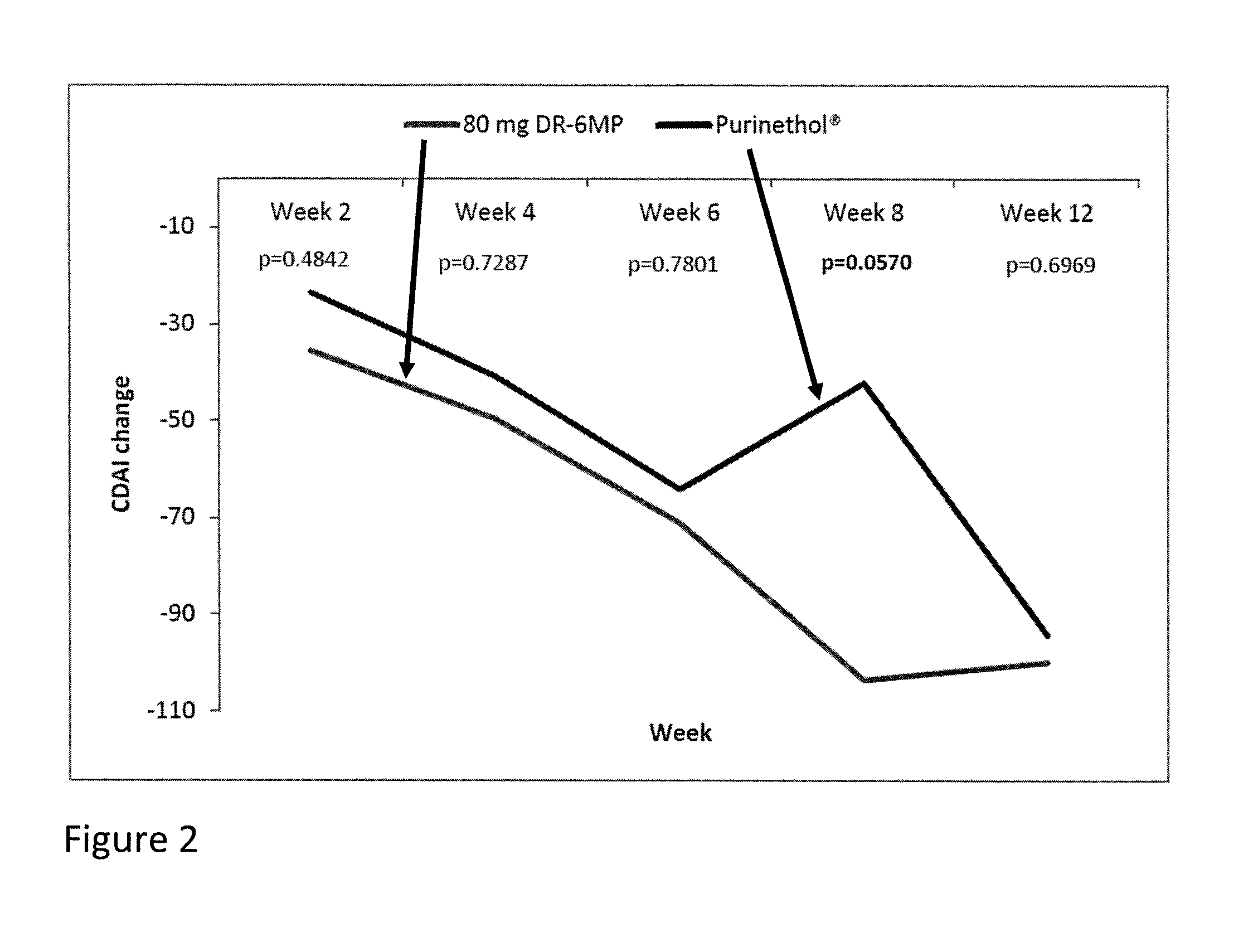
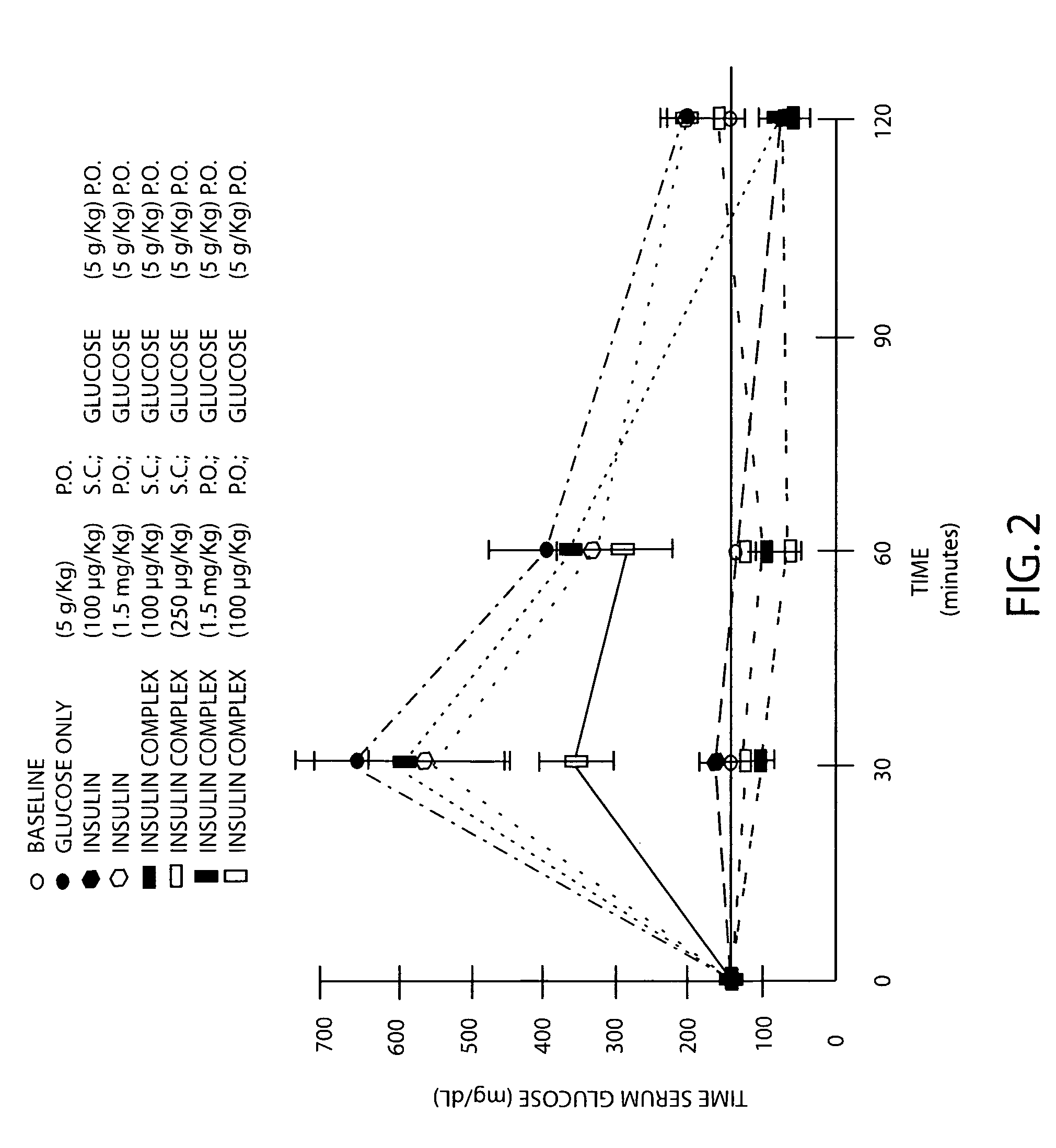
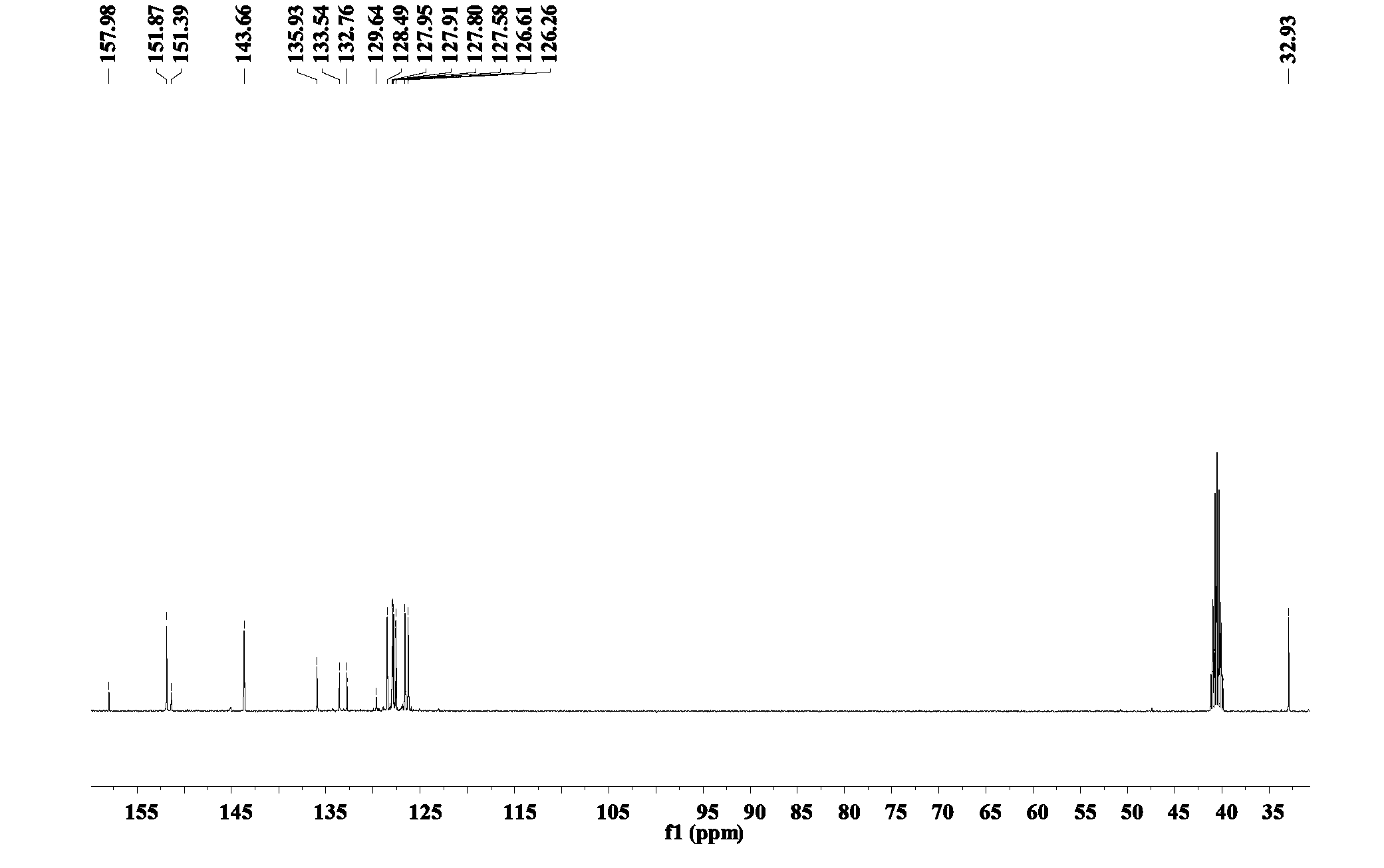
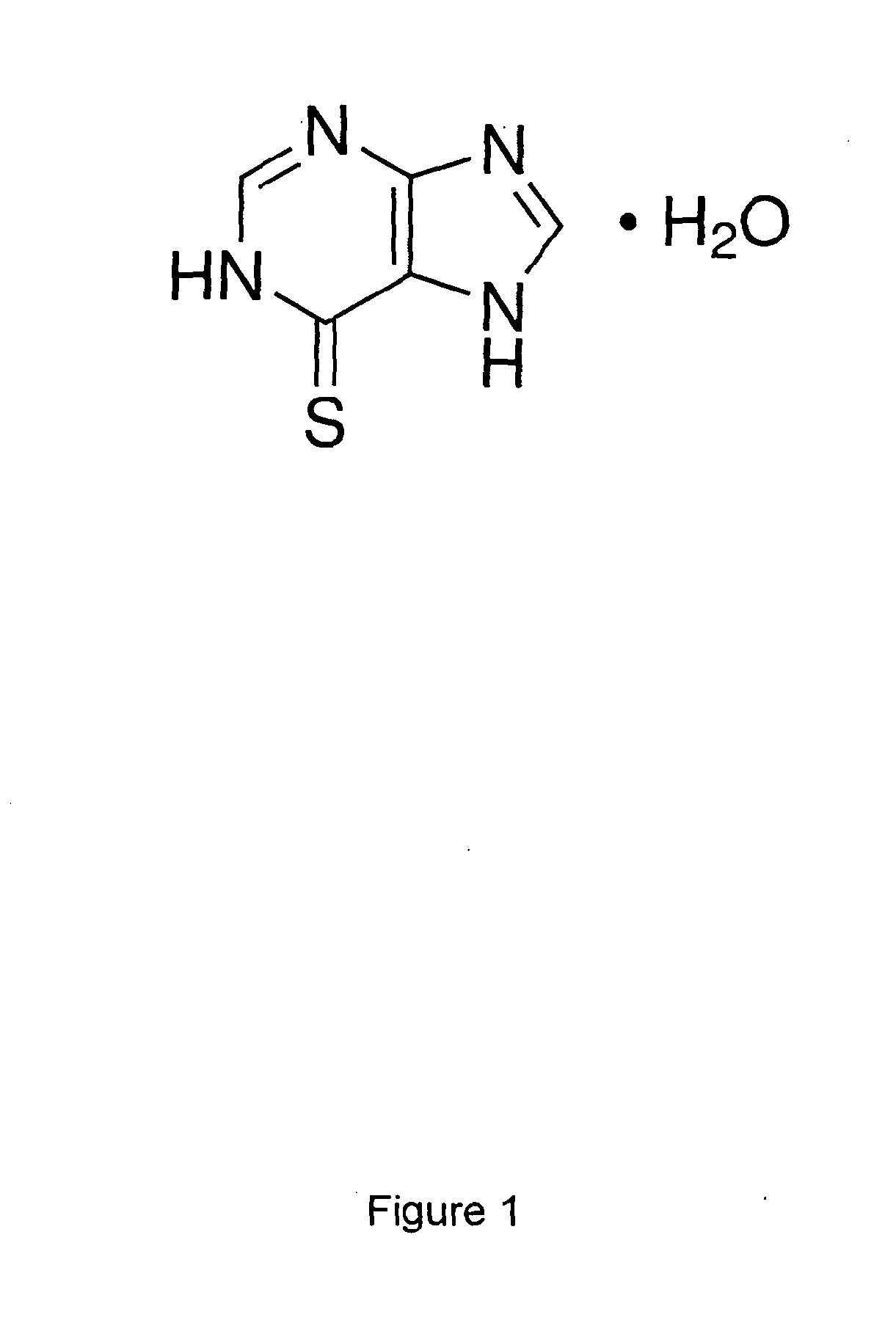
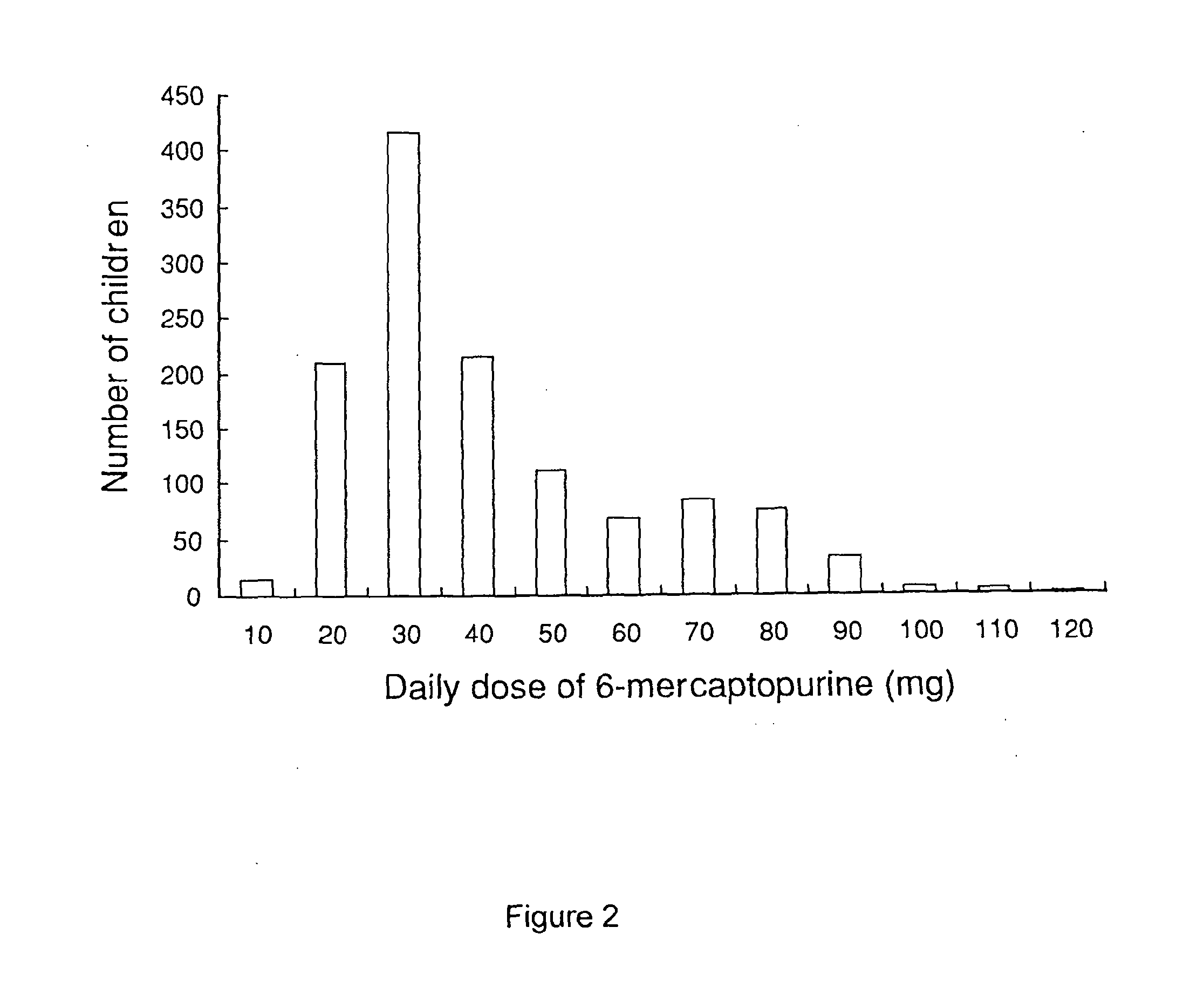
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
71 results about "Mercaptopurine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used with other drugs to treat a certain type of cancer (acute lymphocytic leukemia).
