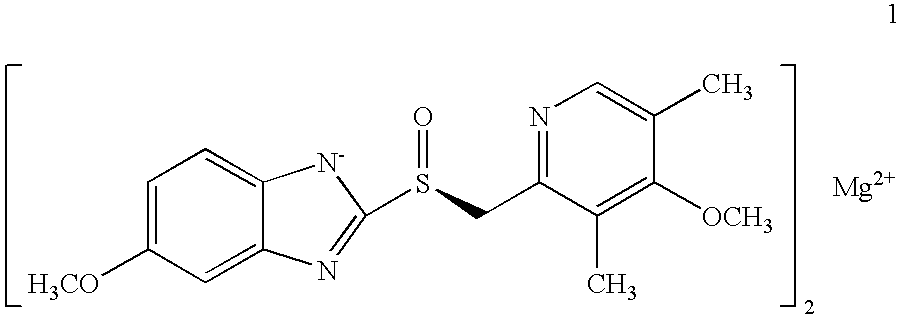
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
86 results about "Rabeprazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
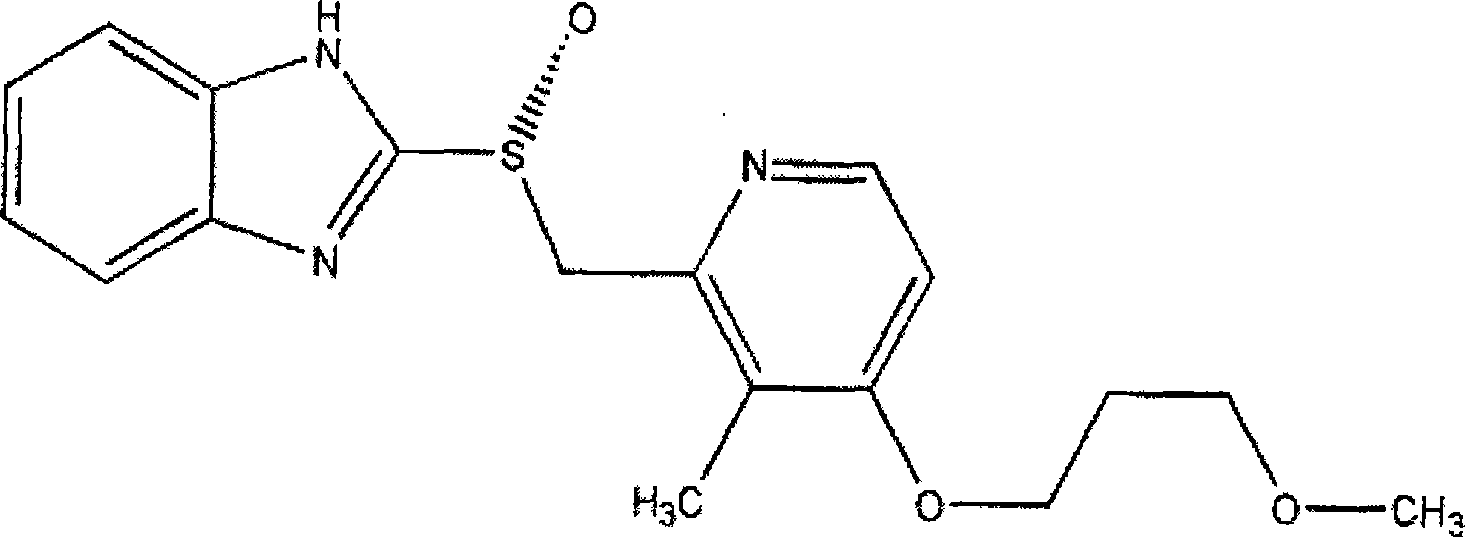
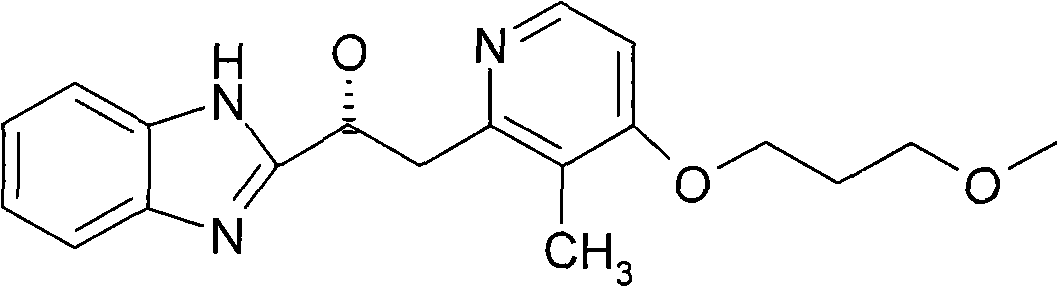
Rabeprazole is used to treat certain stomach and esophagus problems (such as acid reflux, ulcers).
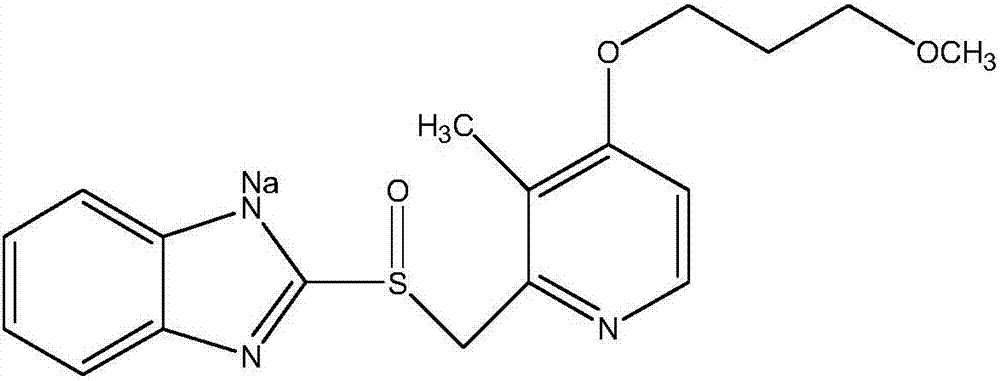
High-purity sodium rabeprazole compound
InactiveCN101704811AOvercome purityOvercome the disadvantages of difficult purificationOrganic chemistryOrganic solventRabeprazole
The invention relates to a high-purity sodium rabeprazole compound, belonging to the technical field of medicine. The method includes the following steps: dissolving crude sodium rabeprazole synthesized by the reaction of rabeprazole and sodium hydroxide in water, adjusting pH value to be faintly acid to neutral by using solid acid salt, and collecting precipitated solid; after dissolving the solid with organic solvent, conducting elution and purification by using eluting agent through macroporous adsorption resin, and collecting eluent; and adjusting the pH value of the eluent to be alkaline, and collecting the precipitated solid to obtain the pure sodium rabeprazole.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
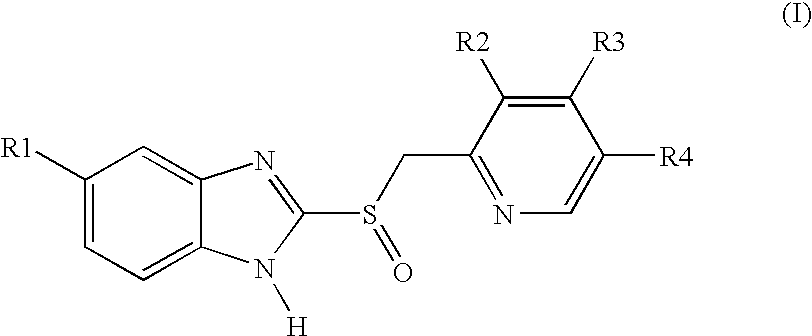
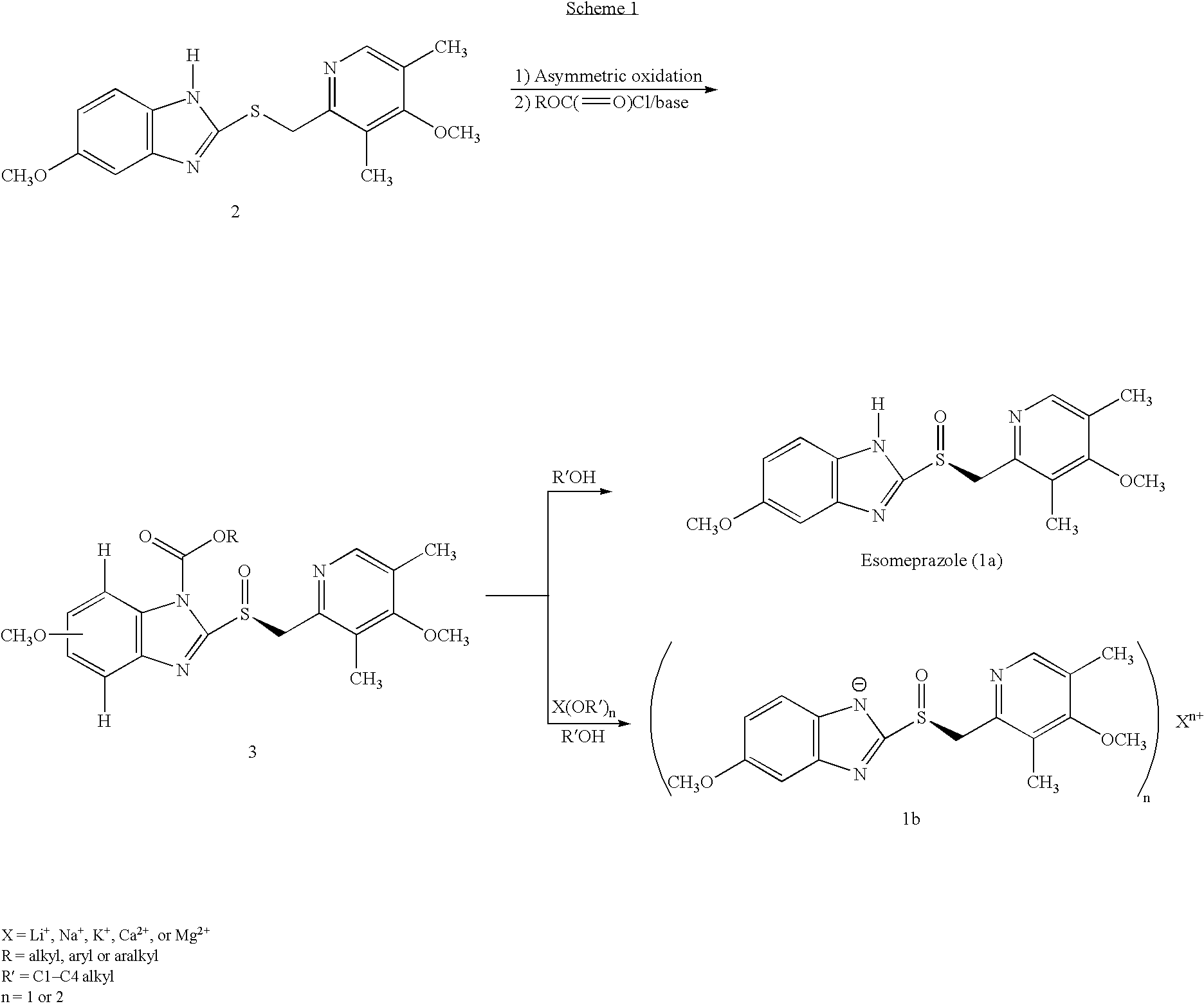
