Improved preparation and separated purification method of benzimidazole type proton pump inhibitors and precursor thereof
A technology of benzimidazole and compound, which is applied in the field of preparation of benzimidazole-type proton pump inhibitors and their precursors, can solve problems such as unstable yield and product purity, cumbersome post-treatment, difficult crystallization, etc., and achieve reaction efficiency and Good selectivity, simple and easy post-processing, and solve the cumbersome effect of post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
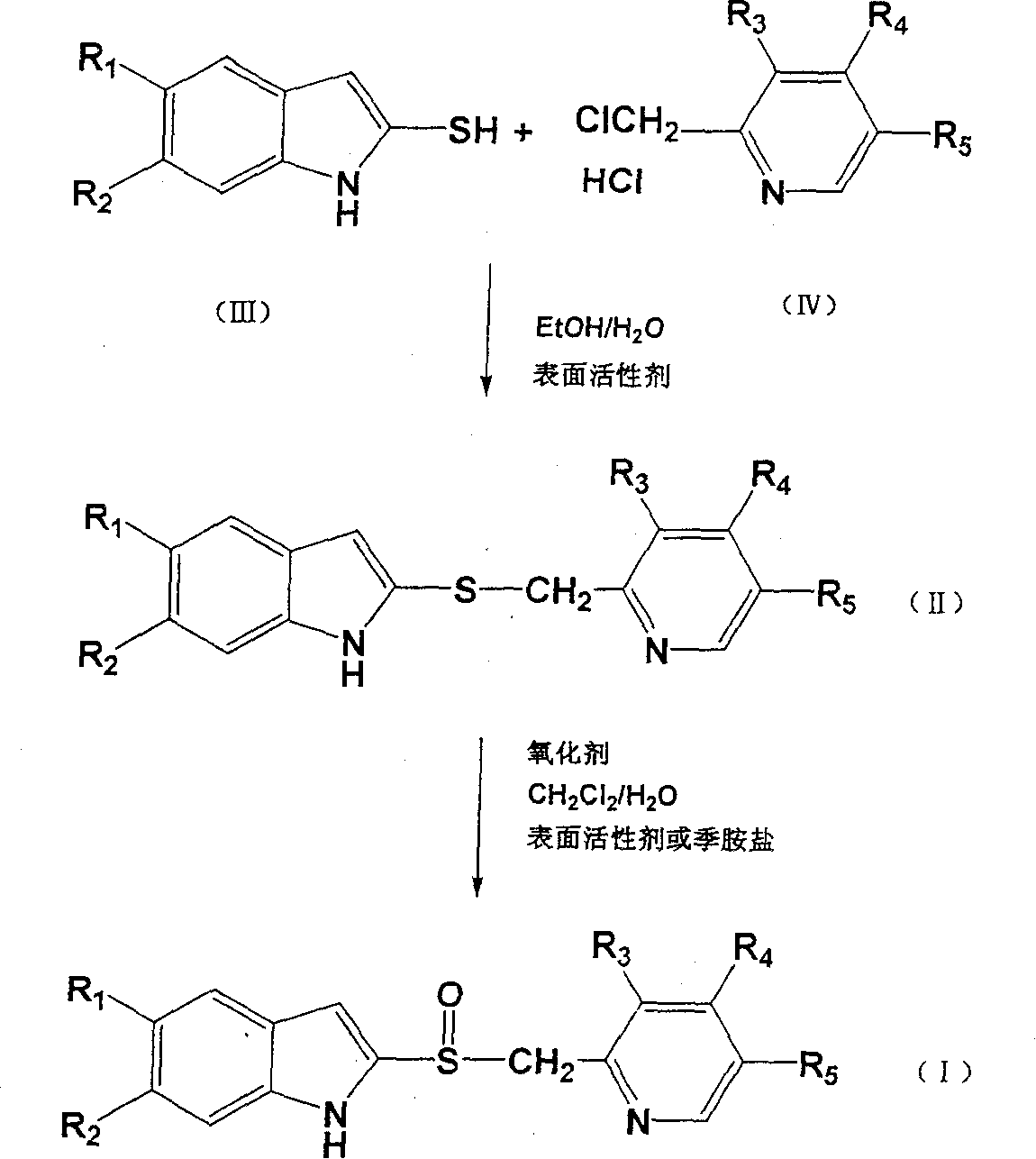
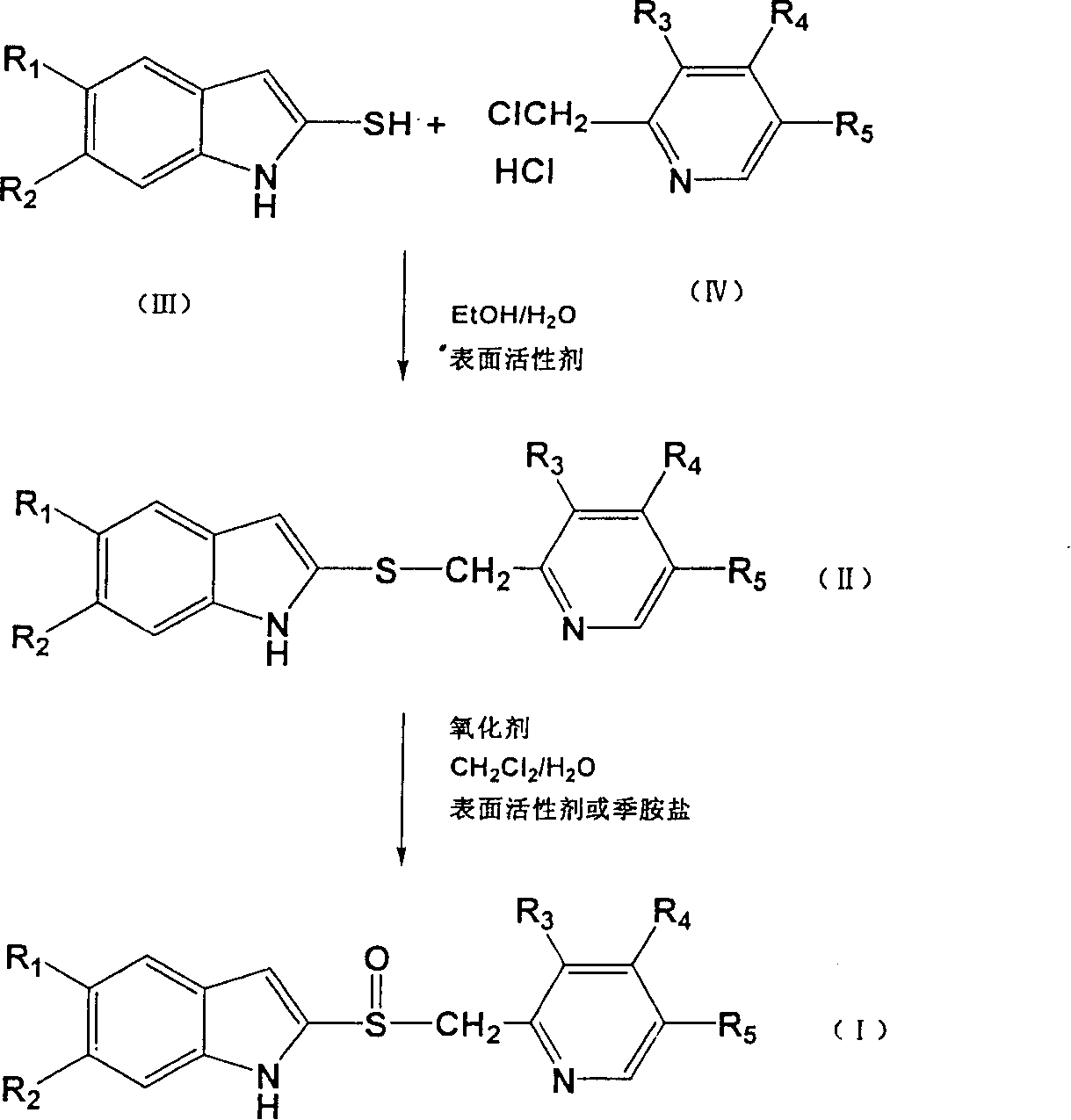
Image
Examples
Embodiment 1
[0052] Add 3.62g (0.02 moles) of 5-methoxy-2-mercapto-1H-benzimidazole, 6 mL of water, 1.63 g (0.04 moles) of sodium hydroxide, and 30 mL of ethanol into the reaction flask. After stirring and dissolving, add 3% Molar equivalent of SDS, add 4.44g (0.02 mole) 2-chloromethyl-4-methoxy-3,5-lutidine hydrochloride, heat and stir to reflux for 2 hours. After the reaction is complete, filter, add water to the filtrate, adjust the pH to 7 with 36% acetic acid, extract with dichloromethane, wash the organic layer with water, evaporate the organic layer to dryness, add acetone, add petroleum ether, stir well, freeze, filter, and dry , to obtain 4.47 g of 5-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridyl)-methylthio]-1H-benzimidazole, yield 69.7%, The content is 95.6%.