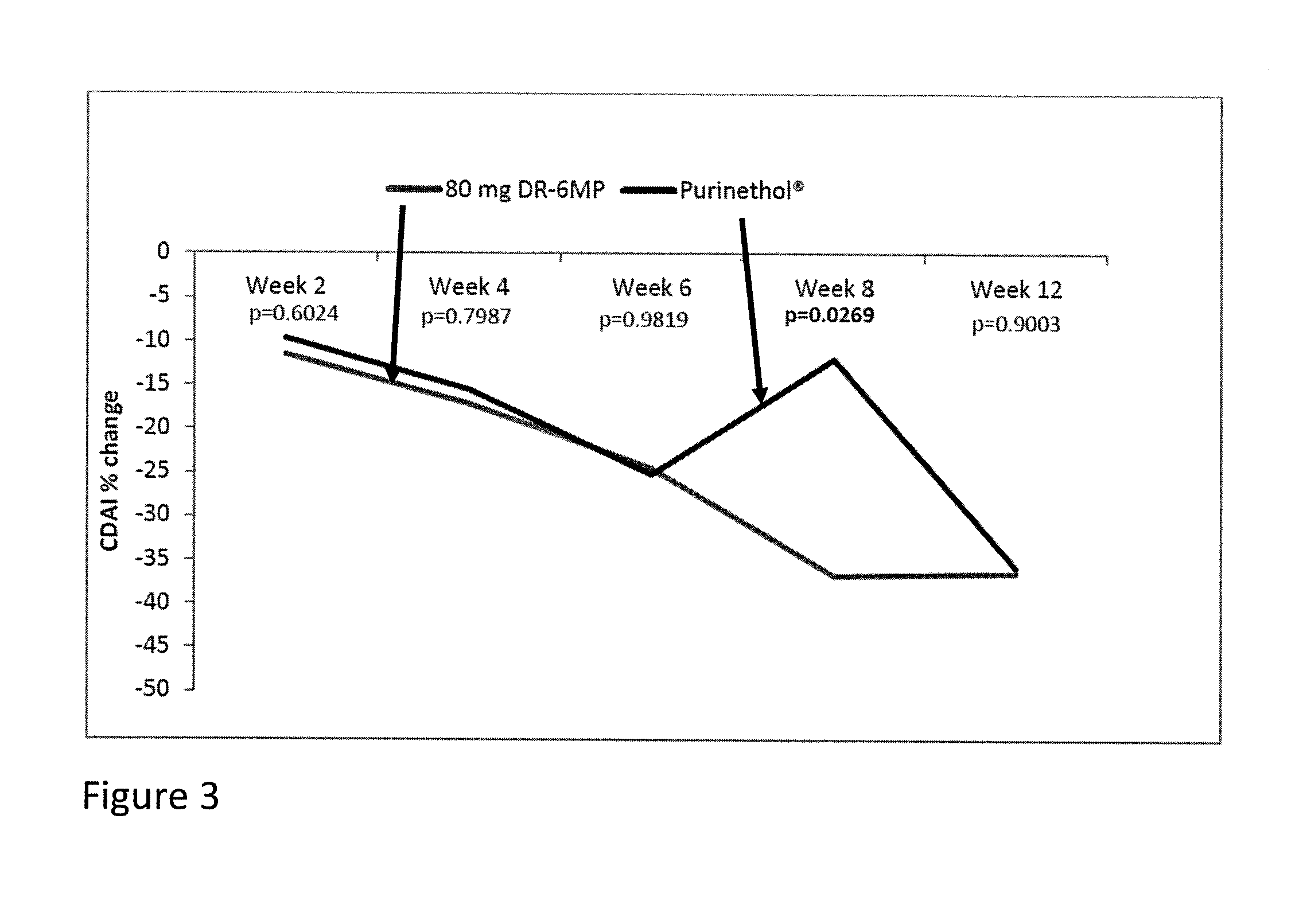
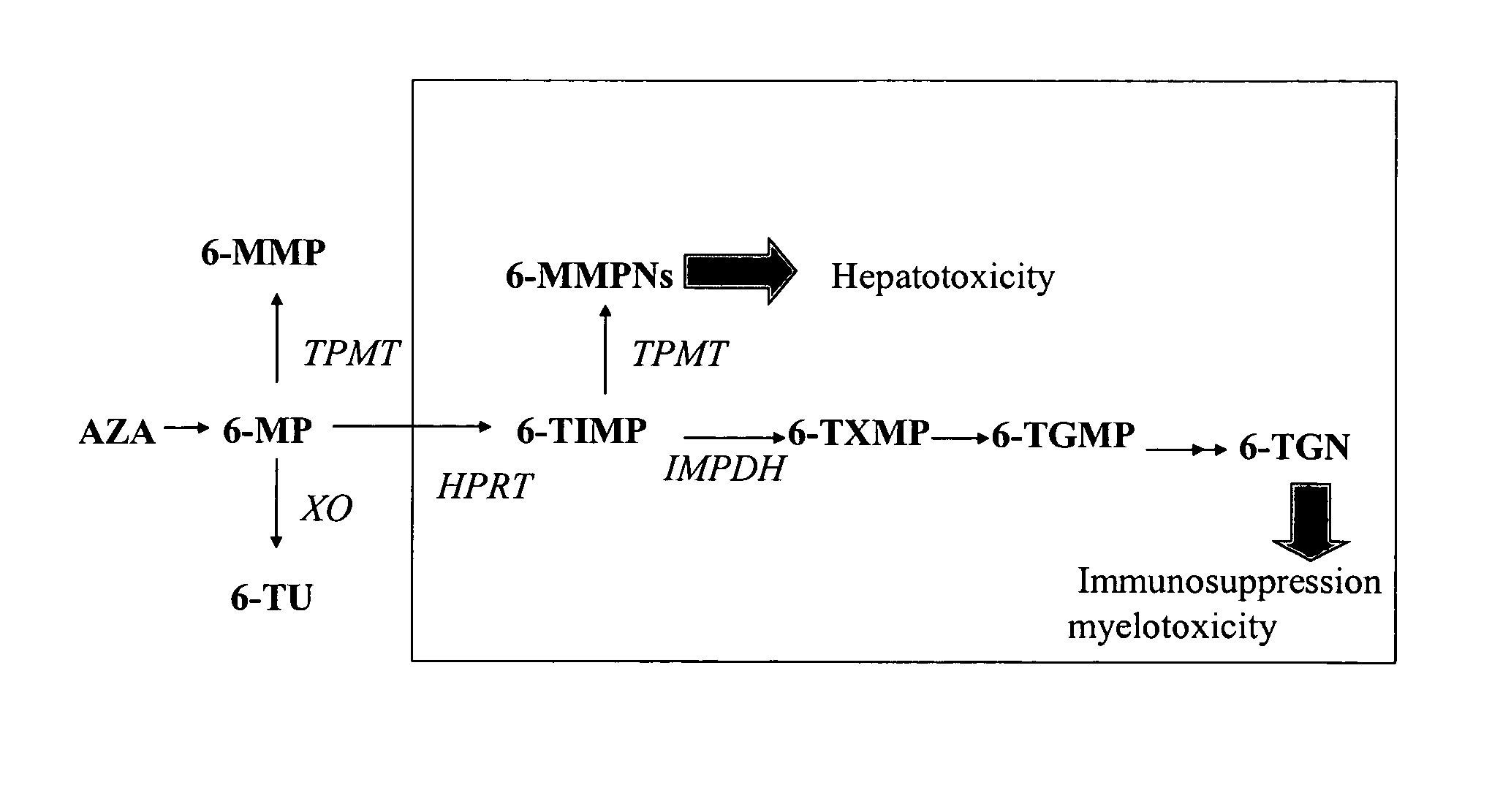
Method of treating IBD/Crohn's disease and related conditions wherein drug metabolite levels in host blood cells determine subsequent dosage
The present invention provides a method of optimizing therapeutic efficacy and reducing toxicity associated with 6-mercaptopurine drug treatment of an immune-mediated gastrointestinal disorder such as inflammatory bowel disease. The method of the invention includes the step of determining the level of one or more 6-mercaptopurine metabolites in the patient having an immune-mediated gastrointestinal disorder.
Owner:HOPITAL SAINTE JUSTINE
Personal care compositions and methods for the beautification of mammalian skin and hair
Personal care composition comprising from about 0.05% to about 5% of at least one aquaporin-stimulating compound selected from the group consisting of xanthine, caffeine; 2-amino-6-methyl-mercaptopurine; 1-methyl xanthine; 2-aminopurine; theophylline; theobromine; adenine; adenosine; kinetin; p-chlorophenoxyacetic acid; 2,4-dichlorophenoxyacetic acid; indole-3-butyric acid; indole-3-acetic acid methyl ester; beta-naphthoxyacetic acid; 2,3,5-triiodobenzoic acid; adenine hemisulfate; n-benzyl-9-(2-tetrahydropyranyl)adenine; 1,3-diphenylurea; 1-phenyl-3-(1,2,3-thiadiazol-5-yl)urea; zeatin; indole-3-acetic acid; 6-benzylaminopurine; alpha-napthaleneacetic acid; 6-2-furoylaminopurine; green tea extract; white tea extract; menthol; tea tree oil; ginsenoside-RB1; ginsenoside-RB3; ginsenoside-RC; ginsenoside-RD; ginsenoside-RE; ginsenoside-RG1; ginseng root extract; ginseng flower extract; pomegranate extract, extracts from Ajuga turkestanica; extracts from viola tricolor and combinations thereof; an additional ingredient selected from the group consisting of niacinamide, glycerin and mixtures thereof, and a dermatologically-acceptable carrier.