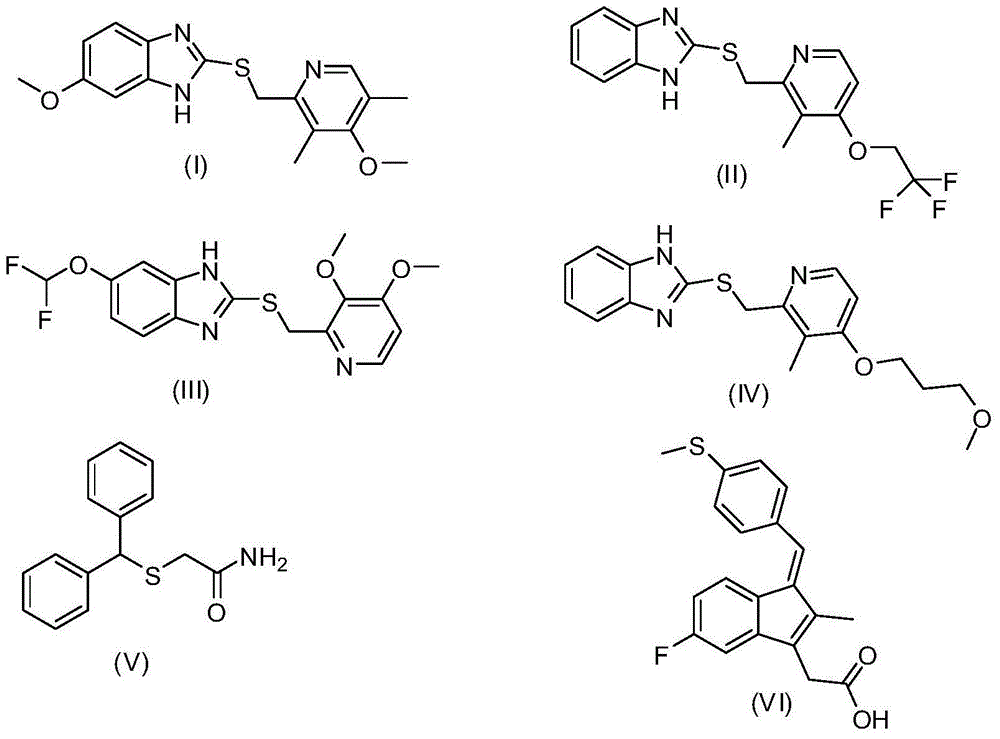
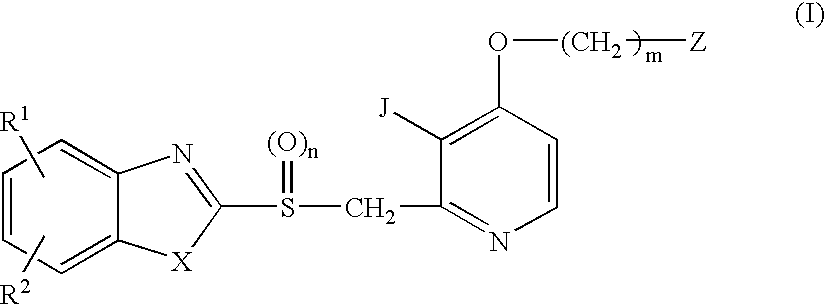
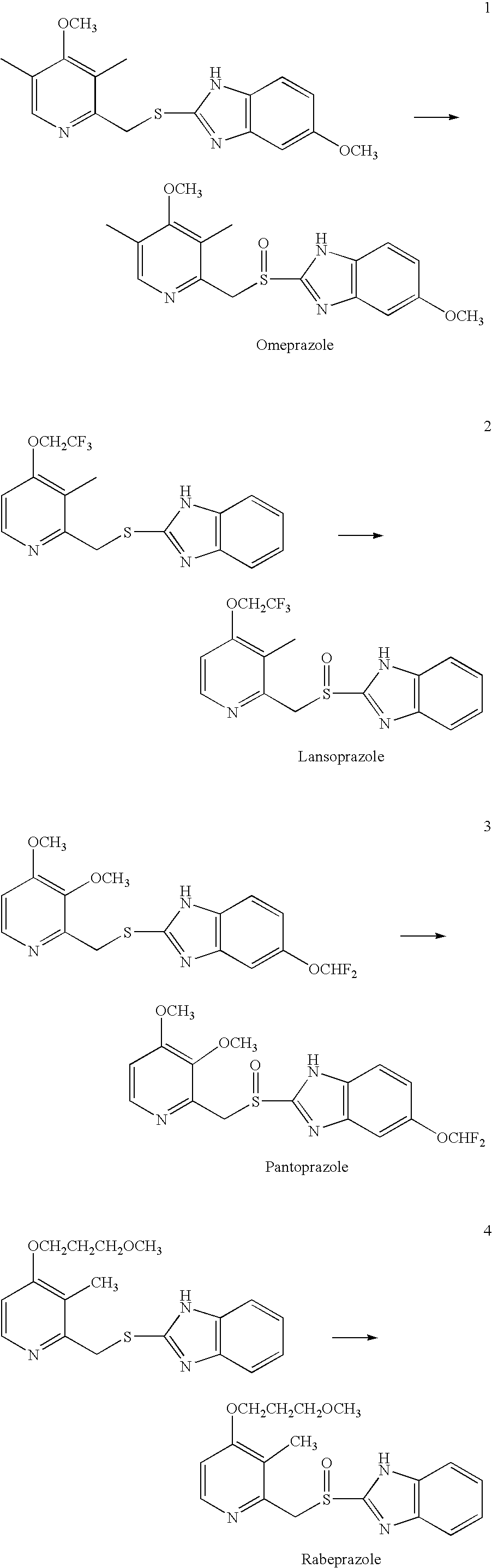
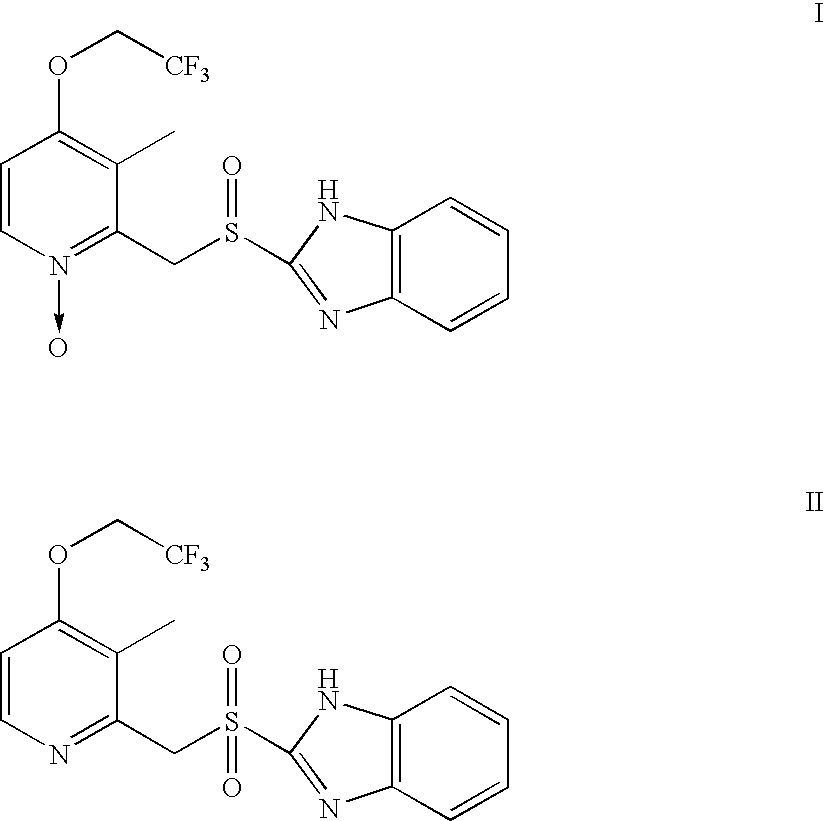
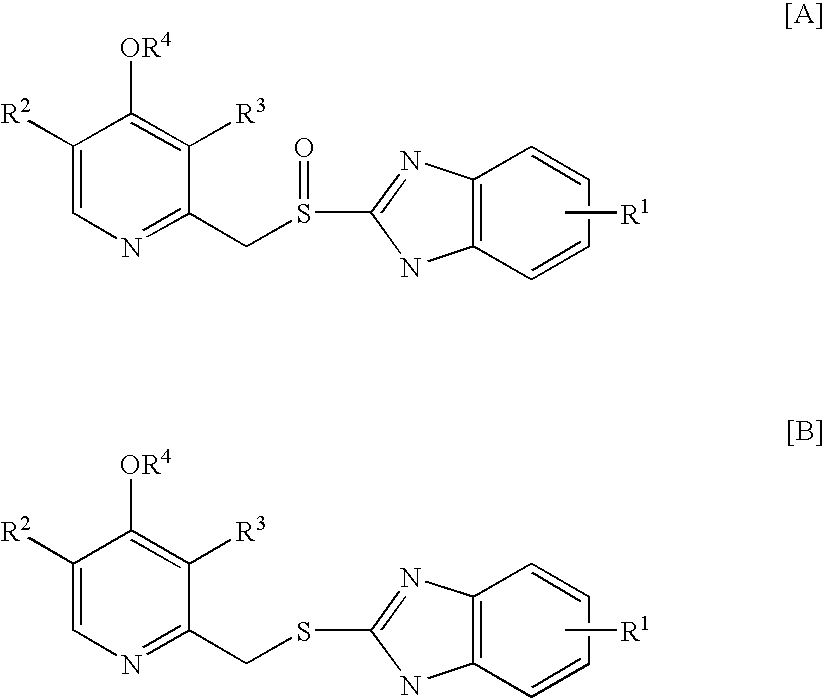
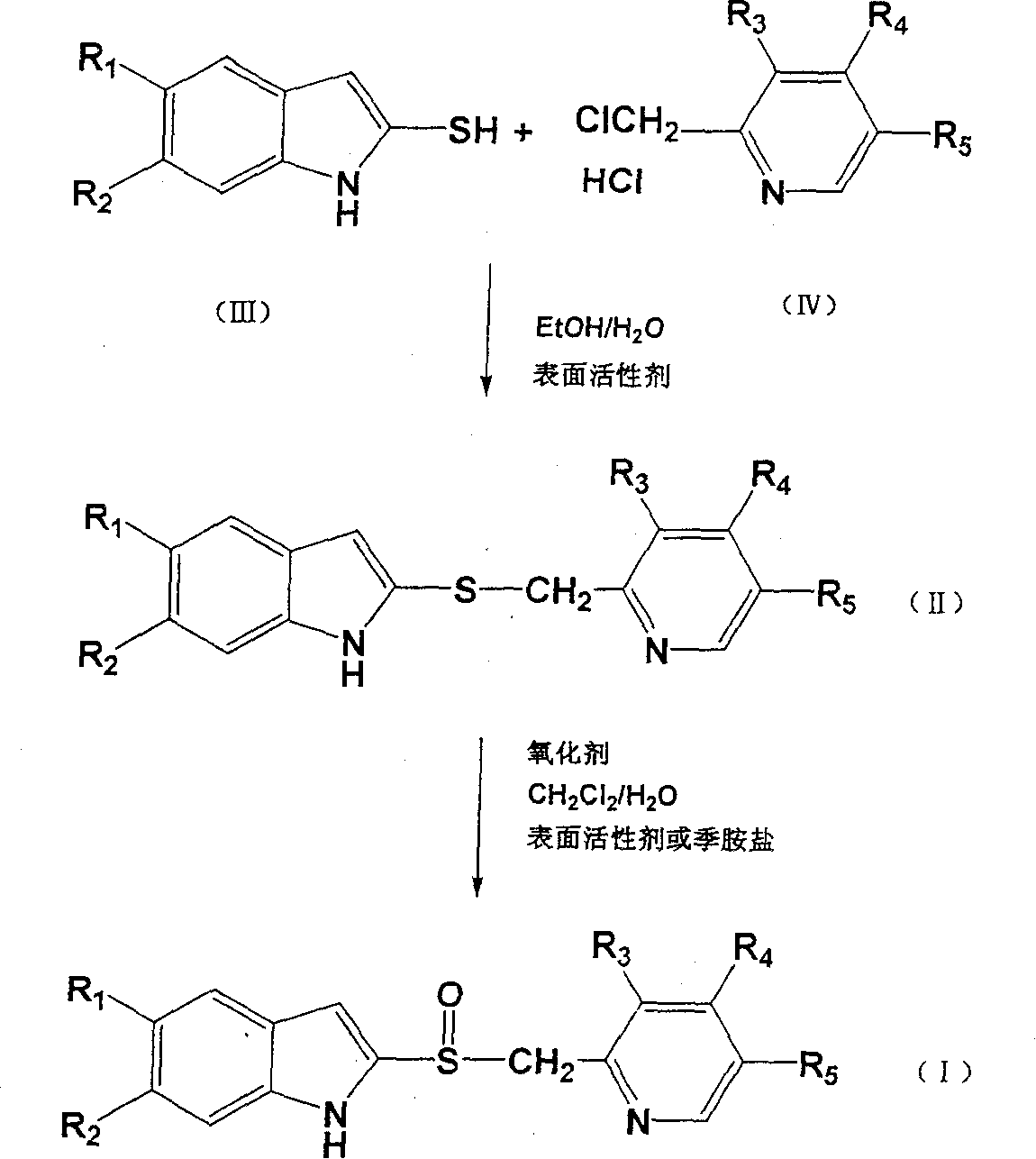
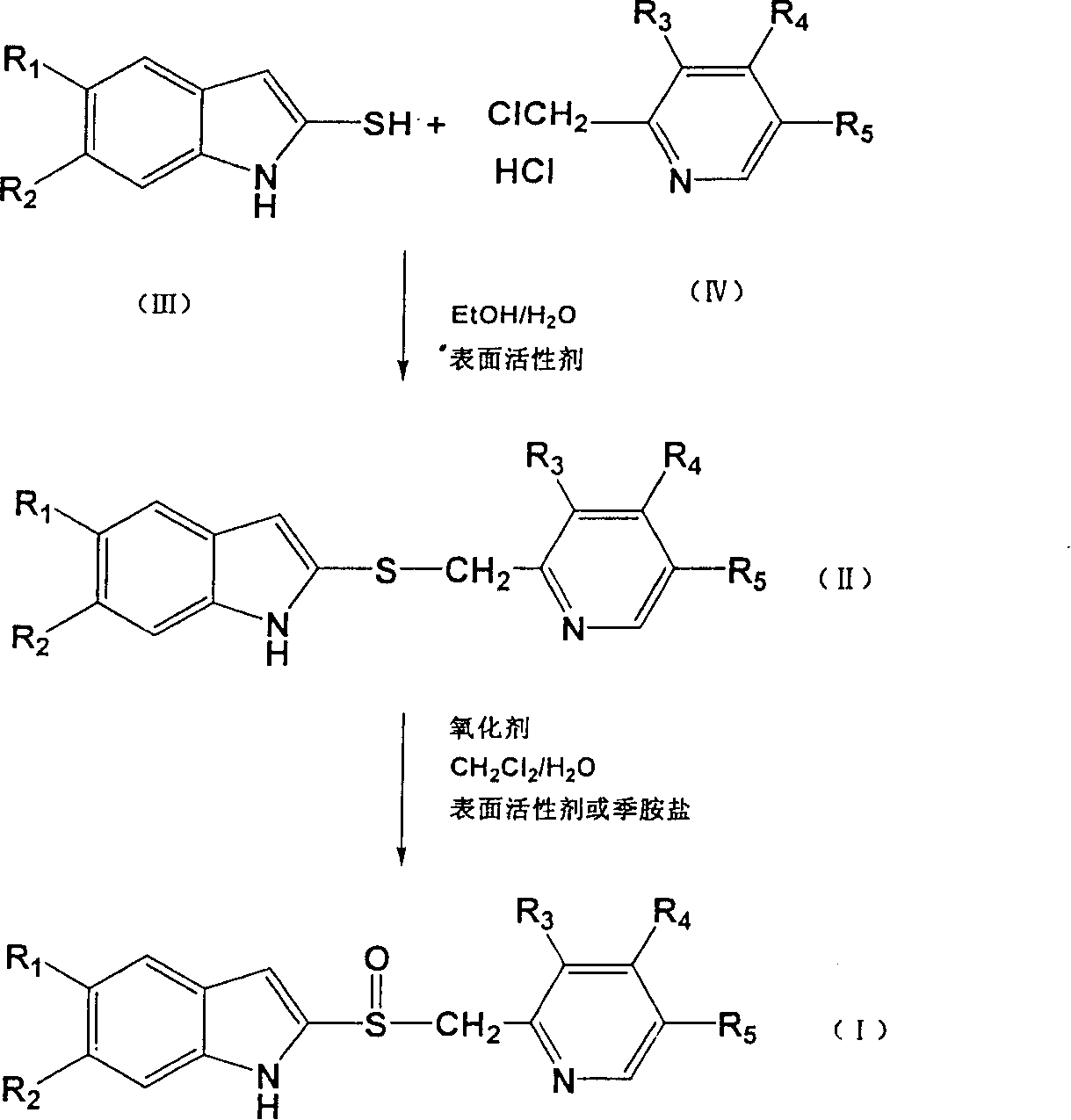
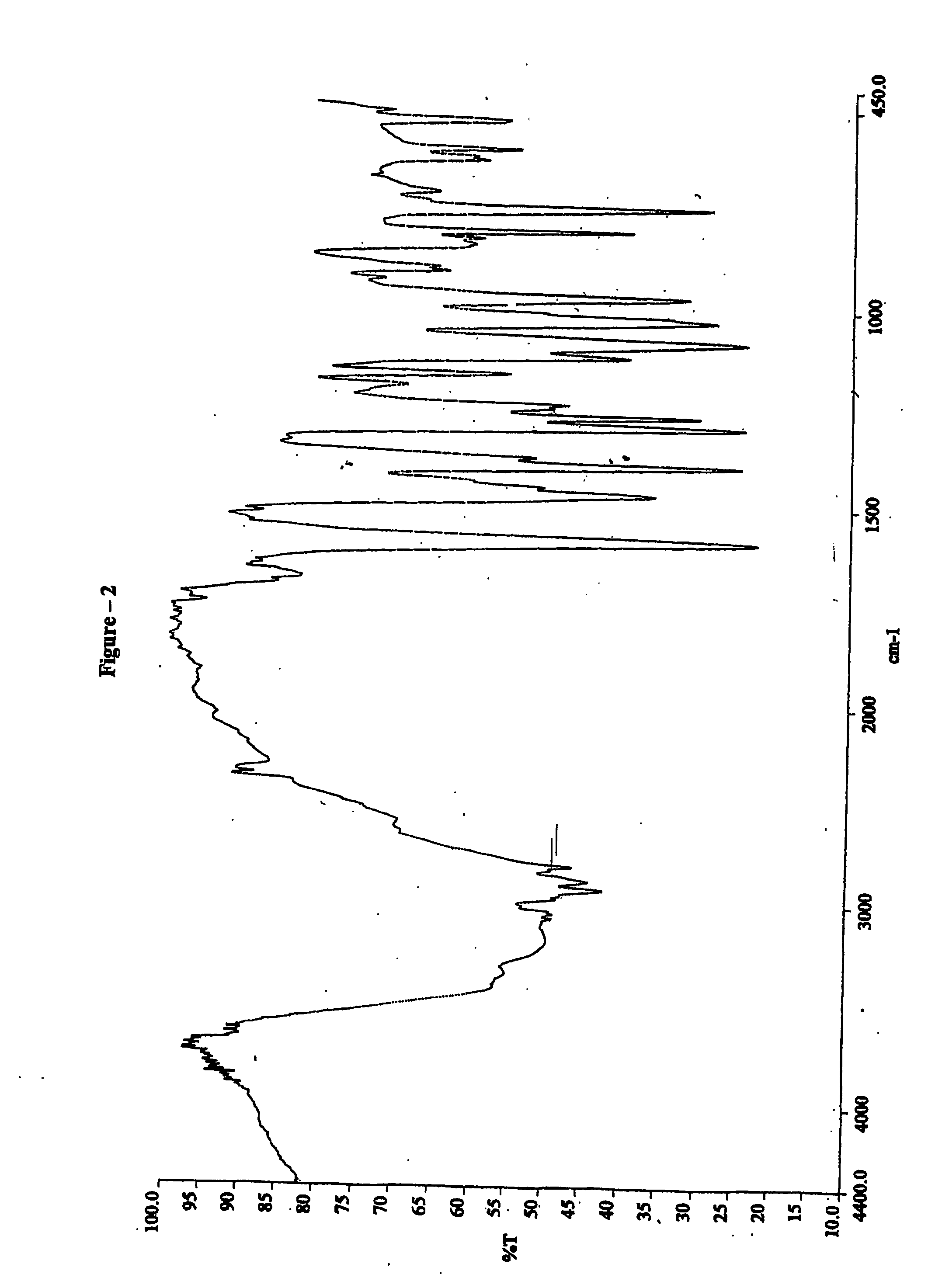
Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole
The present invention provides a process comprising admixing a thioether with about 1.05 to about 1.6 molar equivalents of an active chlorine-containing oxidant, preferably sodium hypochlorite, and about 2.5 to about 5.0 molar equivalents of an alkali metal base; and recovering a sulfoxide that is preferably pantoprazole, lansoprazole, omeprazole, or rabeprazole. The process may further comprise contacting the sulfoxide with a source of sodium ions, preferably sodium hydroxide, to produce the sodium salt of the sulfoxide. The invention also relates to novel chlorinated derivatives of pantoprazole including 5(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)-chloromethyl]sulfinyl]-1H- benzimidazole and 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)chlorohydroxymethyl] sulfinyl]-1H-benzimidazole and processes for making them. The invention also relates to processes of quantifying and identifying a compound other than pantoprazole in a mixture of pantoprazole and at least one other compound.
