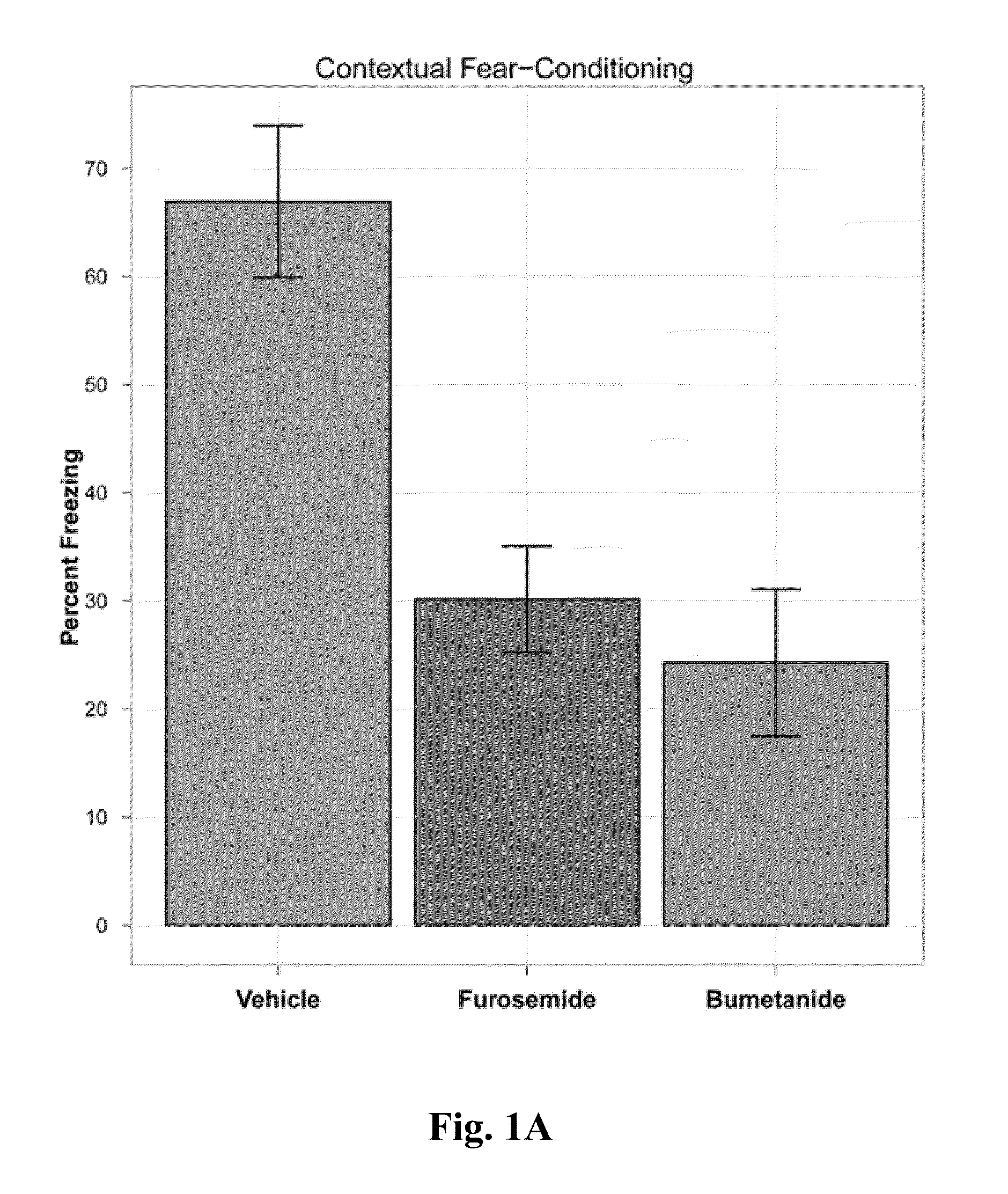
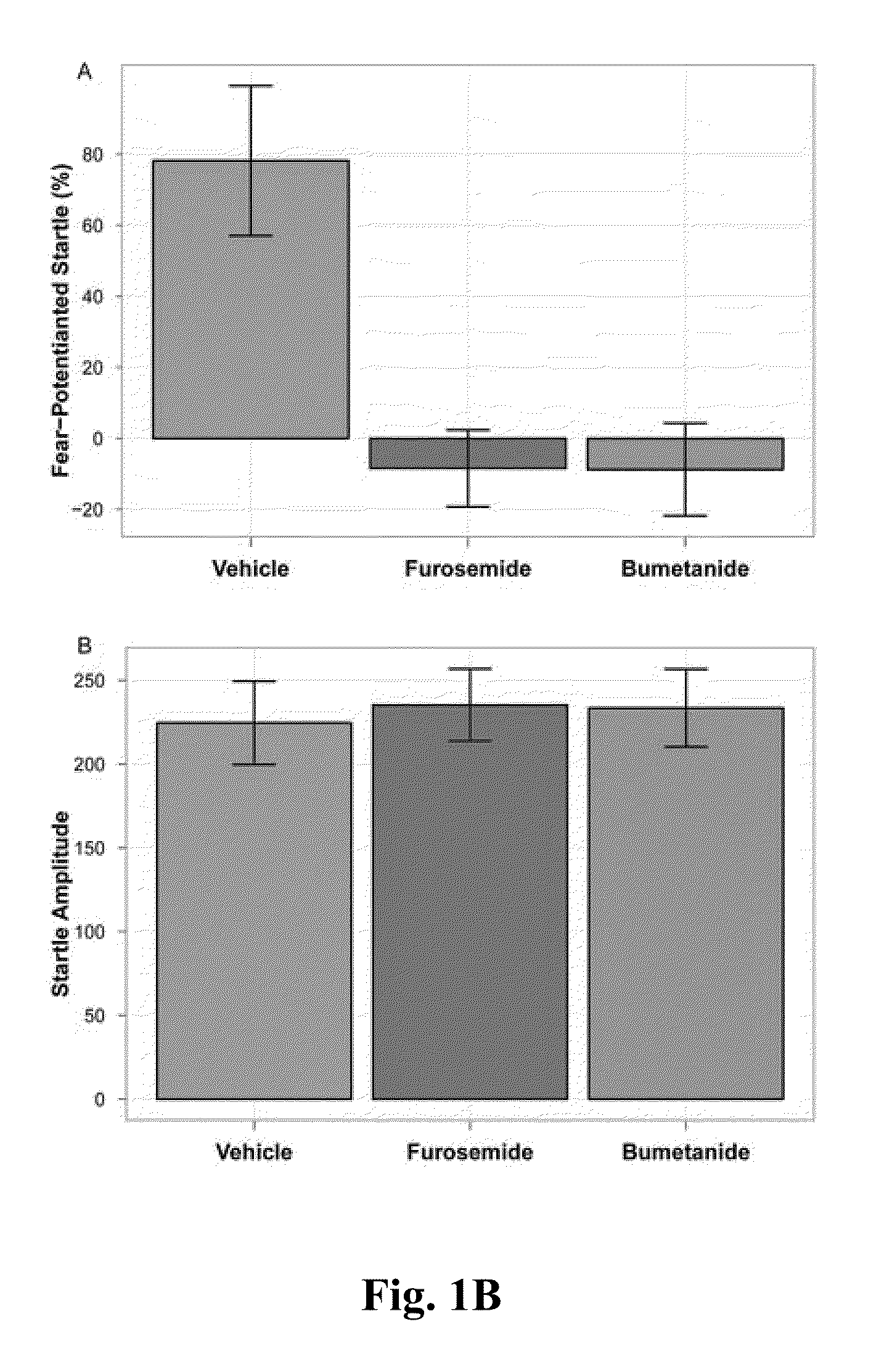
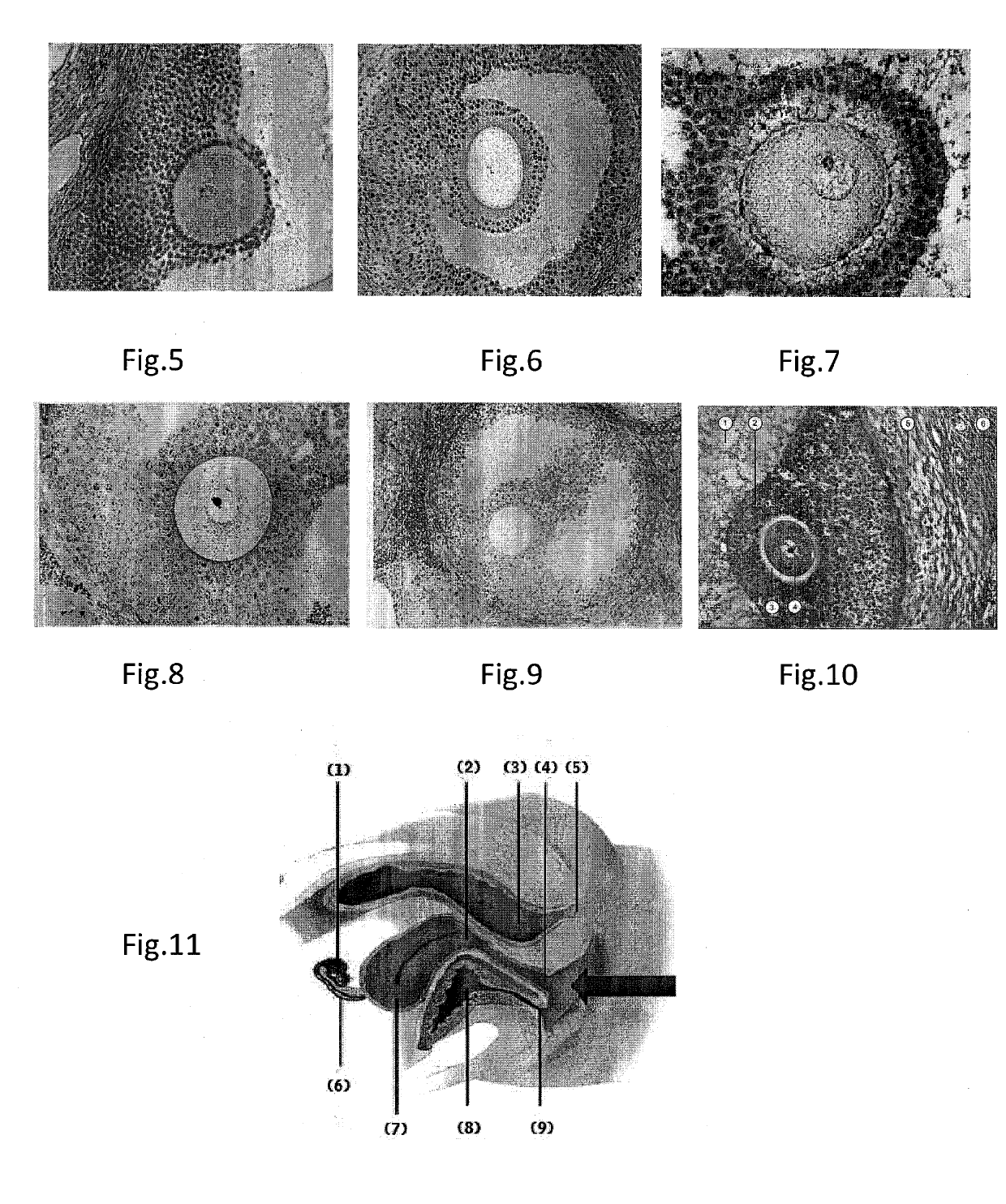
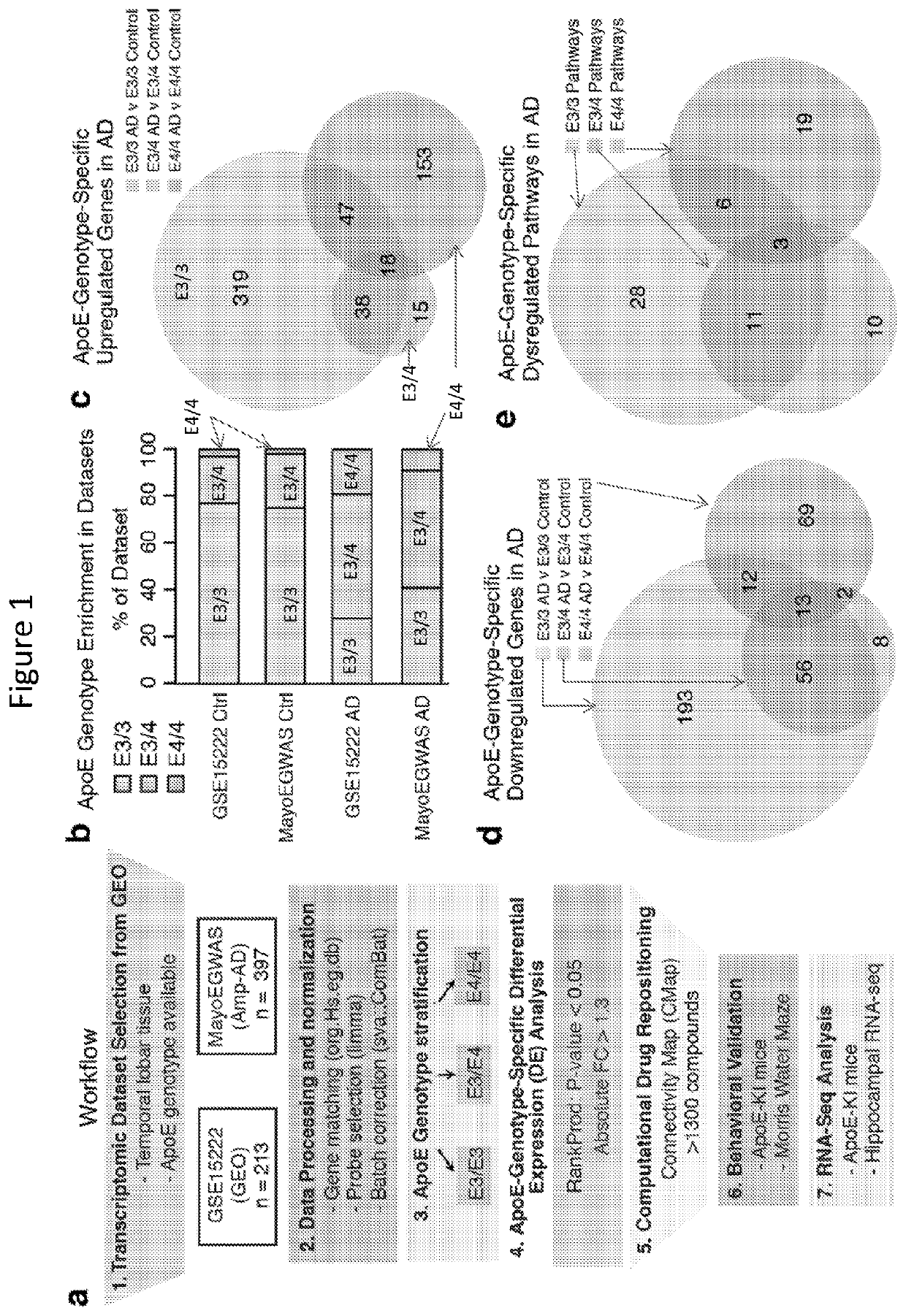
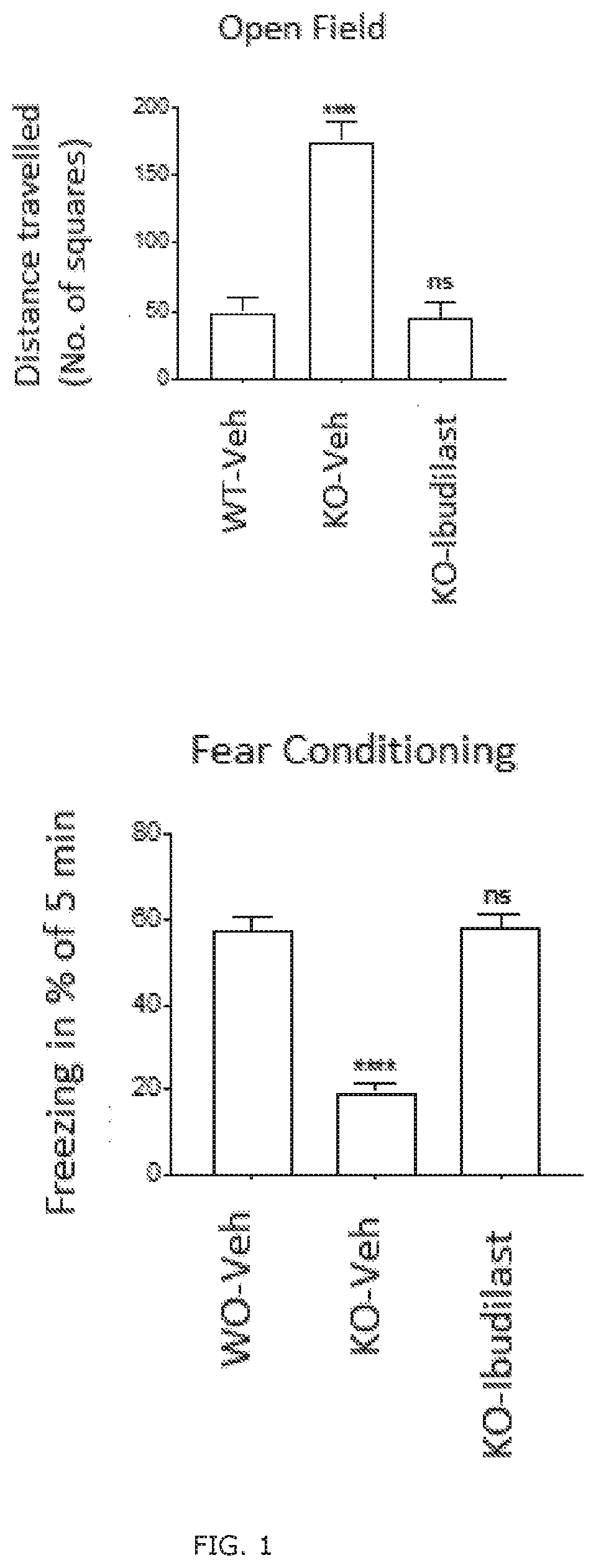
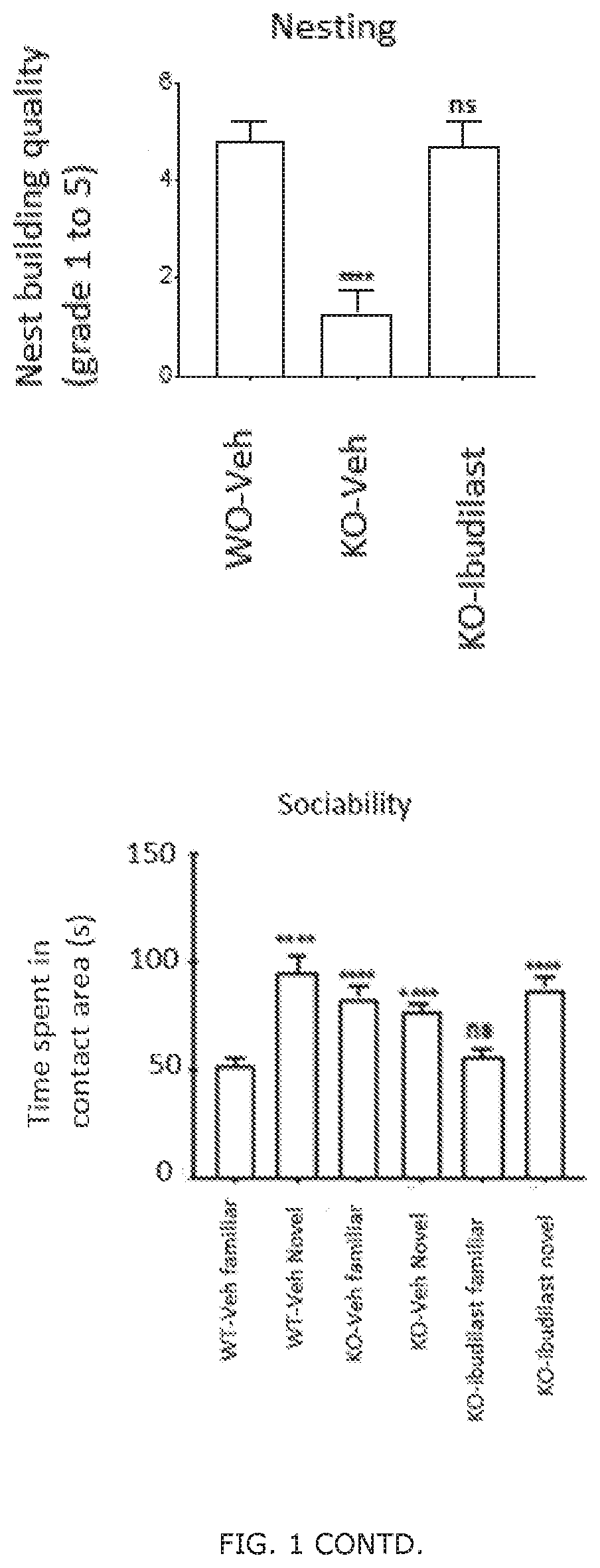
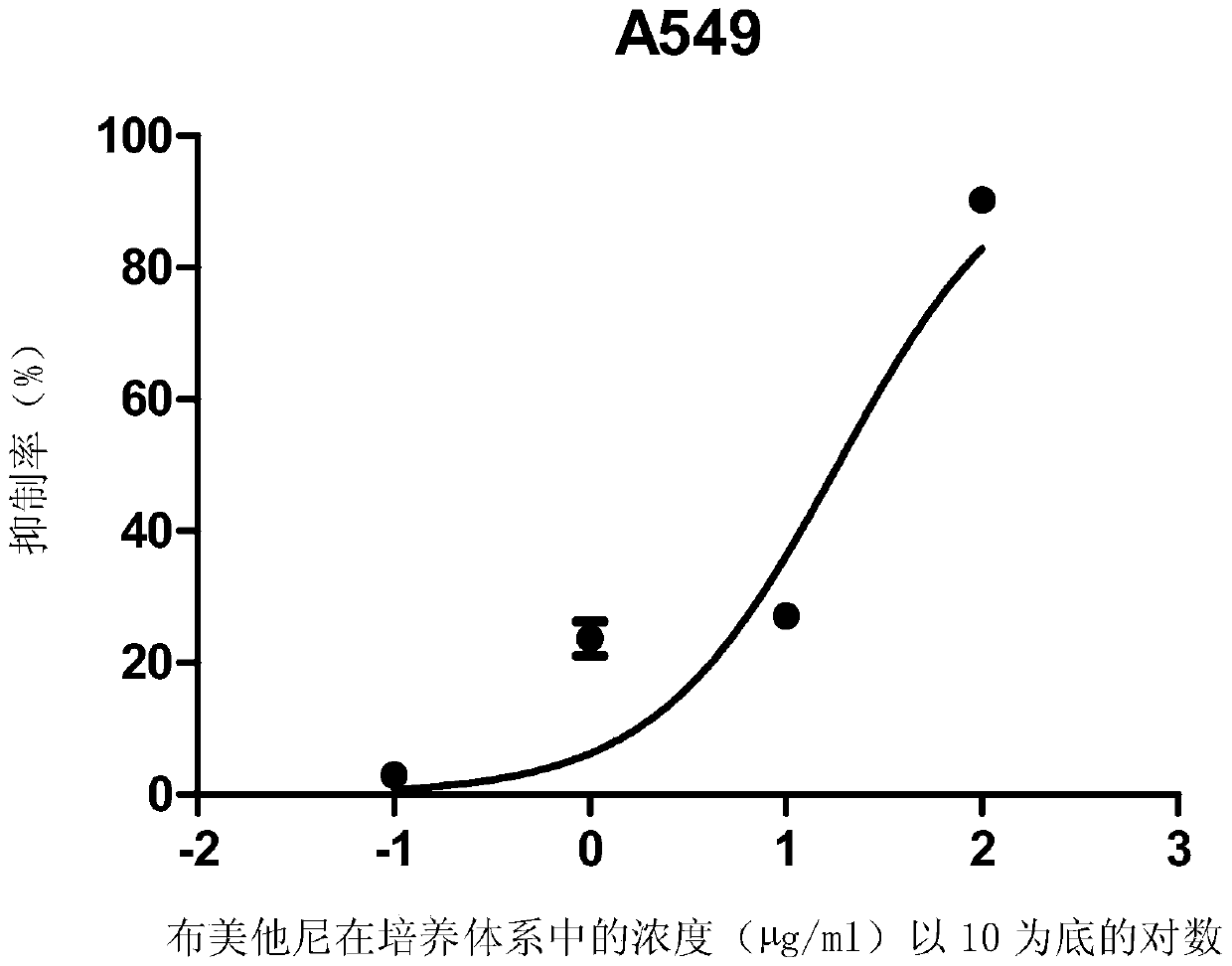
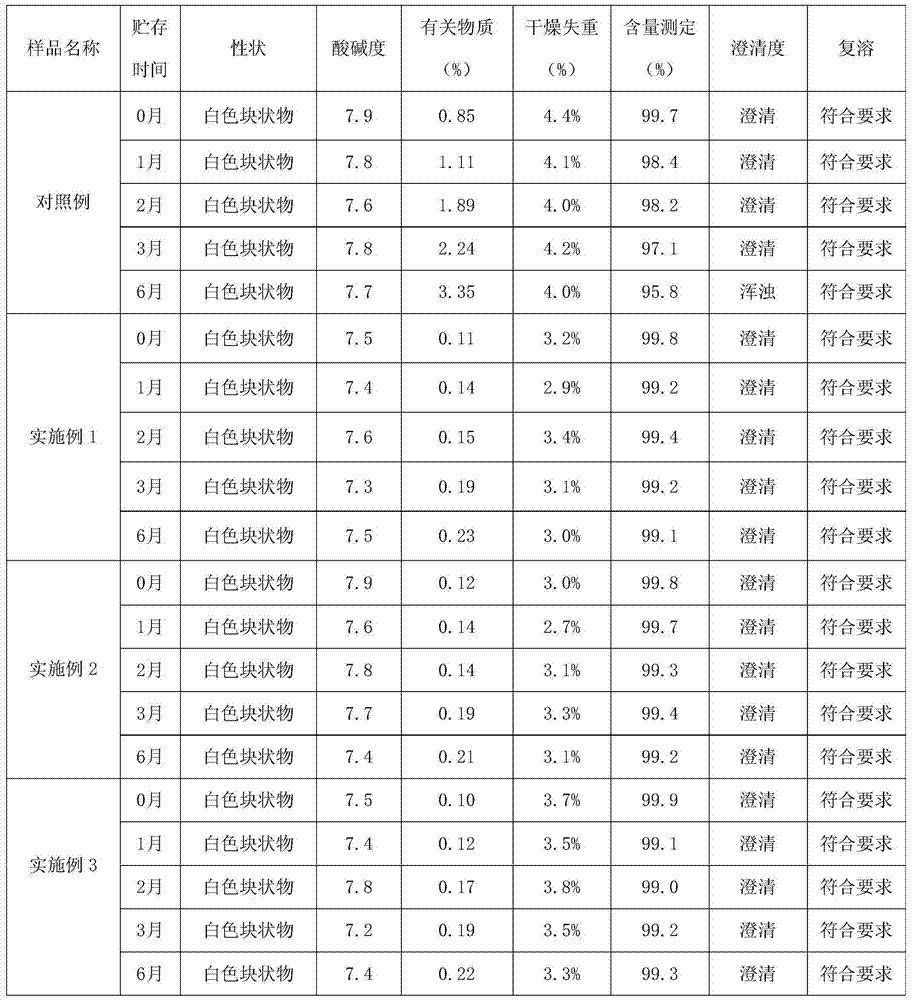
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Bumetanide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bumetanide is used to reduce extra fluid in the body (edema) caused by conditions such as heart failure, liver disease, and kidney disease.
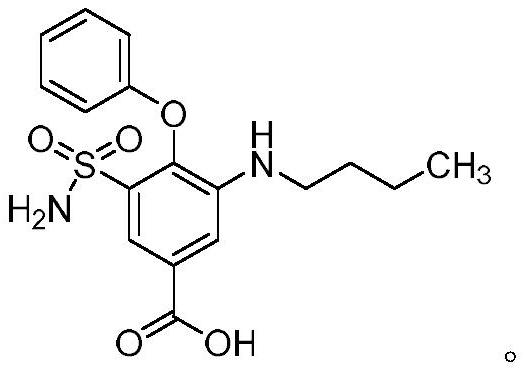
Analogs and prodrugs of bumetanide; compositions and methods of use
InactiveUS20140066504A1Improve lipophilicityReduced diuretic effectBiocideSenses disorderBumetanideRisk stroke
Novel analogs and prodrugs of the loop diuretic bumetanide are described. Pharmaceutical compositions containing bumetanide analogs and prodrugs are also described. These analogs and prodrugs are particularly useful for the treatment and / or prophylaxis of conditions that involve the NKCC cotransporter family (NKCC1 and NKCC2), or the KCC cotransporter family (KCC1, KCC2, KCC3, KCC4), or GABAa receptors. Such conditions include, but are not limited to anxiety disorders, epilepsy, migraine, non-epileptic seizures, sleep disorders, obesity, eating disorders, autism, depression, edema, glaucoma, stroke, ischemia, neuropathic pain, addictive disorders, schizophrenia, psychosis, and tinnitus.
Owner:DARYL W HOCHMAN +1
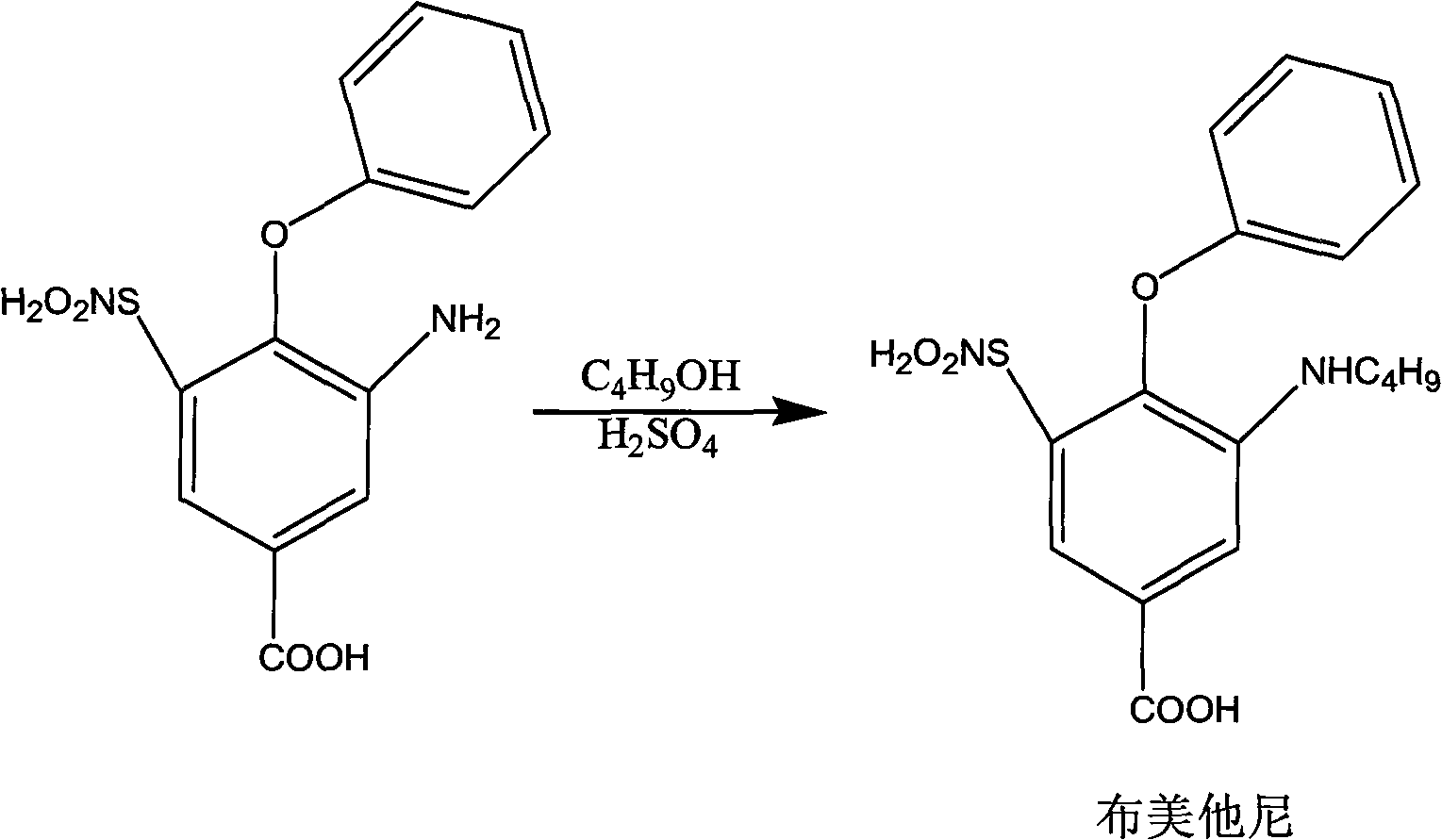
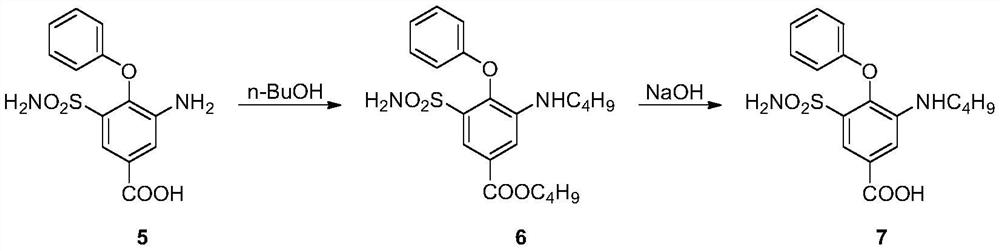
Method for preparing bumetanide
