Methods for treating apoe4/4-associated disorders
A technology for Alzheimer's disease, objects, applied in chemical instruments and methods, biochemical equipment and methods, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 3
[0200] Data Integration. Search the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) and National Institute of Aging's Accelerating Drug Collaboration - Accelerating Alzheimer's Disease Transcriptome AD data from the Medicines Partnership-Alzheimer's Disease (AMP-AD) database from temporal lobe tissues for which apoE genotype information was available. From the NCBI GEO database in available series matrix file format 24 Direct download from Webster et al, 2009 17 Microarray experiments, accession number GSE15222. The data has been described as 17,25 Perform rank-invariant normalization. The prenormalization procedure created negative values in the series matrix file, which can be eliminated by adding a constant to the expression matrix 26 . Since the fold change (FC) calculated after this addition was underestimated, all subsequent FC estimates were conservative with respect to the threshold. The Log2 transformation is then applied to...
Embodiment 4 to 6
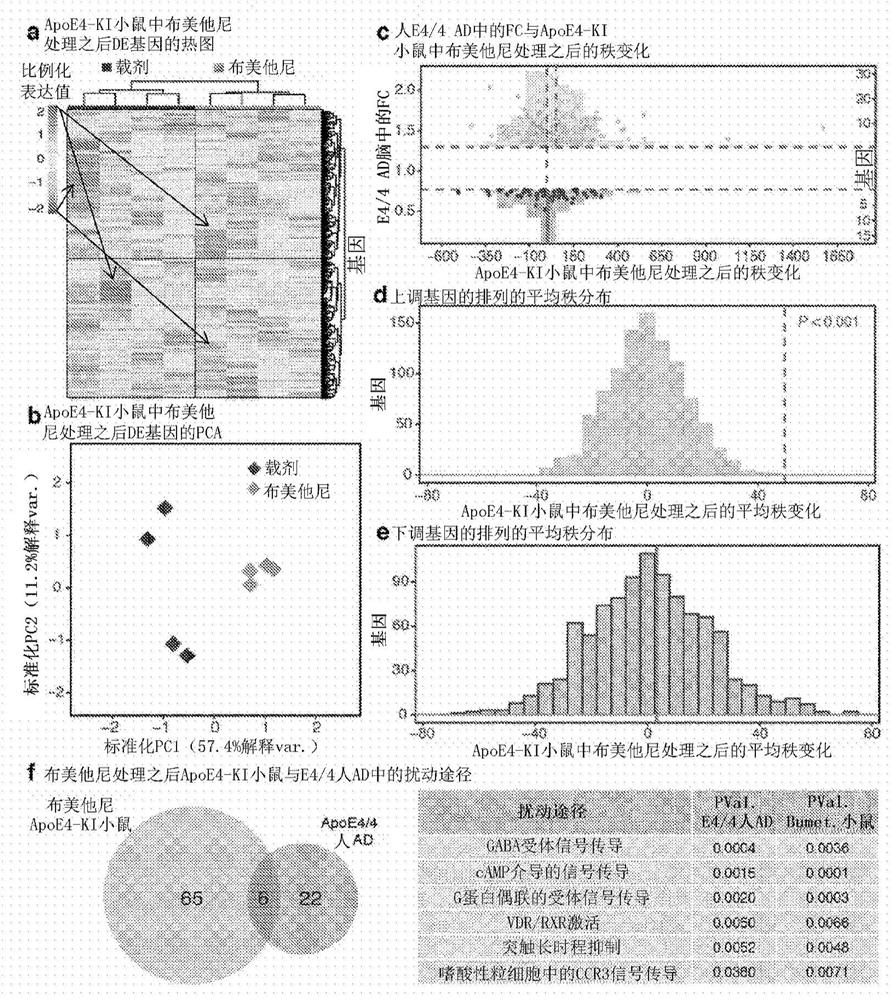
[0218] Brain slice electrophysiological recording and data analysis. For long-term potentiation (LTP) recording studies, apoE4-KI mice were randomly assigned to vehicle (n=6, age 15.2±0.56 months) and bumetanide (n=3, age 15.1 ±0.06) treatment group. ApoE3-KI mice were similarly assigned to vehicle (n=6, age 15.5±0.11) and bumetanide (n=3, age 15.57±0.19) treated groups. For input / output recording studies, apoE4-KI mice were randomly assigned to vehicle (n=3, age 15.1±0.06 months) and bumetanide (n=3, age 15.1±0.06) treatments Group. ApoE3-KI mice were similarly assigned to vehicle (n=7, age 15.3±0.06) and bumetanide (n=3, age 15.57±0.29) treated groups. All mice were dosed for 6 to 8 weeks and slice recording days and dosing times were randomly assigned between groups to allow for equal dosing times while the experiments were performed. The final bumetanide injection was performed one hour before sacrifice. All mice were 16 to 17 months of age at the time of recording. ...
Embodiment 1
[0231] Computerized screening and identification of bumetanide
[0232] The study protocol is described and the results are shown here.
[0233] Described herein is an apoE genotype-specific drug repositioning method for screening drugs for the treatment of AD and / or diseases and disorders associated with the apoE4 / 4-genotype.
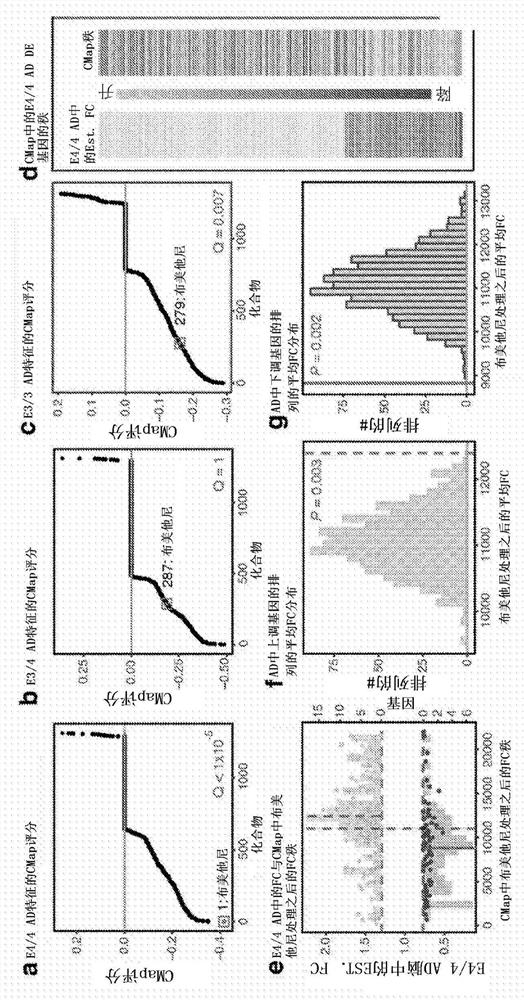
[0234] An apoE genotype-specific transcriptome signature of AD was established by gene expression meta-analysis of 610 human temporal lobe samples obtained from public databases and used to query a validated transcriptome perturbation signature comprising 1300 existing drugs The Connectivity Map (CMap) database was used to identify drugs that perturb entire gene expression networks away from apoE genotype-driven disease states toward normal states.
[0235] The ApoE genotype-specific transcriptome signature of AD from publicly available databases was first analyzed and the first CMap database search using the ApoE genotype-specific signature of AD w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com