Owner:THE PROCTER & GAMBLE COMPANY
Methods of optimizing drug therapeutic efficacy for treatment of immune-mediated gastrointestinal disorders
InactiveUS20010006970A1Good curative effectLow toxicityBiocideDigestive systemMetaboliteGastrointestinal disorder
The present invention provides a method of optimizing therapeutic efficacy and reducing toxicity associated with 6-mercaptopurine drug treatment of an immune-mediated gastrointestinal disorder such as inflammatory bowel disease. The method of the invention includes the step of determining the level of one or more 6-mercaptopurine metabolites in the patient having an immune-mediated gastrointestinal disorder.
Owner:HOPITAL SAINTE JUSTINE
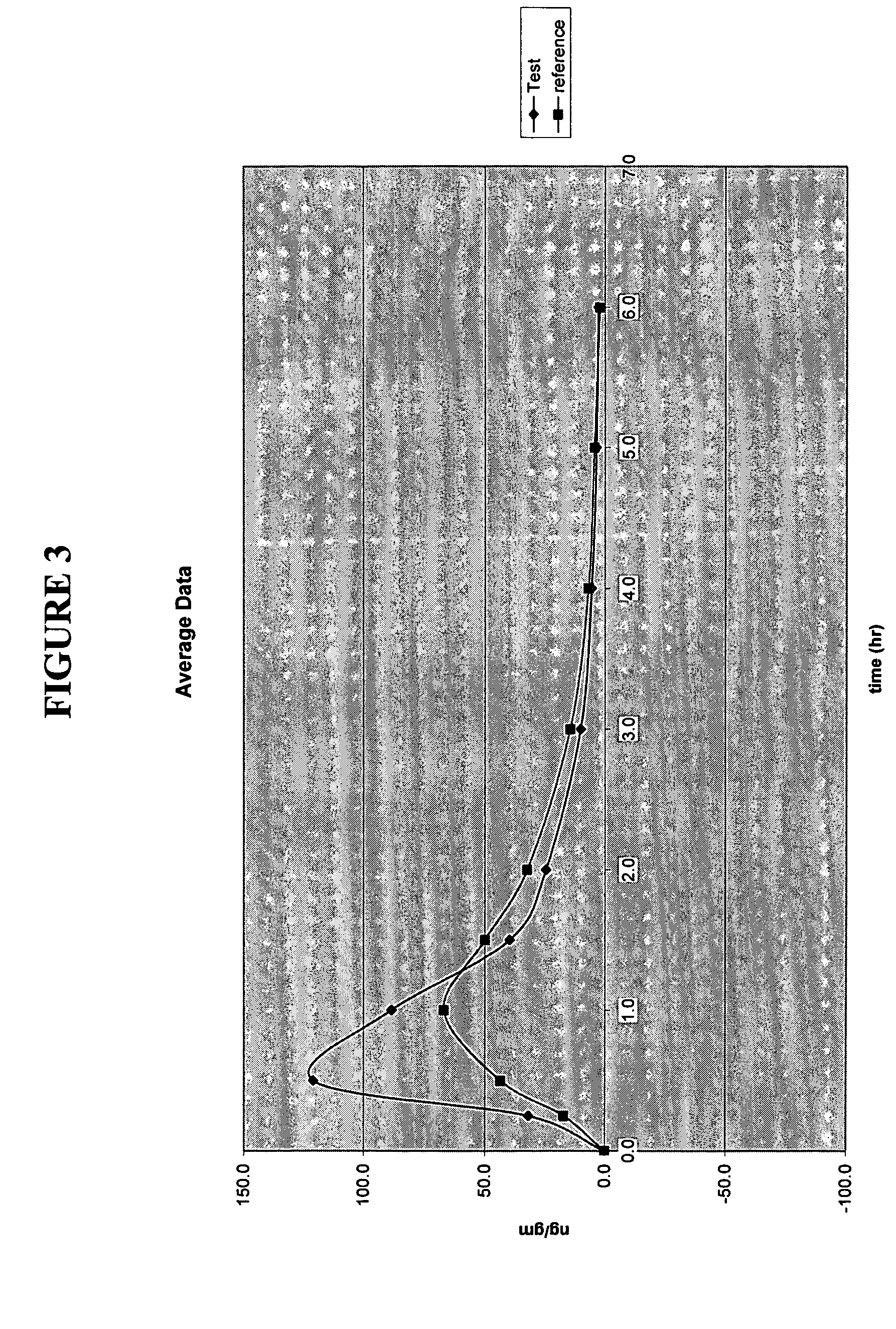
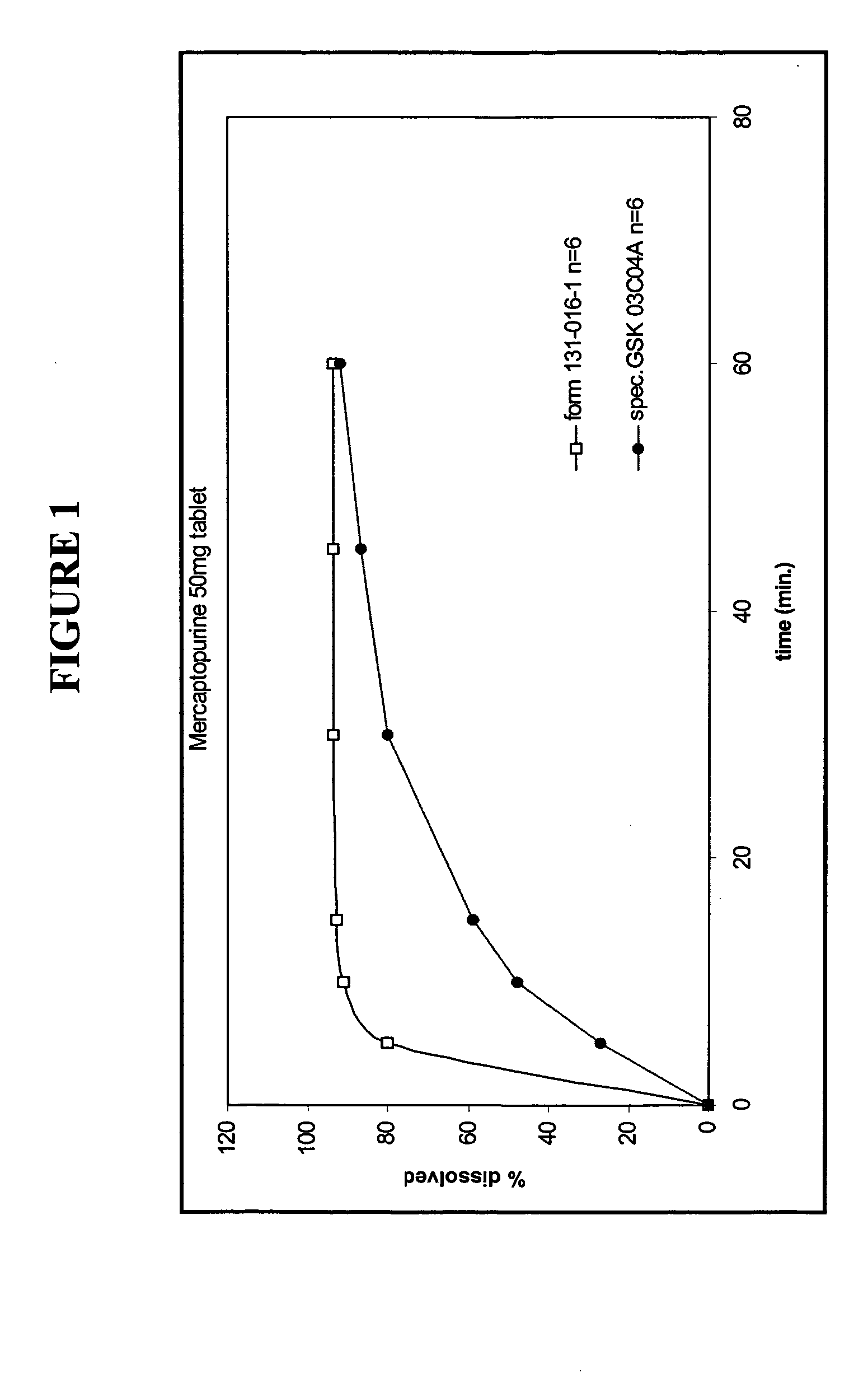
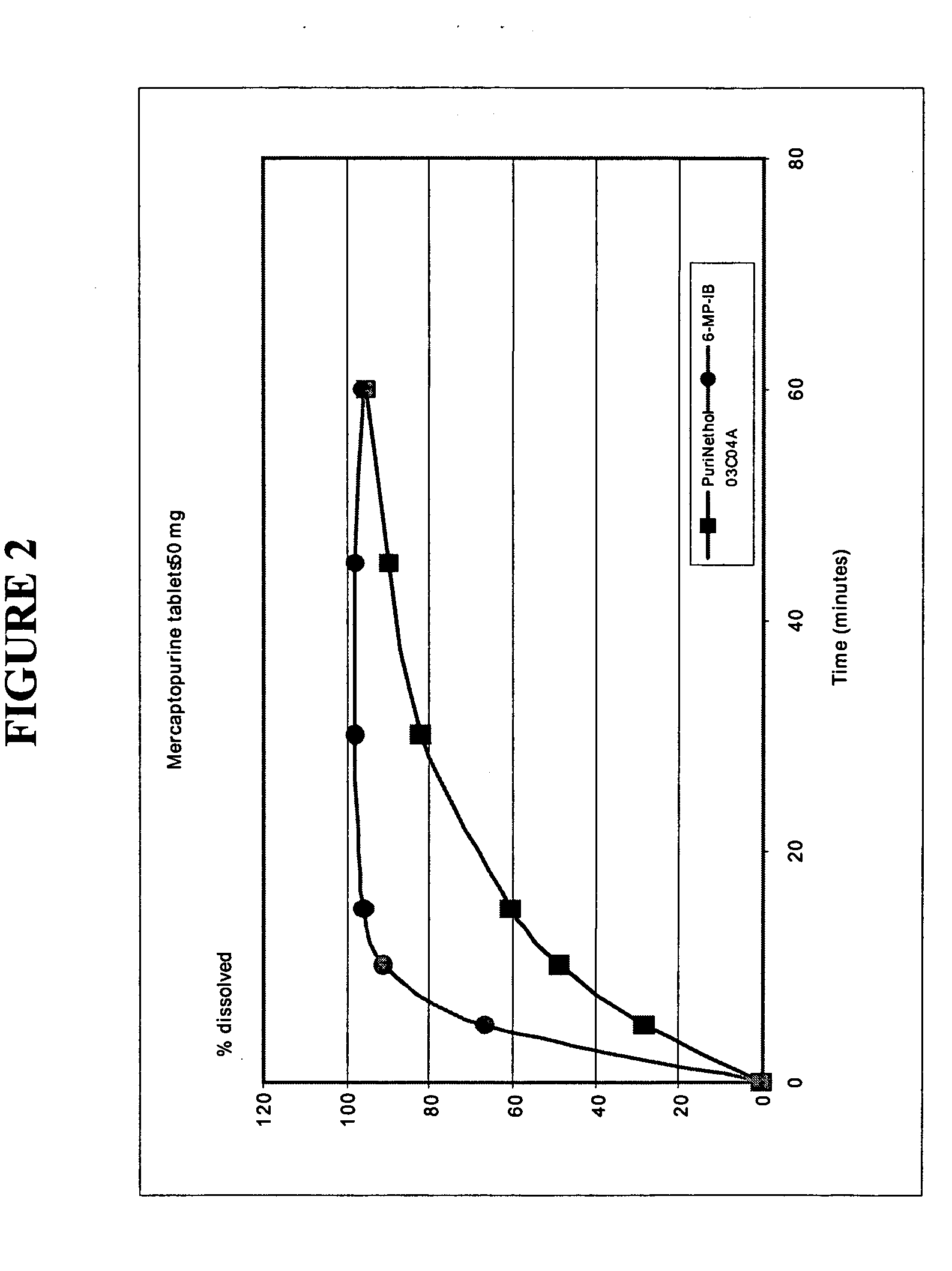
Delayed release formulations of 6-mercaptopurine
InactiveUS20060008520A1Inhibition releaseReduce releaseBiocideDigestive systemBioavailabilityMercaptopurine
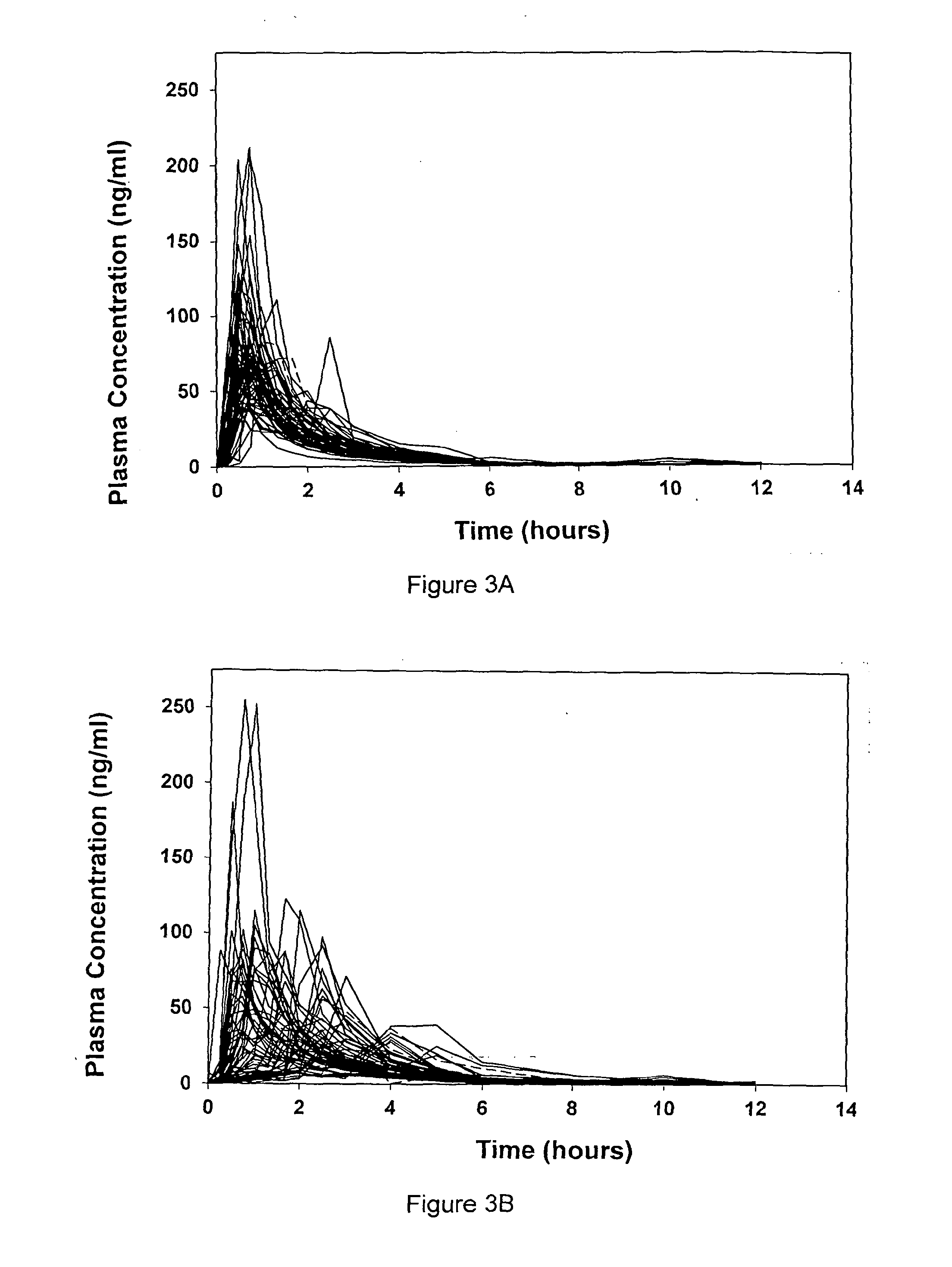
The present invention provides enterically coated formulations of 6-mercaptopurine that exhibit a delay in release of the 6-mercaptopurine such that substantial release of 6-mercaptopurine does not occur until after passage through the stomach. Optionally, the formulations also comprise a delay coating in addition to the enteric coating that provides an even further delay such that substantial release of 6-mercaptopurine does not occur until after a certain period of time following passage through the stomach. Such a period of time is preferably at least one hour after passage through the stomach. Following the delay imparted by the enteric coating and optional delay coating, the formulations exhibit better bioavailability and faster dissolution than previous formulations.
Owner:TEVA PHARM USA INC
Treatment of crohn's disease with delayed-release 6-mercaptopurine