Owner:TEVA PHARMA IND LTD
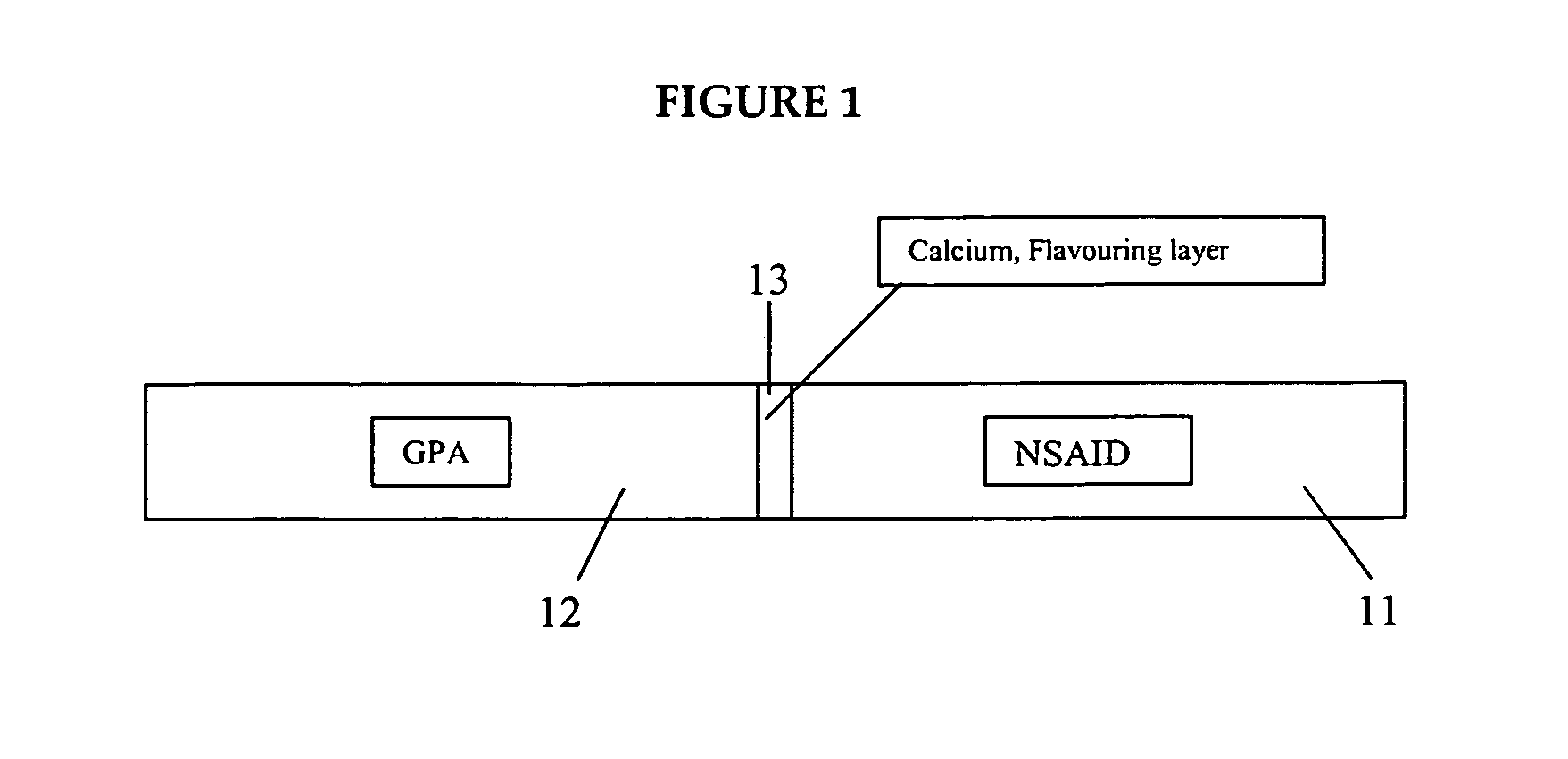
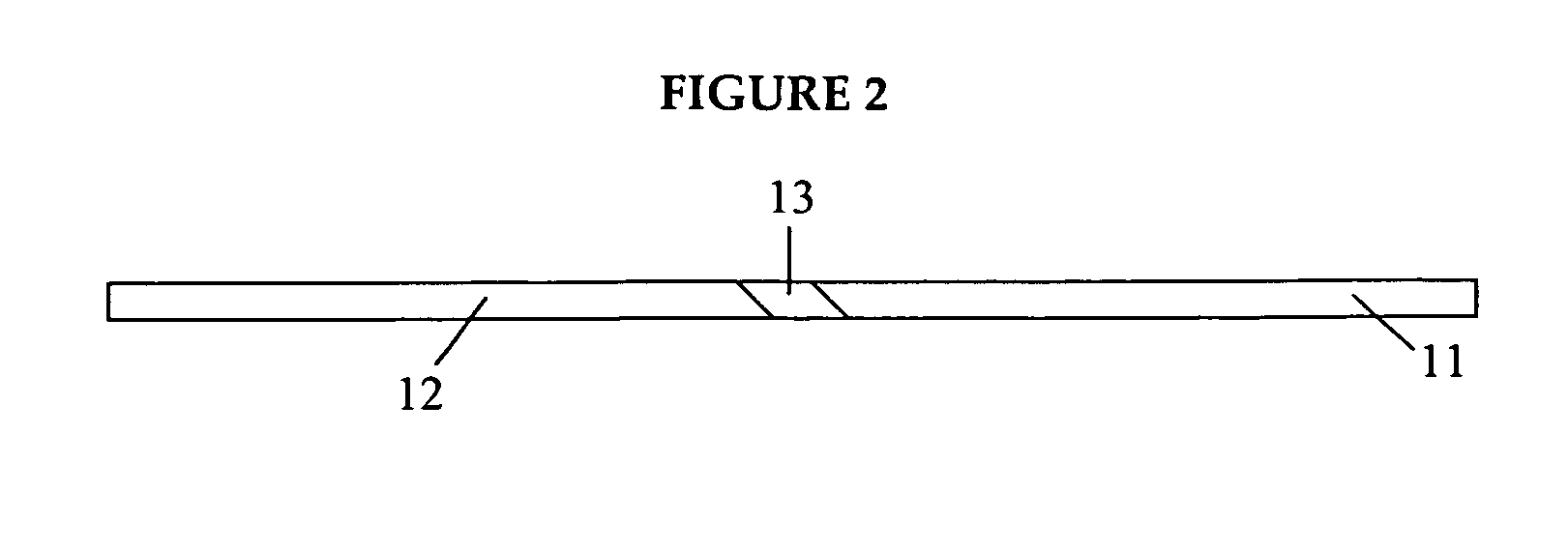
Medicated gumstick for treatment in anti-inflammatory conditions and prophylaxis against NSAID gastropathy
InactiveUS20070003490A1Promote absorptionMinimize contactAntipyreticAnalgesicsDiseaseMedicated chewing-gum
A stick of gum is provided containing therapeutic benefits of non-steroid anti-inflammatory drugs for inflammation in conditions such as arthritis, and also alleviates subsequent side effects of NSAID administration, as well as antacid effects from compounds such as an H2 antagonist (ranitidine, cimetidine, famotidine) and / or a proton pump inhibitor (such as lansoprazole, pantoprazole, omeprazole, esomeprazole or rabeprazole) and / or an acid pump antagonist selected from the group of soraprazan, AZD0865, YH1885 and CS-526.
Owner:MEDICAL FUTURES
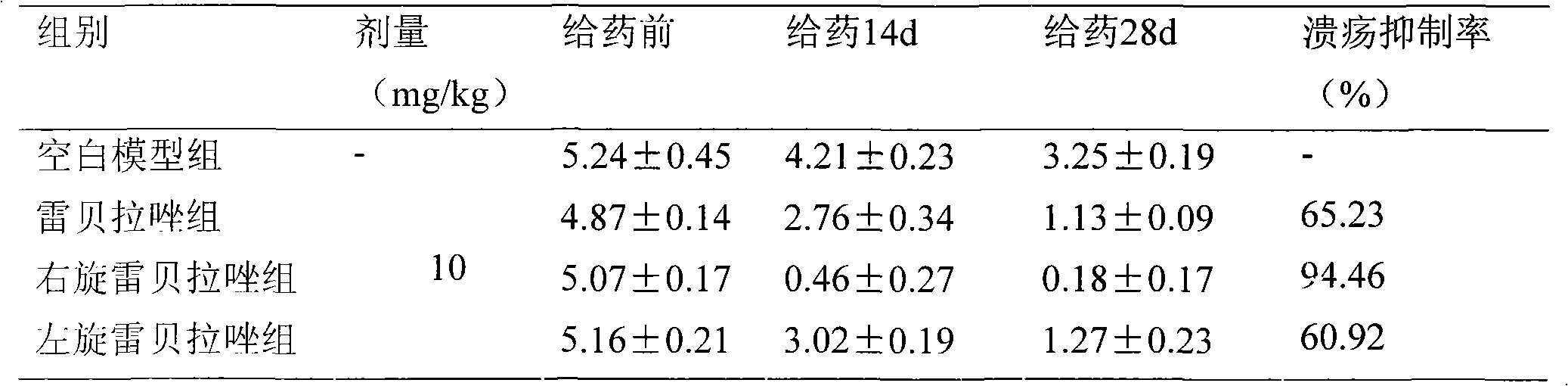
Freeze-dried powder injection containing dextrogyrate rabeprazole and salts thereof, preparing technology thereof
The invention relates to a freeze-dry powder injection containing dextral rabeprazole and salt of the dextral rabeprazole and a preparation technique thereof. The dextral rabeprazole and the salt thereof are taken as medical active components and mixed with a plurality of pharmaceutically acceptable supplements to form a medical composition. The preparation method is that the dextral rabeprazole and the salt thereof are taken as raw materials, a plurality of supplements with special type and proportion are added, and the freeze-dry powder injection preparation for intravenous injection are prepared and developed according to the technical method described by the invention. The invention can be applied in urgent treatments of tachycardia type arrhythmia (including the atrial fibrillation, atrial flutter and sinus tachycardia) happened during operation.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
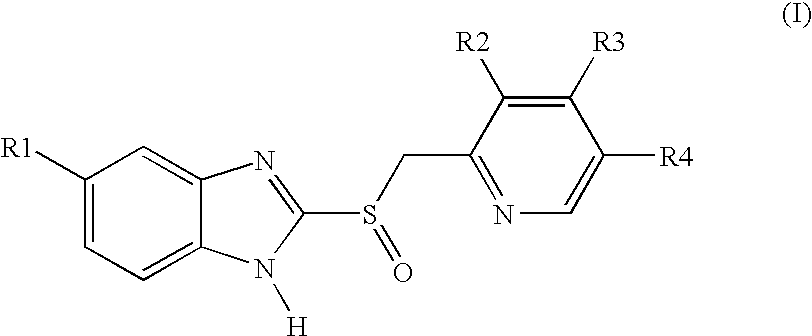
Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole
The present invention provides a process comprising admixing a thioether with about 1.05 to about 1.6 molar equivalents of an active chlorine-containing oxidant, preferably sodium hypochlorite, and about 2.5 to about 5.0 molar equivalents of an alkali metal base; and recovering a sulfoxide that is preferably pantoprazole, lansoprazole, omeprazole, or rabeprazole. The process may further comprise contacting the sulfoxide with a source of sodium ions, preferably sodium hydroxide, to produce the sodium salt of the sulfoxide. The invention also relates to novel chlorinated derivatives of pantoprazole including 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)-chloromethyl]sulfinyl]-1H-benzimidazole and 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)-chlorohydroxymethyl]sulfinyl]-1H-benzimidazole and processes for making them. The invention also relates to processes of quantifying and identifying a compound other than pantoprazole in a mixture of pantoprazole and at least one other compound.
