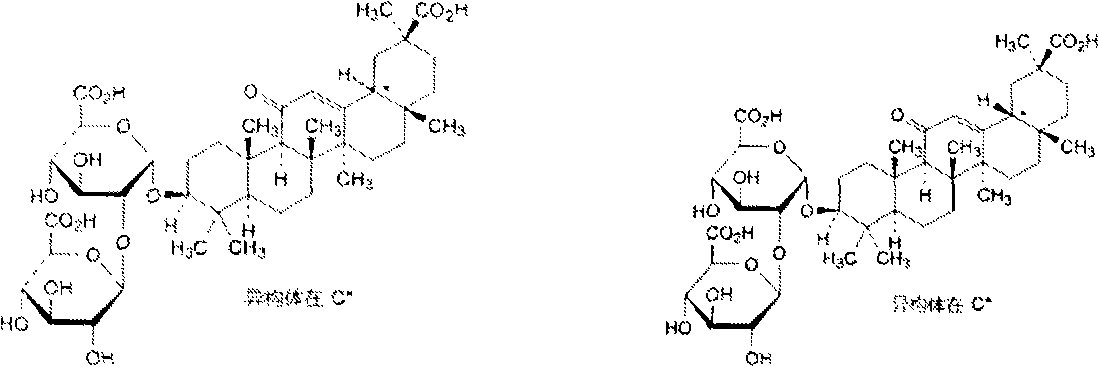
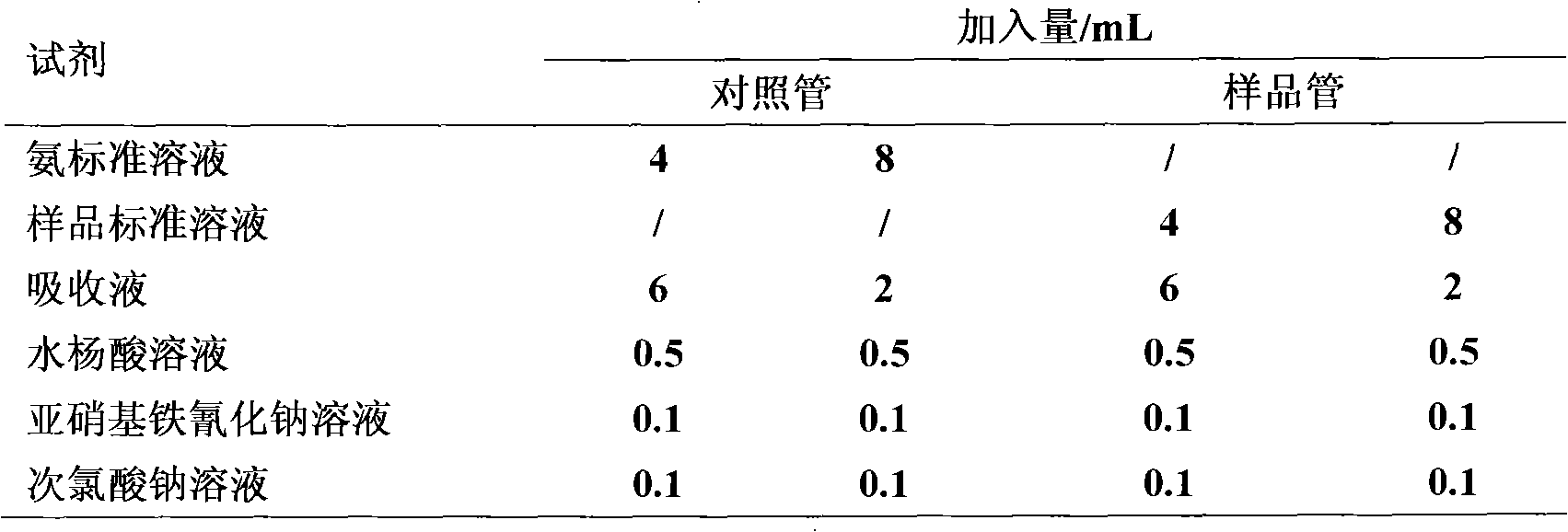
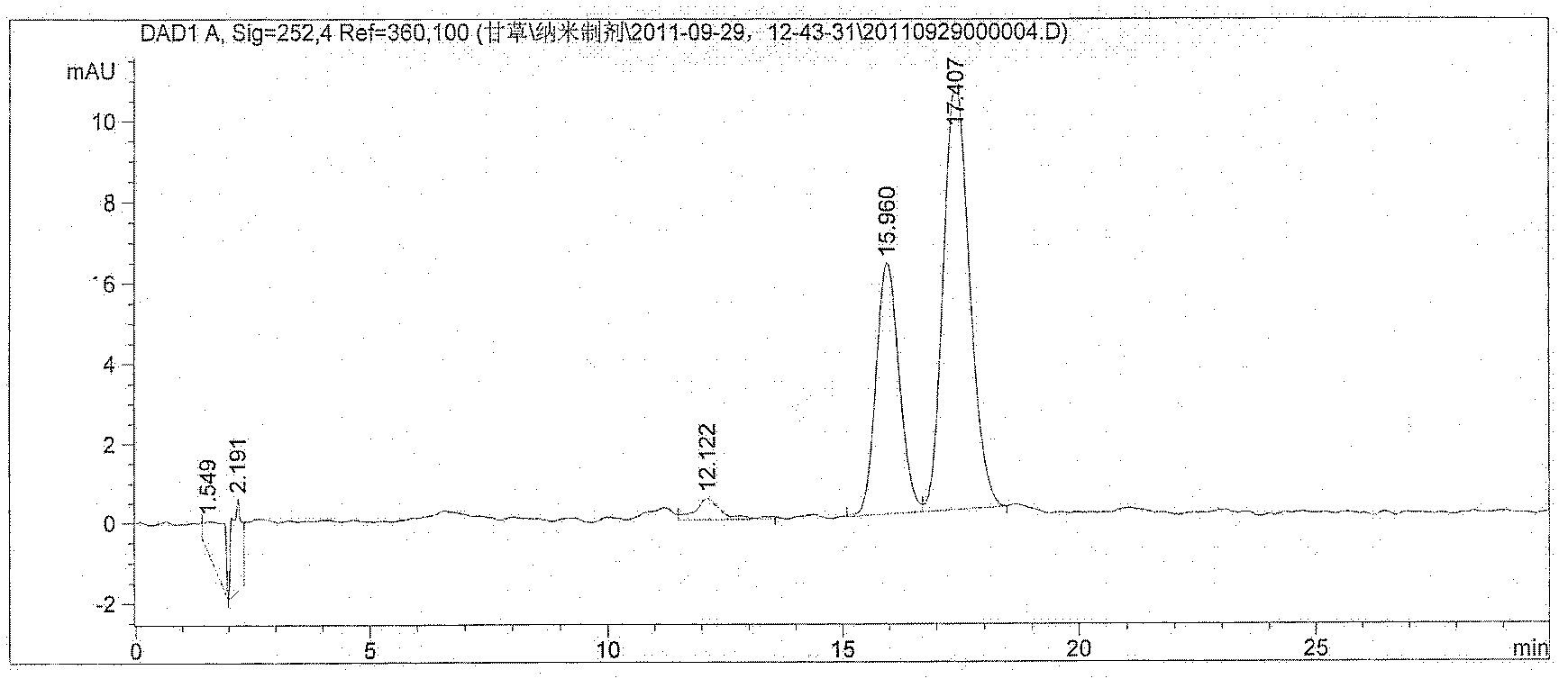
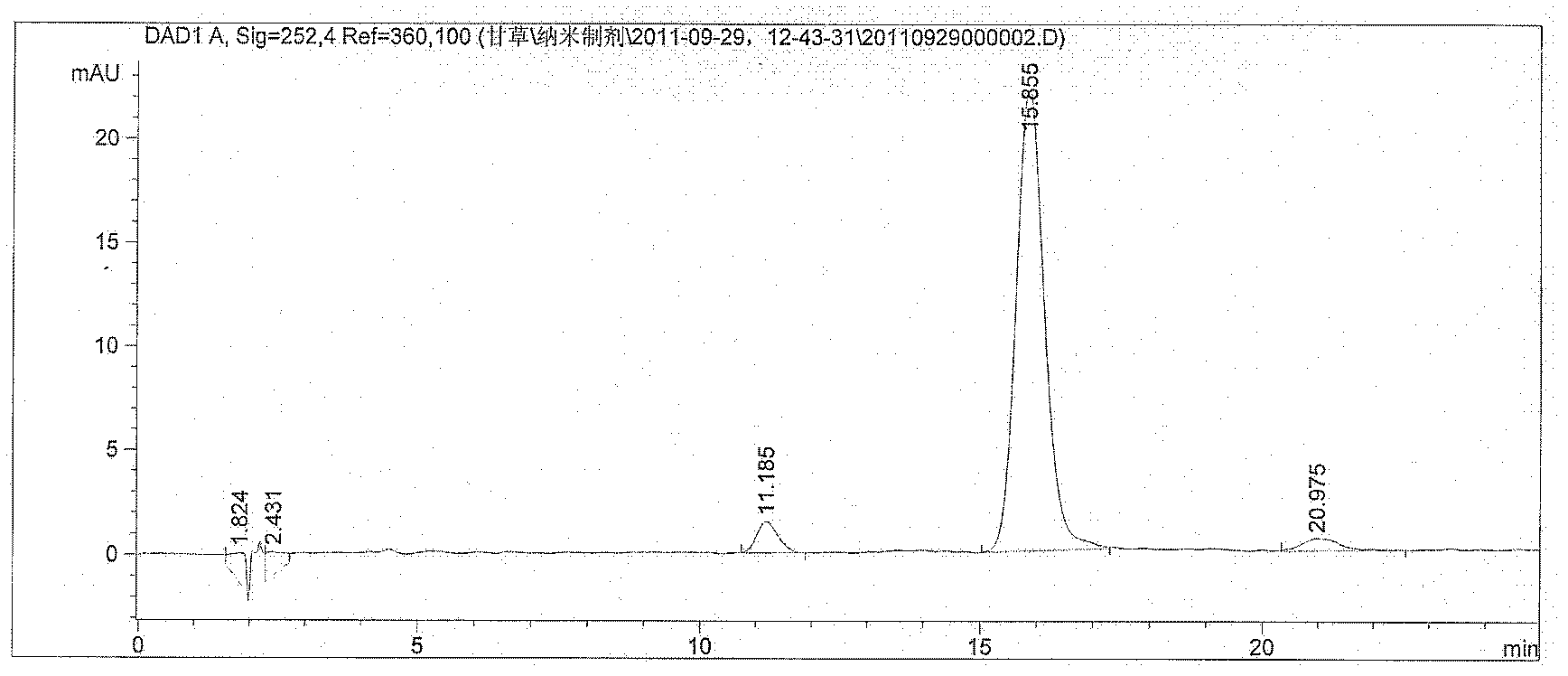
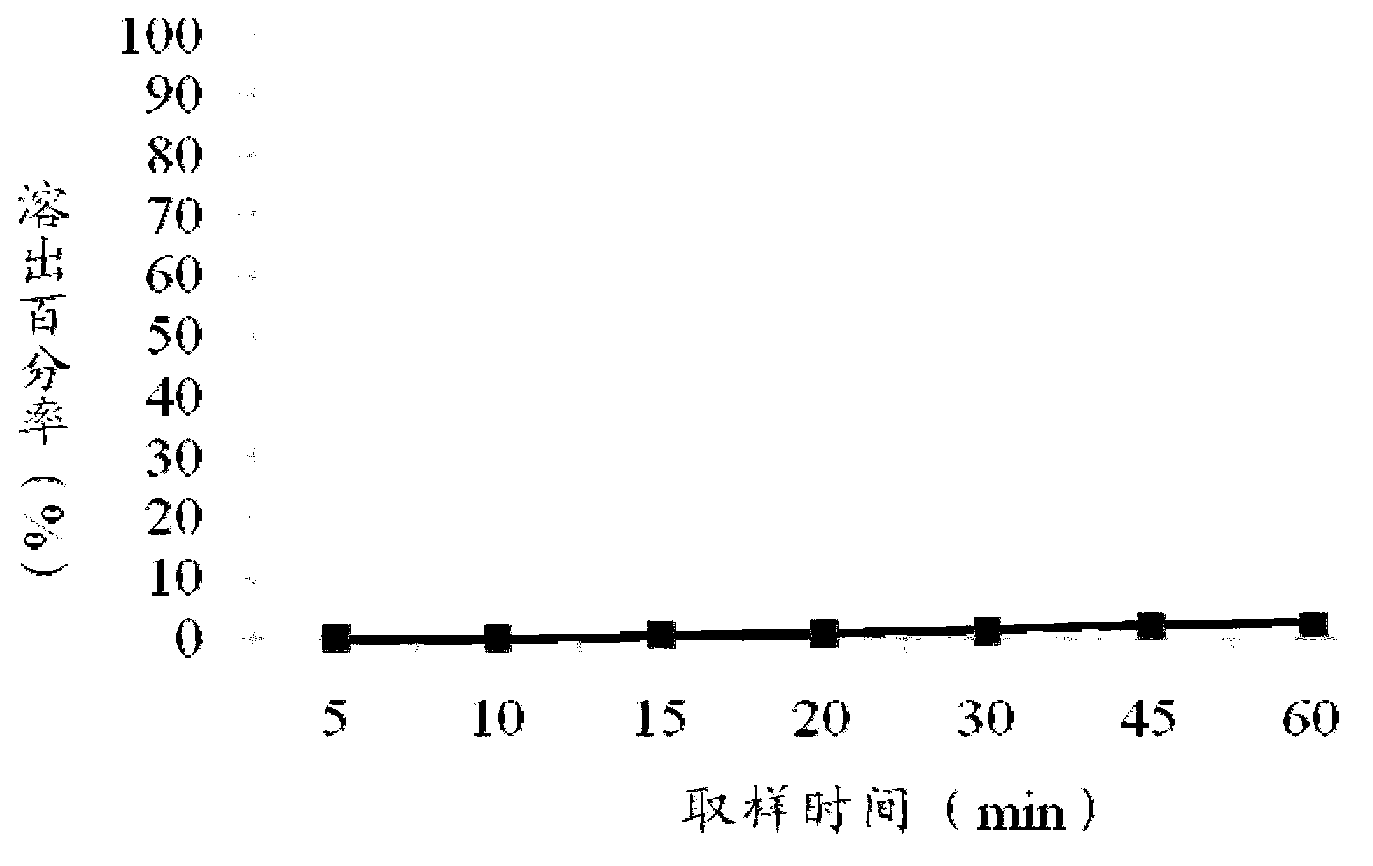
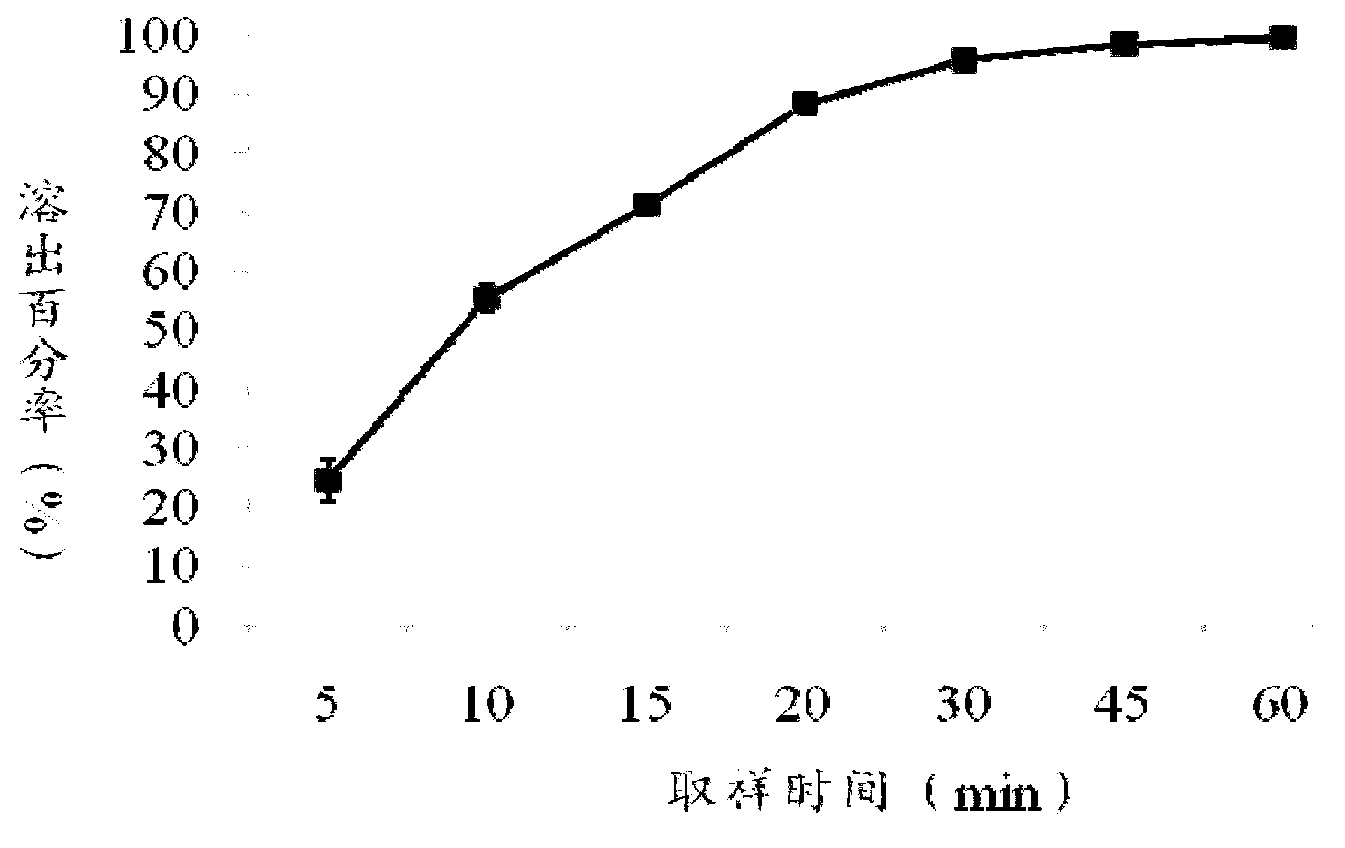
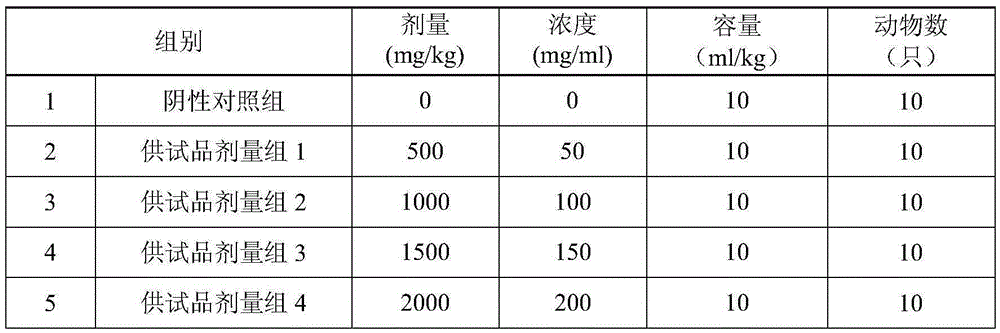