Methods of treating patients suffering from Crohn's disease or ulcerative colitis who did not experience a clinical response to previous thiopurine administration, or suffered side effects from previous thiopurine administration, by administering a delayed release pharmaceutical composition comprising 6-mercaptopurine are disclosed. Methods of treating patients suffering from Crohn's disease or ulcerative colitis who are also being administered a steroid, 5-aminosalicylic acid, or an antibiotic by adjunctively administering a delayed release pharmaceutical composition comprising 6-mercaptopurine are also disclosed.
Owner:VALE LIMITED
Formulations of 6-mercaptopurine
ActiveUS8188067B2High dissolution rateImprove bioavailabilityBiocideGranular deliveryDissolutionBioavailability
The present invention provides improved formulations of 6-mercaptopurine that exhibit better bioavailability and faster dissolution than previous formulations.
Owner:TEVA PHARM USA INC +1
Formulations of 6-mercaptopurine
ActiveUS20060009473A1High dissolution rateImprove bioavailabilityBiocideGranular deliveryDissolutionBioavailability
The present invention provides improved formulations of 6-mercaptopurine that exhibit better bioavailability and faster dissolution than previous formulations.
Owner:TEVA PHARM USA INC +1
Treatment of inflammatory bowel disease with 6-mercaptopurine
InactiveUS20090263482A1Reduce morbidityImprovement of immunological statusBiocideDigestive systemSide effectWhole body
Methods of administering a delayed release 6-mercaptopurine pharmaceutical composition to patients suffering from inflammatory bowel disease which provide for release of the 6-mercaptopurine after passage of the pharmaceutical composition through the stomach are disclosed. The methods result in significant clinical improvement despite leading to very little systemic absorption of 6-mercaptopurine and also result in very few undesirable side effects.