Owner:TEVA PHARM USA INC
Preparation method of chiral sulfoxide medicament though catalysis of asymmetric oxidation of sulfides compound
ActiveCN104447692AEasy to synthesizeRaw materials are easy to getOrganic chemistryOrganic compound preparationManganeseSulfide compound
The invention provides a preparation method of a chiral sulfoxide medicament though catalysis of asymmetric oxidation of sulfides compounds. A chiral complex formed by quadridentate nitrogen organic ligand and metal manganese compound as a catalyst and hydrogen peroxide as an oxidant are used for asymmetric catalytic oxidation of prochiral thioether compound, so as to obtain the corresponding chiral sulfoxide medicament compounds including S-omeprazole, S-lansoprazole, S-pantoprazole, S-rabeprazole, R-Modafinil and R-sulindac. The reaction has the advantages of cleaness, mild reaction conditions, high conversion rate and antipodal selectivity, and shows industrial prospects.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
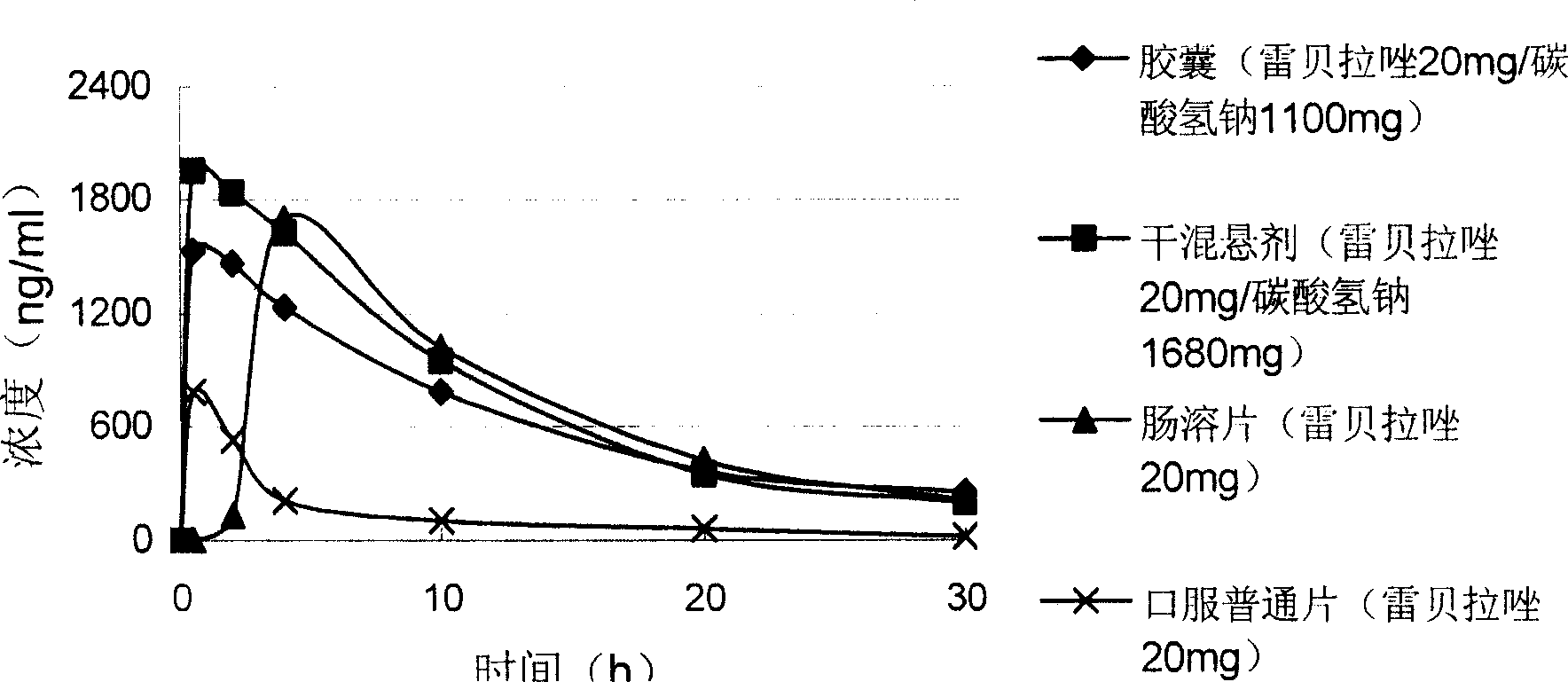
Preparations containing rebeprazole and sodium bicarbonate and method for preparing the same
ActiveCN101134036AAvoid deficienciesOrganic active ingredientsDigestive systemSodium bicarbonateRabeprazole
The present invention provides one kind of medicine composition comprising rabeprazole in 10-80 weight portions and sodium bicarbonate in 100-5000 weight portions. The medicine composition with both quickly released medicine and slowly released medicine is superior to enteric preparation, and has short peak reaching time and medicine acting time as long as 12-20 hr.
Owner:SHANGHAI SINE PHARMA LAB
Compositions and methods to treat gastrointestinal disorders
InactiveUS20050014797A1Reduce needReducing or eliminating a patient's need for dilationBiocideAnimal repellantsRabeprazoleGastrointestinal disorder
The invention provides safe and effective methods for treating and preventing dysphagia, lower esophageal mucosal rings, esophageal strictures, achalasia, gastric mucosal injuries, and bacterial infections. The methods comprise administering at least one proton pump inhibitor, optionally in combination with antibacterial compounds. In one embodiment, the proton pump inhibitor is rabeprazole, a pharmaceutically acceptable salt thereof and / or a stereoisomer thereof.
Owner:EISAI CO LTD
Medicine for treating gastroesophageal reflux disease and functional dyspepsia
InactiveCN101143143AImprove toleranceHigh synergistic effectDigestive systemSolution deliveryLansoprazoleRabeprazole
A combination preparation for remedying the gastroesophageal reflux disease (GERD) and the functional dyspepsia is characterized in that the prescription of the combination preparation consists of a proton pump depressor and a gastrointestinal power drug of itopride; the proton pump depressor is selected from one of a Pantoprazole, a Omeprazole, a Esomeprazole, a Lansoprazole, a Rabeprazole, a Tenatoprazole and a Leminorazole, wherein the Pantoprazole is preferential, and at the same time the neutral form of the basic salt of the proton pump depressor is also included, such as Naplus, Mg2plus, Ca2plus, Kplus or Li plus salt and a pure optical stereoisomer of the proton pump depressor or an active metabolite of the proton pump depressor; the gastrointestinal power drug is the itopride and a ramification of the itopride or one of the medicinal salts of the itopride; in the combination preparation, the weight ratio of the Pantoprazole and the itopride is 2 to 5 to 2 to 7. The invention has important affect for remedying the gastroesophageal reflux disease and the functional dyspepsia, and the preparation method of the invention is simple and convenient; the cost is low; the invention is fit for being orally taken by the patient; the invention has good conformance performance, high curative effect, low recrudescence rate and little adverse reaction.
Owner:沈阳东宇药业有限公司
Preparation methods of optically pure rabeprazole and sodium salt thereof
The invention discloses preparation methods of optically pure rabeprazole and a sodium salt thereof. The preparation method of optically pure rabeprazole specifically comprises the steps of dissolving an S-(-)-rabeprazole or R-(+)-rabeprazole crude product in an organic solvent, extracting by using ammonium hydroxide, removing an organic layer, adjusting a pH value of the ammonium hydroxide extract to be 8.5-10.5 by using acetic acid, extracting by using a ketone type solvent, washing the obtained organic layer by using a buffering solution with the pH value of 9-11, and cooling and crystallizing to obtain optically pure rabeprazole. According to the preparation method of optically pure rabeprazole, the chiral rabeprazole solids can be obtained at lower temperature without being subjected to high-temperature concentration; the obtained chiral rabeprazole has good crystal form, high chemical purity and high chiral purity.
Owner:燃点(南京)生物医药科技有限公司
Method for preparing sodium rabeprazole
The invention relates to a method for preparing sodium rabeprazole. The method is not subjected to concentration, extraction or lyophilization and comprises the steps of reacting rabeprazole with alkaline substances containing sodium ions at 50-55 DEG C and then adding an alkane solvent, thus dissolving out solid sodium rabeprazole.
Owner:JIANGSU HANSOH PHARMA CO LTD
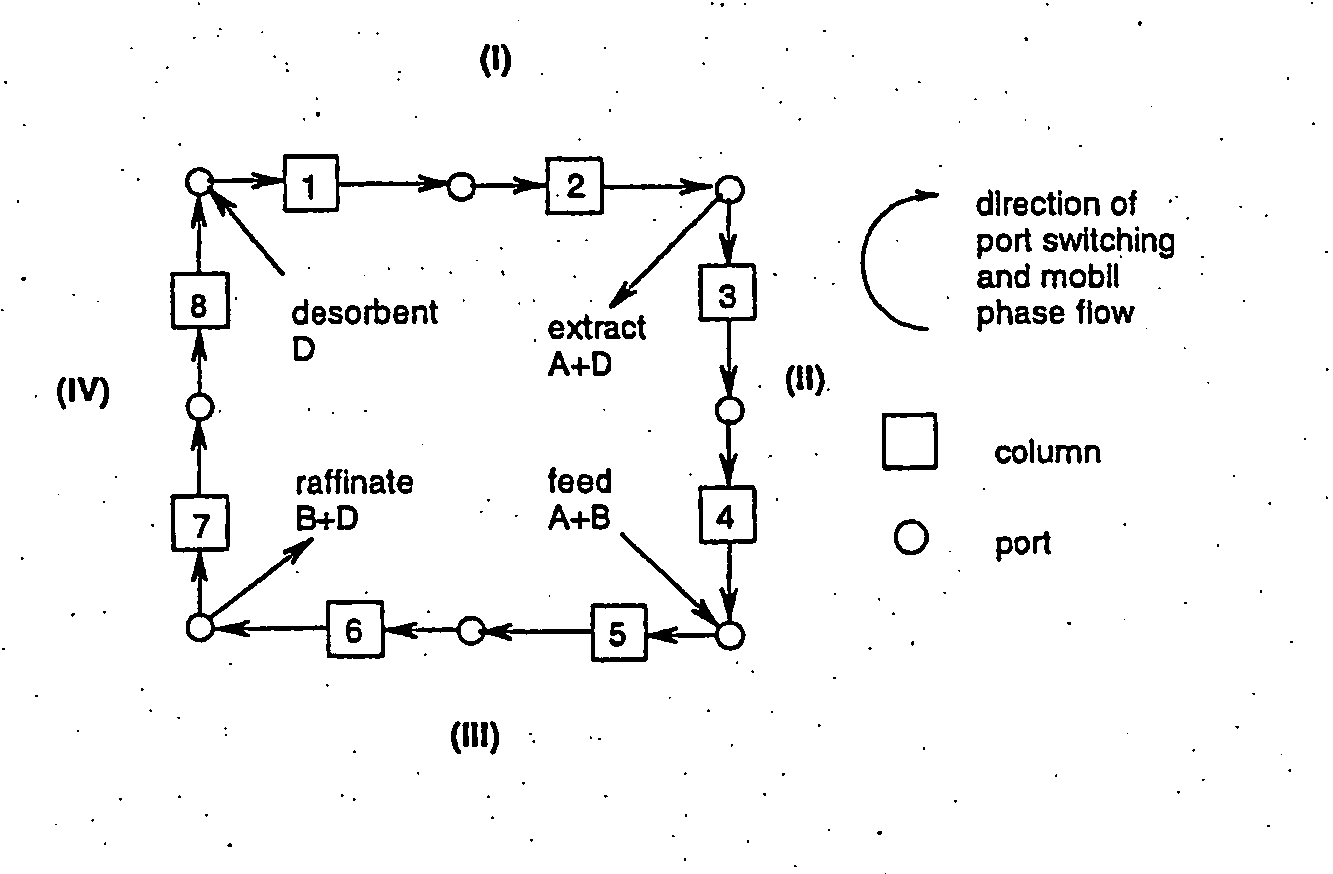
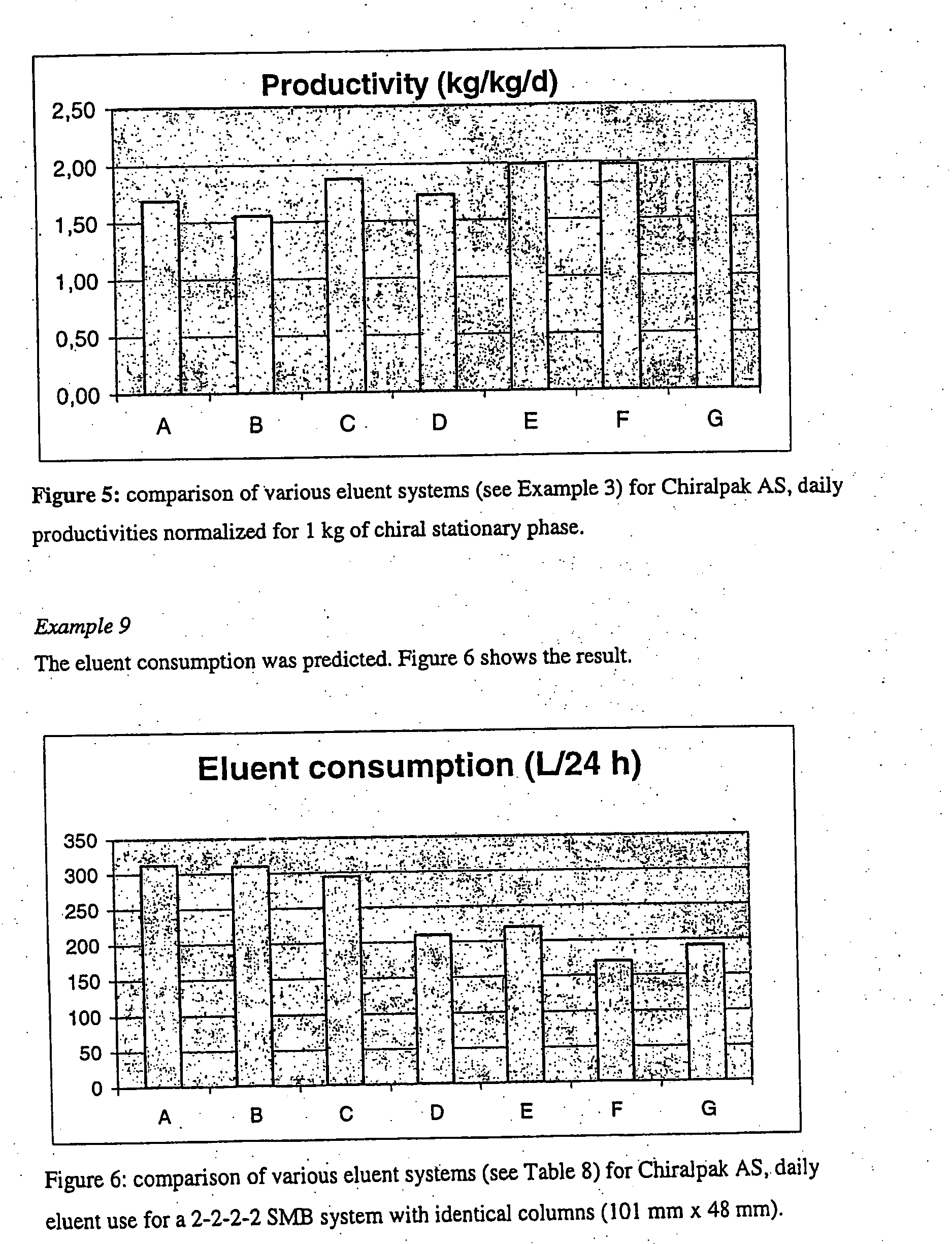
Process
The present invention relates to a process for the preparation of certain 2-pyridinyl)methyl]sulfinyl]-1H-benzimidazoles compounds. More specifically it relates to the preparation of an enantiomerically pure or optically enriched enantiomer of either omeprazole, pantoprazole, lansoprazole, or rabeprazole from a mixture containing the same using means for simulated moving bed chromatography.
