Bumetanide freeze-dried powder preparation for injection and preparation method thereof
A technology of bumetanide and freeze-dried powder injection, which is applied in the field of medicine, can solve problems such as complicated process, increased impurities, and uncontrollable quality, and achieve the effects of reducing solvent usage, good resolubility, and increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Prepare one thousand bottles of bumetanide freeze-dried powder preparation for injection with a total amount of 2000ml:
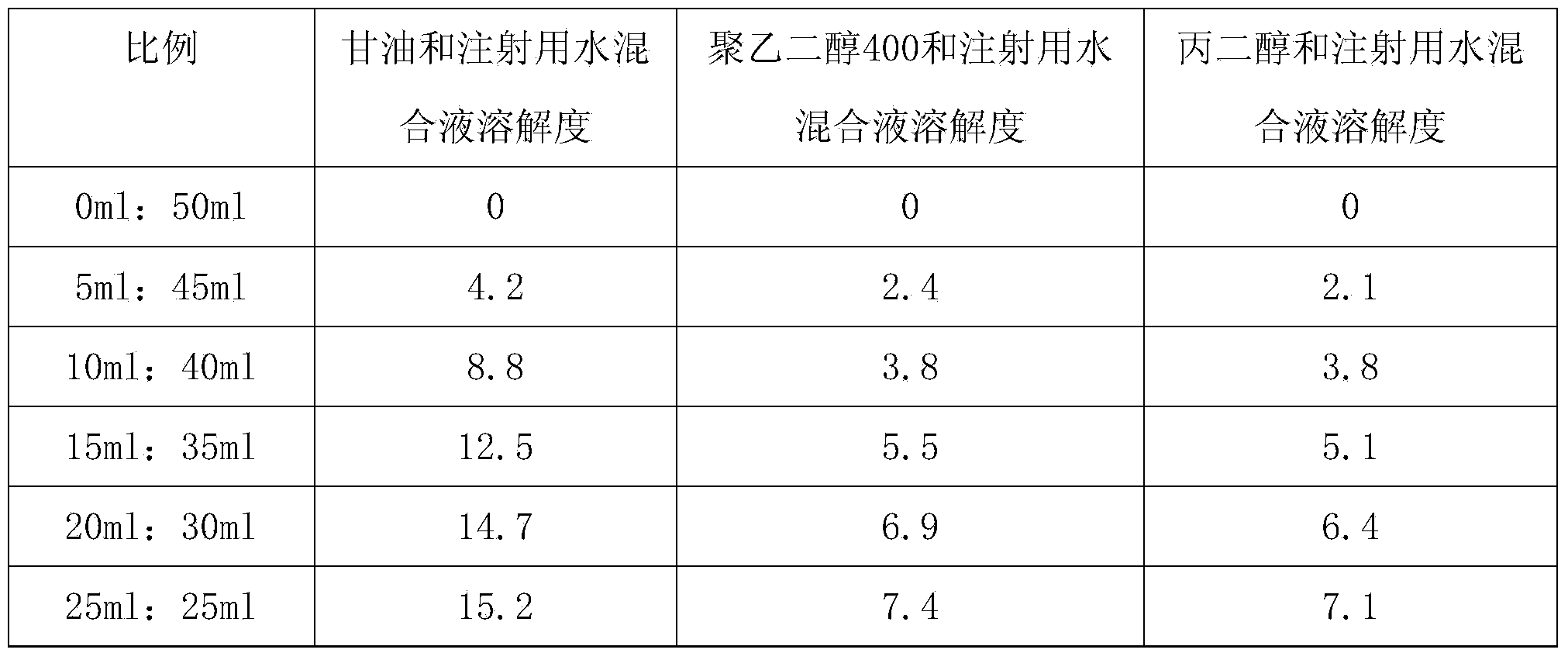
[0015] 1. Measure 2ml of glycerin and add it to 8ml of water for injection, stir to obtain a latent solvent;
[0016] 2. Weigh 0.5g of bumetanide, add it to the latent solvent, and stir in the dark to completely dissolve the bumetanide, add activated carbon whose mass is 0.03% of the latent solvent volume, stir at room temperature for 30 minutes, filter until clarity, and obtain Solution A, standby;
[0017] 3. Take 200g of mannitol, add about 80% of the total amount of water for injection, stir to dissolve, add activated carbon with a mass of 0.03% of the volume of the mixed solution, stir at room temperature for 30 minutes, filter until clear, and obtain solution B for later use;
