Anti-tumor active polymer with pH and glutathione sensitivity and preparation method thereof
An anti-tumor activity, glutathione technology, applied in the direction of anti-tumor drugs, organic active ingredients, medical preparations of non-active ingredients, etc., can solve the problems of easy generation of drug resistance and high doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0055] The synthesis of the anti-tumor active polymer DOX@5-FU@ZnO@CdSe@Dex-PTA-PBA of the present invention will be further described below through specific examples.
[0056] (1) Preparation of 3-(7H-purin-6-ylthio)acrylic acid (PTA)
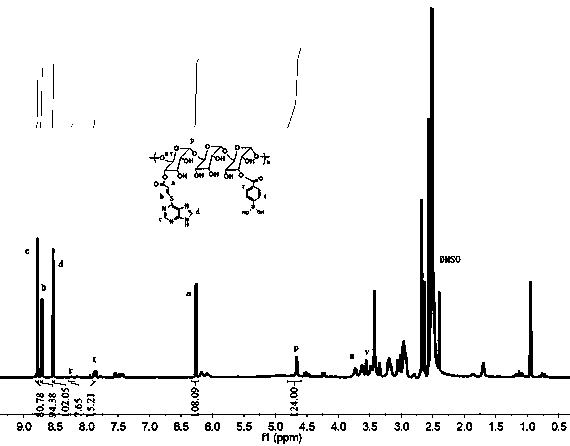
[0057] 6-MP (0.17 g, 1 mmol), sodium methoxide (0.2 g, 3.7 mmol) and propiolic acid (0.07 g, 1 mmol) were added to 20 mL of anhydrous methanol solution under stirring, and the mixture was refluxed for 24 hours before adding 4 mL water to quench the reaction. Excess HCl solution (1 M) was then added to precipitate the product; the precipitate was redissolved in NaOH (1 M) and reprecipitated in HCl (1 M) to give brown-yellow PTA (0.12 g, 54%). 1 H NMR (600 MHz, DMSO- d 6 ) : δ 8.80 (d, J =19.9Hz,1H)-COOH-CH-CH-S-C-N-C H -N,8.77(d,10.1Hz,1H)-COOH-CH-C H -S-C-N-CH-N,8.57(s,1H)-COOH-CH-CH-S-C-C-NH-C H -N,6.29 (d, J =10.1Hz,1H)-COOH-CH -CH-S-C-N-CH-N.
[0058] (2) Preparation of Dex-PTA-PBA
[0059] PTA (0.10g, 0.45mmol), PBA (0.05g, 0.30m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com