Owner:ASTRAZENECA AB
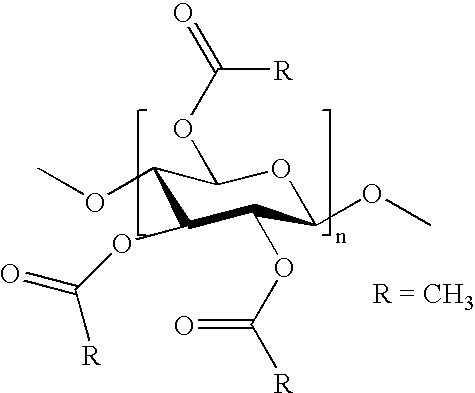
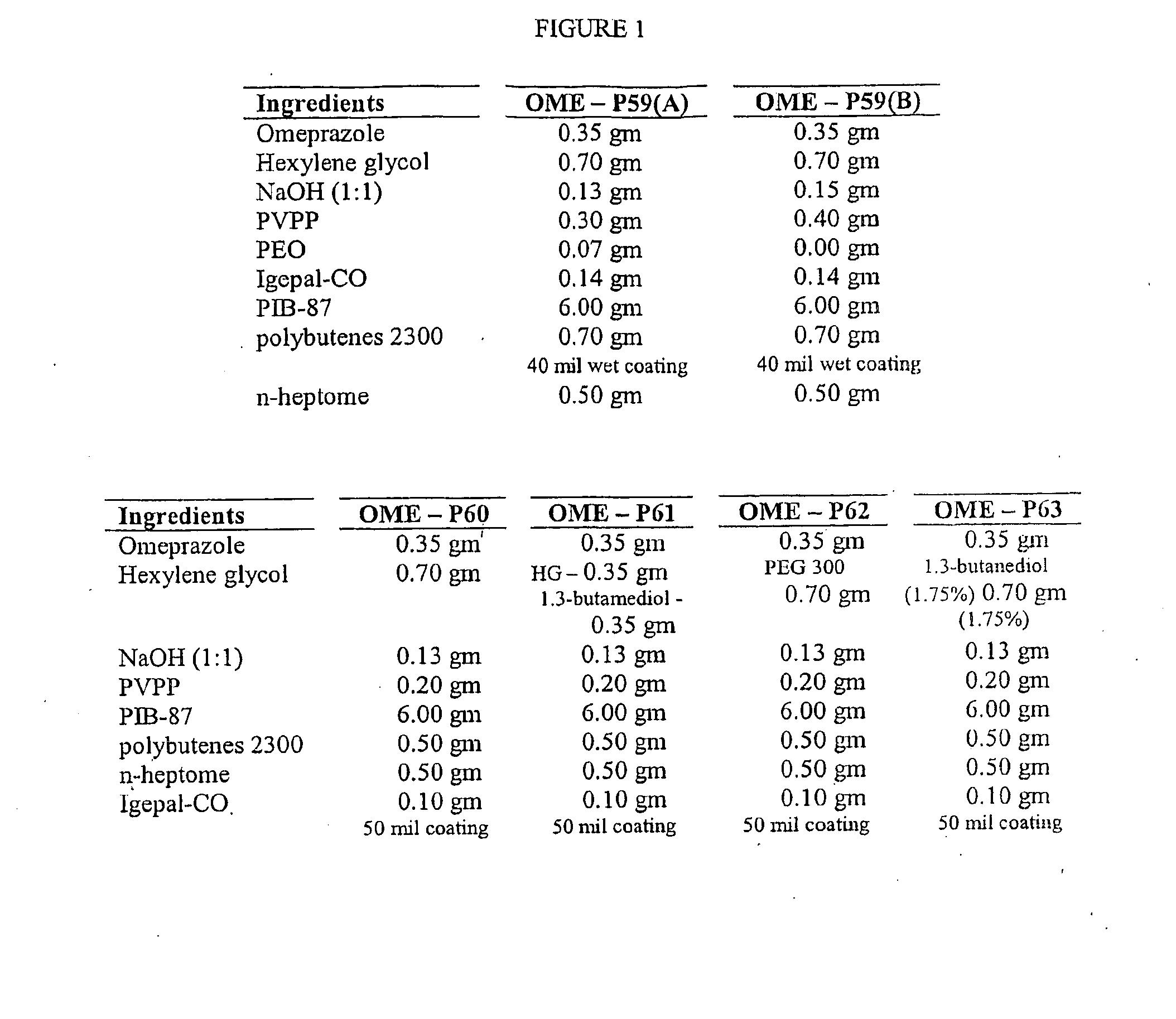
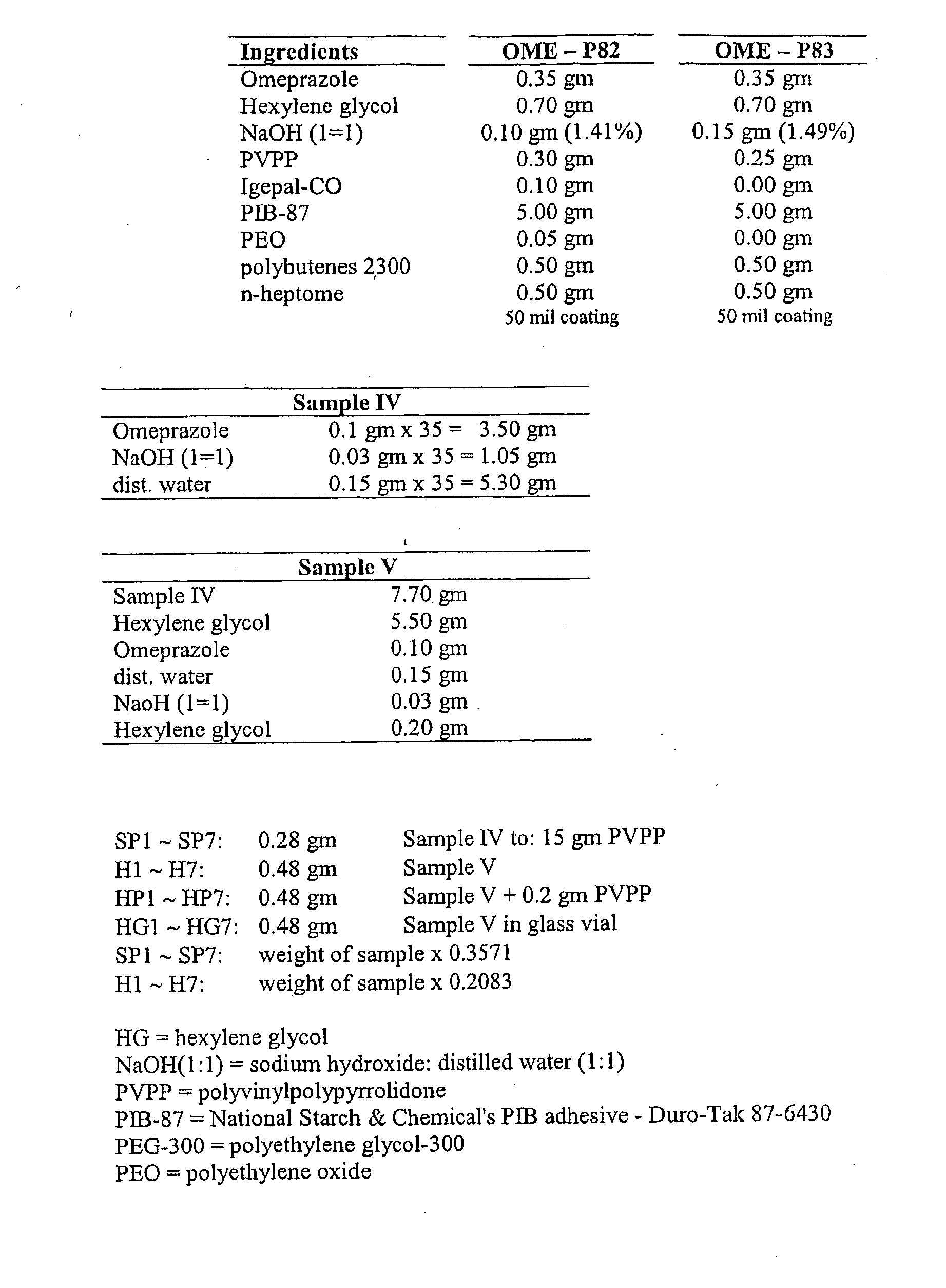
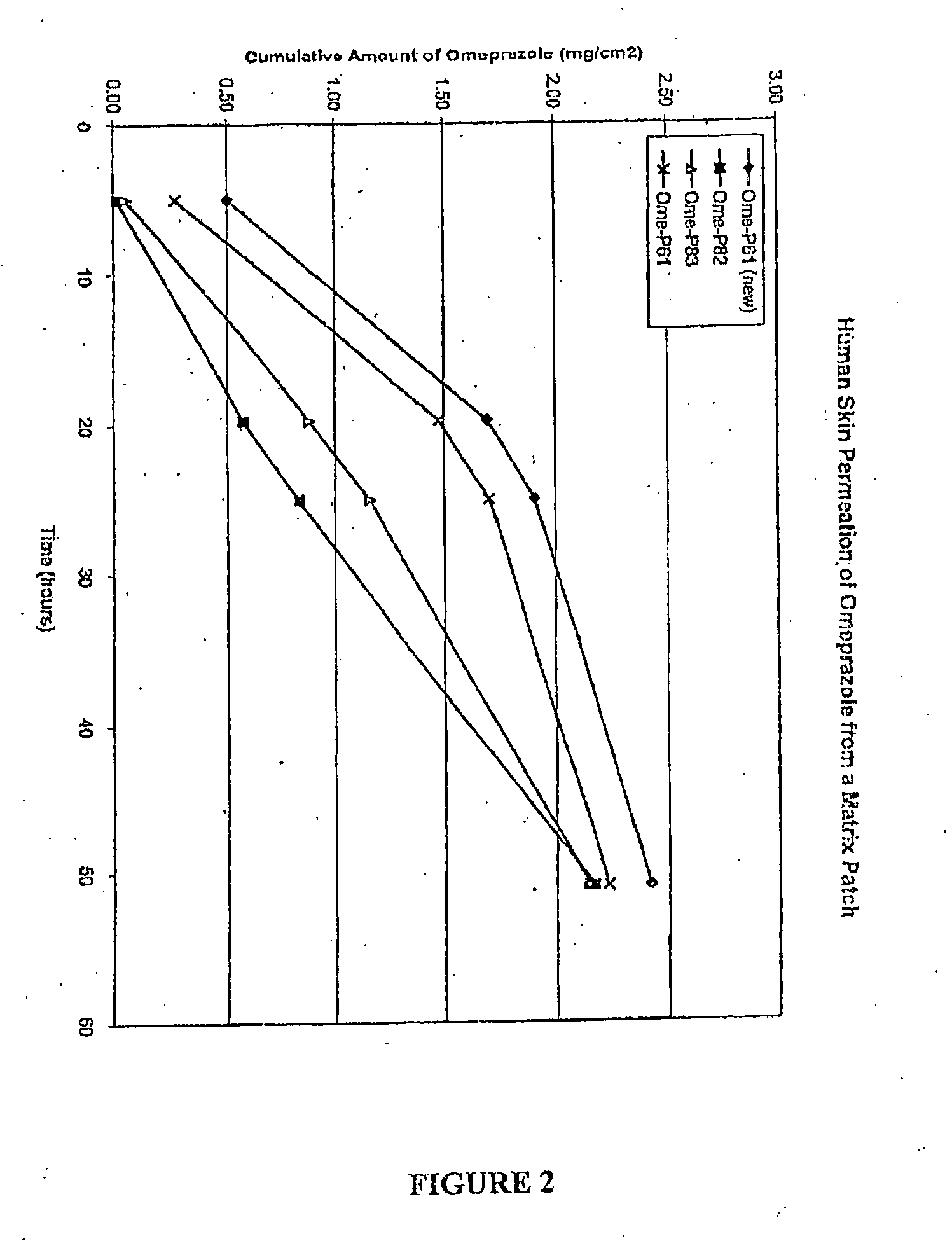
Transdermal Administration of Proton Pump Inhibitors
InactiveUS20080287502A1Increase ratingsImprove throughputBiocidePharmaceutical non-active ingredientsDepressantTransdermal
A method and composition for the transdermal administration of proton pump inhibitors such as substituted pyridyl methylsulfinyl benzimidazoles, and in particular, omeprazole, lansoprazole, esomeprazole, pantoprazole and raberprazole. The method and composition include the use of a hydroxide-releasing agent as a permeation enhancer to increase the flux of the protein pump inhibitor through a patient's skin or mucosal tissues and optionally also include the use of a carrier such as 1,3-butanediol, dipropylene glycol, and hexylene glycol.