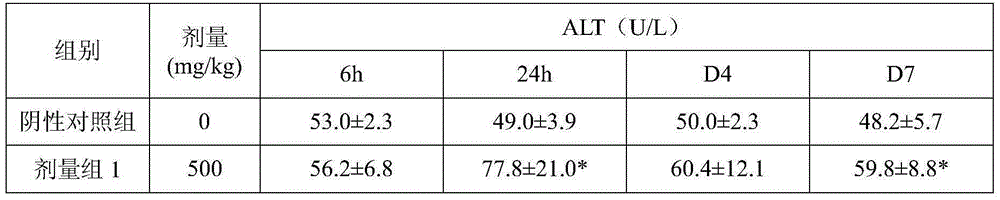
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
80 results about "Diammonium Glycyrrhizinate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
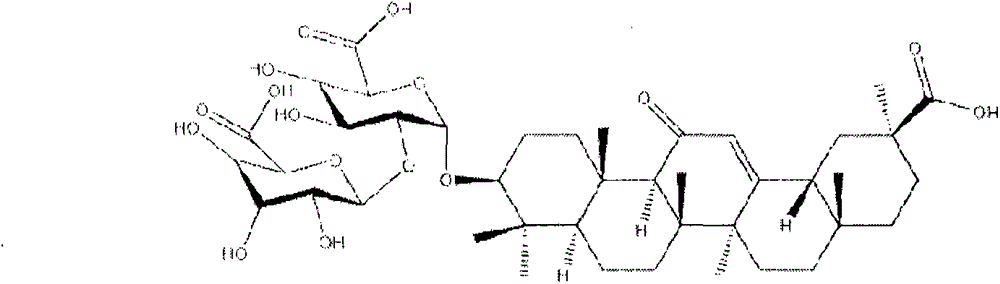
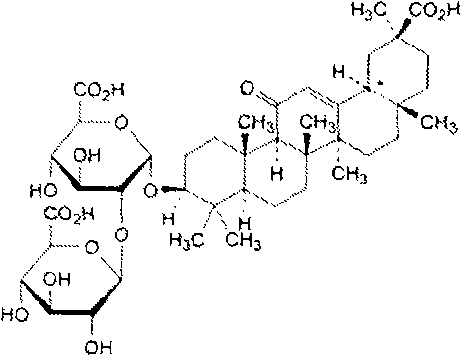
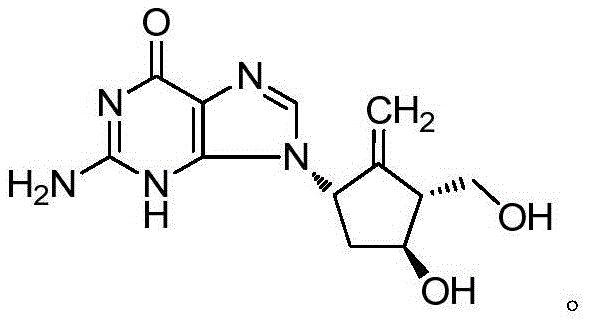