InactiveCN101591276ALower requirementEasy to operateOrganic active ingredientsSulfonic acid amide preparationBumetanideEther
The invention relates to a method for preparing bumetanide, which comprises that: 3-amino-4-phenoxy-5-sulfonylaminobenzoic acid and n-butanal are used as raw materials; firstly, the raw materials are subjected to condensation-dehydration reaction in the presence of a boron trihalide ether catalyst to generate 3-butylimine-4-phenoxy-5-sulfonylaminobenzoic acid; and then, the 3-butylimine-4-phenoxy-5-sulfonylaminobenzoic acid is catalyzed by palladium carbon and hydrogenated to form the bumetanide. The method has the advantages of simple operation, short reaction time and low equipment requirement, and is suitable for industrialized production; and the yield of a target product can reach more than 90 percent.
Owner:SUZHOU LIXIN PHARMA
Diabetes treating compound medicine for reducing side effect of rosiglitazone and preparation method thereof
The invention discloses a diabetes treating compound medicine for reducing side effect of rosiglitazone and a preparation method thereof, and belongs to the field of medicine preparation. The compound medicine consists of rosiglitazone and a diuretic medicine, wherein the ratio of rosiglitazone to the diuretic medicine by weight is 1:(0.04-1). The diabetes treating compound medicine is compounded and matched with any one of spirolactone, amiloride, compound amiloride, ergosterol, triamterene, ethacrynic acid, hydroflumethiazide and bumetanide which can better eliminate side effect of rosiglitazone with rosiglitazone so as to form the compound medicine which not only can reduce the side effect of rosiglitazone, but also better treats diabetes.