Owner:DERMATRENDS INC
Method for preparing 2- (2-pyridinylmethylsulfinyl) benzimidazoles
The present invention provides a method for preparing an antiulcer agent, 2-(2-pyridinylmethylsulfinyl)benzimidazoles, such as Omeprazole, Lansoprazole, Pantoprazole and Rabeprazole, which includes oxidizing an intermediate having a linkage of methylthio group (—CH2S—) to methylsulfinyl (—CH2S(O)—) in the presence of an oxidation catalyst of an alkali metal salt of tungstate at a temperature of 10-50° C.
Owner:SYN TECH CHEM & PHARM
Medical composition containing dextro-Rabeprazole and preparation method thereof
The invention relates to a medical composition containing dextro-Rabeprazole and a preparation method thereof. The medical composition is prepared by taking dextro-Rabeprazole as the main active constituent and mixing dextro-Rabeprazole with pharmaceutically acceptable auxiliary materials. The composition can be prepared into various oral preparations according to the requirements, such as tablets, capsules, granules, dispersible tablets, chewable tablets, buccal tablets, effervescent tablets, effervescent granules and any other solid formulation, wherein, the unit preparation content of dextro-Rabeprazole is 2-50mg, preferably 10-40mg. The composition of the invention can be used for treating various digestive tract diseases, in particular to peptic ulcer.
Owner:北京利乐生制药科技有限公司
Improved preparation and separated purification method of benzimidazole type proton pump inhibitors and precursor thereof
ActiveCN100393712CSimple post-processingGood reproducibilityOrganic chemistryLansoprazolePurification methods
The provided preparation, separation and purification method for benzimidazole-type proton pump inhibitor and its precursor, such as omeprazole, rabeprazole, lansoprazole, and ilaprazole comprises: for the first time, adding surfactant or phase-transfer catalyst in the synthesis process to speed up and promote the reaction more complete. This invention has well reaction efficiency and selectivity to overcome the defects in prior art and benefit to post-treatment and crystallization.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI +1
An enteric-coated tablet containing D-rabeprazole or a pharmaceutically acceptable salt thereof, and a preparation method therefor
InactiveCN103202818AUniform alkaline environmentReduce dosageOrganic active ingredientsDigestive systemRabeprazoleEnteric coated tablets
The present invention relates to an enteric-coated tablet containing D-rabeprazole or a pharmaceutically acceptable salt thereof, and a preparation method therefor. The tablet comprises a tablet core and a coating layer. The tablet core is prepared by using the D-rabeprazole or the pharmaceutically acceptable salt thereof, and pharmaceutically acceptable excipients through a wet granulation process, wherein the binder used in the wet granulation process has a pH of 9-14. The tablet of the invention has good stability, and the preparation method is simple and easy to operate, thus being suitable for industrial production.
Owner:SHANDONG NEWTIME PHARMA
Long-circulating rabeprazole liposome composition and preparation method and application thereof
InactiveCN104546718AGood resolubilityHigh encapsulation efficiencyOrganic active ingredientsPowder deliveryCholesterolMetal chelate
The invention relates to the field of medicinal preparations, specifically to a long-circulating rabeprazole liposome composition and its preparation method and application. The composition comprises rabeprazole, lecithin, cholesterol, Phosphatidylethanolamine Pegol, an antioxidant, metal chelate and a freeze-drying protective additive. The liposome injection provided by the invention has good re-dissolubility. After redissolution, particle size is uniform and stability is excellent. Quality of a preparation product is enhanced, half-life period of rabeprazole in the blood circulation system is prolonged, and bioavailability is higher.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
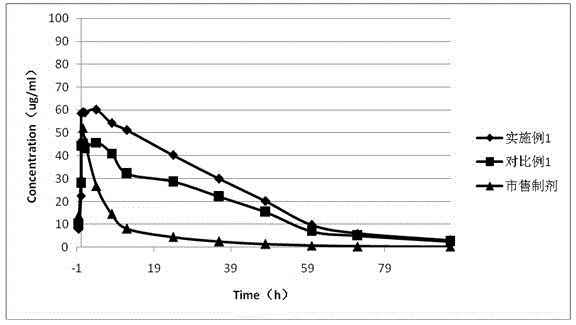
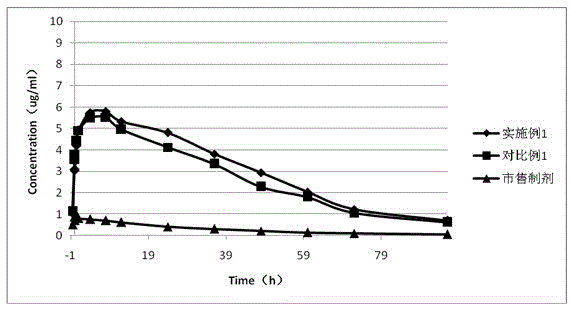
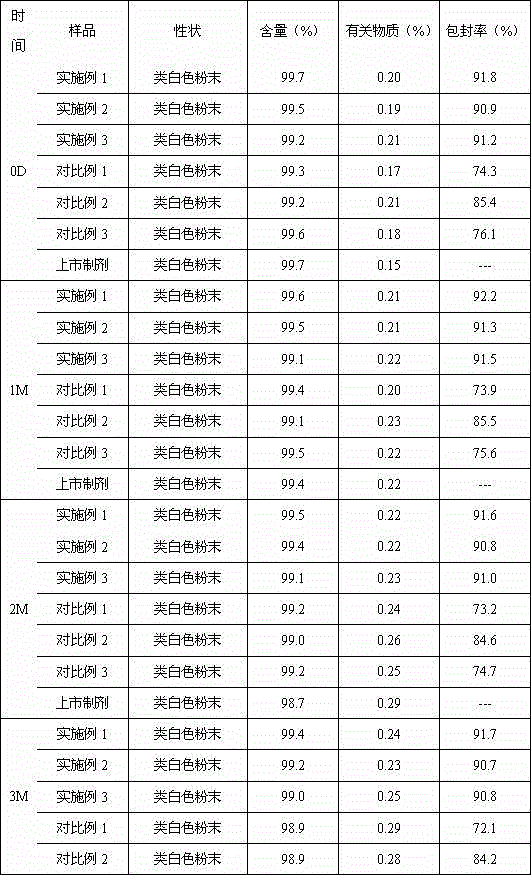
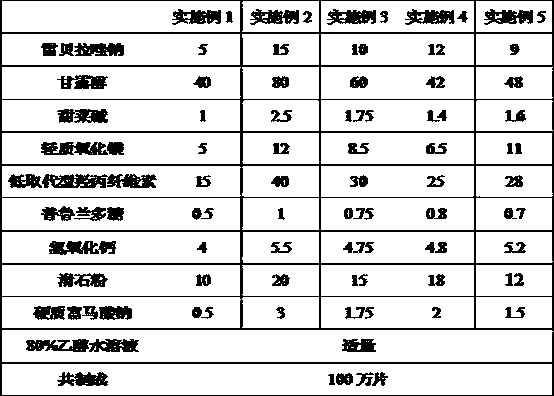
Rebeprazole sodium plain tablets, rebeprazole sodium enteric tablets and preparation method of rebeprazole sodium enteric tablets
ActiveCN110507628AHigh hardnessImprove stabilityOrganic active ingredientsDigestive systemCellulosePullulan
The invention discloses rebeprazole sodium plain tablets. The rebeprazole sodium plain tablets comprise the following components in parts by weight of 5-15 parts of rebeprazole sodium, 40-80 parts ofmannitol, 1-2.5 parts of betaine, 5-12 parts of light weight magnesium oxide, 15-40 parts of low substituted hydroxypropy cellulose, 0.5-1 part of pullulan polysaccharide, 4-5.5 parts of calcium hydroxide, 10-20 parts of talcum powder and 0.5-3 parts of sodium stearyl fumarate. The invention provides rebeprazole sodium enteric tablets containing the rebeprazole sodium plain tablets and a preparation method of the rebeprazole sodium enteric tablets. The preparation method comprises the following steps of S1, preparing the rebeprazole sodium plain tablets; S2, performing protective layer coating; S3, performing isolating layer coating; and S4, performing enteric layer coating. Rebeprazole sodium components and coating thereof are improved, dissolution and stability of products are promoted,and the technical problem that tablet core dissolution of the rebeprazole sodium coating tablets or enteric tablets is poor, so that the bioavailability of the rebeprazole sodium is low can be solved.
Owner:双鹤药业(海南)有限责任公司
Composition of oryzanol and proton pump inhibitor
InactiveCN103041392AGreat potential for synergiesGood curative effectOrganic active ingredientsDigestive systemLansoprazoleRabeprazole
The invention provides a composition of oryzanol and a proton pump inhibitor. The composition includes a clinical effective dose of proton pump inhibitor and an effective dose of oryzanol which are existent in different drug release units respectively; in a unit preparation, the clinical effective dose of proton pump inhibitor is 10-100 mg and the effective dose of oryzanol is 100-500 mg; the proton pump inhibitor in the composition is selected from omeprazole, lansoprazole, pantoprazole, rabeprazole, esomeprazole or pharmacologically suitable racemes, alkaline salt forms or single corresponding isomer forms thereof; and the drug release unit where the proton pump inhibitor is also includes an alkalizer. The composition can be used for treating peptic ulcer.
Owner:BEIJING KEYUAN CHUANGXIN TECH
Rabeprazole calcium
InactiveUS20060122233A1Treating and preventingBiocideOrganic chemistryRabeprazoleGastrointestinal ulcers
The present invention relates to calcium salts of rabeprazole and processes for preparing rabeprazole calcium. The invention also relates to pharmaceutical compositions that include the rabeprazole calcium and use of said compositions for the treatment or prevention of gastrointestinal ulcers
Owner:TOKYO OHKA KOGYO CO LTD +1
Purifying method for rabeprazole
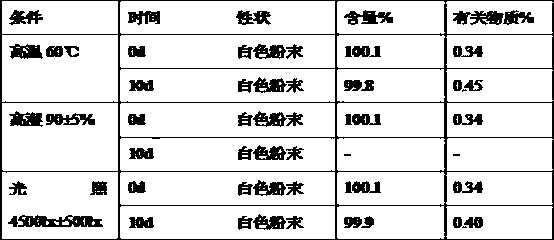
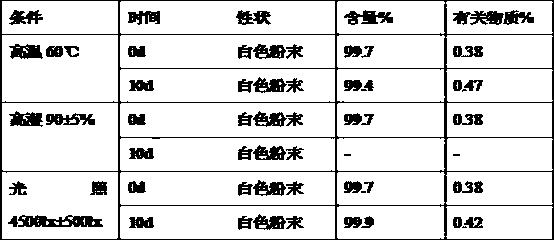
The invention relates to a purifying method for rabeprazole, which includes the steps of: 1) dissolving a rabeprazole crude product, which contains more than 0.1% of a rabeprazole peroxide impurity, in a solvent, adding an adsorbent, stirring the solution for 2-3 h, and filtering the solution to remove the adsorbent; 2) concentrating the filtrate to dry, adding a crystallized solvent to the concentrate, heating the liquid to 35-40 DEG C, stirring the liquid for clarification, reducing temperature to 0-10 DEG C, stirring the solution for 2 h, and performing suction filtration to prepare purified rabeprazole, which is more than 99.9% in purity and is less than 0.1% in content of the peroxide impurity. The purifying method is simple, wherein the solvent can be recovered and is low in consumption, so that the method is low in environment pollution and is high in product purity. The method has important practical application significance in industrial production.