Owner:TEVA PHARM USA INC
Treatment of inflammatory bowel disease with 6-mercaptopurine
InactiveUS20130280328A1Reduce morbidityImprovement of immunological statusBiocideDigestive systemSide effectInflammatory Bowel Diseases
Methods of administering a delayed release 6-mercaptopurine pharmaceutical composition to patients suffering from inflammatory bowel disease which provide for release of the 6-mercaptopurine after passage of the pharmaceutical composition through the stomach are disclosed. The methods result in significant clinical improvement despite leading to very little systemic absorption of 6-mercaptopurine and also result in very few undesirable side effects.
Owner:ROSENBERGER VERED +3
Compositions Useful For The Treatment Of Gastrointestinal Disorders
ActiveUS20140287001A1Increase water transportInduce cGMP productionNervous disorderAntipyreticCyclaseLung Inflammations
This invention provides novel peptides and methods to prevent, control, and treat an inflammation, cancer and other disorders, particularly of the gastrointestinal tract and the lung by administering at least one agonist of guanalyte cyclase receptor either alone or in combination with a compound selected from i) 5-aminosalicyclic acid (5-ASA) or a derivative or a pharmaceutically acceptable salt thereof; ii) mercaptopurine; or iii) an anti-TNF therapy.
Owner:BAUSCH HEALTH IRELAND LTD
Method of determining thiopurine methyltransferase acitivity
InactiveUS20030199015A1Material analysis by observing effect on chemical indicatorComponent separationThiopurine methyltransferase activityFluorescence
The present invention provides a method of determining thiopurine methyltransferase (TPMT) activity in a subject. The method includes the steps of reacting sample obtained from the subject with a thiopurine derivative that is not 6-mercaptopurine to produce a methylated purine product; contacting the reacted sample with acid, thereby precipitating proteinaceous material from the reacted sample; separating supernatant from the precipitated proteinaceous material; and detecting in the supernatant the methylated purine product, where the amount of the:methylated purine product indicates a level of thiopurine methyltransferase activity in the subject. In a method of the invention, the subject can be, for example, an inflammatory bowel disease patient. In one embodiment, the acid used to precipitate proteinaceous material is perchloric acid, for example, 70% perchloric acid. In another embodiment, the thiopurine derivative used as a substrate is 6-thioguanine. In a further embodiment, the methylated purine product is detected by fluorescence, which can be combined, if desired, with high performance liquid chromatography (HPLC).
Owner:NESTEC SA +1
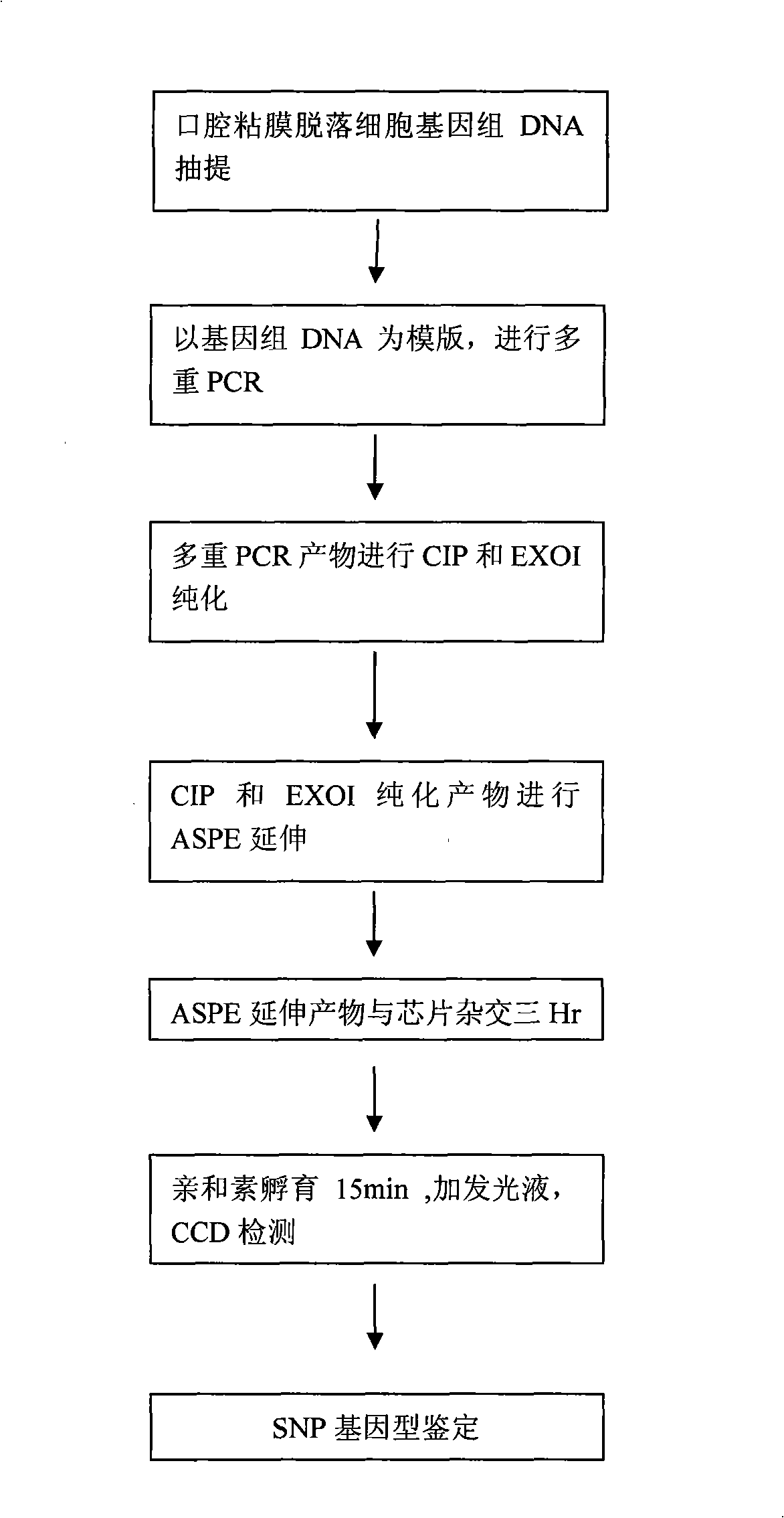
Purine medicaments insensitive gene detecting kit
ActiveCN101333560ATo achieve the purpose of parallel detectionThe result is accurateMicrobiological testing/measurementPurineDrugs sensitivity
The invention provides a purine drug sensitivity gene chip detection kit belonging to the gene chip technique field of clinical detection, which can be used to parallelly and economically detect genes related to purine drug sensitivity. The kit comprises an extraction solution, a hybridization buffer solution, a washing liquor, an amplifying solution, Taq enzyme and a gene chip, the amplifying solution contains a primer for amplifying genes of three mutant sites on mercaptopurine methyltransferase (TPMT), and the three mutant sites are respectively 238, 460 and 719. The purine drug is used for treating acute lymphoblastic leukemia, and the detection kit can be used to detect the three site mutation conditions of a TMPT gene sensitive to the purine drug.
Owner:上海裕隆生物科技有限公司
Method for optimizing thiopurine efficacy and toxicity using mass spectrometry
InactiveUS20060216726A1Low toxicityMicrobiological testing/measurementDisease diagnosisMedicineCurative effect
The present invention relates to methods for optimizing therapeutic efficacy or reducing toxicity in a subject receiving a drug providing 6-thioguanine nucleotide. The methods provide determining 6-thioguanine and 6-methyl-mercaptopurine nucleotide concentration levels using an analytical technique having an ionizing source.
Owner:NESTEC SA
Compositions useful for the treatment of gastrointestinal disorders
This invention provides novel peptides and methods to prevent, control, and treat an inflammation, cancer and other disorders, particularly of the gastrointestinal tract and the lung by administering at least one agonist of guanalyte cyclase receptor either alone or in combination with a compound selected from i) 5-aminosalicyclic acid (5-ASA) or a derivative or a pharmaceutically acceptable salt thereof; ii) mercaptopurine; or iii) an anti-TNF therapy.
Owner:BAUSCH HEALTH IRELAND LTD
Kit for detecting mercaptopurine methyltransferase genetype and method thereof
ActiveCN101210268AHigh amplification efficiencyStrong specificityMicrobiological testing/measurementMaterial analysis by electric/magnetic meansGene technologyMethyltransferase
The invention relates to a method and a kit for detecting genotype of thiopurine methyltransferase (TPMT), belonging to gene technology field. The method comprises the following steps of: performing polymerase chain reaction to a sample DNA using specific primers of TPMT gene polymorphism sites, digesting and amplifying the product with restriction endonuclease, and determining genotype according to the length polymorphism of the digested fragments, wherein the primers at least include a specific primer for detecting the TPMT G460A polymorphism site and a specific primer for detecting the A719G polymorphism site. The inventive method and the kit for genotype detection can provides guidance for the individualized administration of purine drugs; and has the advantages of good stability and reliability, low price, rapidness, and applicability to clinic application.
Owner:深圳泰乐德医疗有限公司
Reductively degradable mercaptopurine nanometer micellar prodrug with controllable drug release and application thereof
InactiveCN102335190ASmall toxicityLong-lasting stable releaseOrganic active ingredientsAntineoplastic agentsSolubilityDrug release rate
The invention discloses a reduction-sensitive mercaptopurine nanometer micellar prodrug with controllable drug release and application thereof. According to the invention, considering deficiency of small molecular mercaptopurine drugs, mercaptopurine and polyethylene glycol derivatives are grafted on main chains of imide polyasparate through a ring-opening reaction and a mercaptan-disullfide exchange reaction so as to synthesize the high-molecular micellar prodrug which is capable of molecular self-assembly at nanometer scale in human bodies, has a particle size of about 160 nm, a narrow distribution scope and long-acting cycles in blood, is targeted to cancer tissue, and has a controllable drug release rate. The objectives of the invention are to improve water-solubility of original mercaptopurine, to minimize toxic and side effects of original mercaptopurine and enhance curative effects and bioavailability simultaneously so as to overcome the bottleneck of undesirable clinical therapeutic effects on acute lymphocytic leukemia at present.