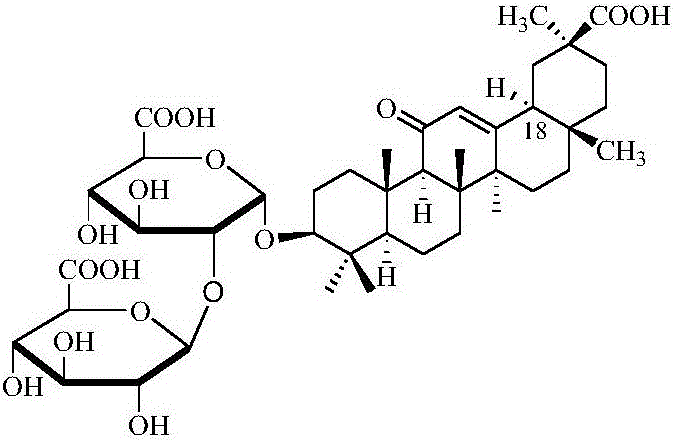
Diammonium glycyrrhizinate is a widely used anti-inflammatory agent isolated from the licorice root. It is metabolized to GLYCYRRHETINIC ACID, which inhibits 11-BETA-HYDROXYSTEROID DEHYDROGENASES and other enzymes involved in the metabolism of CORTICOSTEROIDS.