Owner:罗国安
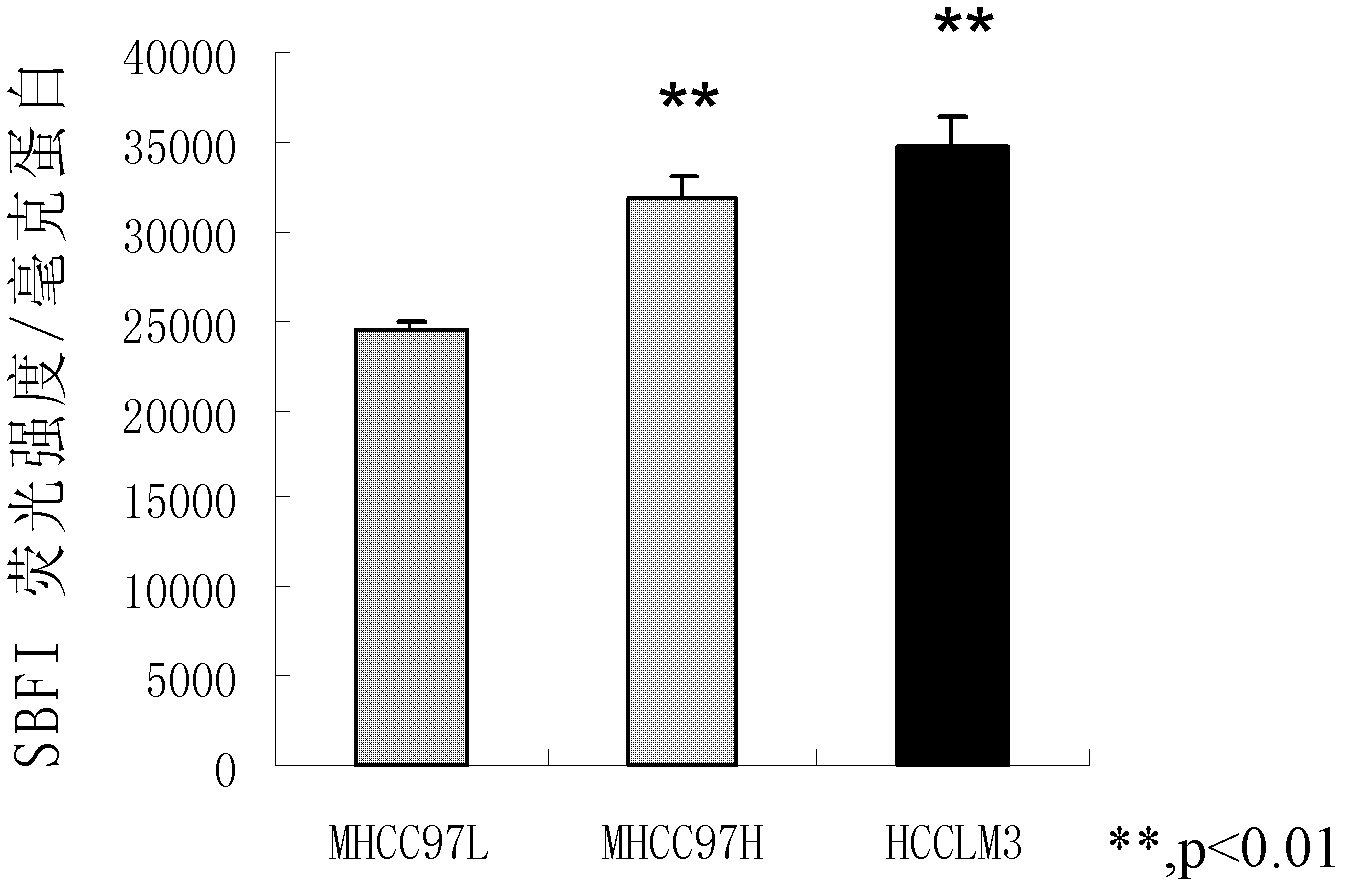
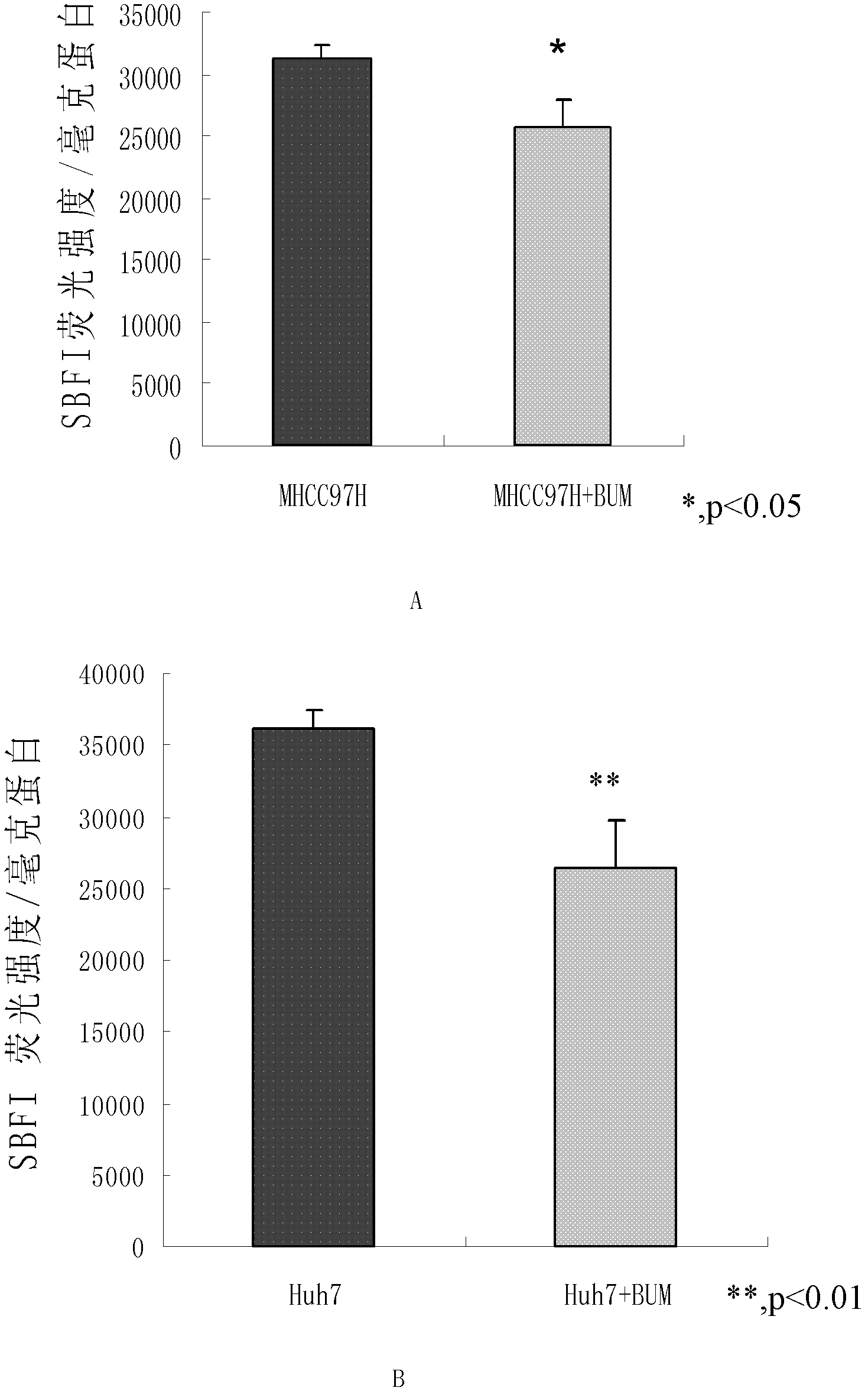
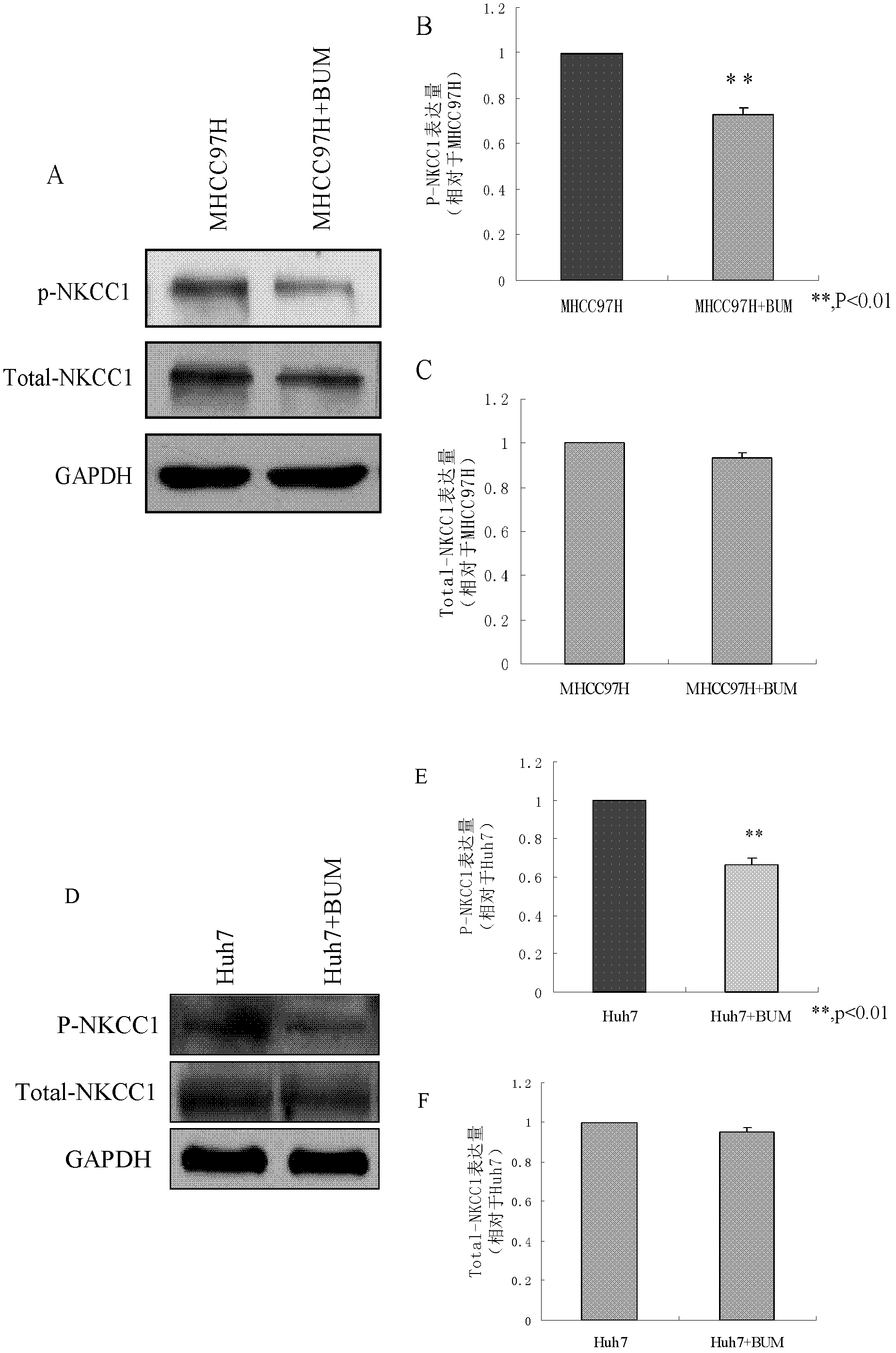
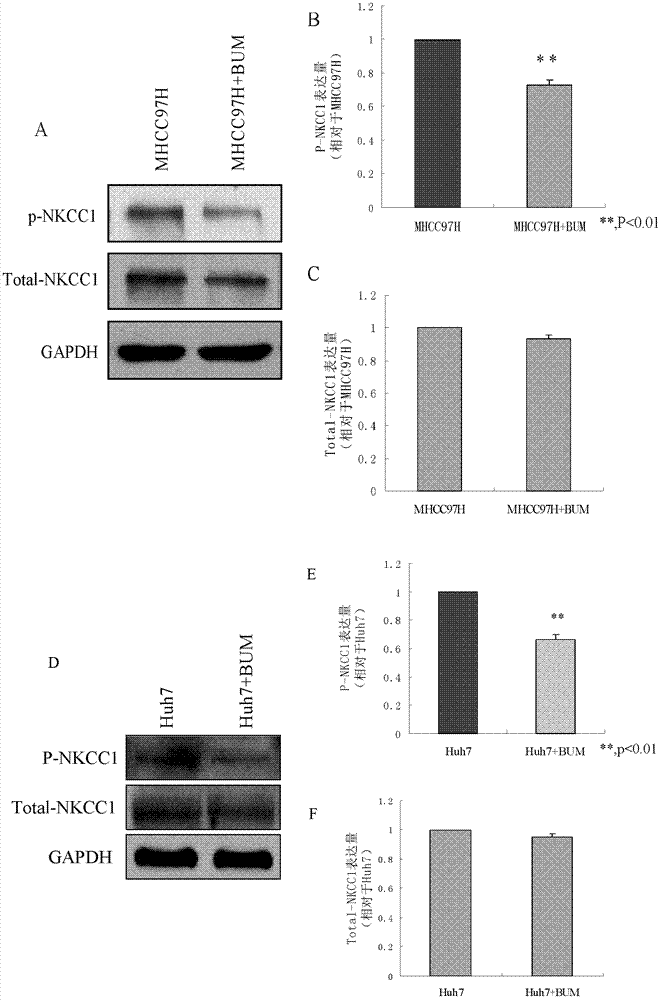
Application of bumetanide in inhibition of hepatoma cell transfer
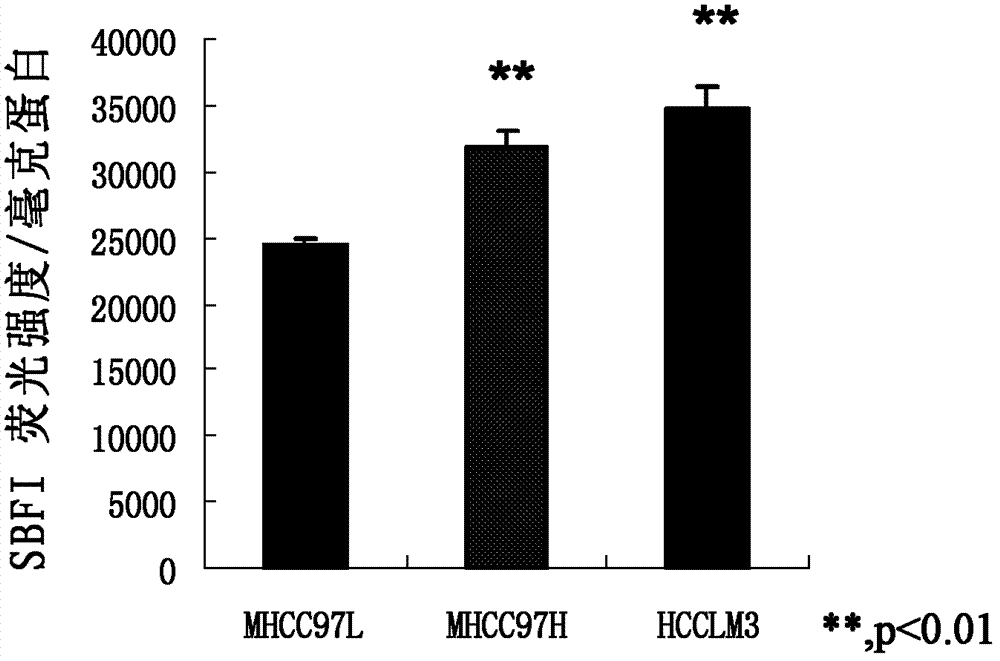
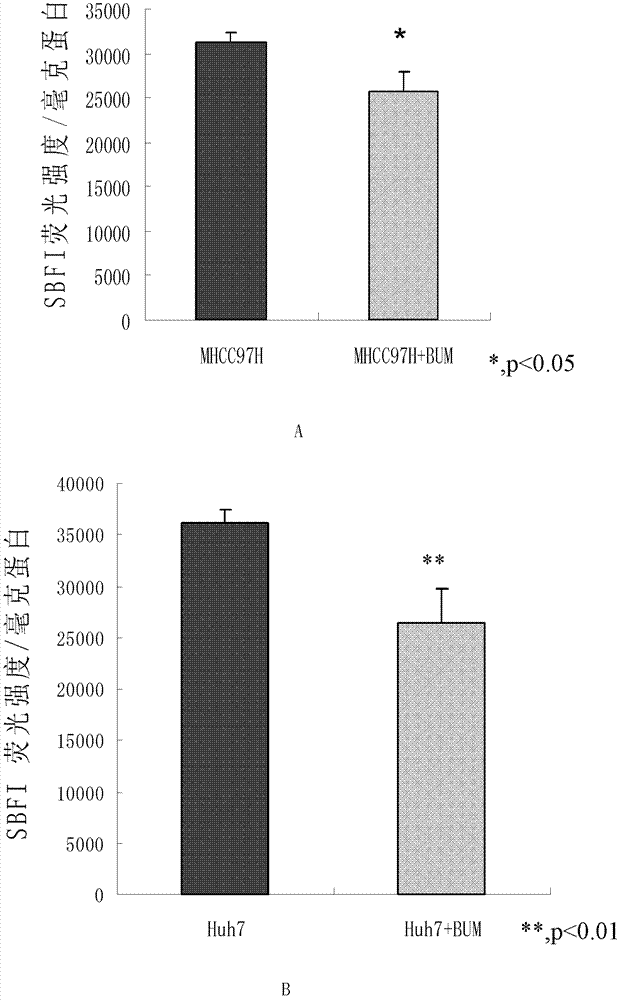
ActiveCN102813643BReduce contentPrevent proliferationOrganic active ingredientsTumor/cancer cellsBumetanidePhosphorylation
The invention discloses application of bumetanide in inhibition of hepatoma cell transfer. Bumetanide can be used for preparing a medicine which is used for inhibiting hepatoma cell multiplication and / or hepatoma cell attack and / or hepatoma cell transfer, can be used for preparing a medicine which is used for curing hepatoma cells and can be used for preparing a product which is used for reducing phosphorylation NKCC1 protein content in hepatoma cells. The bumetanide can be used for reducing the phosphorylation NKCC1 protein content in hepatoma cells, so that hepatoma cell multiplication, attack and transfer are inhibited. The application has significant value for hepatoma cell cure.