Owner:EAST CHINA NORMAL UNIVERSITY
Treatment of non-alcoholic fatty liver disease or non-alcoholic steatohepatitis with delayed-release 6-mercaptopurine
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT
Copper electroplating solution for heterojunction solar cell and preparation method of copper electroplating solution
The invention belongs to the technical field of metal surface treatment, and particularly relates to a copper electroplating solution for a heterojunction solar cell and a preparation method of the copper electroplating solution. The copper electroplating solution for the heterojunction solar cell contains copper salt, a complexing agent, conductive salt, a crystallization refiner, a destressing agent, a brightening agent and a pH stabilizer, wherein the crystallization refiner is prepared from saccharin, sodium propynylsulfonate and polyvinyl alcohol in the mass ratio of (8-10):(4-6):(1-2), and the destressing agent is prepared from 2-amino-6-mercaptopurine, 1,4-butynediol and polyethyleneimine in the mass ratio of (11-13):(4-7):1. After the heterojunction solar cell is electroplated by adopting the copper electroplating solution, the deposition stress of an obtained copper-plated film is small, and the reliability of a plated part is remarkably improved; and the copper-plated film obtained after the heterojunction solar cell is electroplated by adopting the copper electroplating solution is uniform, compact and bright, and the quality of the plated layer is remarkably improved.
Owner:GUANGZHOU SANFU NEW MATERIALS TECH
Preparation method for 6-mercaptopurine molecularly-imprinted electrochemical sensor
InactiveCN105510418AHigh affinityIncreased sensitivityMaterial analysis by electric/magnetic meansButanediolElectrochemistry
The invention discloses a preparation method for a 6-mercaptopurine molecularly-imprinted electrochemical sensor. The preparation method is characterized by comprising the following steps: modifying a glassy carbon electrode with a silane coupling agent and nanometer platinum; then adding, by mass percentage concentration, 10 to 20% of dimethacrylic acid-1,4-butanediol ester, 8 to 18% of methyl acrylate, 8 to 18% of maleic anhydride, 50 to 60% of N,N-dimethyl formamide 1.0 to 3.0% of dimethyl azodiisobutyrate and 1.0 to 3.0% of 6-mercaptopurine into a reactor, introducing argon for 5 min to remove oxygen and preparing a 6-mercaptopurine molecularly-imprinted polymer in an argon atmosphere; and coating the modified electrode with the polymer and removing template molecule so as to obtain the 6-mercaptopurine molecularly-imprinted electrochemical sensor. The sensor has the advantages of high 6-mercaptopurine recognition performance, low cost, high sensitivity, good specificity, rapid detection, reusability and improved response to 6-mercaptopurine.
Owner:UNIV OF JINAN
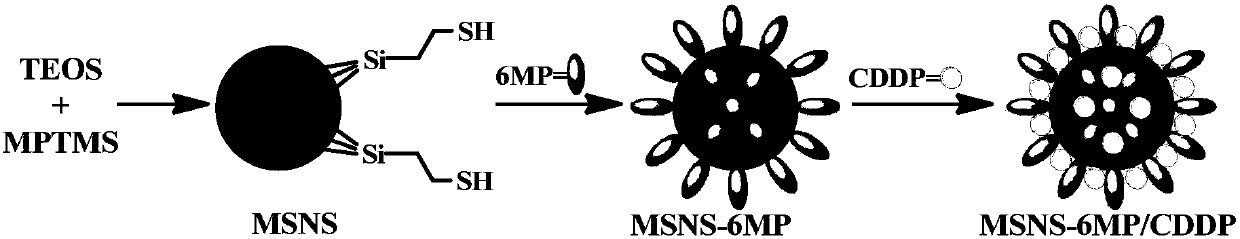
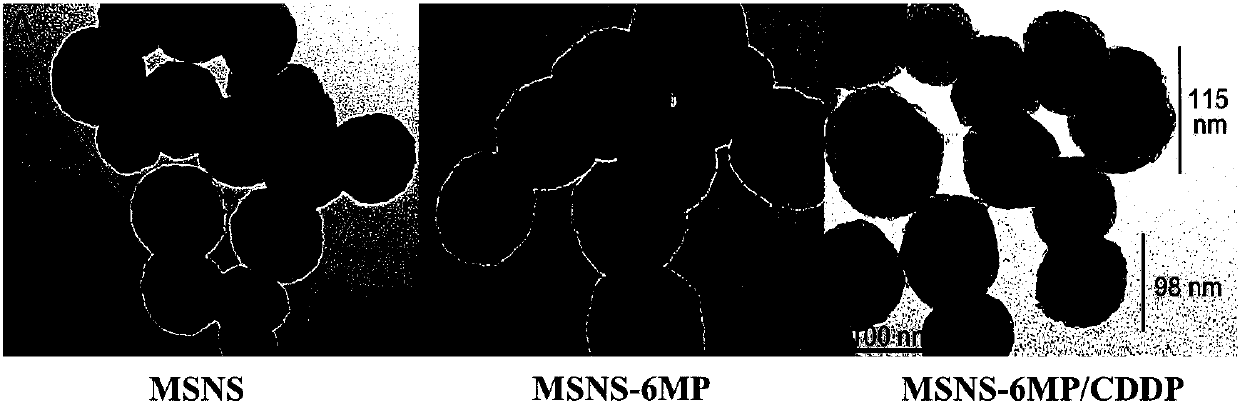
Mesoporous silicon dioxide-6-mercaptopurine-cisplatin nanoparticles as well as preparation, activity and application thereof
InactiveCN107684626AGrowth inhibitionPowder deliveryHeavy metal active ingredientsCisplatinWhole body
The invention discloses a nano-scale mesoporous silica-6-mercaptopurine (6MP) / cisplatin (CDDP) nano-delivery system (MSNS-6MP / CDDP). Its preparation method is disclosed, that is, modifying mercapto groups on mesoporous silica, linking 6MP through disulfide bonds to form mesoporous silica nanoparticles (MSNS‑6MP) connected to 6MP, loading CDDP in the channel to form a link 6MP, CDDP-loaded mesoporous silica nanoparticles; disclosed its nanostructure; disclosed its role in inhibiting tumor growth in S180 mice, compared with conventional 6MP and CDDP combined application, MSNS‑6MP / CDDP significantly prolongs S180 Survival time of mice, improved efficacy and reduced systemic toxicity. Therefore, the invention discloses its application in the preparation of tumor treatment drugs, which has a good application prospect.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Delayed release formulations of 6-mercaptopurine
The present invention provides enterically coated formulations of 6-mercaptopurine that exhibit a delay in release of the 6-mercaptopurine such that substantial release of 6-mercaptopurine does not occur until after passage through the stomach. Optionally, the formulations also comprise a delay coating in addition to the enteric coating that provides an even further delay such that substantial release of 6-mercaptopurine does not occur until after a certain period of time following passage through the stomach. Such a period of time is preferably at least one hour after passage through the stomach. Following the delay imparted by the enteric coating and optional delay coating, the formulations exhibit better bioavailability and faster dissolution than previous formulations.
Owner:TEVA PHARM USA INC
Gold nanocluster-based 6-mercaptopurine detection method and kit
ActiveCN107589099AEasy to manufactureHigh quantum yieldFluorescence/phosphorescenceUltimate tensile strengthBuffer solution
The invention discloses a gold nanocluster-based 6-mercaptopurine detection method and a kit. With use of specific interaction of carboxylated chitosan-dithiothreitol-gold nanoclusters and 6-mercaptopurine, fluorescence of the carboxylated chitosan-dithiothreitol-gold nanoclusters is quenched, and a carboxylated chitosan-dithiothreitol-gold nanocluster solution is added to a phosphate buffer solution containing 6-mercaptopurine; with increasing of the concentration of 6-mercaptopurine, the fluorescence intensity value F650 of the carboxylated chitosan-dithiothreitol-gold nanoclusters at 650 nmis decreased, so that the content of 6-mercaptopurine is determined. The invention provides a new detection method for 6-mercaptopurine, wherein the method has the advantages of high test sensitivity, good specificity, good accuracy, simple detection process, good stability, simple operation, short detection time, high sensitivity, strong specificity and the like.
Owner:FUJIAN MEDICAL UNIV
Amphiphilic oligomers
InactiveUS20050181976A1Promote absorptionGood componentPeptide/protein ingredientsHydrolasesAmpicillinDaunorubicin
A therapeutic formulation comprising a microemulsion of a therapeutic agent in free and / or conjugatively coupled form, wherein the microemulsion comprises a water-in-oil (w / o) microemulsion including a lipophilic phase and a hydrophilic phase, and has a hydrophilic and lipophilic balance (HLB) value between 3 and 7, wherein the therapeutic agent may for example be selected from the group consisting of insulin, calcitonin, ACTH, glucagon, somatostatin, somatotropin, somatomedin, parathyroid honnone, erythropoietin, hypothalamic releasing factors, prolactin, thyroid stimulating hormones, endorphins, enkephalins, vasopressin, non-naturally occurring opioids, superoxide dismutase, interferon, asparaginase, arginase, arginine deaminease, adenosine deaminase, ribonuclease, trypsin, chymotrypsin, papain, Ara-A (Arabinofuranosyladenine), Acylguanosine, Nordeoxyguanosine, Azidothym id ine, Didesoxyadenosine, Dideoxycytidine, Dideoxyinosine Floxuridine, 6-Mercaptopurine, Doxorubicin, Daunorubicin, or I-darubicin, Erythromycin, Vancomycin, oleandomycin, Ampicillin; Quinidine and Heparin. In a particular aspect, the invention comprises an insulin composition suitable for parenteral as well as non-parenteral administration, preferably oral or parenteral administration, comprising insulin covalently coupled with a polymer including (i) a linear polyalkylene glycol moiety and (ii) a lipophilic moiety, wherein the insulin, the linear polyalkylene glycol moiety and the lipophilic moiety are conformationally arranged in relation to one another such that the insulin in the composition has an enhanced in vivo resistance to enzymatic degradation, relative to insulin alone. The microemulsion compositions of the invention are usefully employed in therapeutic as well as non-therapeutic, e.g., diagnostic, applications.