Owner:LIVZON GROUP CHANGZHOU KONY PHARMA
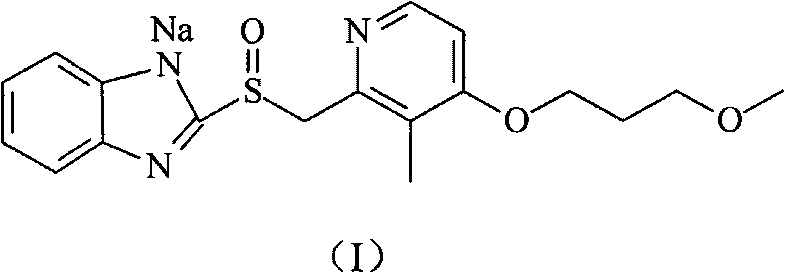
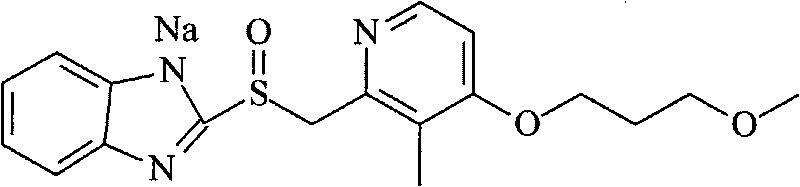
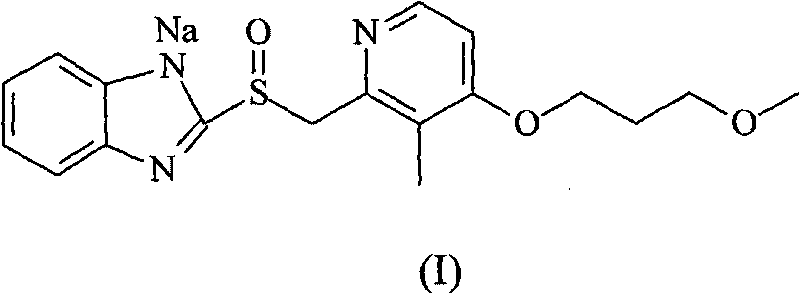
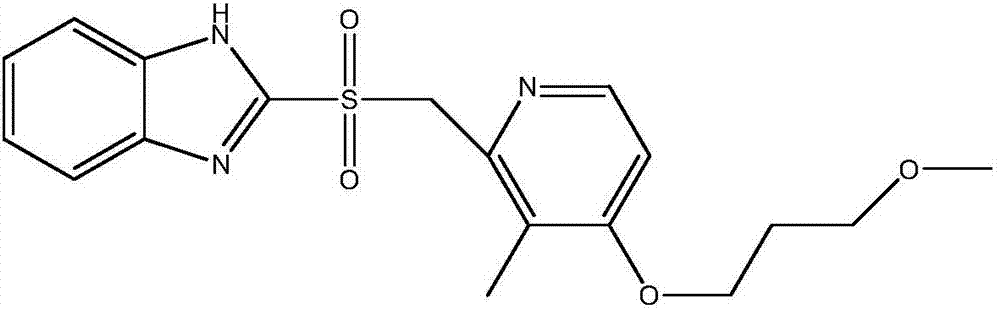
Preparation method of rabeprazole chloride and intermediate thereof
According to an existing method, acetic anhydride is adopted for a reaction when a formula III is synthesized from a formula IV, the reaction temperature is high, the reaction time is long, a large number of isomer byproducts can be generated in the reaction process, and the product purity is low. When the compound shown in the formula I is synthesized from free alkali of a compound shown in a formula II, halogenated alkane is used as a solvent and thionyl chloride is used as a chlorination reagent for reaction, alkali quenching, water washing extraction and salt formation after concentrationare needed for aftertreatment, the operation is complex, and the production period is long. According to the invention, p-toluenesulfonic acid is added for catalysis when the compound shown as the formula III is prepared, so that the reaction can be carried out at a relatively low temperature, the side reaction is greatly reduced, and the purity of the product is improved. When the compound shownin the formula I is prepared, ethyl acetate is used as a solvent, a product is gradually separated out along with the reaction, the product can be obtained through direct filtration after the reactionis completed, aftertreatment is simple, the product purity is high, and the method is suitable for industrial production.
Owner:珠海润都制药股份有限公司 +1
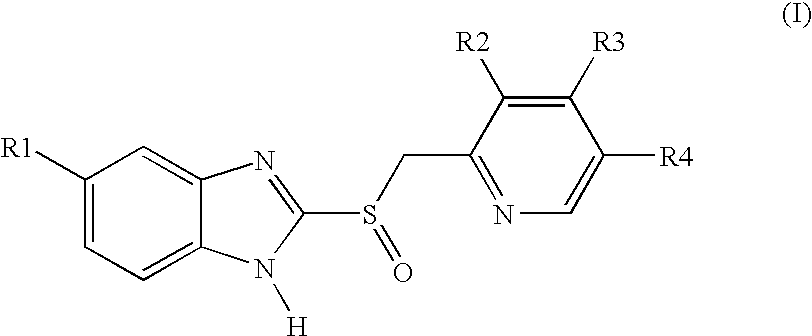
Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole
The present invention provides a process comprising admixing a thioether with about 1.05 to about 1.6 molar equivalents of an active chlorine-containing oxidant, preferably sodium hypochlorite, and about 2.5 to about 5.0 molar equivalents of an alkali metal base; and recovering a sulfoxide that is preferably pantoprazole, lansoprazole, omeprazole, or rabeprazole. The process may further comprise contacting the sulfoxide with a source of sodium ions, preferably sodium hydroxide, to produce the sodium salt of the sulfoxide. The invention also relates to novel chlorinated derivatives of pantoprazole including 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)-chloromethyl]sulfinyl]-1H-benzimidazole and 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)-chlorohydroxymethyl]sulfinyl]-1H-benzimidazole and processes for making them. The invention also relates to processes of quantifying and identifying a compound other than pantoprazole in a mixture of pantoprazole and at least one other compound.
Owner:BRAUDE VIVIANA +6
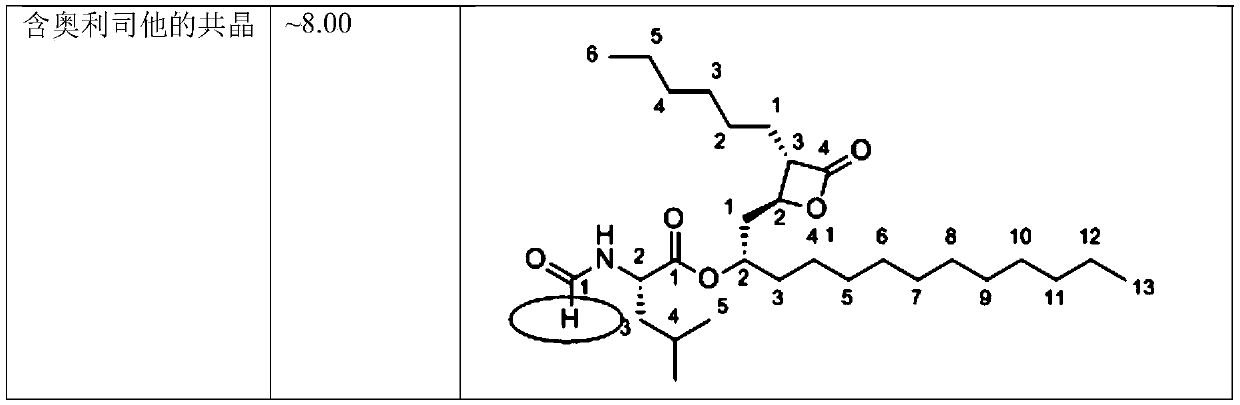
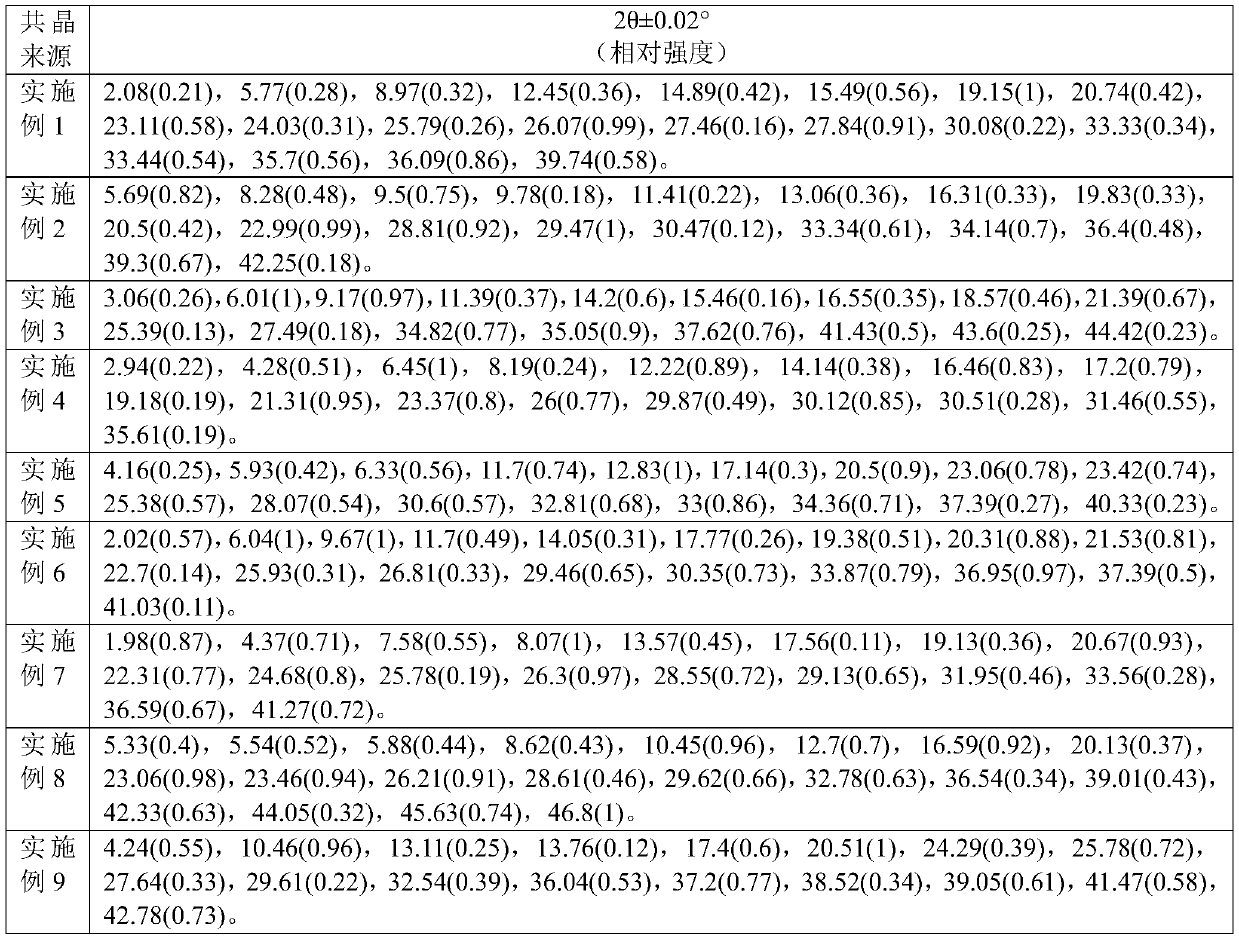
Eutectic crystal formed by orlistat and proton pump inhibitor, composition thereof, and application of eutectic crystal or composition
The invention provides a eutectic crystal formed by orlistat and a proton pump inhibitor. The proton pump inhibitor is selected from one of omeprazole, esomeprazole, pantoprazole, rabeprazole and lansoprazole, or a pharmaceutically acceptable salt thereof. The eutectic crystal can simultaneously generate synergistic antibacterial and antitumor effects.
Owner:ZHONGSHAN WANHAN PHARM CO LTD
Synthesizing method of 2-pyridine methyl sulfide and synthesizing process of related drugs
ActiveCN109134354AStrong position selectivityHigh yieldOrganic chemistryLithium bromidePyridine-N-oxide
The invention relates to a simple synthesizing method of 2-pyridine methyl sulfide and related drugs. The method is characterized in that 2-methyl pyridine n-oxide is used as the raw material and pyridine n-oxide or dichloromethane is used as the solvent to have reaction with trifluoroacetic anhydride to obtain a trifluoroacetate intermediate, purification is not needed, the trifluoroacetate intermediate is allowed to have reaction with thiophenol under the catalysis of lithium bromide or tetrabutyl ammonium bromide and by using toluene or ethyl acetate as the solvent to generate the 2-pyridine methyl sulfide. The method is simple to operate, cheap in reagents, easy in reagent obtaining, mild in reaction conditions, wide in substrate applicability, good in position selectivity, high in yield and the like. In addition, the method is successfully applied to the synthesizing of omeprazole sulfide and rabeprazole sulfide, and the synthesizing method does not need catalysts.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Pharmaceutical composition for treating helicobacter pylori infection and application of pharmaceutical composition
ActiveCN106729719AReduce usageReduced responseAntibacterial agentsDigestive systemBismuth / pectinColloidal bismuth subcitrate
The invention discloses a pharmaceutical composition for treating helicobacter pylori infection and application of the pharmaceutical composition. The pharmaceutical composition is prepared from, by weight, 7.5-40 parts of proton pump inhibitor, 150-1000 parts of antibiotic, 220-300 parts of bismuth agent and 60-140 parts of volatile extracts, wherein the proton pump inhibitor is selected from one of omeprazole, lansoprazole, esomeprazole, pantoprazole and rabeprazole; the antibiotic is selected from one of amoxicillin, clarithromycin, levofloxacin, metronidazole, furazolidone, tetracycline and minocycline; the bismuth agent is selected from one of bismuth potassium citrate, colloidal bismuth subcitrate and colloidal bismuth pectin; the volatile extracts are volatile components extracted from chenopodium ambrosioides and twigs and leaves of Pilular Adina. By means of the pharmaceutical composition, the eradication rate of helicobacter pylori can be improved, the effective rate is increased, and the drug resistance rate and the rate of adverse reactions are reduced.
Owner:DONGZHIMEN HOSPITAL OF BEIJING UNIV OF CHINESE MEDICINE
Pellet formulations of acid-labile benzimidazonle compounds
InactiveUS20070042043A1Short manufacturing timeEconomic savingsPowder deliveryBiocidePotassiumOleic Acid Triglyceride
They comprise insert granules of sugar / starch which are: initially coated with a non-alkaline active layer having the benzimidazole compound (omeprazole, lansoprazole, pantoprazole, rabeprazole, etc.), sodium and / potassium salts of acids of formula R—O—SO3H wherein R is an alkyl radical of a (C6-C20)-fatty acid (preferably sodium lauryl sulfate), (C6-C20)-fatty acids (preferably oleic acid), sodium and / or potassium salts of (C6-C20)-fatty acids (preferably potassium oleate), sodium carboxymethyl starch and polyvinylpyrrolidone; secondly coated with a non-alkaline barrier layer having hydroxypropylmethylcellulose; and finally coated with an enteric layer. The preferred molar ratio (sodium lauryl sulfate):(oleic acid+potassium oleate) is between 4:1 and 6:1. All coatings are done with aqueous solutions, suspensions or dispersions at a relatively high temperature, and all dryings are done at a relatively low temperature and for a relatively short time. They are stable over time and useful for oral administration.