Owner:BEIJING PROTEOME RES CENT +1
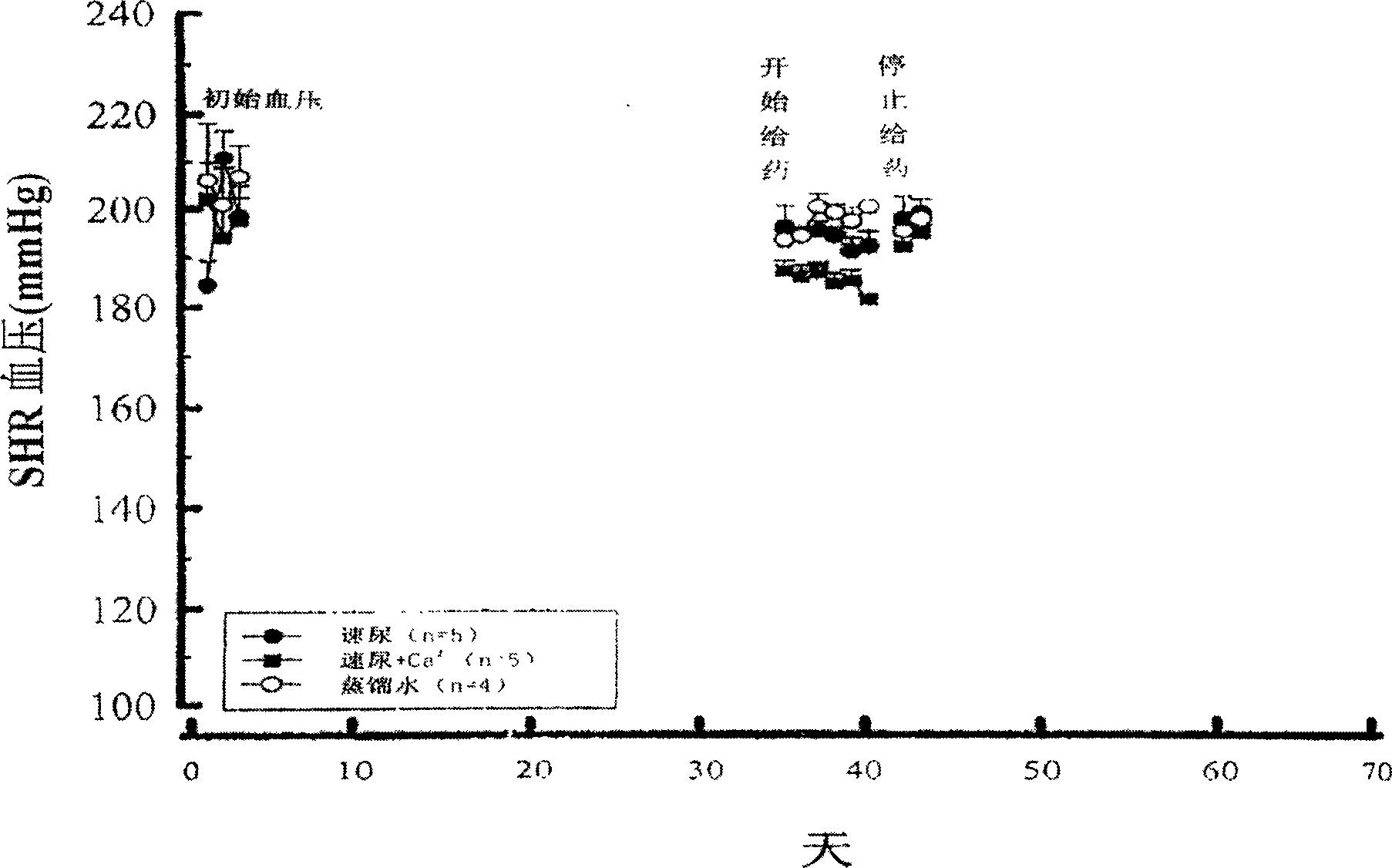
Compsn. of medication for treating high blood pressure
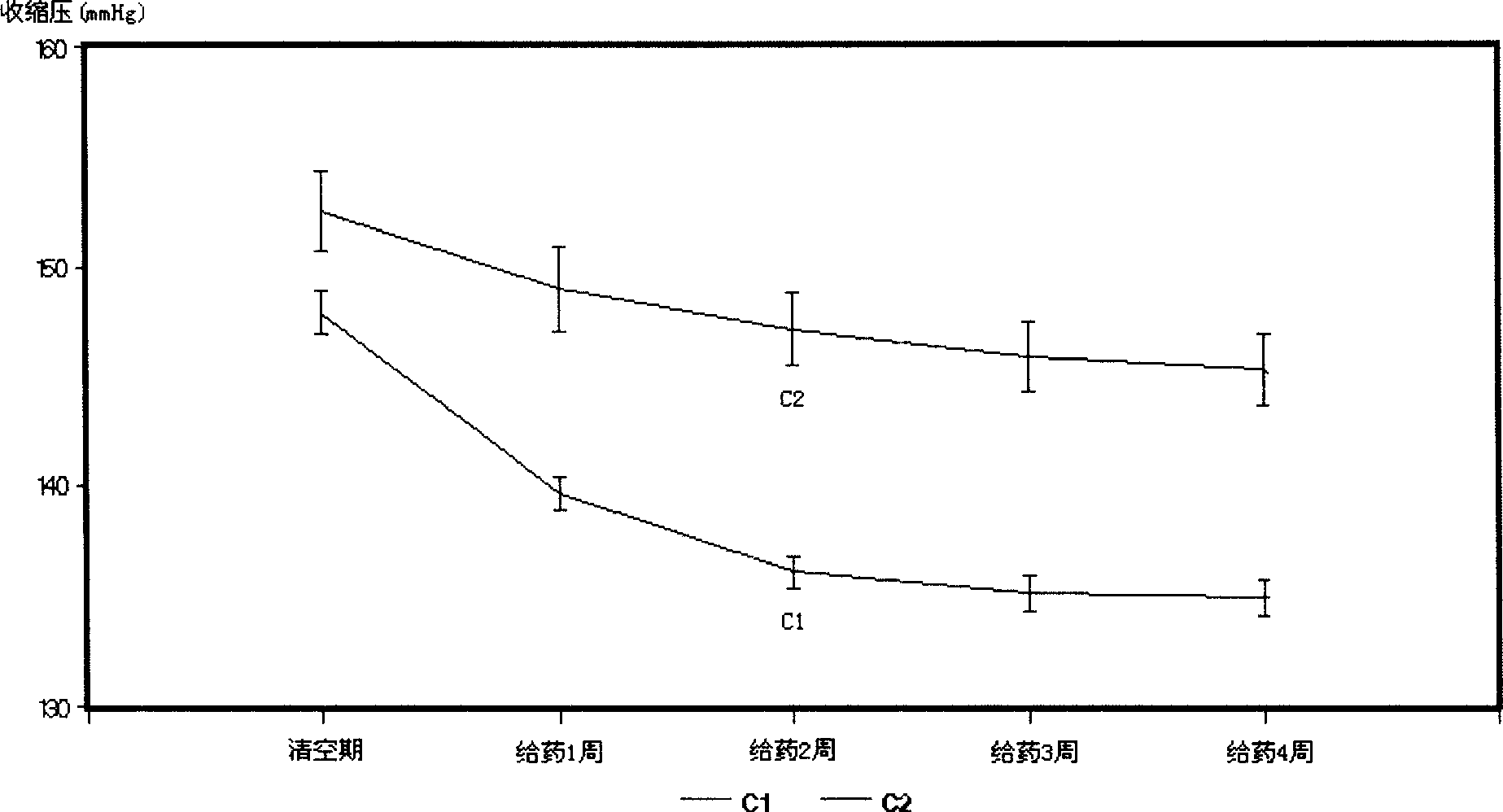
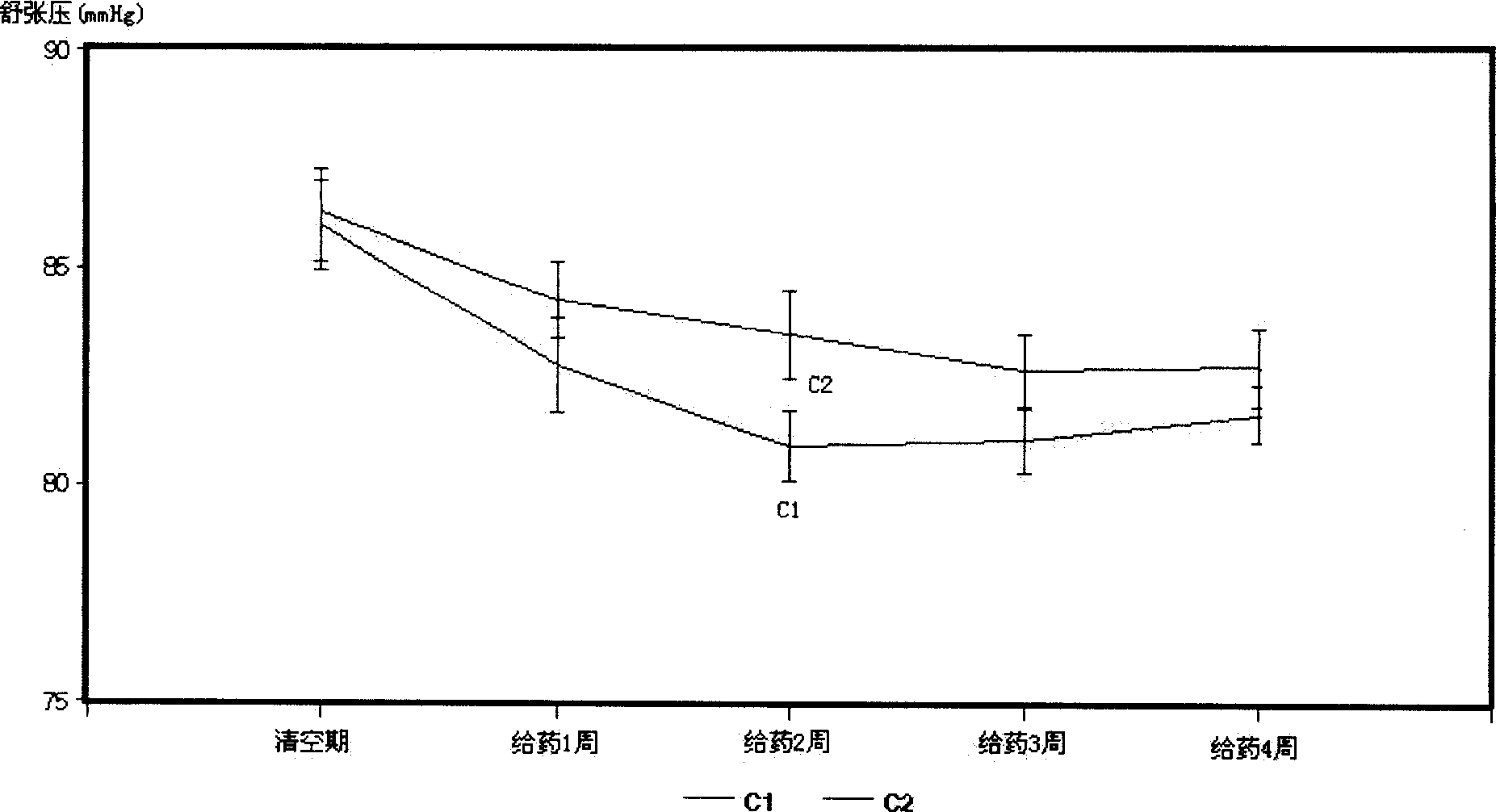
InactiveCN1695738ALower blood pressureOrganic active ingredientsCardiovascular disorderBlood pressure fallBumetanide
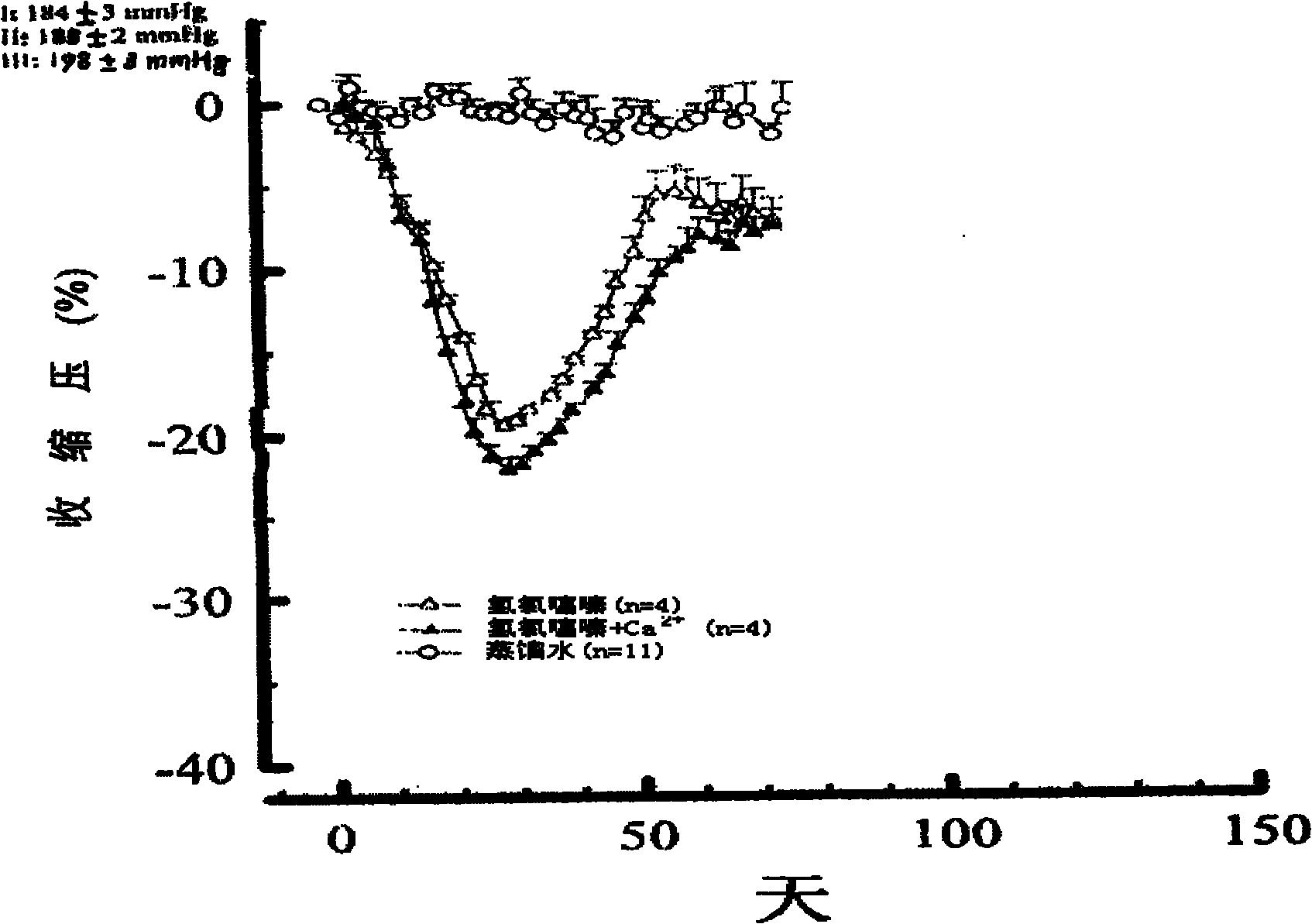
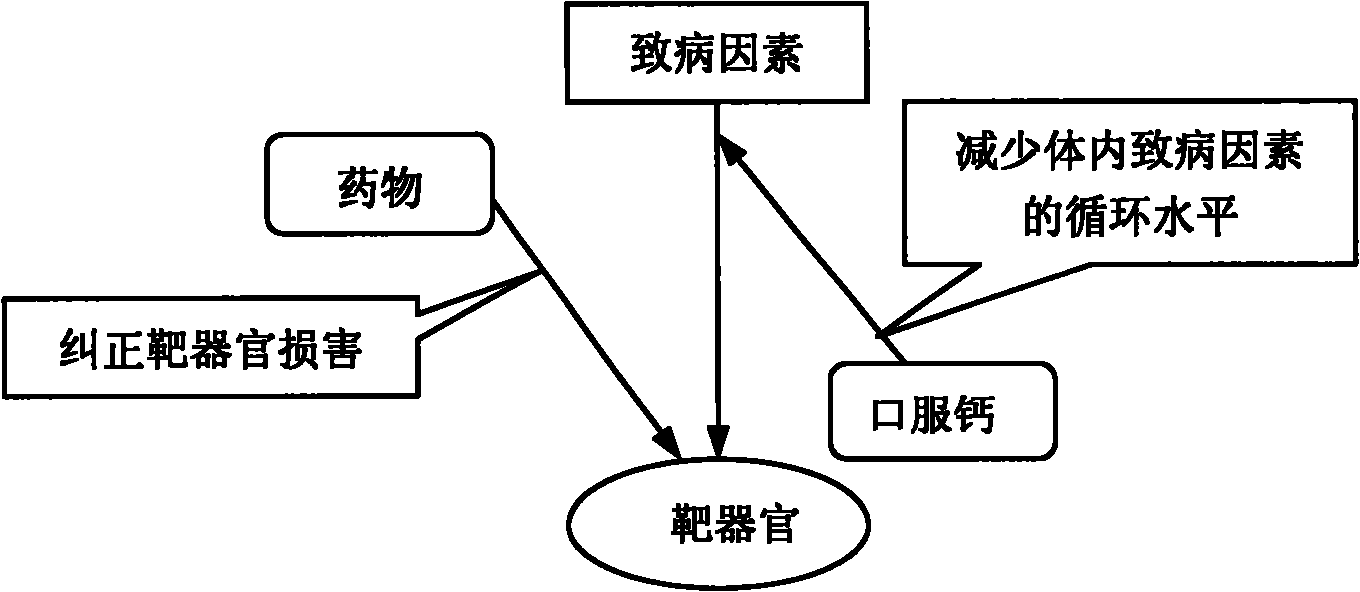
A composite medicine for treating hypertension contains the diuretic chosen from errolon, ethacrynic acid, bumetanide, chlorothiazinde, hydrochlorothiazinde, etc a Ca-contained component chosen from shell, inorganic Ca salt, organic Ca salt, etc, and the pharmacologically acceptable additives.
Owner:苏金平
Application of bumetanide in inhibition of hepatoma cell transfer
ActiveCN102813643AReduce contentPrevent proliferationOrganic active ingredientsTumor/cancer cellsBumetanidePhosphorylation
The invention discloses application of bumetanide in inhibition of hepatoma cell transfer. Bumetanide can be used for preparing a medicine which is used for inhibiting hepatoma cell multiplication and / or hepatoma cell attack and / or hepatoma cell transfer, can be used for preparing a medicine which is used for curing hepatoma cells and can be used for preparing a product which is used for reducing phosphorylation NKCC1 protein content in hepatoma cells. The bumetanide can be used for reducing the phosphorylation NKCC1 protein content in hepatoma cells, so that hepatoma cell multiplication, attack and transfer are inhibited. The application has significant value for hepatoma cell cure.
Owner:BEIJING PROTEOME RES CENT +1
Application of C188-9, Venetoclax and Bumetanide in drugs for fibrotic diseases
The invention provides an application of C188-9, Venetoclax and Bumetanide in drugs for fibrotic diseases from perspective of clinic practice. A RNA recognition sequence (RNA recognition motif, RRM) region of LARP6 protein is taken as a docking target, and subject to computer virtual docking and simulated screening. Finally it is found by cellular and animal experiments that C188-9, Venetoclax andBumetanide (a potent quick-acting diuretic commonly used in clinic practice) combine with the RRM region of LARP6 protein to inhibit stability of collagen mRNA, thereby inhibiting collagen productionand further inhibiting fibrosis-related diseases in the process of occurrence and development of injury-induced fibrosis. The study of the invention enriches basic research on the pathogenesis of fibrosis-related diseases, and provides a new idea for deep exploration of essential causes of the fibrotic diseases. Meanwhile, the C188-9, Venetoclax and Bumetanide can be considered as a new potentialdrug for clinical treatment of the fibrotic diseases.
Owner:TIANJIN MEDICAL UNIV
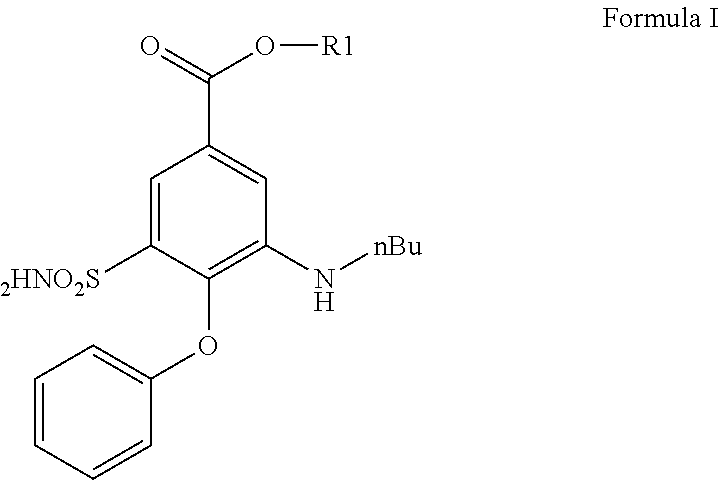
Bumetanide Derivatives for the Therapy of Stroke and Other Neurological Diseases/Disorders Involving NKCCs
PendingUS20210163406A1Improve propertiesImprove lipophilicityNervous disorderOrganic chemistryNervous systemBumetanide
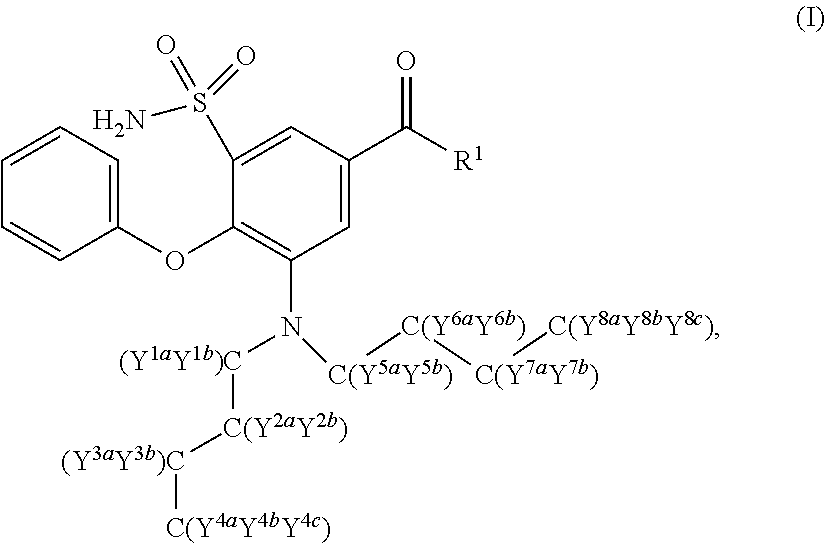
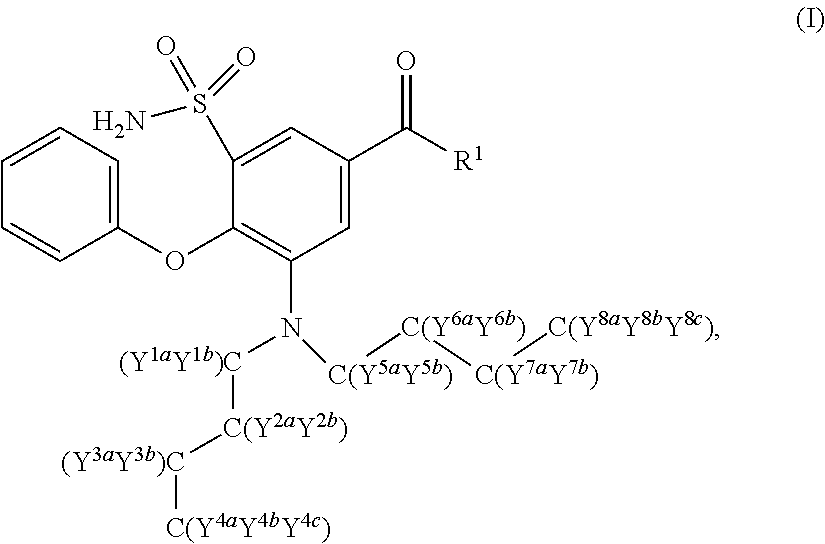
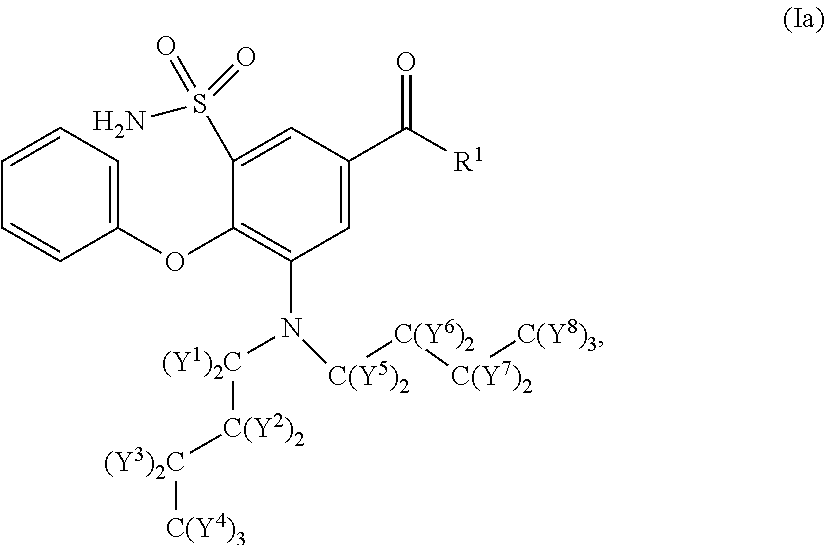
The present invention relates to bumetanide derivatives of formula (I) as well as pharmaceutical compositions comprising these compounds for use in the treatment or prevention of neurological diseases / disorders involving Na+-K+-20Γ-cotransporters (NKCCs), such as stroke, traumatic brain injury (TBI), spinal cord injury (SCI), peripheral nerve injury (PNI), brain edema, or glioma, and particularly for use in the treatment or prevention of stroke. The invention likewise relates to a method of treating or preventing a neurological disease or disorder involving an NKCC, such as stroke, TBI, SCI, PNI, brain edema, or glioma, the method comprising administering a compound of formula (I) to a subject in need thereof.