Combination medicament of ilaprazole sodium and preparation process thereof
InactiveCN102058593AEliminate adverse reactionsGood regeneration performanceOrganic active ingredientsDigestive systemCoated tabletsFreeze-drying
The invention provides a combination medicament of ilaprazole sodium. The medicament is characterized by comprising the following raw materials in percentage by weight: 5-10 percent of ilaprazole sodium, 25-35 percent of reduction composition containing glutathione and tiopronin with a weight ratio of 1:10 and 45-55 percent of diammonium glycyrrhizinate. The invention also provides a preparation process of the medicament. According to a pharmaceutically acceptable dosage of the ilaprazole sodium, the combination medicament can be respectively prepared into medical preparations of the dosage forms of injections of the combination medicament of the ilaprazole sodium, freeze-drying injections of combination medicament of the ilaprazole sodium, coated tablets of the combination medicament of the ilaprazole sodium, enteric capsules, sprays, and the like and is used for treating gastric ulcer.
Owner:吴赣英
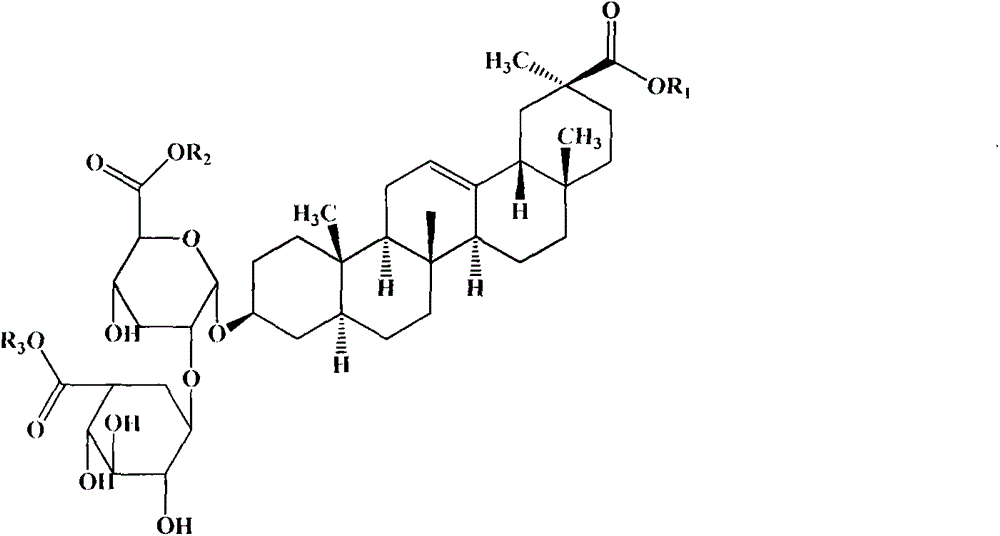
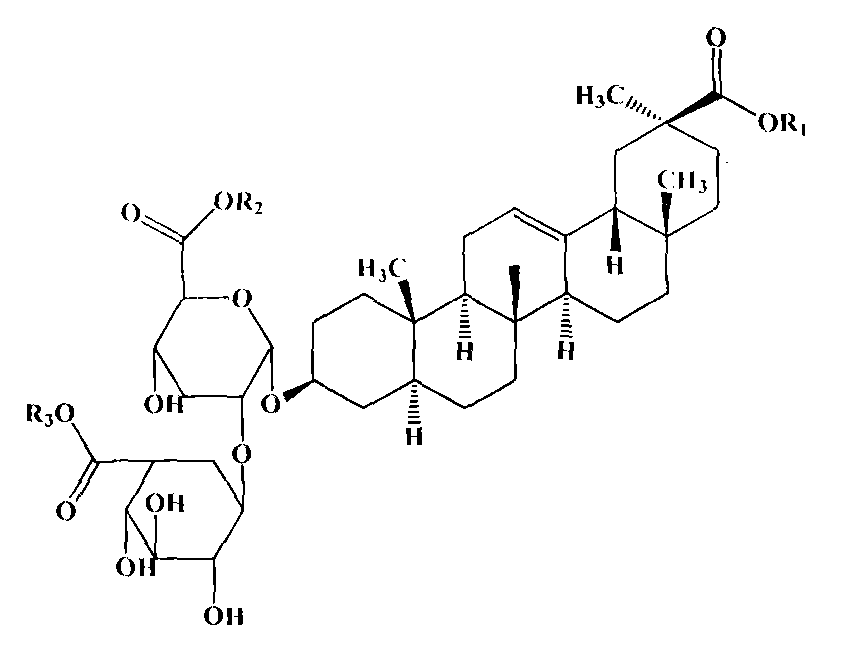
Preparation method of ammonium 18alpha,beta-H-glycyrrhetate and hydrate thereof
The invention relates to a preparation method of ammonium 18alpha,beta-H-glycyrrhetate and a hydrate thereof. The method selecting 75% crude glycyrrhizic acid as a raw material comprises the following steps: purifying the crude glycyrrhizic acid to obtain purified glycyrrhizic acid with the content of above 98%, converting 18alpha,beta-H-glycyrrhizic acid into 18alpha-H-glycyrrhizic acid, and controlling different pH values of the 18alpha-H-glycyrrhizic acid to generate the following different salts: diammonium 18alpha-H-glycyrrhetate, triammonium 18alpha-H-glycyrrhetate and hydrate thereof, and converting 18beta-H-glycyrrhizic acid to generate monoammonium 18beta-H-glycyrrhetate and hydrate thereof. The method has the advantages of simple operation, mild reaction condition, easy control of the quality, high conversion rate, and suitableness for large scale preparation.
Owner:李玉山
Preparation method of diammonium glycyrrhizinate salt capable of accurately controlling ammonium radical content
ActiveCN101914126AHigh yieldSolve the problem of wrong root contentSugar derivativesSteroidsDiammonium GlycyrrhizinateProtic solvent
The invention relates to a preparation method of a diammonium glycyrrhizinate salt capable of accurately controlling ammonium radical content, which is characterized by comprising the following steps of: using a mono-ammonium glycyrrhizinate salt as an initiative raw material; dissolving by adding a protic solvent; regulating a pH value as 4.5-9.0 by adding ammonia; drying by using a suitable method; and monitoring the ammonium radical content by adopting indophenol blue colorimetry to consequently prepare the diammonium glycyrrhizinate salt with correct ammonium radical content. A preparation technology and a monitoring method of the invention solve the problem of wrong ammonium radical content of the diammonium glycyrrhizinate salt prepared by the prior art in a breakthrough mode, reduce crystallizing steps at the same time, greatly increase the diammonium salt yield and are suitable for industrialized production. The prepared diammonium glycyrrhizinate salt also has definite18alpha and 18beta configurational compositions.
Owner:BEIJING UNION PHARMA FACTORY
Diammonium glycyrrhizinate salt preparation method
The invention discloses a diammonium glycyrrhizinate salt preparation method. According to the method, according to the special physical and chemical properties of glycyrrhizic acid, the diammonium glycyrrhizinate salt with characteristics of good color, good character, high content and meeting of the industry standard is produced by using an ammonium glycyrrhizinate salt as a raw material. Compared to the method in the prior art, the method of the present invention has significant advantages of novel process, simple operation, energy saving, environmental protection, strong practicality, mild reaction condition, high conversion rate, easy quality control, reduction of the use of other solvents, reduction of impurity introduction, good product color, simple operation process, low equipment and material cost, high treatment capacity, good product chrominance, good product yield, high content, excellent product color, uniform crystal and good quality, and is suitable for industrial production.
Owner:JIANGSU TIANSHENG PHARMA
Combined medicine of diammonium glycyrhetate and its preparing method
InactiveCN1709272AHinder inactivationInhibition releaseOrganic active ingredientsSulfur drugAlcoholisms
The present invention relates to a compound medicine formed from diammonium glycyrrhizinate and one or several kinds of halfcystine, glycine, methionine and vitamin B1. Its therapeutic action is extensive, and side effect is low. Said compound medicine can be used for curing the liver diseases of acuter, chronic and virus hepatitises, cirrhosis and fatty liver, etc. and can be used for improving function of liver and cholestasis in the liver. Besides, it also can be used for auxiliary therapy of alcoholism and barbitones and sulfonamides drug poisoning.