Owner:LAB BELMAC
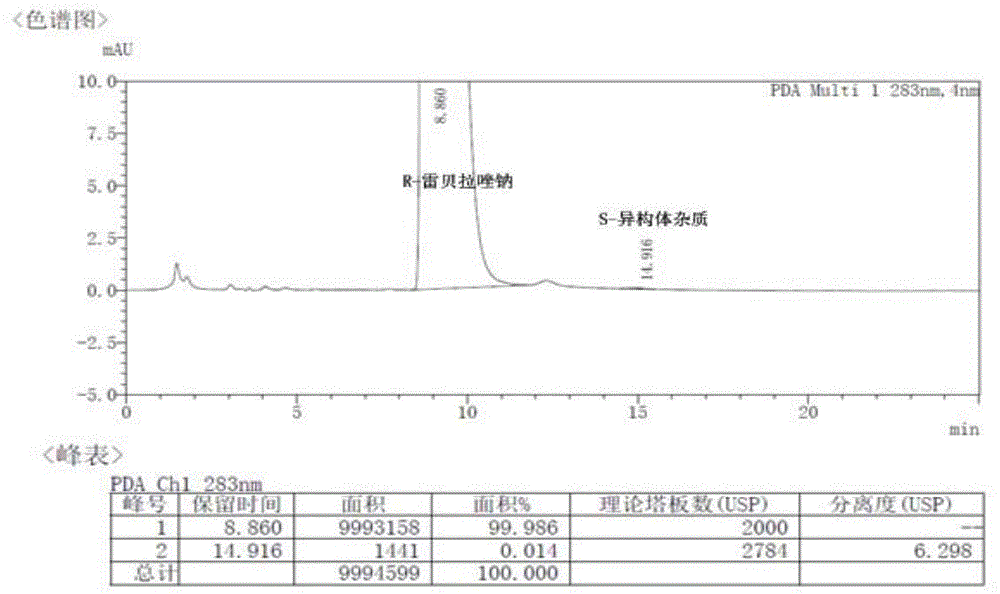
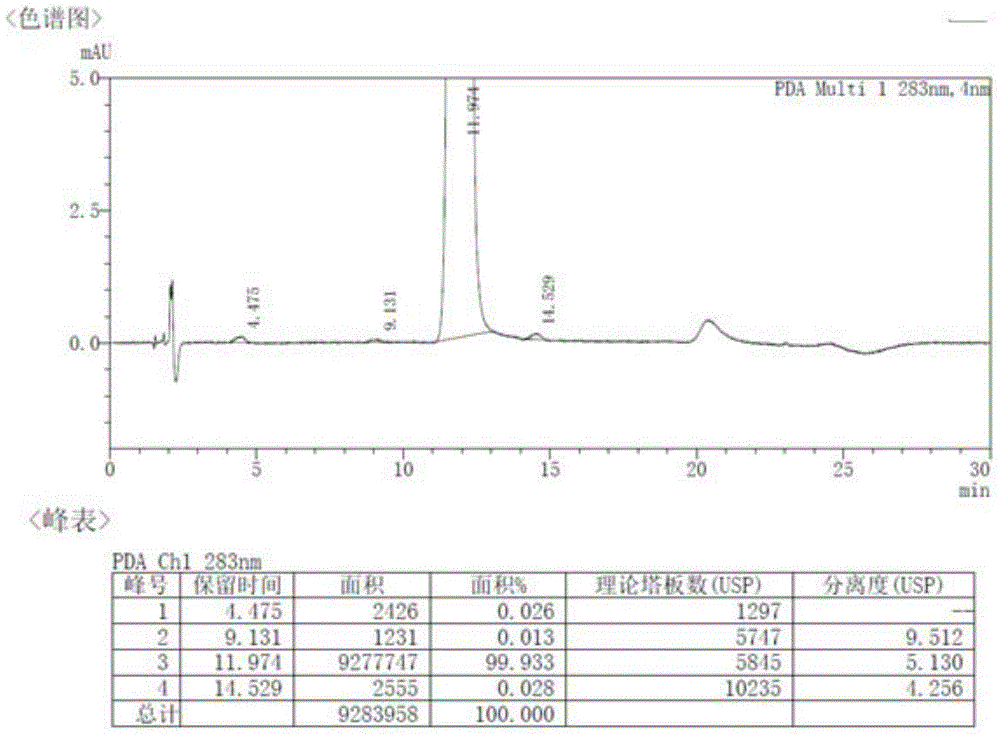
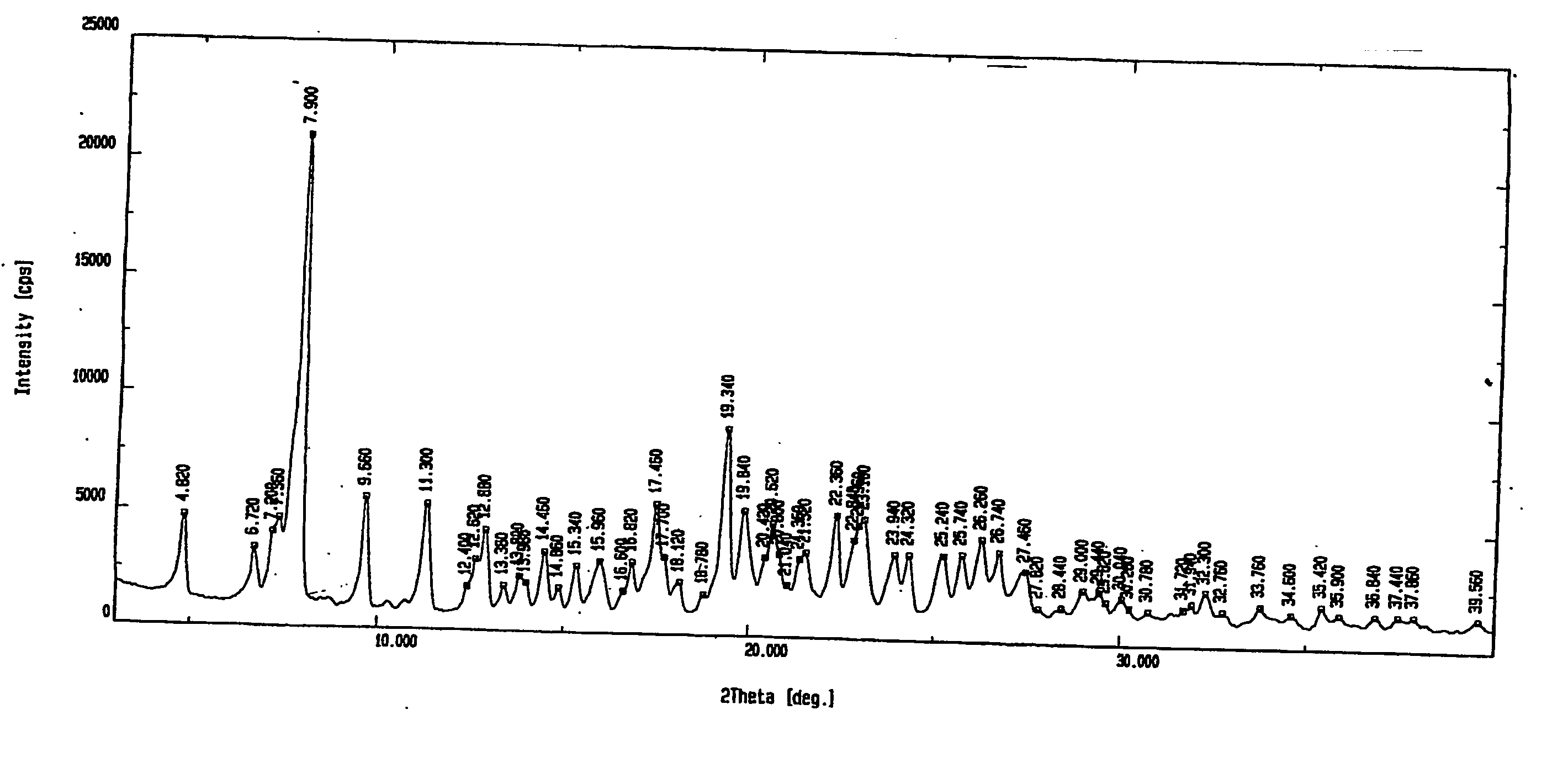
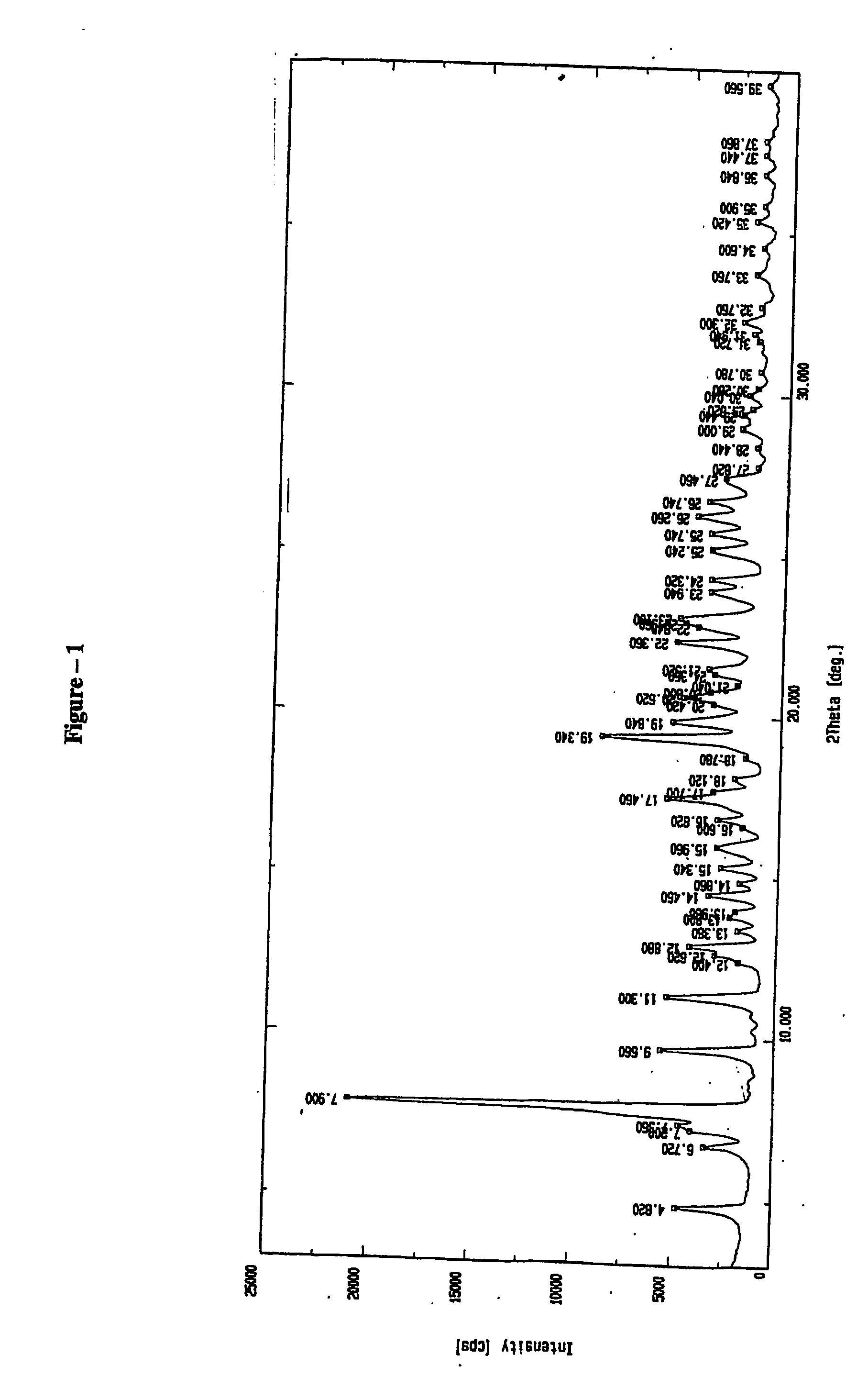
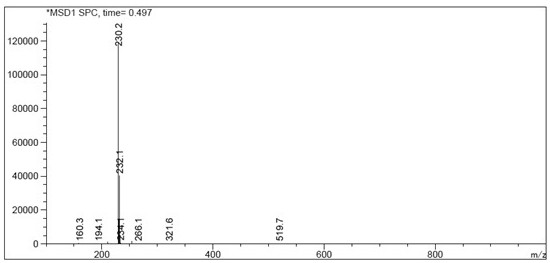
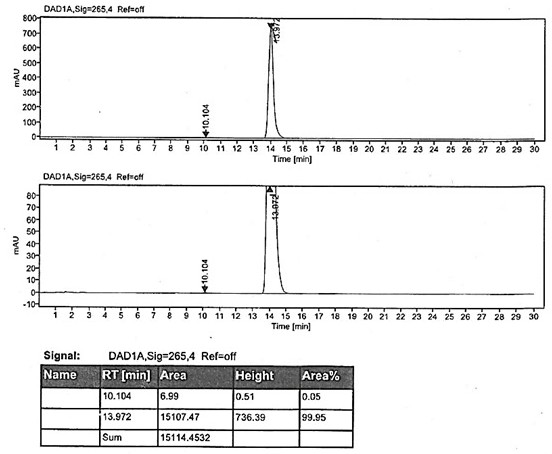
Method for preparing rabeprazole impurities through HPLC method
The invention discloses a method for preparing rabeprazole impurities through an HPLC method, and belongs to the fields of organic chemistry and medicine synthesis. The preparation method has the advantages of being simple, convenient to use, short in reaction time, high in target substance purity and helpful to the study of the rabeprazole impurities. The active pharmaceutical ingredient of rabeprazole is taken as the raw material, the HPLC method is adopted to prepare the target impurities, the chemical property of the impurities is analyzed, and bases are provided for the evaluation of thequality, safety and efficiency of the active pharmaceutical ingredient of the rabeprazole.
Owner:珠海润都制药股份有限公司
Process for the preparation of esomeprazole and salts thereof
InactiveUS7786309B2Promote effectiveImprove chemical yieldOrganic chemistryBulk chemical productionAlkaline earth metalEnantiomer
Owner:APOTEX PHARMACHEN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a8916e2e-db80-4459-98c4-e5f80d1ab692/A20048002223900401.PNG)
![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a8916e2e-db80-4459-98c4-e5f80d1ab692/A20048002223900411.PNG)
![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a8916e2e-db80-4459-98c4-e5f80d1ab692/A20048002223900421.PNG)
![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f276dec5-fdf8-4bc3-88d5-ce5cab9c91ec/US20050075370A1-20050407-D00001.png)
![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f276dec5-fdf8-4bc3-88d5-ce5cab9c91ec/US20050075370A1-20050407-D00002.png)
![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f276dec5-fdf8-4bc3-88d5-ce5cab9c91ec/US20050075370A1-20050407-D00003.png)










![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/acf1e396-3616-46aa-9520-b9311b6d3bf3/US20080004319A1-20080103-D00001.png)
![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/acf1e396-3616-46aa-9520-b9311b6d3bf3/US20080004319A1-20080103-D00002.png)
![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/acf1e396-3616-46aa-9520-b9311b6d3bf3/US20080004319A1-20080103-D00003.png)