Owner:UNIVERSITY OF PITTSBURGH +2
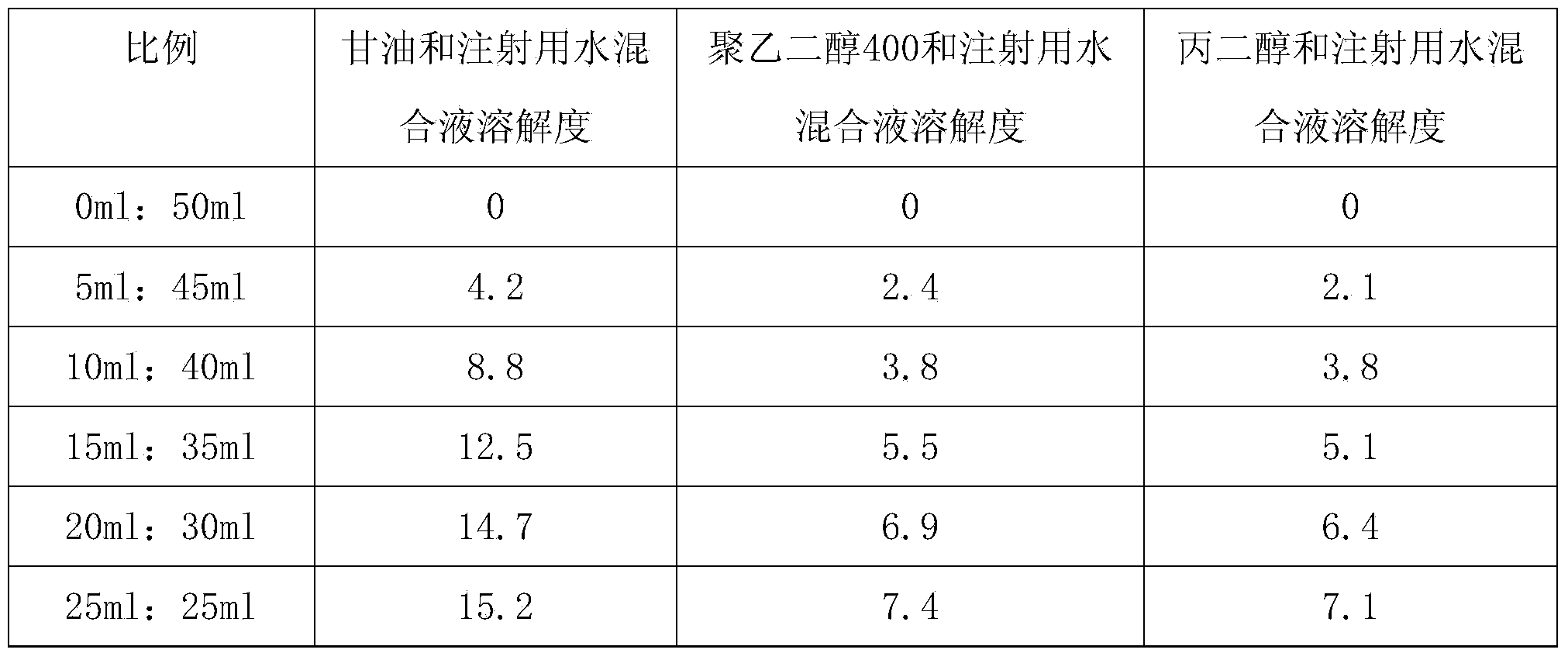
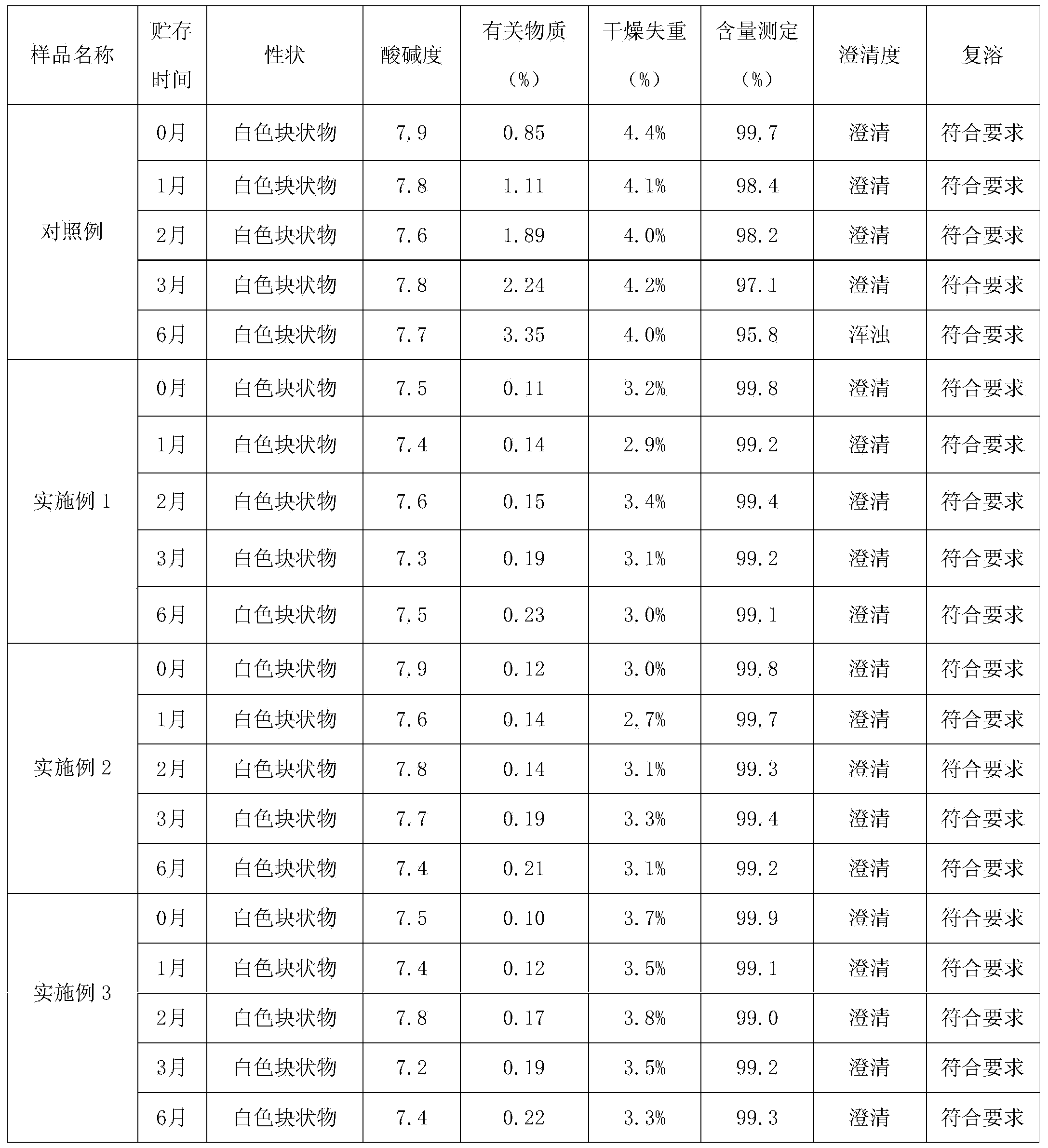
Bumetanide freeze-dried powder preparation for injection and preparation method thereof
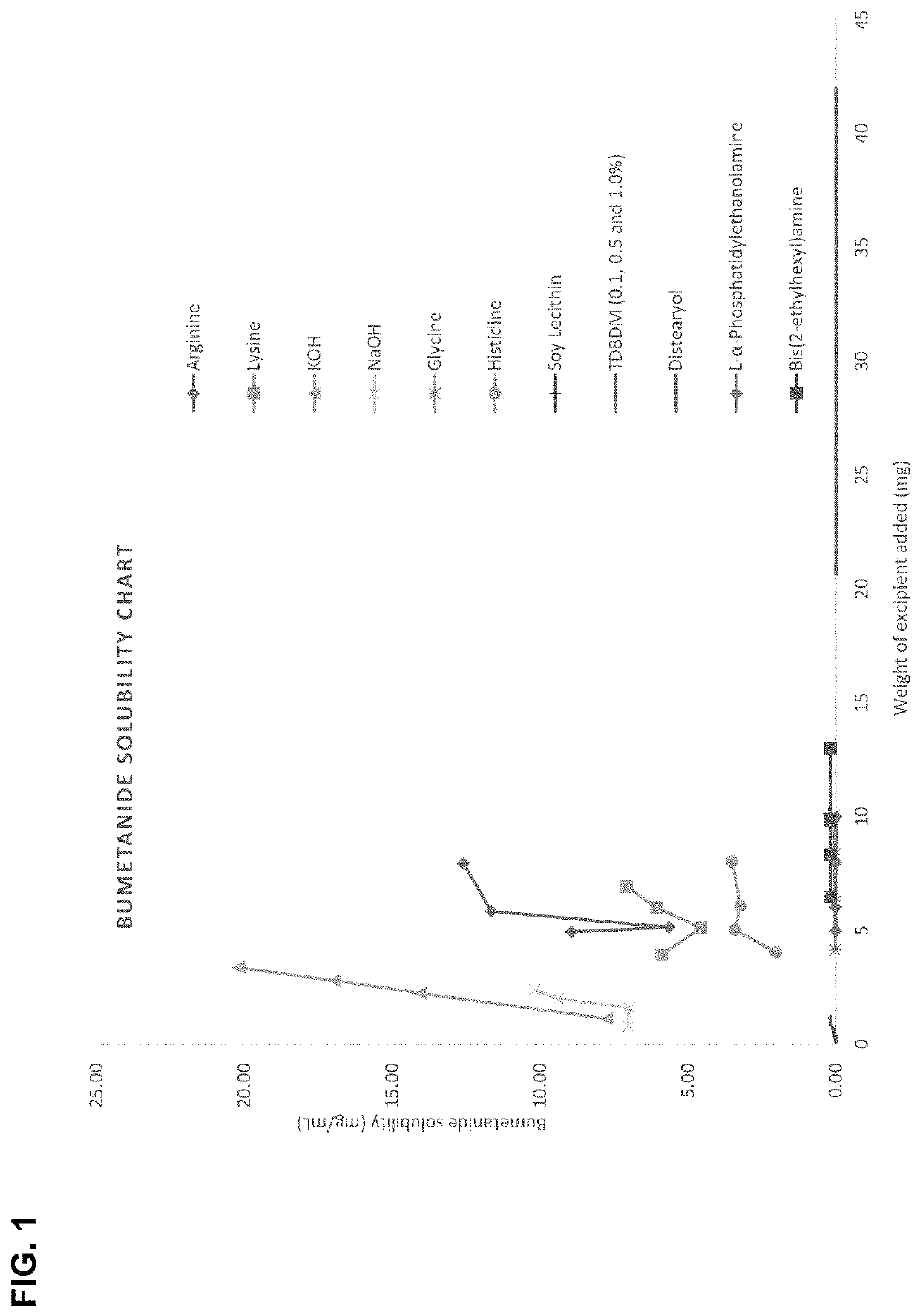
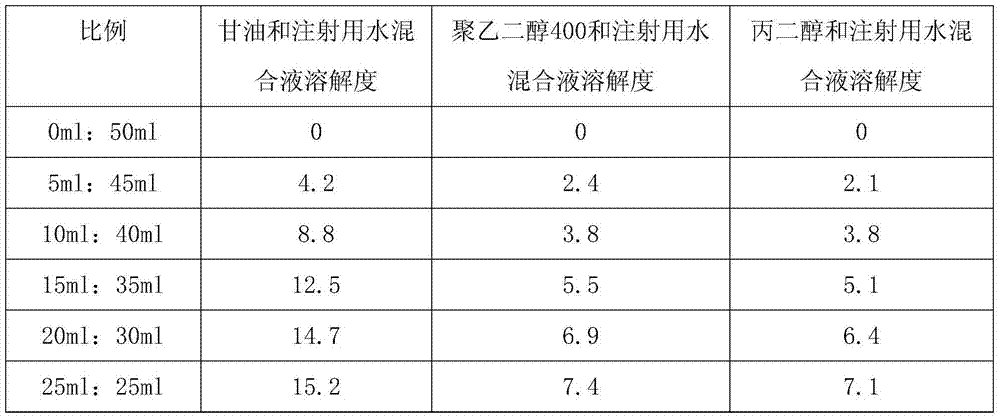
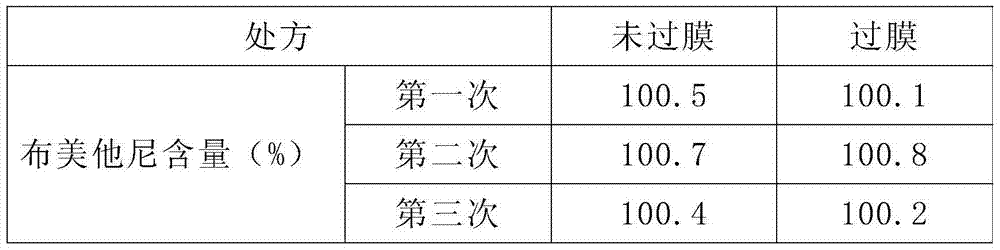
ActiveCN103520122AImprove solubilitySolve the problem of insoluble in waterPowder deliveryOrganic active ingredientsBumetanideFreeze-drying
The invention provides a bumetanide freeze-dried powder preparation for injection and a preparation method thereof. The method consists of: weighing 10ml of glycerol and water for injection in a volume ratio of 1:4-2:3, and stirring them to obtain a latent solvent; weighing 0.5g of bumetanide and dissolving it in the latent solvent, conducting filtering to clarity, thus obtaining a solution A; weighing 200g of mannitol, adding water for injection accounting for 80% of the total amount, carrying out stirring and filtering to clarity, thus obtaining a solution B; mixing the solution A and the solution B evenly, then adding the remaining water for injection to a total amount of 2000ml, performing degerming filtering, then subjecting the product to filling into 1000 10ml tube-type bottles respectively according to a dosage of 2.0ml / bottle; subjecting the tube-type bottles to heat preservation for 4h under -45DEG C; conducting vacuum pumping to a vacuum degree of not greater than 20pa, raising the slab temperature to -5DEG C at a rate of 2DEG C / h, and carrying out heat preservation for 18h; raising the slab temperature to 30DEG C at a rate of 7DEG C / h, and carrying out heat preservation for 2h, thus obtaining the bumetanide freeze-dried powder preparation for injection. According to the invention, the product stability is effectively ensured, the content of impurities is reduced, and the re-dissolubility of the product is good.
Owner:HAINAN GENERAL & KANGLI PHARMA
Compound preparation for treating cholestatic jaundice and preparing method thereof
InactiveCN105998052AAnti-inflammatoryCompatibility is reasonableDigestive systemEther/acetal active ingredientsTherapeutic effectBile fluid
The invention discloses a compound preparation for treating cholestatic jaundice and a preparing method thereof. The compound preparation is prepared from ademetionine 1,4-butanedisulfonate, diammonium glycyrrhizinate, dihydroxydibutylether, baicalin, methionine, anethol trithione, potassium magnesium aspartate, crocetin dimethyl ester, glucurolactone, cholestyramlne, hydroxymethylnicotinamide, trimethoprim lactate, bumetanide, piperazine citrate and azathioprine. The compound preparation has the effects of resisting bacteria and diminishing inflammations, clearing dampness and heat, soothing the liver and benefiting the gallbladder, removing the toxicity and eliminating jaundice and strengthening the spleen and the stomach, can reduce stasis of bile in liver cells, promote bile excretion, improve the liver functions and the tissue cell respiration function, reduce accumulation of fat in the liver and promote jaundice elimination and liver function recovery and is significant in treatment effect, safe and few in side effect when the compound preparation is used for treating the cholestatic jaundice and complications thereof.