Owner:张哲峰
Diammonium glycyrrhizinate freeze drying powder for injection and its prepn
InactiveCN1526399AGood effectImprove stabilityOrganic active ingredientsPowder deliveryFreeze-dryingSodium Chloride Injection
The present invention relates to medicine technology. The diammonium glycyrrhizinate freeze drying powder for injection includes the following components: diammonium glycyrrhizinate 10-100 wt%, solabilizer 0-90 wt% and excipient 0-80 wt%. Its preparation process includes dissolving the above said components, regulating pH value, filtering, packing and freeze drying. The medicine is used clinically in intravenous dripping for treating chronic organizing hepatitis accompanied with raised glutamic pyruvic transaminase and chronic mobile hepatitis. It has stable quality, long storage period and fast dissolution in water, and may be matched with glucose injection and / or sodium chloride injection clinically.
Owner:肖广常 +1
Preparation method for trans-glycyrrhizic acid
ActiveCN102584928AIncrease profitSimple processSugar derivativesSteroidsAcid catalysisDiammonium Glycyrrhizinate
The invention relates to a preparation method for trans-glycyrrhizic acid. Diammonium glycyrrhizinate is directly placed in carbinol, a proper amount of protonic acid is added for catalysis, and under room temperature conditions, trans-glycyrrhizic acid methyl ester is selectively combined in automatic coherent type multicomponent reactive mode so as to change physical and chemical properties of a glycyrrhizic acid stereoisomer mixture, enable trans-glycyrrhizic acid methyl ester with weak polarity to be dissolved out from reaction liquid and filtered and obtain trans-glycyrrhizic acid methyl ester to be separated from cis-glycyrrhizic acid. Then, single-component trans-glycyrrhizic acid is prepared through saponification, acid neutralization and the like, and cis-glycyrrhizic acid is recycled. The preparation method resolves the technical problem that the glycyrrhizic acid stereoisomer mixture cannot be separated and has the advantages of being simple, low in energy consumption and high in yield and improving utilization ratio of natural glycyrrhizic acid.
Owner:杭州市第六人民医院
Environment-friendly equipment for producing liver protection drug diammonium glycyrrhizinate
PendingCN108568342AEnvironmental protectionPromote environmental protectionDispersed particle filtrationTransportation and packagingEngineeringDiammonium Glycyrrhizinate
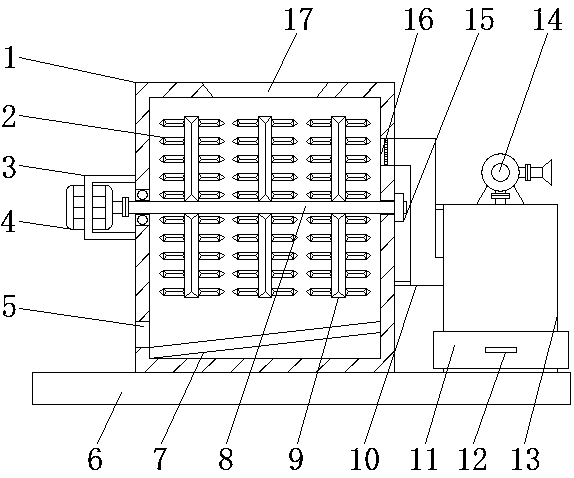
The invention discloses environment-friendly equipment for producing a liver protection drug diammonium glycyrrhizinate. The environment-friendly equipment comprises a bottom plate, wherein a crushingbox is fixedly connected with the left side of the top of the bottom plate; a feeding opening is formed in the center of the top of an inner cavity of the crushing box; a discharging opening is formed in the bottom of the left side of the inner cavity of the crushing box. According to the environment-friendly equipment disclosed by the invention, an air induction pipe, a sealing plate, a pull handle, a dust collection box, an air exhausting machine, an air suction opening, a vibration motor, a supporting spring, an air inlet, a storage frame, a rail, a sliding rail, a supporting plate, a filtering frame, an air outlet and a filter screen are matched to use, and dust generated in a liquorice root crushing process can be recycled, so that the utilization of diammonium glycyrrhizinate production equipment is more environmentally friendly; the problems in a production process of diammonium glycyrrhizinate production equipment that the dust generated in the liquorice root crushing processcannot be recycled and a lot of dust is discharged into the external environment so that the environment-friendly effect of the diammonium glycyrrhizinate production equipment is relatively poor are solved.
Owner:HENAN HUIJIN PHARMA
Rabeprazole sodium combined medicament and preparation process thereof
InactiveCN102091070AEliminate adverse reactionsGood regeneration performanceOrganic active ingredientsDigestive systemDrugs preparationsRabeprazole Sodium
The invention provides a rabeprazole sodium combined medicament and a preparation process thereof. The rabeprazole sodium combined medicament is characterized by comprising the following raw materials as components in proportion by weight: 5-10 of ilaprazole sodium, 20-30 of composition of reduced glutathione and hepatocyte growth-promoting factors in proportion by weight of 1:10, and 50-60 of diammonium glycyrrhizinate. According to a pharmaceutically allowable dose of rabeprazole sodium, pharmaceutical preparations in dosage forms of injection, lyophilized powder injection, enteric coated tablets, enteric coated capsules, spray and the like of the rabeprazole sodium combined medicament are respectively prepared for treating gastric ulcer.
Owner:吴赣英
Primary hepatocyte cryopreservation solution, hepatocyte cryopreservation method and hepatocyte resuscitation method
ActiveCN110463689AReduced activityReduce adhesionCulture processDead animal preservationRecovery methodHydroxyethyl starch
The invention discloses a primary hepatocyte cryopreservation solution, a hepatocyte cryopreservation method and a hepatocyte recovery method, and belongs to the field of primary hepatocyte cryopreservation and recovery. The primary hepatocyte cryopreservation solution comprises, by volume, 45% of a solution A, 10-45% of fetal bovine serum, 10% of dimethyl sulfoxide, and 0-35% of water, and the solution A comprises D-glucose, hydroxyethyl starch, lactobionic acid, polyvinylpyrrolidone, D-raffinose pentahydrate, potassium hydroxide, potassium dihydrogen phosphate, magnesium sulfate heptahydrate, adenosine, reduced glutathione, allopurinol, tauroursodeoxycholic acid, and diammonium glycyrrhizinate. The invention also discloses the hepatocyte cryopreservation method and the hepatocyte recovery method. The primary hepatocyte cryopreservation solution can reduce the damages of the primary hepatocytes in the cryopreservation process and improve the vitality and adherence efficiency of the primary hepatocytes after thawing, and can be widely used in basic research and new drug development for livers.