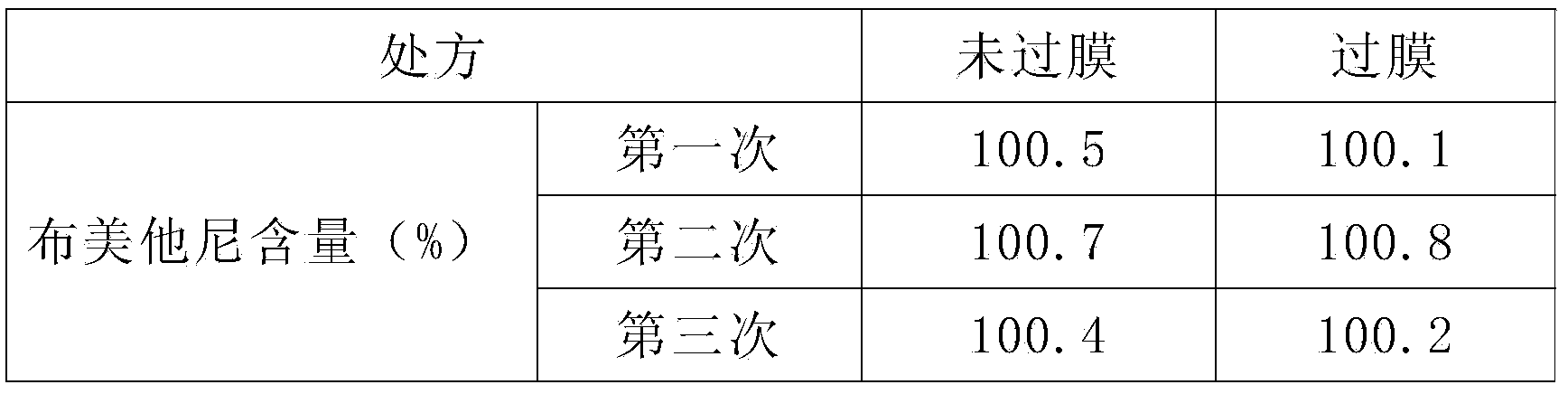
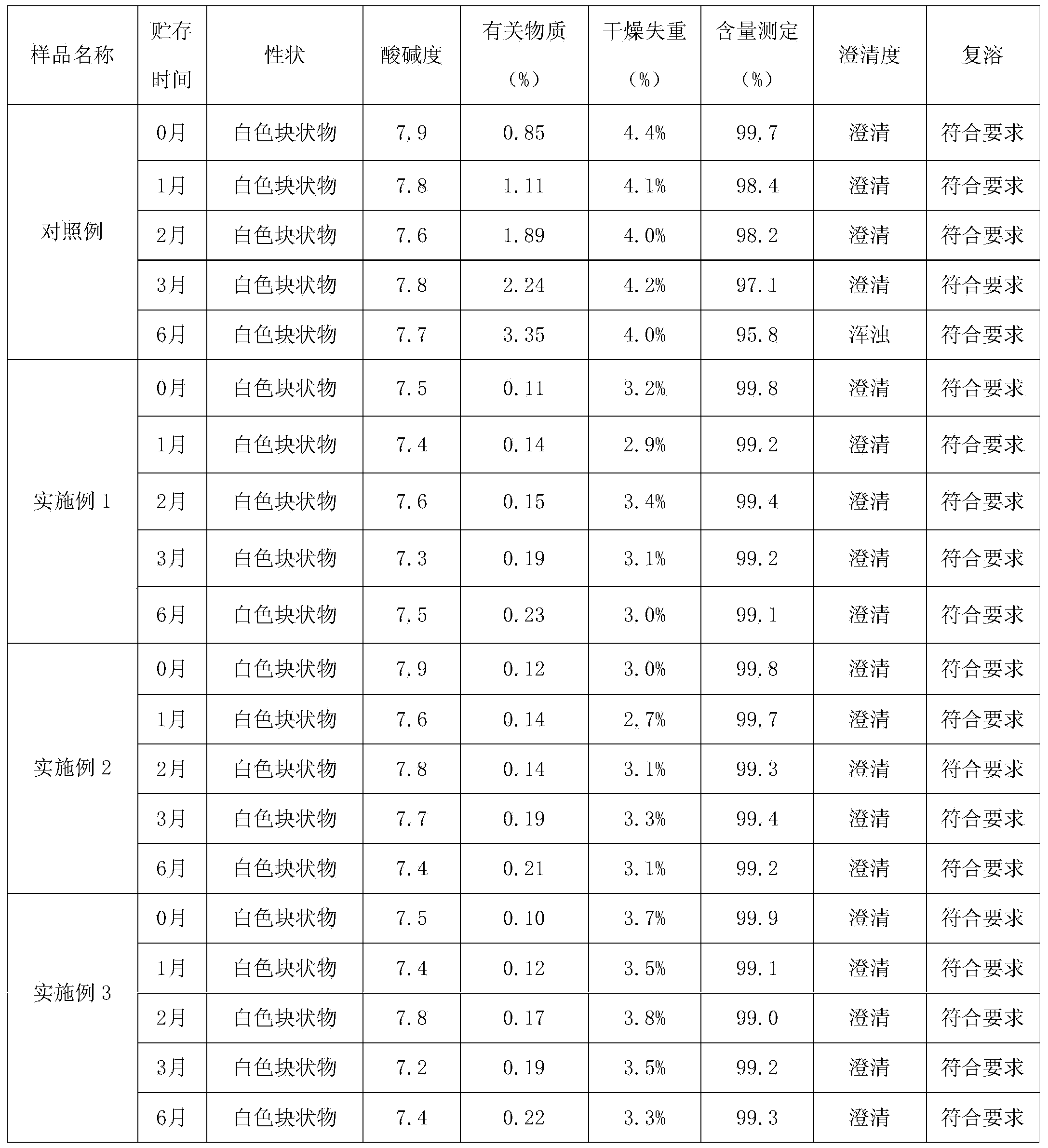
[0018] 4. Mix solution A and solution B, add the remaining water for injection to a total of 2000ml, filter the solution until it is clear, check the content of bumetan...
Embodiment 2
[0024] Prepare one thousand bottles of bumetanide freeze-dried powder preparation for injection with a total amount of 2000ml:
[0025] 1. Measure 3ml of glycerin and add it to 7ml of water for injection, stir to obtain a latent solvent;
[0026] 2. Weigh 0.5g of bumetanide, add it to the latent solvent, and stir in the dark to dissolve the bumetanide completely. Add activated carbon whose mass is 0.03% of the latent solvent volume, stir at room temperature for 30 minutes, and filter until clear. Get solution A, for subsequent use;
[0027] 3. Take 200g of mannitol, add about 80% of the total amount of water for injection, stir to dissolve, add activated carbon whose mass is 0.03% of the volume of latent solvent, stir at room temperature for 30 minutes, filter until clear, and obtain solution B for later use;
[0028] 4. Mix solution A and solution B, add the remaining water for injection to a total of 2000ml, filter the solution until it is clear, check the content of bumeta...
Embodiment 3
[0034] Prepare one thousand bottles of bumetanide freeze-dried powder preparation for injection with a total amount of 2000ml:
[0035] 1. Measure 4ml of glycerin and add it to 6ml of water for injection, stir to obtain a latent solvent;
[0036] 2. Weigh 0.5g of bumetanide, add it to the latent solvent, and stir in the dark to dissolve the bumetanide completely. Add activated carbon whose mass is 0.03% of the latent solvent volume, stir at room temperature for 30 minutes, and filter until clear. Get solution A, for subsequent use;
[0037] 3. Take 200g of mannitol, add about 80% of the total amount of water for injection, stir to dissolve, add activated carbon whose mass is 0.03% of the volume of latent solvent, stir at room temperature for 30 minutes, filter until clear, and obtain solution B for later use;
[0038] 4. Mix solution A and solution B, add the remaining water for injection to a total of 2000ml, filter the solution until it is clear, check the content of bumeta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com