Owner:徐海燕
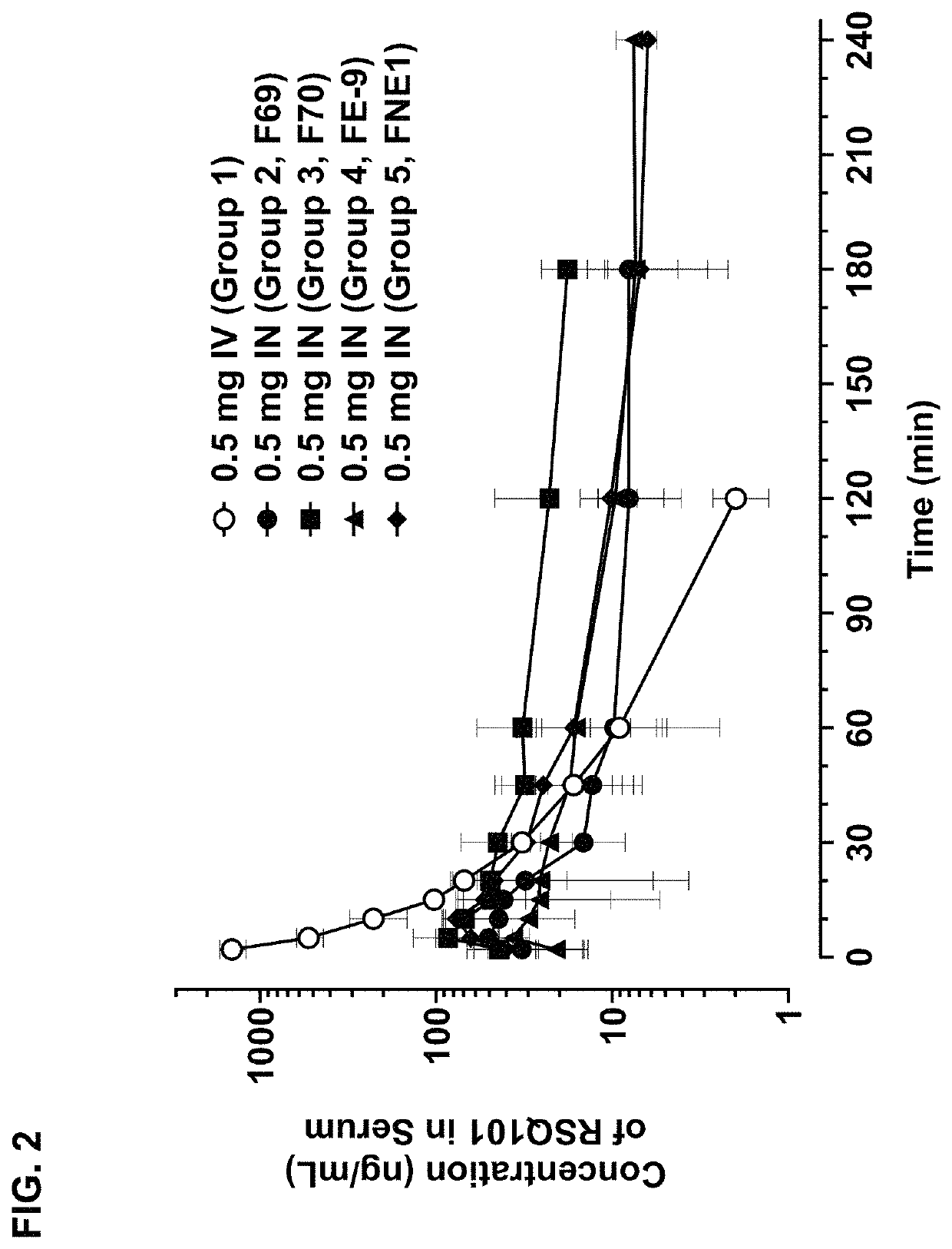
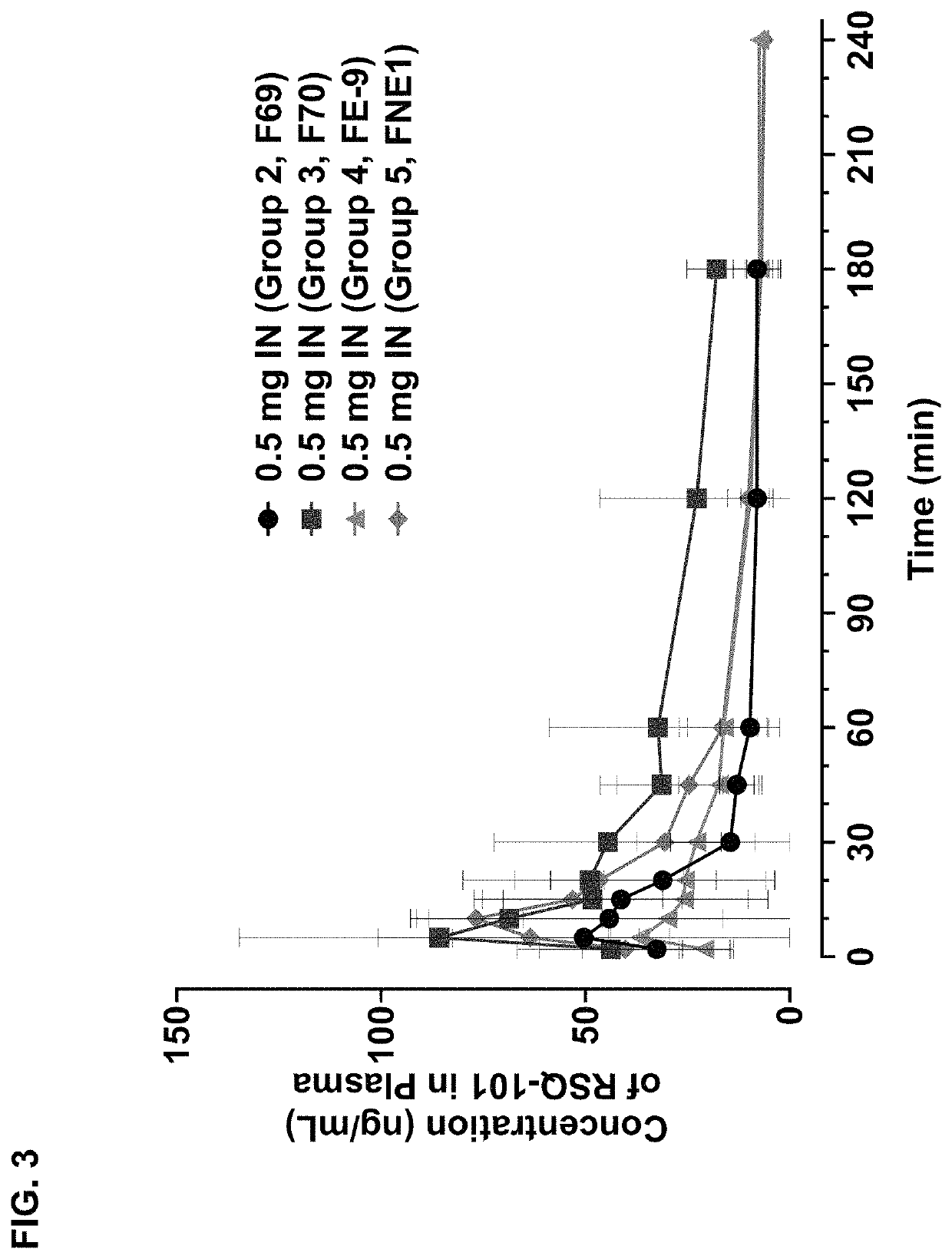
Methods and compositions for treating edema refractory to oral diuretics
ActiveUS20210169833A1Subject is at riskOrganic active ingredientsPharmaceutical non-active ingredientsBumetanideMedicinal chemistry
The present invention features methods and compositions for the intranasal, sublingual, and subcutaneous administration of bumetanide for the treatment of subjects suffering from edema refractory to oral diuretics.
Owner:RESQ PHARMA LLC
Deuterated n-butyl bumetanide
Owner:CONCERT PHARMA INC
Synthetic method of bumetanide
ActiveCN106748906AShort process stepsShort reaction timeSulfonic acid amide preparationBumetanideRoom temperature
The invention provides a synthetic method of bumetanide. The synthetic method comprises the following specific steps: 3-amino-4-phenoxy-5- sulfamoylbenzoic acid and n-butyl alcohol are added to a reactor and stirred at room temperature, a Lewis acid catalyst is added, a reaction is performed for 2-10 h, and pure bumetanide is obtained through post-processing after the reaction. The method is short in reaction step, short in reaction time and high in efficiency, the product yield and purity are higher, reaction conditions are mild, equipment corrosion is avoided, and industrial production is facilitated.
Owner:枣庄市润安制药新材料有限公司
Synthesis method of intermediate of bumetanib
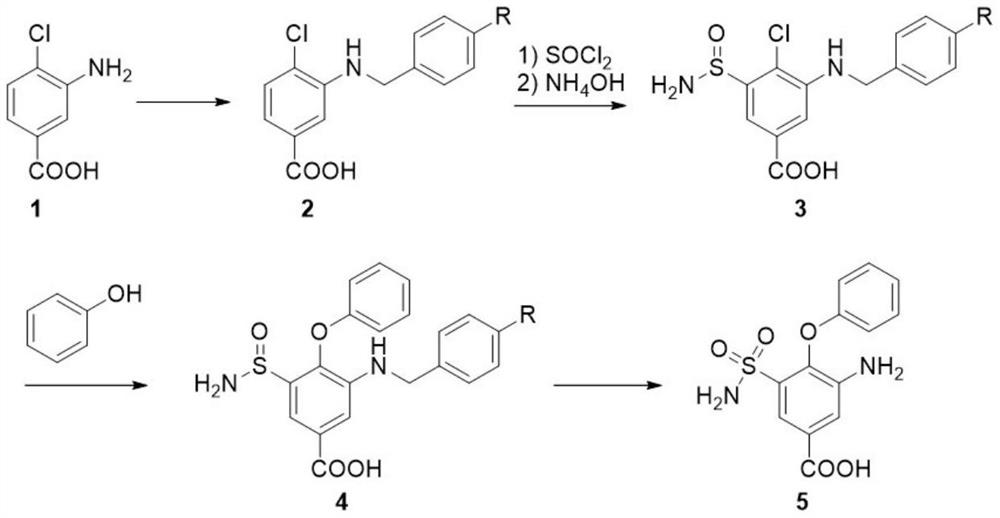
PendingCN113735744AReduce usageAvoid nitrificationOrganic compound preparationSulfonic acid amide preparationBenzoic acidChlorosulfuric acid
The invention discloses a synthesis method of an intermediate of bumetanib. The comprises the following steps: protecting amino in 3-amino-4-chlorobenzoic acid as shown in a formula 1 by using a compound containing a benzyl protecting group to obtain a compound as shown in a formula 2; reacting the compound as shown in the formula 2 with thionyl chloride, and treating with ammonia water to obtain a compound as shown in a formula 3; reacting the compound as shown in the formula 3 with phenol to obtain a compound as shown in a formula 4; and reacting the compound as shown in the formula 4 with an oxidizing agent to obtain a bumetanib intermediate as shown in a formula 5, namely 3-amino-4-phenoxy-5-sulfamoyl benzoic acid. Compared with the prior art, the method provided by the invention has the advantages that the use of highly toxic chlorosulfonic acid is avoided, the nitration reaction is avoided, the production organization is facilitated, and the production safety is improved.
Owner:南京卓康医药科技有限公司
Pharmaceutical compound preparation and application of pharmaceutical compound preparation in preparing medicines for treating hypertension with coronary heart disease
InactiveCN107595851ALittle side effectsSignificant effectEster active ingredientsCardiovascular disorderCoronary artery diseaseSide effect
The invention relates to a pharmaceutical compound preparation and application of pharmaceutical compound preparation in preparing medicines for treating hypertension with coronary heart disease. Thepharmaceutical compound preparation comprises the following components in parts by weight: 15-20 parts of lovastatin, 12-20 parts of bumetanide, 15-30 parts of metolazone, 5-15 parts of acid protective agent, 5-15 parts of an antioxidant, 25-55 parts of a filling agent, 15-30 parts of a bonding agent, 10-35 parts of a disintegrating agent and 2-10 parts of a lubricant. According to the pharmaceutical compound preparation, the lovastatin, the bumetanide and the metolazone are combined for treating the hypertension with the coronary heart disease, so that the curative effect is very obvious, theaction of synergistic interaction and advantage complementation are shown, and the side effects of all the medicines also can be reduced.
Owner:GUILIN HAOXIN TECH SERVICE
Composition of medication for treating high blood pressure
InactiveCN1695738BLower blood pressureOrganic active ingredientsCardiovascular disorderBumetanideHigh pressure
Owner:苏金平
Pharmaceutical co-crystal of gefitinib and bumetanide and preparation method thereof
ActiveCN111454221ASmall toxicityReduce dosageOrganic active ingredientsOrganic chemistryCancer cellSide effect
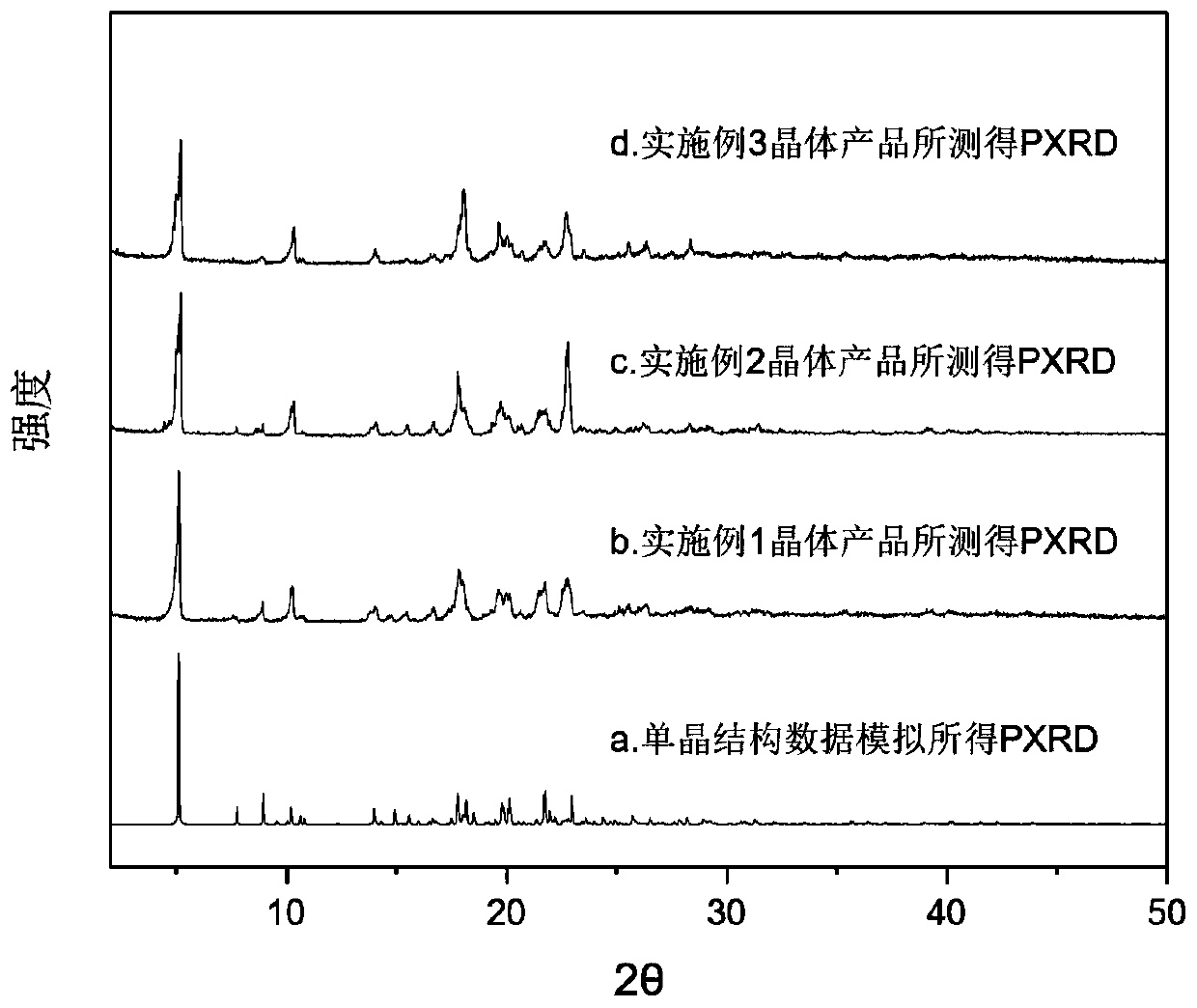
The invention discloses a pharmaceutical co-crystal of gefitinib and bumetanide and a preparation method thereof. A molar ratio of gefitinib to bumetanide in the pharmaceutical co-crystal is 1: 1. Inan X-ray diffraction pattern represented by 2theta, characteristic peaks occur when the value of 2theta is equal to 5.08 + / - 0.2 degrees, 8.88 + / - 0.2 degrees, 10.26 + / - 0.2 degrees, 10.56 + / - 0.2 degrees, 17.78 + / - 0.2 degrees, 19.29 + / - 0.2 degrees, 19.68 + / - 0.2 degrees, 20.04 + / - 0.2 degrees, 21.47 + / - 0.2 degrees and 22.70 + / - 0.2 degrees. Compared with gefitinib technical, the pharmaceuticalco-crystal of the invention has better storage stability, better solubility and dissolution rate. Cytotoxicity experiment results show that the pharmaceutical co-crystal has a better inhibition effect on cancer cells and obviously reduced toxic and side effects on normal cells. The preparation method of the pharmaceutical co-crystal is friendly to environment, stable in yield and easy to industrialize.