Owner:周明
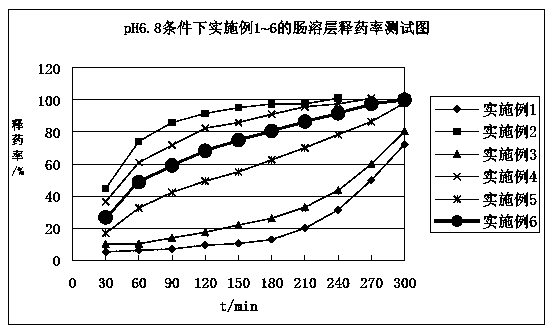
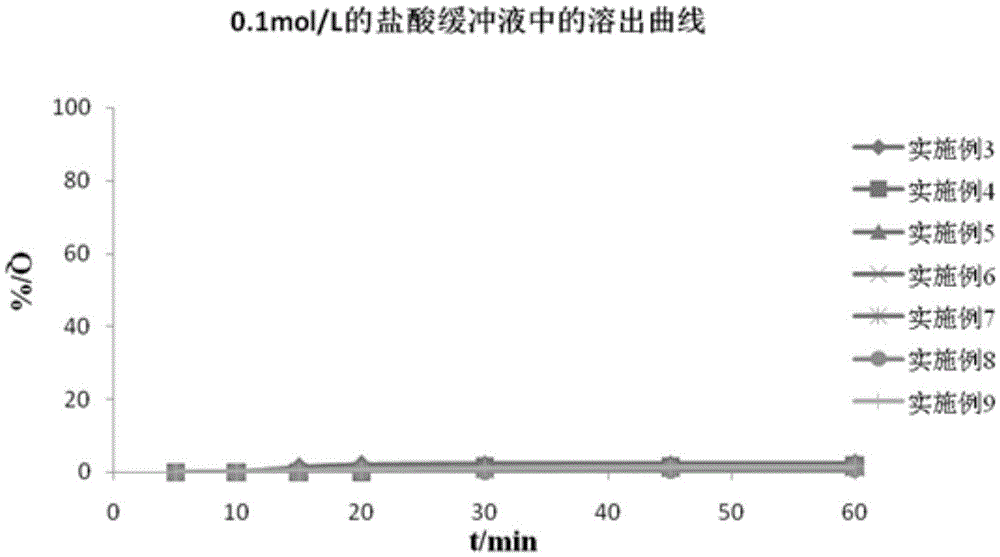
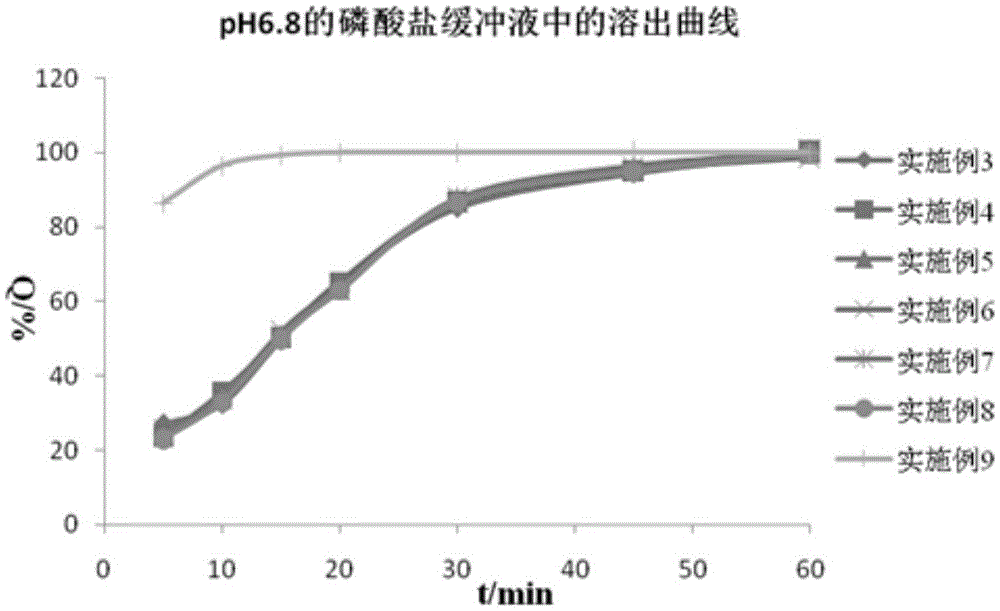
Diammonium glycyrrhizinate enteric-coated pellet as well as preparation method and preparation thereof
ActiveCN103301074AAvoid destructionGuaranteed stabilityOrganic active ingredientsDigestive systemMedicineDiammonium Glycyrrhizinate
The invention relates to the technical field of medicines and in particular relates to a diammonium glycyrrhizinate enteric-coated pellet as well as a preparation method and preparation thereof. The diammonium glycyrrhizinate enteric-coated pellet comprises diammonium glycyintestinal tractrrhizinate, excipient, adhesive and coating material in a mass ratio of 1:(0.30-8):(0.02-0.1):(0.2-3.2). The diammonium glycyrrhizinate enteric-coated pellet is table in gastric juice and good in dissolution rate in intestinal tract. The damages of the gastric juice to the diammonium glycyrrhizinate are avoided, so that the stability of the diammonium glycyrrhizinate is ensured, and the biological utilization rate is improved.
Owner:CHENGDU HUASUN GRP INC LTD
Diammonium glycyrrhizinate freeze dried powder ampoul agent and its preparation method
InactiveCN1493294AImprove stabilityVarious dosage formsPowder deliveryOrganic active ingredientsMANNITOL/SORBITOLFreeze-drying
A freeze dried powder injection of diammonium glycyrrhetate is prepared from diammonium glycyrrhetate and mannitol or sodium chloride or glucose in ratio of 1:(0.5-2) through dissolving the diammonium glycyrrhetate in water, adding mannitol, stirring for dissolving, regulating pH value, adding carbon for injection, heating to 80 deg.C and holding the temp for 30 min, cooling to 45 deg.C, decarbonizing, adding the water for injection, fine filter, pouring in bottles, and freeze drying. Its advantage is high curative effect.
Owner:NANJING KEFEIPING MEDICINE SCI & TECH
Medicine composition of glycyrrhizic acid or its salt, ginseng and astragalus root
InactiveCN1985873AMeet urgent clinical needsOrganic active ingredientsDigestive systemHepatitisTraditional medicine
The present invention belongs to the field of medicine technology, and is especially one medicine composition for treating hepatitis and its preparation process and use. The medicine composition consists of glycyrrhizic acid or its pharmaceutically acceptable salt 4-800 weight portions, ginseng 250-16000 weight portions, and astragalus root 5000- 80000 weight portions. It may consist of glycyrrhizic acid or its pharmaceutically acceptable salt, the extract of ginseng and the extract of astragalus root. It may be prepared into different forms. The medicine composition has the synergistic effect of the components, high stability, high effect and wide application.
Owner:海安江理工技术转移中心有限公司
Pharmaceutical composition of acetaminophen and glycyrrhizic acid or salt thereof or derivative thereof
PendingCN106806376ALessen liver damageEasy to useOrganic active ingredientsAntipyreticDiammonium GlycyrrhizinateDISODIUM GLYCYRRHIZATE
The invention relates to a pharmaceutical composition, in particular to a pharmaceutical composition of acetaminophen and glycyrrhizic acid or medicinal salt thereof or medicinal derivative thereof. The weight ratio of acetaminophen to glycyrrhizic acid or medicinal salt thereof or medicinal derivative thereof is (40:1)-(1:1). Glycyrrhizic acid medicinal salt or glycyrrhizic acid medicinal derivative is selected from mono-ammonium glycyrrhizinate, diammonium glycyrrhizinate, monopotassium glycyrrhizinate, dipotassium glycyrrhizinate, disodium glycyrrhizate, trisodium glycyrrhetate, magnesium isoglycyrrhizinate, glycyrrhetinic acid or glycyrrhizin. The invention further relates to an oral drug prepared from the pharmaceutical composition, and the oral drug can be tablets, capsules or particles. By the adoption of the pharmaceutical composition, liver injury caused by acetaminophen can be reduced to a certain extent, using convenience is ensured, and therefore a solution is provided for safer and more efficient use of acetaminophen.
Owner:COSCI MED TECH CO LTD
Medicine composition of glycyrrhizic acid or its salt and reduced glutathione
InactiveCN1985987BMeet urgent clinical needsRaise the ratioOrganic active ingredientsDigestive systemDiseaseHepatic Diseases
The present invention belongs to the field of medicine technology, and is especially one medicine composition for treating liver diseases and its preparation process and use. The medicine compositionconsists of glycyrrhizic acid or its pharmaceutically acceptable salt 4-800 weight portions, and reductive glutathione 75- 1500 weight portions. The pharmaceutically acceptable salt of glycyrrhizic acid may be metal salt or organic nitrogen salt. The medicine composition may be prepared into different forms. The medicine composition has the synergistic effect of the components in treating liver diseases, high stability, and wide application.