Owner:BIOCON LTD
Antitumor composition and application thereof in preparation of antitumor medicine or medicine for inhibiting cancer cells, as well as antitumor medicine
ActiveCN109453176AEnhanced inhibitory effectImprove anti-tumor activityAntineoplastic agentsHeterocyclic compound active ingredientsCancer cellDihydroartemisinin
The invention relates to an antitumor composition and application thereof in preparation of an antitumor medicine or a medicine for inhibiting cancer cells, as well as the antitumor medicine, and belongs to the technical field of medicine preparation. The antitumor composition involved in the invention comprises a first antitumor component and a second antitumor component; the first antitumor component is one or two of mercaptopurine and thioguanine or a salt or salts of one or two of the mercaptopurine and the thioguanine; the second antitumor component is dihydroartemisinin, and the molar ratio of the first antitumor component to the dihydroartemisinin is 1:(1-4); or the second antitumor component is an artemisinin derivative, and the molar ratio of the first antitumor component to artemisinin or the dihydroartemisinin generated through metabolism of the second antitumor component is 1:(1-4); and an antitumor compound capable of generating the dihydroartemisinin through metabolism isone or a combination of several of artesunate, artemether and arteether. The two antitumor components of the antitumor composition involved in the invention have a synergy effect.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
6-(beta-alkylnaphthy)mercaptopurine compound, and synthesis method and application thereof
InactiveCN103319485AThe synthesis steps are simpleHigh yieldOrganic chemistryAntineoplastic agentsCancer cellSynthesis methods
The invention discloses a 6-(beta-alkylnaphthy)mercaptopurine compound of which the structural formula is disclosed in the specification. The 6-(beta-alkylnaphthy)mercaptopurine compound can inhibit growth of cancer cells. The invention also discloses a synthesis method and application of the 6-(beta-alkylnaphthy)mercaptopurine compound. The synthesis method has the advantages of simple synthesis steps and higher yield, and is suitable for industrial production. The 6-(beta-alkylnaphthy)mercaptopurine compound disclosed by the invention is used for inhibiting cancer cells, and has obvious effect.
Owner:JILIN PROVINCE SCI & TECH EVALUATION
Oral Suspension
InactiveUS20140294972A1Accurate measurementImprove accuracyPowder deliveryBiocideOral suspensionsIntrathecal
A liquid pharmaceutical composition for use in the treatment of acute lymphoblastic leukaemia (ALL) comprising 6-mercaptopurine or a salt, hydrate or solvate thereof and a pharmaceutically-acceptable excipient, wherein the composition is a suspension for oral administration, a kit of parts for the accurate dosing and administration of the liquid pharmaceutical composition, and a method for the treatment of ALL in a human patient comprising administration of a therapeutically effective amount of the liquid pharmaceutical composition.
Owner:NOVA BIO PHARMA TECH LTD
Kit and method for detecting gene polymorphism capable of influencing mercaptopurine personalized medications by means of pyro sequencing method
InactiveCN102676667AQuick analysisAccurate analysisMicrobiological testing/measurementDrug utilisationNucleotide
The invention discloses a kit and a method for detecting a genetic polymorphism capable of influencing mercaptopurine personalized medications by means of a pyro sequencing method. The genetic polymorphism specifically relates to a thiopurine s-methyltransferase (TPMT) (rs1142345) single nucleotide polymorphism. The kit contains primers shown by SEQID NO.3-6. The kit is capable of achieving accurate, rapid and high throughput detection of the TPMT (rs1142345), so that safe, reasonable and efficient personalized dosing of the mercaptopurine medications can be achieved.
Owner:周宏灏
Mercaptopurine composition freeze-drying tablet and preparation method thereof
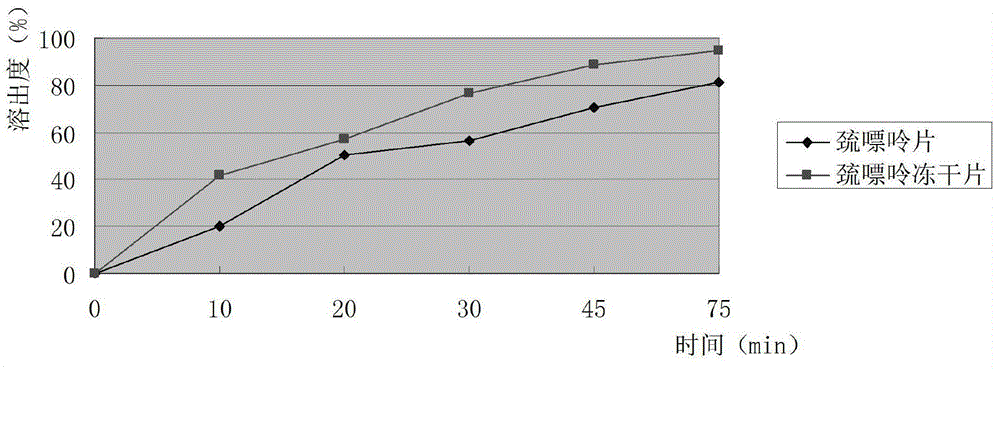
InactiveCN104546766AGood molding effectHigh dissolution ratePill deliveryPharmaceutical non-active ingredientsFreeze-dryingCurative effect
The invention provides a mercaptopurine composition freeze-drying tablet and a preparation method thereof, and relates to the technical fields of medicines and medicine production. The mercaptopurine composition freeze-drying tablet contains mercaptopurine, starch and cane sugar, wherein the starch and the cane sugar are used as auxiliary materials, and after heating technology processing is performed on common corn starch, the bonding and disintegrating functions of the starch in the tablet can be improved, and the formability of the tablet is improved; the mercaptopurine composition freeze-drying tablet only needs the starch and the cane sugar as the auxiliary materials. The mercaptopurine composition freeze-drying tablet adopts a two-drop two-rise free-drying technology, twice temperature drop and twice temperature rise can enable the tablet to be better formed, and the dissolution rate of the tablet can be increased, so that the biological availability of the tablet is improved; the tablet disclosed by the invention overcomes the defects of a common mercaptopurine tablet, and has the characteristics that the kinds and the dosage of the auxiliary materials in the mercaptopurine tablet are reduced, and the tablet has a high dissolution rate and high biological availability, so that the curative effect and the safety of clinical medication are guaranteed.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Anti-tumor active polymer with pH and glutathione sensitivity and preparation method thereof
ActiveCN110694074ALow toxicityAvoid damagePharmaceutical non-active ingredientsIn-vivo testing preparationsHigh dosesBackbone chain
The invention provides an anti-tumor active polymer with pH and glutathione sensitivity. Natural polymer glucan is used as a main chain and is linked toan anti-cancer drug 6-mercaptopurine through a glutathione-sensitive carbonyl vinylthioether bond; and through the coordination of quantum dots ZnO with DOX, 5-FU and 6-MP, the delivery of three anticancer drugs is achieved. Meanwhile, by using phenylboronic acid as a targeting substance and through coordination of quantum dots CdSe with 6-MP, fluorescence imaging is performed. In vitro release results show that the polymer can be released in the environment of slight acid and glutathione. In vitro cell experiments show that the simultaneous use of the three anticancer drugs can better reduce the survival rate of B16F10 cells and solve thedisadvantages of high dose and high drug resistance caused by a single drug delivery system. The product of the invention is a drug delivery system with wide application prospects.
Owner:NORTHWEST NORMAL UNIVERSITY
Application of click chemistry and declick chemistry to quantitative synthesis and release of drug
ActiveCN109053621AQuick responseQuick releaseGroup 3/13 element organic compoundsFluorescenceMercaptopurine
The invention provides an application of click chemistry and declick chemistry to quantitative synthesis and release of a drug. On the basis of a template molecule, the quantitative assembling and release of sulfydryl-containing drug molecules such as N-acetyl-L-cysteine (NAC), glutathione (GSH), methimazole, tiopronin, captopril and mercaptopurine can be realized by utilizing the click chemistryand declick chemistry, and the assembling and release process of the sulfydryl-containing drug molecules can be monitored by utilizing the fluorescence variation in the process. The template moleculecan rapidly react with the sulfydryl-containing drug molecules in a ratio of 1:1 in the presence of the surfactant, the template molecule and the molecule after the assembling of the sulfydryl-containing drug molecules can rapidly have declick-chemistry in the presence of a nucleophilic reagent to release the sulfydryl-containing drug molecules. The fluorescence is varied in the sulfydryl-containing drug molecule release process, so that the release of the drug molecules can be conveniently monitored by utilizing the fluorescence variation of the sulfydryl-containing drug molecule.
Owner:ZHENGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com