Owner:SOUTH CHINA UNIV OF TECH
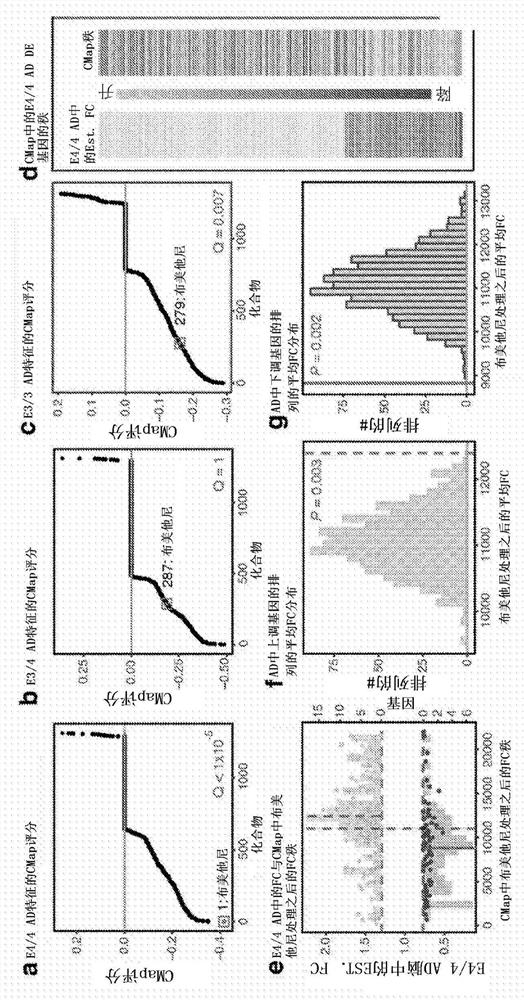
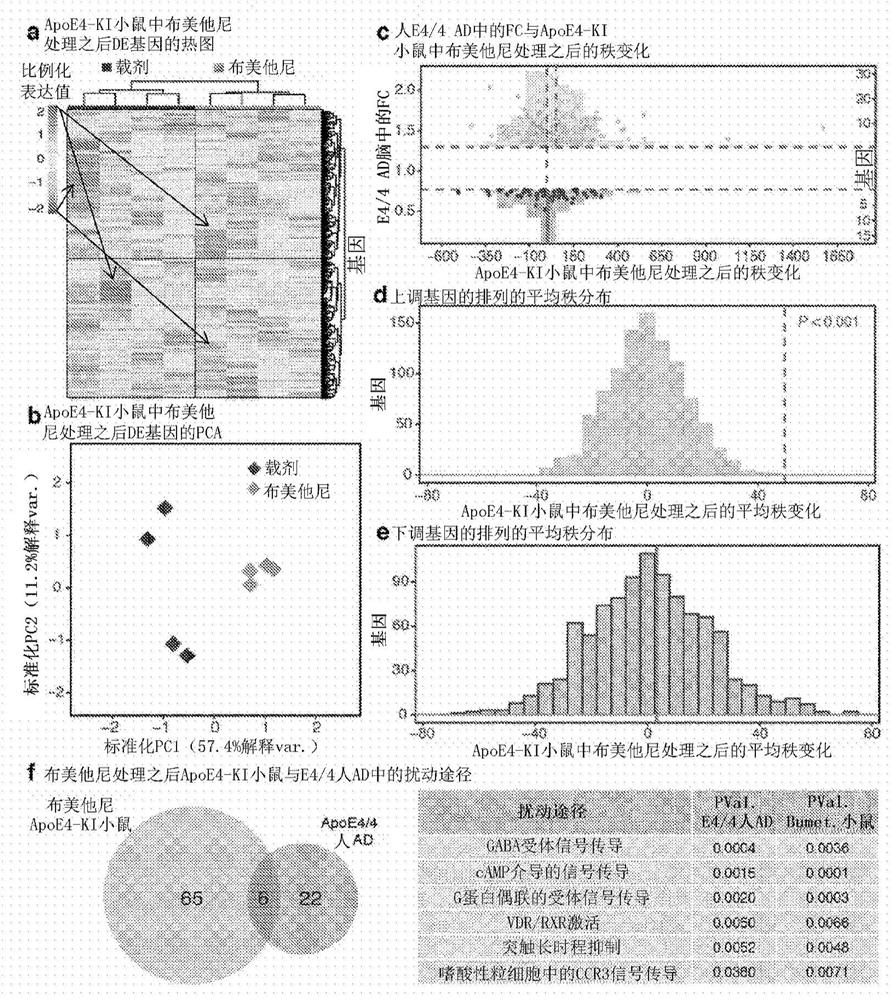
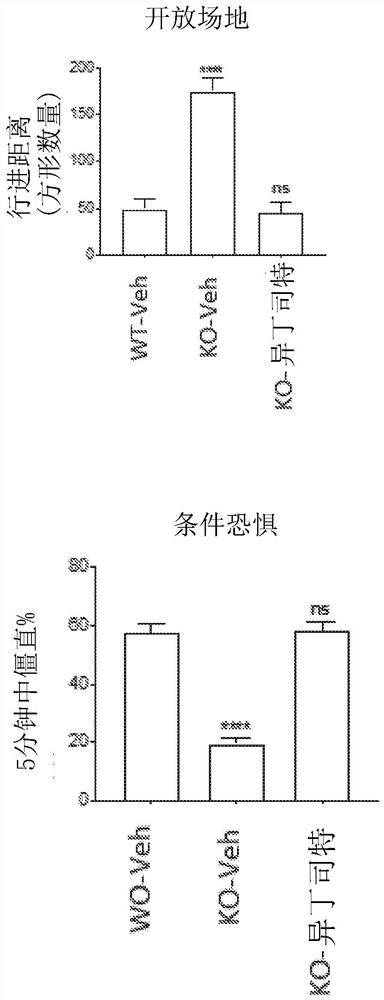
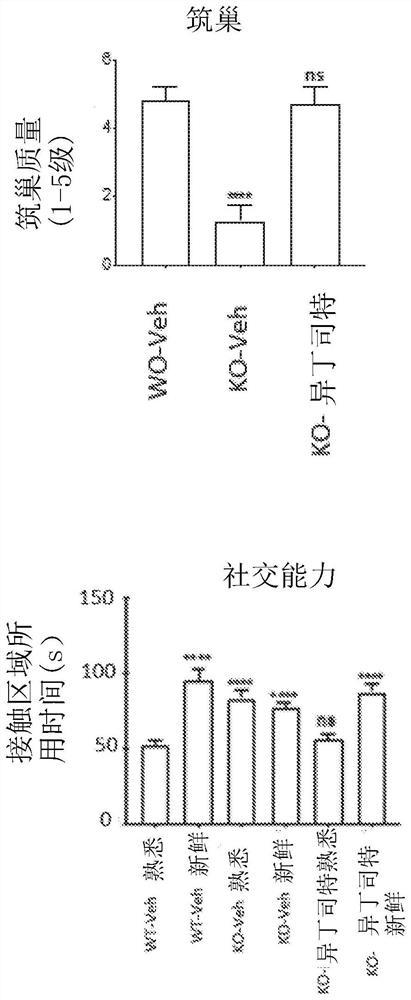
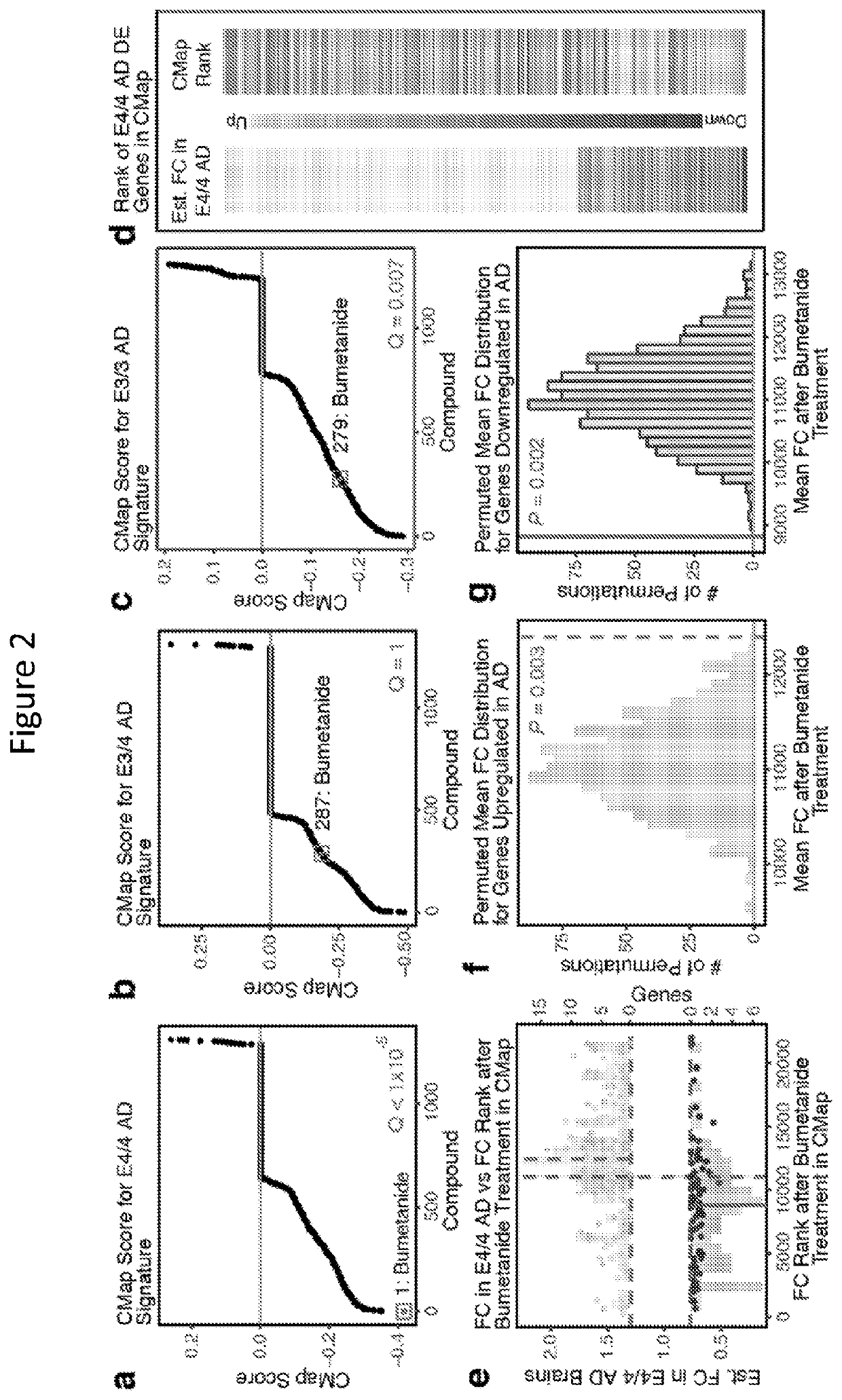
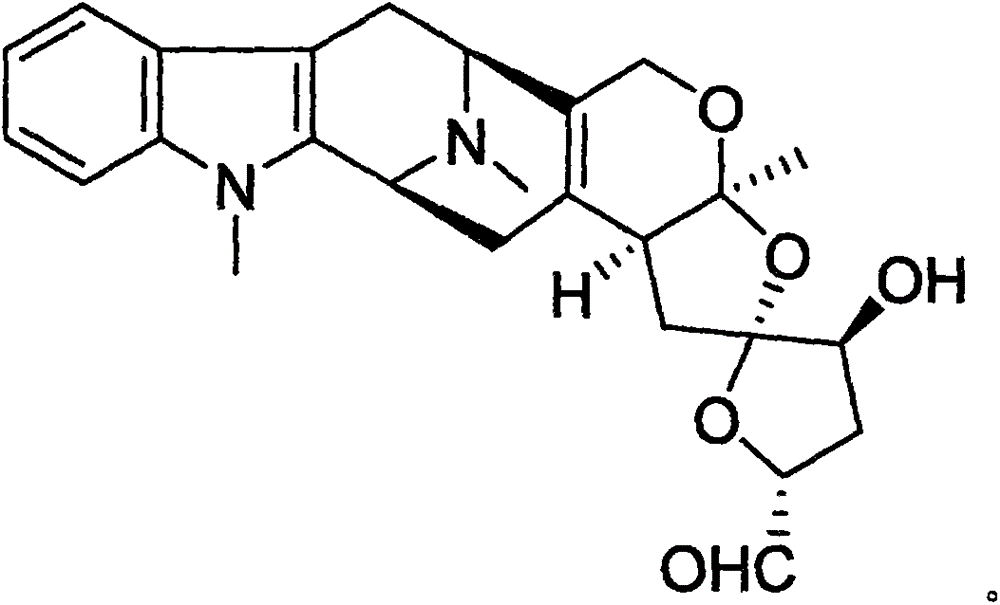
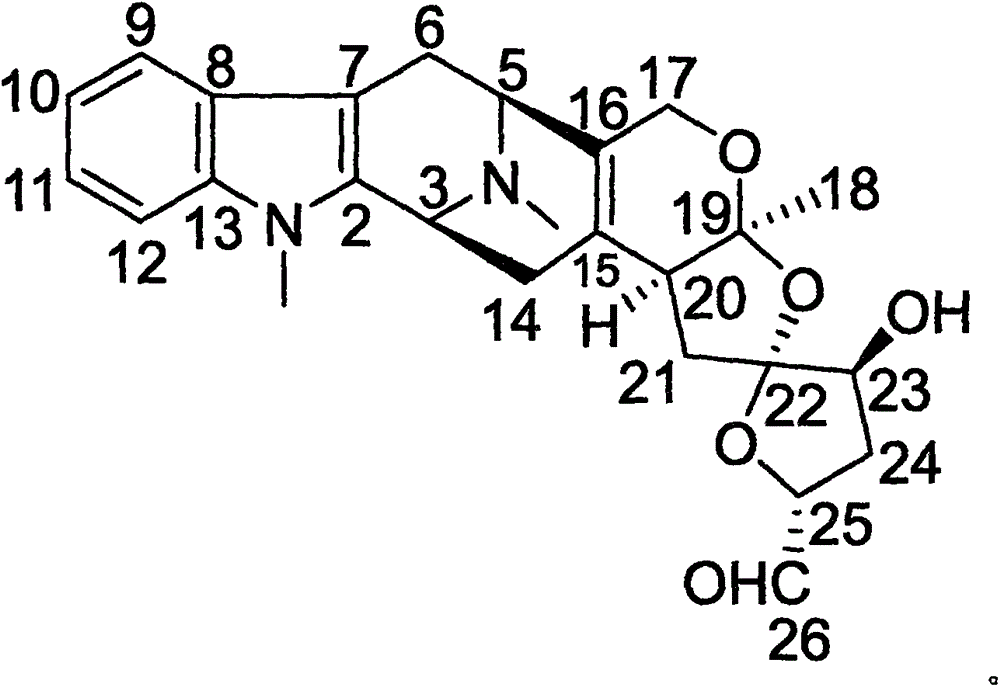
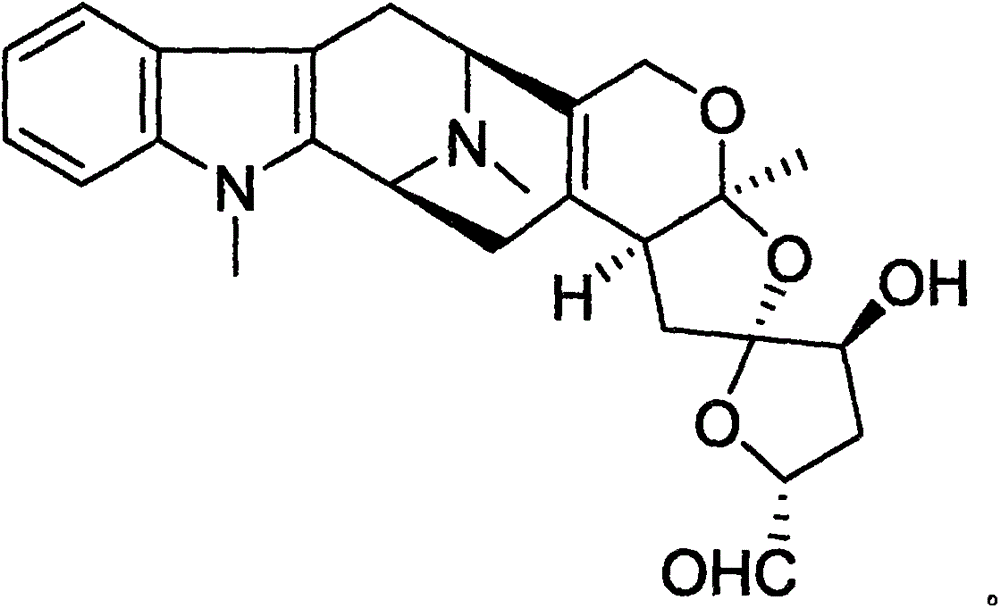
Methods for treating apoe4/4-associated disorders
The present disclosure provides methods of treating Alzheimer's Disease (AD) and rescuing cognitive deficits associated with AD in a subject having an apoE4 / 4 genotype, by administering a therapeutically effective amount of the loop-diuretic bumetanide to the subject. Also disclosed are kits for performing the method, including one or more doses of a bumetanide formulation; and which may also include instructions for treating a patient having an apoE4 / 4 genotype by administering bumetanide, and / or instructions and reagents for testing / identifying a subject having an apoE4 / 4 genotype.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS +1
Compressed capsules for giving birth to males
ActiveUS20190183906A1Satisfies needEasy to swallowOrganic active ingredientsSexual disorderSodium bicarbonateBumetanide
The present invention is related to baby love male capsules including active ingredients such as fludrocortisone, bumetanide, sodium bicarbonates, sodium chloride, and Licourice extract, inactive ingredients such as gelatin, plasticizers, moisture absorbents, and preservatives.The active ingredients are selected according to ovum wall thickness and cell fragility theories. The present invention leads to a breakthrough in the medical field through which the baby gender can be determined and many chronic diseases can be cured.
Owner:RASHWAN EMAD ABD ELAZEEM
Treatment of fragile X syndrome
The present invention relates to a composition comprising ibudilast or a pharmaceutically acceptable salt thereof for use in the treatment of Fragile X Syndrome, wherein the composition does not comprise sulindac or a pharmaceutically acceptable salt thereof or bumetanib or a pharmaceutically acceptable salt thereof.
Owner:HEALX
Methods for treating apoe4/4-associated disorders
The present disclosure provides methods of treating Alzheimer's Disease (AD) and rescuing cognitive deficits associated with AD in a subject having an apoE4 / 4 genotype, by administering a therapeutically effective amount of the loop-diuretic bumetanide to the subject. Also disclosed are kits for performing the method, including one or more doses of a bumetanide formulation; and which may also include instructions for treating a patient having an apoE4 / 4 genotype by administering bumetanide, and / or instructions and reagents for testing / identifying a subject having an apoE4 / 4 genotype.
Owner:RGT UNIV OF CALIFORNIA
Bumetanide medicine composition and anti-inflammatory and abirritation effects thereof
InactiveCN105837589AIncreased anti-inflammatory and analgesic effectsHighlight substantive featuresOrganic active ingredientsOrganic chemistryBelamcanda chinensisNatural product
The invention discloses a bumetanide medicine composition and anti-inflammatory and abirritation effects thereof. The bumetanide medicine composition includes bumetanide and a natural product compound (I) which has a novel structure and is extracted from dry rhizome of belamcanda chinensis. The bumetanide and the compound (I) are not so excellent in anti-inflammatory and abirritation effects when being used separately but are significantly improved in the anti-inflammatory and abirritation effects when being used together, so that the composition can be developed to prepare anti-inflammatory and abirritation drugs. Compared with the prior art, the composition has outstanding substantive features and significant improvement.