Owner:海安江理工技术转移中心有限公司
Xanthosine composition and its production
InactiveCN1981779AMeet urgent clinical needsStable and uniform qualityOrganic active ingredientsDigestive systemXanthosineDiammonium Glycyrrhizinate
A composite medicine for treating hepatism is prepared from scutelloside (60-2000 Wt portions) and glycyrrhizic acid or its pharmacologically acceptable salt (10-800). Its preparing process is also disclosed.
Owner:海安江理工技术转移中心有限公司
Cefoxitin sodium drug combination
InactiveCN101822681AImprove antibacterial propertiesReduce adverse reactionsAntibacterial agentsOrganic active ingredientsAdditive ingredientCurative effect
The invention discloses a cefoxitin sodium drug combination, which consists of the following effective ingredients in part by weight: 1000 to 2000 parts of cefoxitin sodium, 100 to 200 parts of lidocaine, 50 to 120 parts of reduced glutathione and 80 to 120 parts of diammonium glycyrrhizinate. The drug combination cannot cause adverse reactions, and has a high curative effect, and moreover, the preparation method is simple and environment-friendly.
Owner:邓学峰
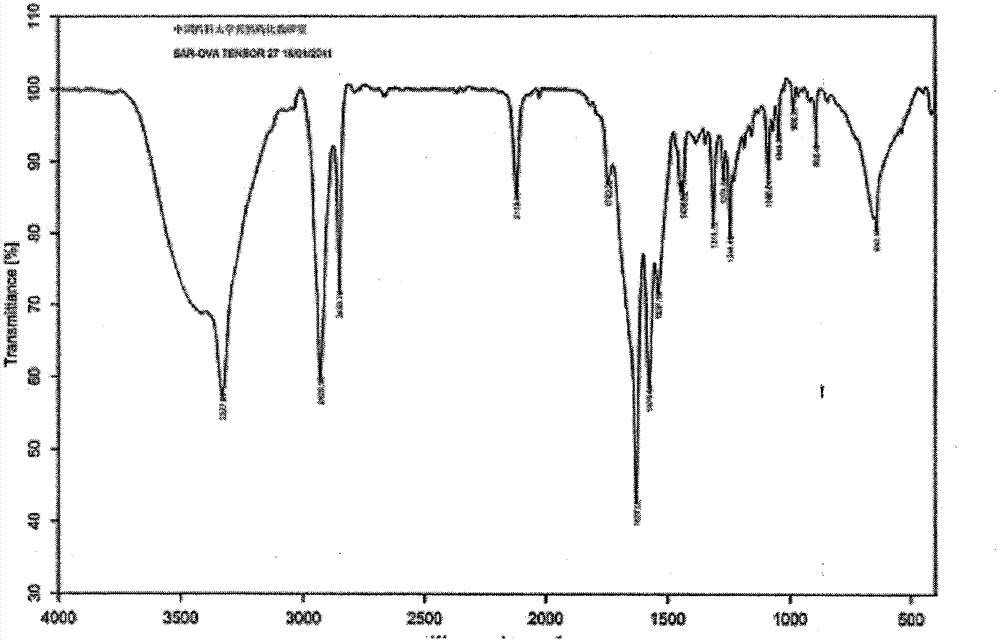
Method for removing solvent residues of diammonium glycyrrhizinate bulk drug
The invention discloses a method for removing solvent residues of a diammonium glycyrrhizinate bulk drug. The method comprises the following steps: using a mixing solvent for performing decolorizing re-crystallization on a synthetic crude product of diammonium glycyrrhizinate; centrifugally filtering; drying under reduced pressure; absorbing water and moisture, and then performing suction filtration, and continuing to dry under reduced pressure, thereby acquiring the diammonium glycyrrhizinate bulk drug. Through detection, the residual solvent ethyl alcohol of the acquired diammonium glycyrrhizinate bulk drug is less than or equal to 2000ppm and is far lower than the quality standard (less than or equal to 5000ppm) for residual solvent of diammonium glycyrrhizinate bulk drug in Chinese pharmacopoeia. The diammonium glycyrrhizinate bulk drug is a white granular crystal in larger crystal size and is high in purity and stability.
Owner:河南豫辰药业股份有限公司
Diammonium glycyrrhizinate enteric-coated sustained-release pellet and preparation method thereof
ActiveCN104288108AImprove bioavailabilityImprove adhesionOrganic active ingredientsAntiviralsCelluloseSustained release pellets
The invention provides a diammonium glycyrrhizinate enteric-coated sustained-release pellet and a preparation method thereof, and belongs to the technical field of pellet preparation. The pellet comprises a pellet core, and a controlled-release layer and an enteric layer which sequentially coat outside the pellet core from inside to outside, wherein the controlled-release layer is prepared from the following components: 88-93 parts of ethyl cellulose, 4-6 parts of albumin and 4-6 parts of amino acid; and the enteric layer is prepared from the following components: 90-100 parts of methacrylate copolymer, 3-6 parts of lecithin, 0.2-0.9 part of sodium hydroxide and 17-22 parts of magnesium stearate. The pellet is prepared by the steps of preparing the pellet core, coating with the controlled-release layer and coating with the enteric layer. The pellet has the functions of solubilizing glycyrrhizic acid and improving the adhesion and permeating effect of glycyrrhizic acid on small intestine wall, and has relatively good intestine absorption effect and sustained-release effect.
Owner:山东世博金都药业有限公司
Compound enteric-coated tablets of entecavir phospholipid complex and diammonium glycyrrhizinate
ActiveCN105582546AImprove bioavailabilityImprove securityDigestive systemAntiviralsAnti virusCurative effect
The invention provides compound enteric-coated tablets of entecavir phospholipid complex and diammonium glycyrrhizinate and also relates to a preparation method of the compound enteric-coated tablets and an application in treatment of hepatitis B. The compound enteric-coated tablets provided by the invention have a slow release effect and can improve the bioavailability, safety and effectiveness of the medicines; and moreover, the entecavir phospholipid complex and diammonium glycyrrhizinate together realize anti-virus and liver protection effects to improve the curative effect on hepatitis B.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Preparation method of 18 alpha type diammonium glycyrrhizinate
ActiveCN106380506ALow reaction temperatureLow costSugar derivativesSteroidsRetention timeReaction temperature
The invention discloses a preparation method of 18 alpha type diammonium glycyrrhizinate. Under a certain alkali concentration, 18 beta type diammonium glycyrrhizinate is converted into 18 alpha type diammonium glycyrrhizinate. The end point is judged according to a principle that under a certain chromatographic condition, the retention time of 18 beta type diammonium glycyrrhizinate and the retention time of 18 alpha type diammonium glycyrrhizinate are different, and high performance liquid chromatography (HPLC) is used to judge the end point. The obtained 18 alpha type diammonium glycyrrhizinate is added into stronger ammonia water, and after refinement, qualified diammonium salt can be obtained. Compared with the conventional technology, the reaction temperature is reduced by about 10 DEG C, the time is also shortened, thus the energy loss of industrial production can be largely reduced; at the same time, the service life of equipment is prolonged, and the cost is reduced. Furthermore, the high temperature of conventional technology will deepen the product color, and the post treatment becomes difficult. The prepared 18 alpha type diammonium glycyrrhizinate has the advantages of good color, high yield and high content. The preparation method is suitable for industrial massive production, the product color is good, the crystals are uniform, and the quality is good.
Owner:JIANGSU TIANSHENG PHARMA
Diammonium glycyrrhizate oral cavity disintegrating agent and its preparation method
InactiveCN1650879ASimple production processEase of mass productionOrganic active ingredientsDigestive systemGlycyrrhizateOrally disintegrating tablet
An orally disintegrating tablet of diammonium glycyrrhetate is proportionally prepared from diammonium glycyrrhetate and them edical auxiliary composed of filler, disintegrant, effervescent agent, lubricant and flouring.
