Pantoprazole sodium enteric-coated tablet and preparation method thereof
A technology of pantoprazole sodium plain tablet and pantoprazole sodium, which is applied in the preparation of pharmaceutical preparations, pantoprazole sodium enteric-coated tablet and the field of preparation thereof, can solve the problems of limited application, easy discoloration of the tablet, and release rate. Instability and other problems, to achieve the effect of easy storage and transportation, stable product quality, and great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 pantoprazole sodium enteric-coated tablet
[0024] formula
[0025] Pantoprazole Sodium Tablets:
[0026] Pantoprazole Sodium 44mg
[0028] Mannitol 80mg
[0029] Microcrystalline Cellulose 18mg
[0030] Crospovidone 15mg
[0031] Povidone 11mg
[0033] Isolation layer:
[0034] Hypromellose 1.8mg
[0035] Sodium carbonate 1.8mg
[0036] Enteric layer:
[0037] Acrylic resin 16mg
[0038] method:
[0039] (1) preparation of pantoprazole sodium tablet:
[0040] The pantoprazole sodium raw material is passed through a 100-mesh sieve, and other auxiliary materials are respectively passed through an 80-mesh sieve, and the raw and auxiliary materials (except magnesium stearate) are mixed evenly and granulated by a high-speed mixing granulator. After boiling and drying at 40-45°C, pass through a 24-mesh sieve, add magnesium stearate, and press into tablets for later use.
[0041] (2) mi...
Embodiment 2
[0046] Embodiment 2: pantoprazole sodium enteric-coated tablet
[0047] formula
[0048] Pantoprazole Sodium Tablets:
[0049] Pantoprazole Sodium 44mg
[0050] Sodium carbonate 10mg
[0051] Mannitol 80mg
[0052] Microcrystalline Cellulose 18mg
[0053] Crospovidone 15mg
[0054] Povidone 11mg
[0055] Magnesium stearate 2mg
[0056] Isolation layer:
[0057] Hypromellose 2.25mg
[0059] Enteric layer:
[0060] Acrylic resin 18mg
[0061] Method is with embodiment 1.
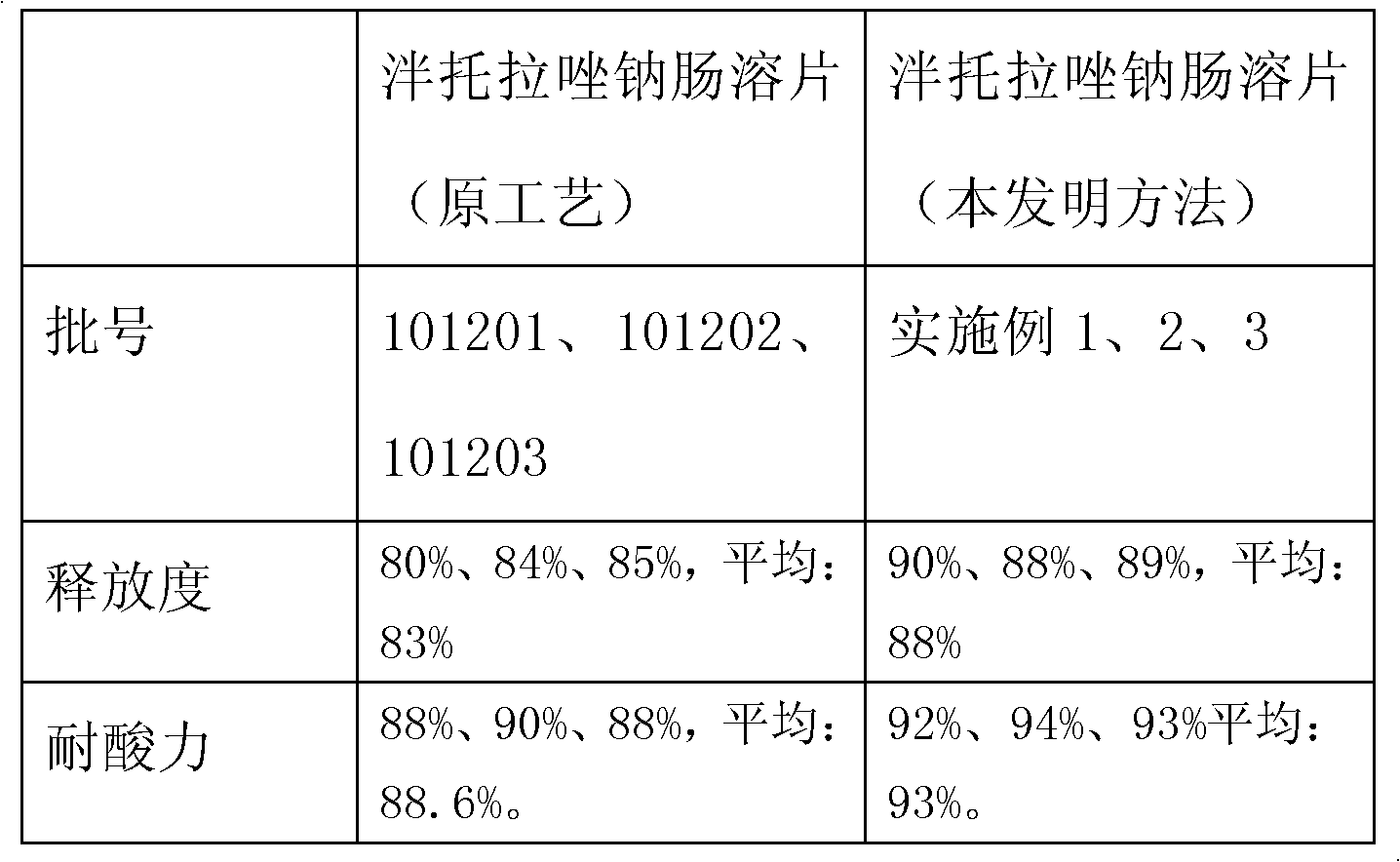
[0062] The acid resistance of the finished product reaches 94%, and the release rate is 88%, which meets and exceeds the requirements of "Chinese Pharmacopoeia" for enteric-coated tablets.
Embodiment 3
[0063] Embodiment 3: pantoprazole sodium enteric-coated tablet
[0064] formula
[0065] Pantoprazole Sodium Tablets:
[0066] Pantoprazole Sodium 44mg
[0067] Sodium carbonate 10mg
[0068] Mannitol 80mg
[0069] Microcrystalline Cellulose 18mg
[0070] Crospovidone 15mg
[0071] Povidone 11mg
[0072] Magnesium stearate 2mg
[0073] Isolation layer:
[0074] Hypromellose 1.8mg
[0075] Sodium bicarbonate 3.6mg
[0076] Enteric layer:
[0077] Acrylic resin 20mg
[0078] Method is with embodiment 1.
[0079] The acid resistance of the finished product reaches 93%, and the release rate exceeds 89%, which meets and exceeds the requirements of "Chinese Pharmacopoeia" for enteric-coated tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com