Owner:薛君
Applications of bumetanide in inhibition of tumor cell proliferation
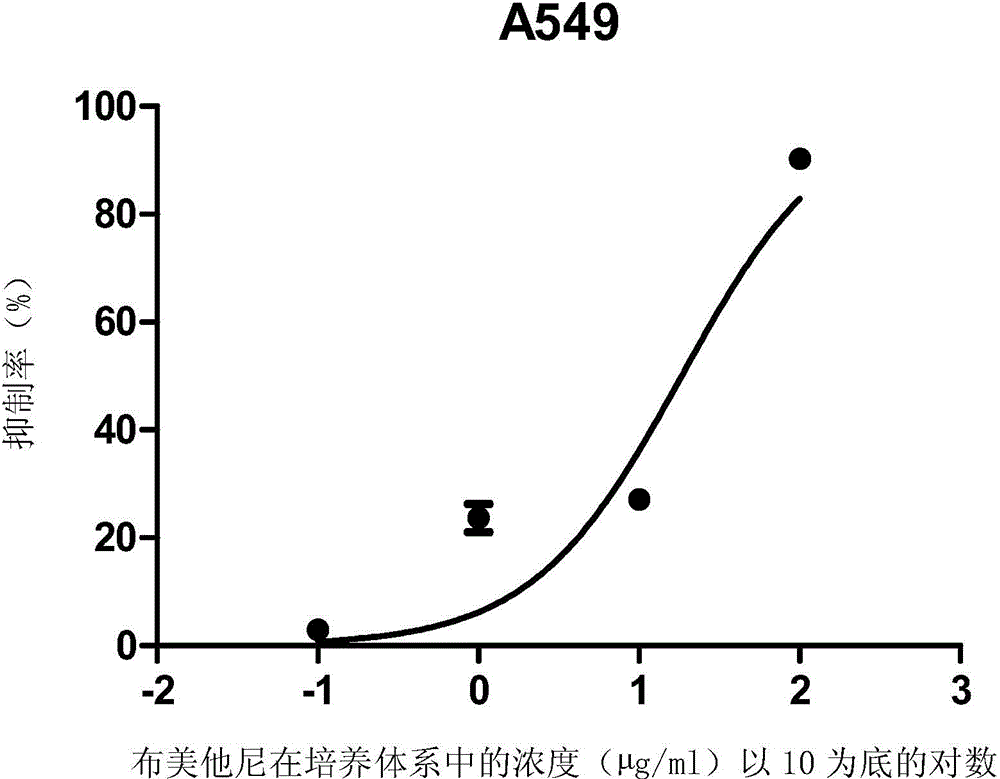
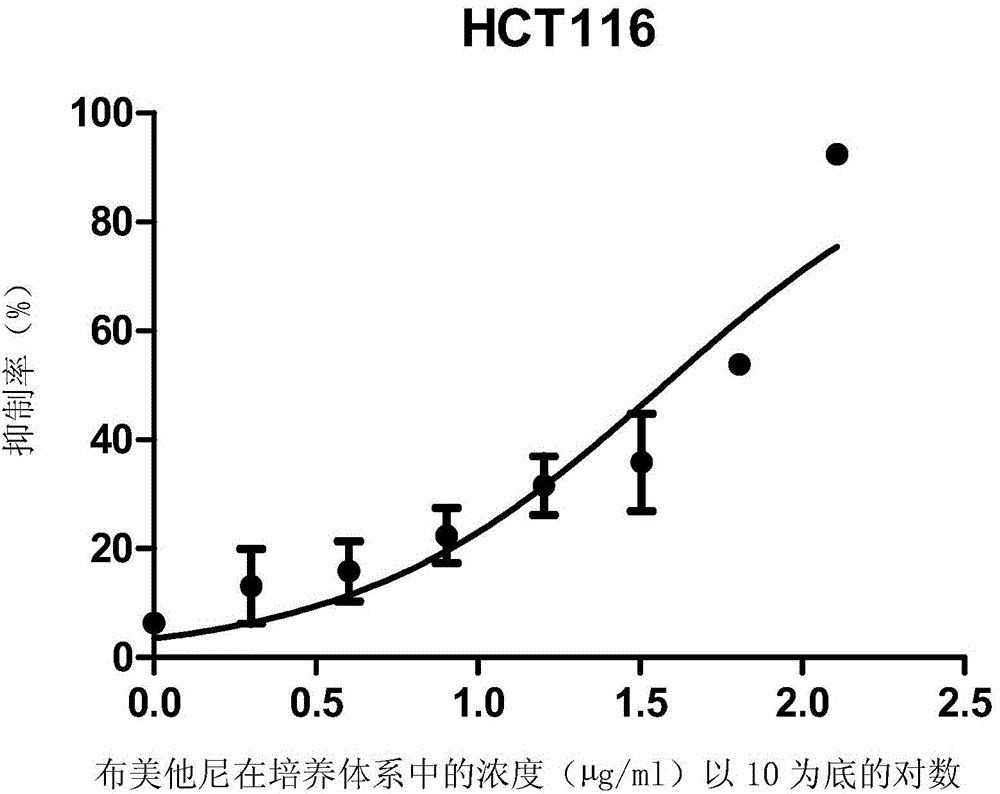
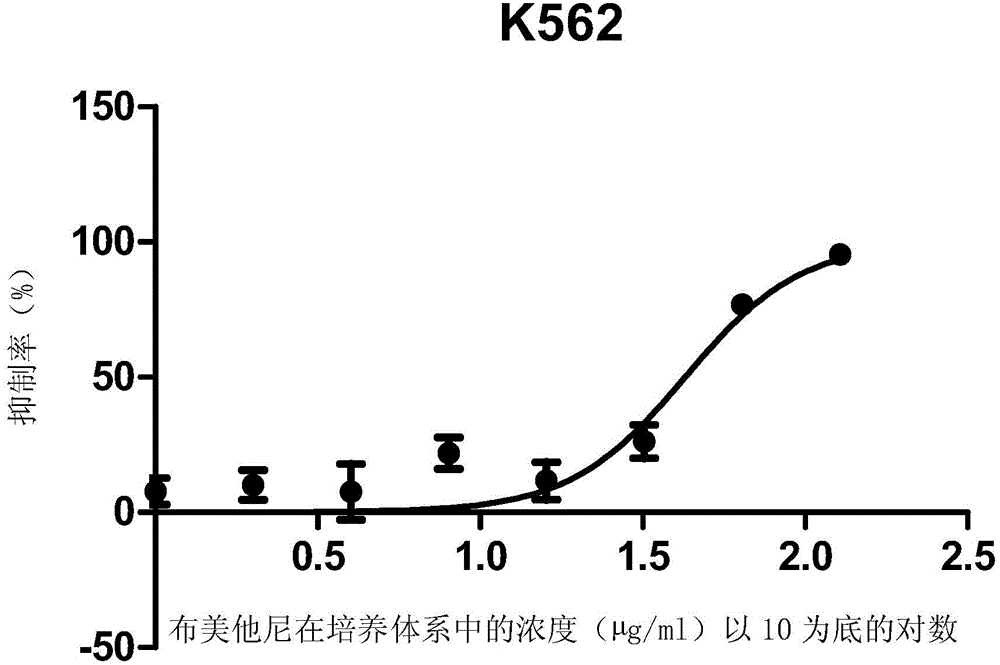
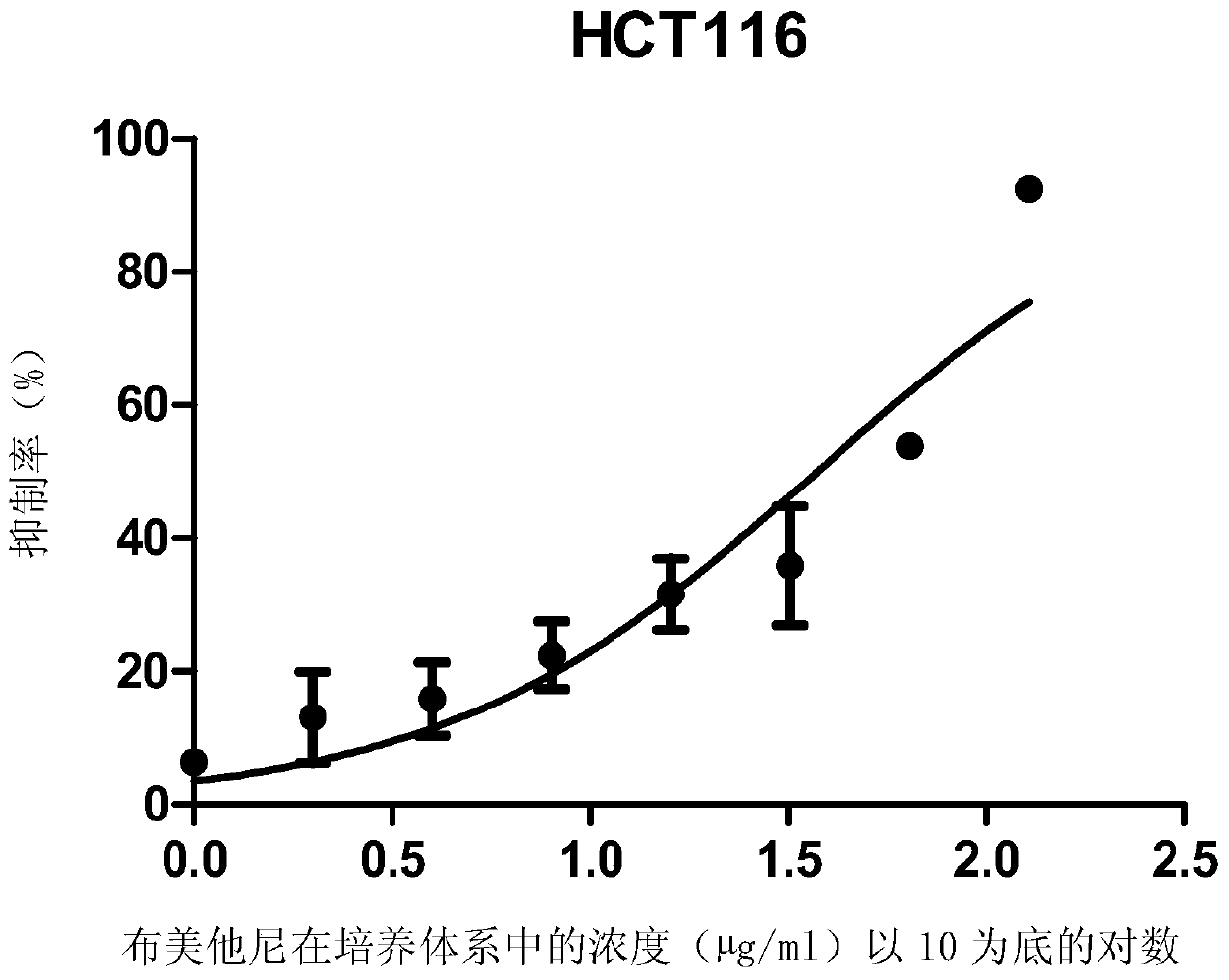
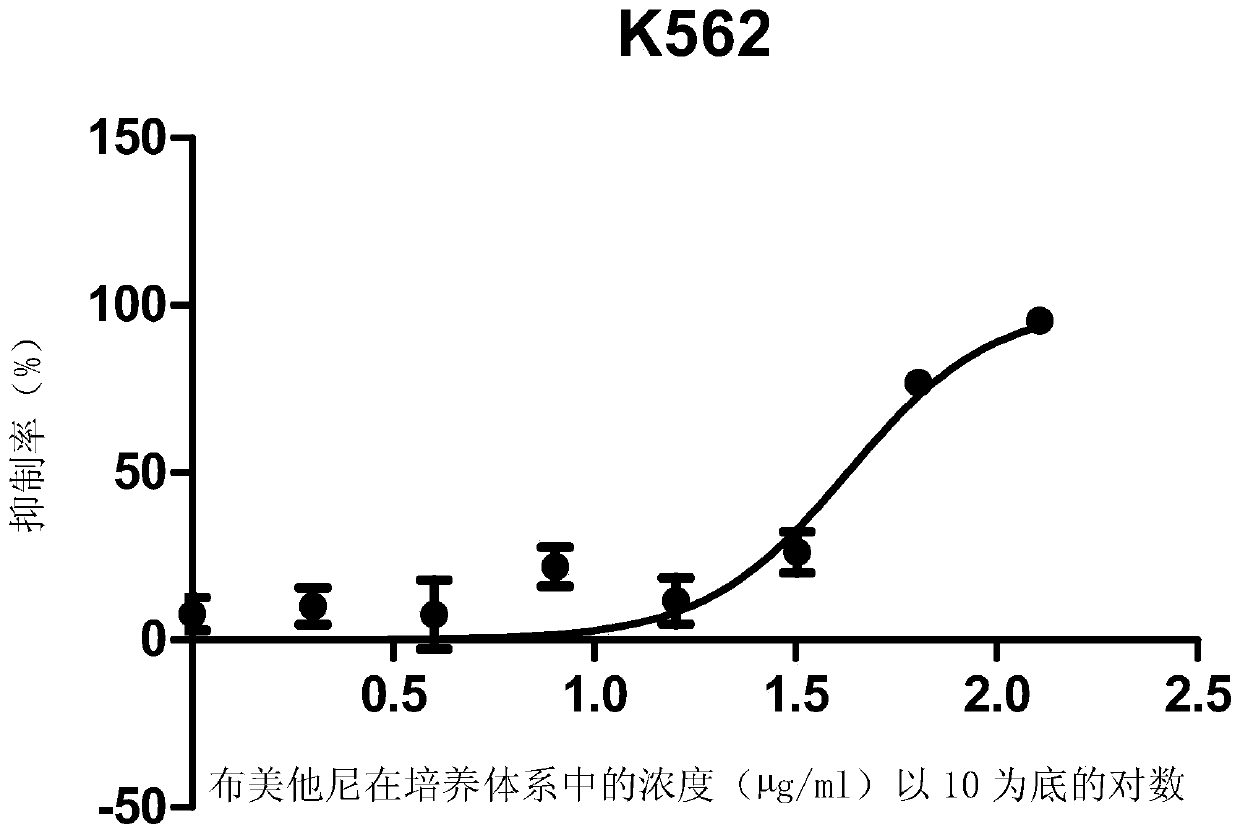
ActiveCN105496995ATherapeutic valueOrganic active ingredientsAntineoplastic agentsHCT116 CellBumetanide
The present invention discloses applications of bumetanide in inhibition of tumor cell proliferation. The present invention provides applications of bumetanide in preparation of drug for inhibition of tumor cells, wherein the tumor cells can be lung adenocarcinoma cells, colon cancer cells, leukemia cells, esophageal cancer cells, cervical cancer cells or breast cancer cells, and can specifically be A549 cells, HCT116 cells, K562 cells, Eca109 cells, Hela cells, Jurkat cells or MCF7 cells. According to the present invention, the great value is provided for tumor treatment.
Owner:BEIJING PROTEOME RES CENT +1
Methods and compositions for treating edema refractory to oral diuretics
ActiveUS11123319B2Organic active ingredientsPharmaceutical non-active ingredientsBumetanideMedicinal chemistry
The present invention features methods and compositions for the intranasal, sublingual, and subcutaneous administration of bumetanide for the treatment of subjects suffering from edema refractory to oral diuretics.
Owner:RESQ PHARMA LLC
Antihypertensive drug composition and application thereof
InactiveCN107213152AGood effectSmall doseSulfonylurea active ingredientsCardiovascular disorderSide effectBumetanide
The invention relates to an antihypertensive drug composition, which comprises gliguidone and bumetanide. A hypoglycemic drug gliguidone is introduced on the basis of a diuretic antihypertensive drug bumetanide, so that collaborative depressurization is achieved and the effect is significantly strengthened. When a fixed compound is formed, the dosage of various single drugs is reduced, and especially the dosage of the bumetanide is greatly reduced, so that the incidence rate of the side effect of the drug is reduced; and protection of the heart is facilitated while hypertension is treated, the damage of the hypertension to the hearth function is significantly reduced, the gliguidone combined with bumetanide has a significant difference in reverse or reduction of left ventricular hypertrophy, and a collaborative treatment effect is obtained.
Owner:YANTAI UNIV
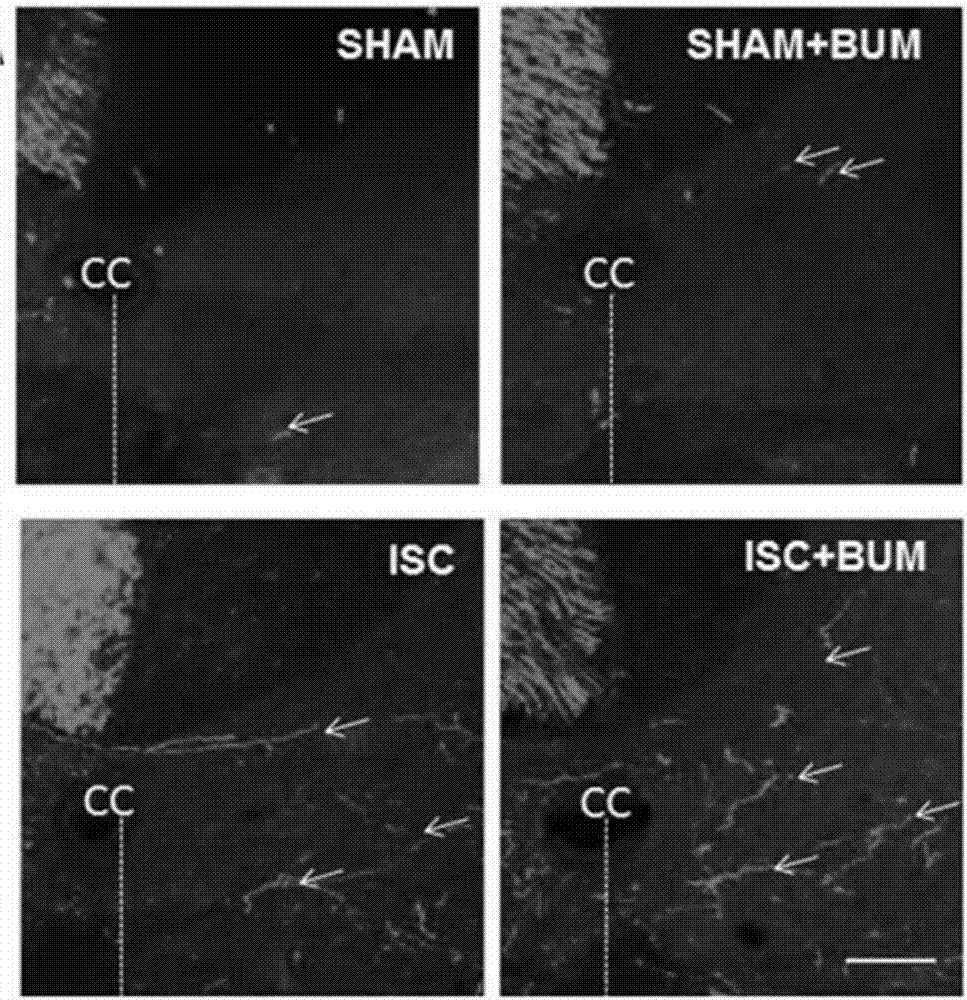
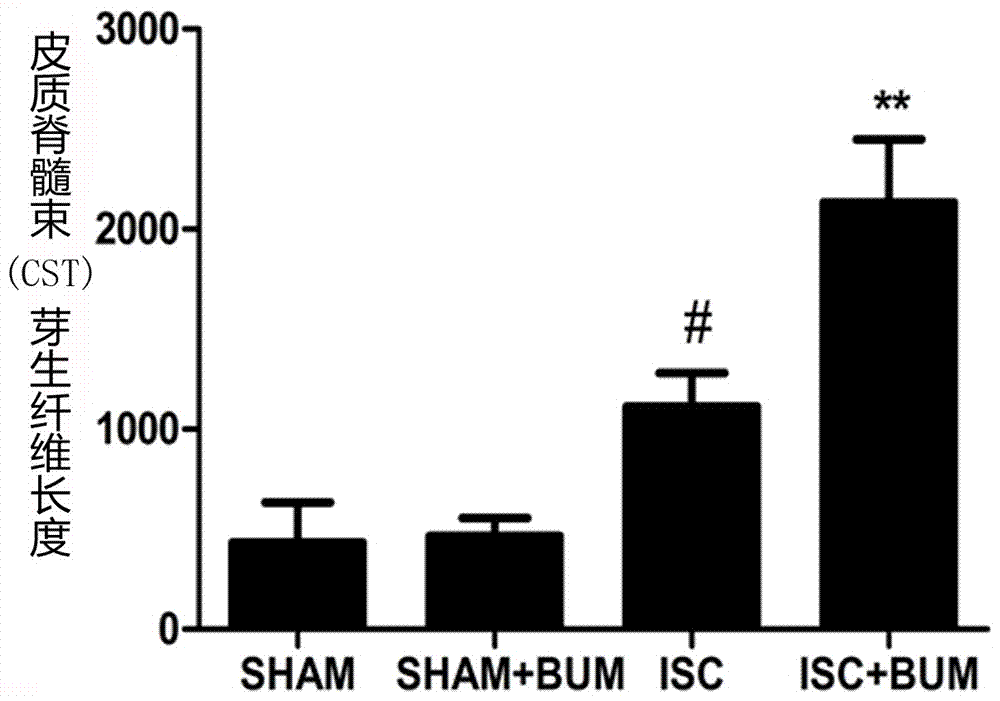
Application of bumetanide in promoting neural functional reconstruction in recovery period after ischemic stroke
InactiveCN107308148APromote remodelingAvoid damageOrganic active ingredientsNervous disorderBumetanideFunction recovery
The invention belongs to the field of biomedicine and particularly relates to application of bumetanide in promoting neural functional reconstruction in the recovery period after ischemic stroke, particularly the application of bumetanide in neural functional reconstruction drugs for the recovery period after ischemic stroke. Studies discover that bumetanide is capable of promoting regeneration of axons in a rat with ischemic stroke, remodeling of axon synapsis and recovery of motor nerve functions, up-regulating NogoA expression level of the periphery of ischemic cortex, up-regulating BDNF (brain-derived neurotrophic factor) protein expression level of the periphery of the ischemic cortex, and promoting regeneration of axons by up-regulating the BDNF protein expression level of the periphery of the ischemic cortex and down-regulating the NogoA level.
Owner:THE FIRST HOSPITAL OF CHINA MEDICIAL UNIV
Treatment of fragile x syndrome
The present invention relates to a composition comprising ibudilast, or a pharmaceutically acceptable salt thereof, for use in the treatment of Fragile X syndrome, wherein the composition does not comprise sulindac, or a pharmaceutically acceptable salt thereof, or bumetanide, or a pharmaceutically acceptable salt thereof.
Owner:HEALX
Application of bumetanide in inhibiting tumor cell proliferation
ActiveCN105496995BTherapeutic valueOrganic active ingredientsAntineoplastic agentsHCT116 CellBumetanide
The invention discloses the application of bumetanide in inhibiting tumor cell proliferation. The invention provides the application of bumetanide in the preparation of drugs for inhibiting tumor cells. The tumor cells may be lung adenocarcinoma cells, colon cancer cells, leukemia cells, esophageal cancer cells, cervical cancer cells or breast cancer cells. The tumor cells may specifically be A549 cells, HCT116 cells, K562 cells, Eca109 cells, Hela cells, Jurkat cells or MCF7 cells. The invention has great value for the treatment of tumors.
Owner:BEIJING PROTEOME RES CENT +1
Preparation method for Bumetanide freeze-dried powder preparation for injection
ActiveCN103520122BImprove solubilitySolve the problem of insoluble in waterOrganic active ingredientsPowder deliveryBumetanideFreeze-drying
The invention provides a bumetanide freeze-dried powder preparation for injection and a preparation method thereof. The method consists of: weighing 10ml of glycerol and water for injection in a volume ratio of 1:4-2:3, and stirring them to obtain a latent solvent; weighing 0.5g of bumetanide and dissolving it in the latent solvent, conducting filtering to clarity, thus obtaining a solution A; weighing 200g of mannitol, adding water for injection accounting for 80% of the total amount, carrying out stirring and filtering to clarity, thus obtaining a solution B; mixing the solution A and the solution B evenly, then adding the remaining water for injection to a total amount of 2000ml, performing degerming filtering, then subjecting the product to filling into 1000 10ml tube-type bottles respectively according to a dosage of 2.0ml / bottle; subjecting the tube-type bottles to heat preservation for 4h under -45DEG C; conducting vacuum pumping to a vacuum degree of not greater than 20pa, raising the slab temperature to -5DEG C at a rate of 2DEG C / h, and carrying out heat preservation for 18h; raising the slab temperature to 30DEG C at a rate of 7DEG C / h, and carrying out heat preservation for 2h, thus obtaining the bumetanide freeze-dried powder preparation for injection. According to the invention, the product stability is effectively ensured, the content of impurities is reduced, and the re-dissolubility of the product is good.
Owner:HAINAN GENERAL & KANGLI PHARMA
Compressed capsules for giving birth to males
InactiveUS10463679B2Meet actual needsEasy to swallowOrganic active ingredientsSexual disorderSodium bicarbonateAdditive ingredient
The present invention is related to baby love male capsules including active ingredients such as fludrocortisone, bumetanide, sodium bicarbonates, sodium chloride, and Licourice extract, inactive ingredients such as gelatin, plasticizers, moisture absorbents, and preservatives.The active ingredients are selected according to ovum wall thickness and cell fragility theories. The present invention leads to a breakthrough in the medical field through which the baby gender can be determined and many chronic diseases can be cured.
Owner:RASHWAN EMAD ABD ELAZEEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com