Owner:FUKANGREN BIO PHARMA
Novel application of diammonium glycyrrhizinate
InactiveCN111228281AOrganic active ingredientsPeptide/protein ingredientsInflammatory factorsAntioxidant
The invention discloses a novel application of diammonium glycyrrhizinate, and relates to the technical field of biological medicines. The invention relates to a novel application of diammonium glycyrrhizinate, in particular to a novel application of diammonium glycyrrhizinate in preparation of medicines for treating novel coronavirus pneumonia 2019-nCoV or chronic obstructive pulmonary disease, and meanwhile, diammonium glycyrrhizinate can also be used in combination with an immunopotentiator or a medicinal antioxidant. The diammonium glycyrrhizinate can replace hormones to resist inflammatory factors to protect the lung, but has no immunosuppressive effect and osteoporosis effect of the hormones.
Owner:GUIZHOU MAQIKA PHARMA
Sarsasapogenin monoclonal antibody and application
InactiveCN102786594AHigh sensitivityStrong specificitySerum albuminImmunoglobulins against plantsAntiendomysial antibodiesBlood plasma
The invention discloses a preparation method and an application of a Sarsasapogenin (SAR) monoclonal antibody. On the basis of Sarsasapogenin artificial antigen preparation, the anti-Sarsasapogenin monoclonal antibody is prepared by using a hybridomas technology, the cross reaction rate of the monoclonal antibody with liriope muscari baily saponins C, diosgenin and ruscogenin are respectively detected as 29%, 5.7% and 33%, no obvious cross reaction is generated by monoclonal antibody with diammonium glycyrrhizinate, pseudo-ginseng saponins R1 and oleanolic acid. The invention establishes an ELISA analysis method based on the Sarsasapogenin monoclonal antibody, the correlation of the ELISA analysis method and the HPLC method detection result is good, compared with the HPLC method, the ELISA analysis method has characteristic of rapid microscale detection. The Sarsasapogenin monoclonal antibody is suitable for Sarsasapogenin microscale detection in Sarsasapogenin-containing traditional Chinese medicinal materials and biology samples like blood plasma, and can be used for medicine quality control of active components-containing medicines and pharmacokinetics research.
Owner:CHINA PHARM UNIV
Diammonium glycyrrhizinate microemulsion and preparation process thereof
InactiveCN101810573AThe preparation method is novel and reasonableSimple processOrganic active ingredientsDigestive systemHepatic first pass effectAntiviral therapy
The invention discloses diammonium glycyrrhizinate microemulsion and a preparation process thereof, providing a microemulsion preparation for patients with viral hepatitis. The diammonium glycyrrhizinate microemulsion is prepared from a medical raw material and three auxiliary materials; and the preparation method of diammonium glycyrrhizinate microemulsion comprises a step of preparing auxiliary material solution, a step of preparing raw material solution and a step of preparing microemulsion. The preparation method of diammonium glycyrrhizinate microemulsion is scientific and novel; the preparation process is simple and convenient; the diammonium glycyrrhizinate microemulsion has more functions of non-specific anti-inflammatory, anti-allergy, stable effect on lysosome membrane and immunoregulation in clinical application; in addition, the diammonium glycyrrhizinate microemulsion improves the thermodynamic activity, prevents the first-pass effect of liver and is easier to work. Compared with the other diammonium glycyrrhizinate oral liquid preparations, the diammonium glycyrrhizinate microemulsion has higher bioavailability and more remarkable efficiency of antiviral therapy and is more convenient for patients, who have difficulty to swallow solid medicine.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Freeze dried preparation of diammonium glycyrrhizinate and its preparation
ActiveCN1546042AImprove performanceReduce the burden onPowder deliveryOrganic active ingredientsMANNITOL/SORBITOLFreeze-drying
The invention relates to a Diammonium Glycyrrhizinate freeze-drying preparation and process for preparing it, wherein the preparation is prepared from Diammonium Glycyrrhizinate, mannitol, sorbitol, sodium chloride, glucose, cane sugar, lactose, sulfo-aminolactic acid, or pharmacologically accepted expanding material of other amino acids through freeze drying.
Owner:广东宏远集团药业有限公司
Mengjin-1 lonicera japonica yield-increasing planting method
InactiveCN106105716AKeep it brightNot easy to dropPlant cultivationCultivating equipmentsGrowth plantBud
The invention discloses a mengjin-1 lonicera japonica yield-increasing planting method and relates to the technical field of lonicera japonica planting. The method is characterized in that 15% paclobutrazol wettable powder, diammonium glycyrrhizinate, boric acid zinc oxide and 800 times netropsin solution are evenly sprayed onto branches and leaves of lonicera japonica between March and April every spring in the lonicera japonica twig production and vigorous growing period before floral bud emergence, wherein paclobutrazol wettable powder, diammonium glycyrrhizinate, boric acid zinc oxide and netropsin are added according to the ratio of 5:4:1:0.5. By the adoption of the method, plant growth is delayed, stem lengthening is inhibited, plant tillering is promoted, plant adverse resistance is improved, the number and size of flower buds are increased, and flower buds do not fall off easily.
Owner:全椒县岱山金银花种植专业合作社
Compound preparation for treating cholestatic jaundice and preparing method thereof
InactiveCN105998052AAnti-inflammatoryCompatibility is reasonableDigestive systemEther/acetal active ingredientsTherapeutic effectBile fluid
The invention discloses a compound preparation for treating cholestatic jaundice and a preparing method thereof. The compound preparation is prepared from ademetionine 1,4-butanedisulfonate, diammonium glycyrrhizinate, dihydroxydibutylether, baicalin, methionine, anethol trithione, potassium magnesium aspartate, crocetin dimethyl ester, glucurolactone, cholestyramlne, hydroxymethylnicotinamide, trimethoprim lactate, bumetanide, piperazine citrate and azathioprine. The compound preparation has the effects of resisting bacteria and diminishing inflammations, clearing dampness and heat, soothing the liver and benefiting the gallbladder, removing the toxicity and eliminating jaundice and strengthening the spleen and the stomach, can reduce stasis of bile in liver cells, promote bile excretion, improve the liver functions and the tissue cell respiration function, reduce accumulation of fat in the liver and promote jaundice elimination and liver function recovery and is significant in treatment effect, safe and few in side effect when the compound preparation is used for treating the cholestatic jaundice and complications thereof.
Owner:徐海燕
Composition of diammonium glycyrrhizinate
The present invention provides one kind of medicine composition and its medicine preparation. The medicine composition consists of two isomers of diammonium glycyrrhizinate. The present invention provides also the medicine composition and its medicine preparation in treating hepatitis B and its related diseases, especially tumor.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
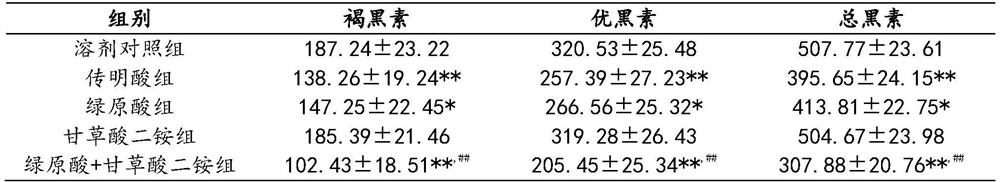
Application of injection composition containing chlorogenic acid in preparation of injection with skin whitening effect
ActiveCN112933102AReduce dosageGood whitening effectOrganic active ingredientsPharmaceutical delivery mechanismChlorogenic acidMedicine
The invention provides application of an injection composition containing chlorogenic acid and diammonium glycyrrhizinate in preparation of an injection with a skin whitening effect, and belongs to the field of medicines. According to the composition disclosed by the invention, the chlorogenic acid is used as a main active component, the diammonium glycyrrhizinate is used as an auxiliary component, and the diammonium glycyrrhizinate can effectively enhance the skin whitening effect of the chlorogenic acid, so that the dosage of the chlorogenic acid is reduced. Pharmacodynamic experiments show that the composition containing chlorogenic acid and diammonium glycyrrhizinate has a remarkable inhibition effect on synthesis of melanin, and the effect of the composition is obviously superior to that of single use of chlorogenic acid or tranexamic acid.
Owner:SICHUAN JIUZHANG BIO TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com