Embodiment 2
[0054] Add 3.62g (0.02 moles) of 5-methoxy-2-mercapto-1H-benzimidazole, 6 mL of water, 1.63 g (0.04 moles) of sodium hydroxide, 30 mL of ethanol into the reaction flask, stir to dissolve, then add 5% To the molar equivalent of CTAB, add 4.44g (0.02 moles) of 2-chloromethyl-4-methoxy-3,5-lutidine hydrochloride, and heat, stir and reflux for 2 hours. After the reaction is complete, filter, add water to the filtrate, adjust the pH to 7 with 36% acetic acid, extract with dichloromethane, wash the organic layer with water, evaporate the organic layer to dryness, add acetone, add petroleum ether, stir well, freeze, filter, and dry , to obtain 4.29 g of 5-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridyl)-methylthio]-1H-benzimidazole, yield 66.9%, Content 94.5%.
Embodiment 3
[0056] Add 3.62g (0.02 moles) of 5-methoxy-2-mercapto-1H-benzimidazole, 6 mL of water, 1.63 g (0.04 moles) of sodium hydroxide, and 30 mL of ethanol into the reaction flask. After stirring and dissolving, add 3% Add 4.44 g (0.02 moles) of 2-chloromethyl-4-methoxy-3,5-lutidine hydrochloride to the molar equivalent neutral surfactant TritonX-100, and heat, stir and reflux for 2 hours. After the reaction is complete, filter, add water to the filtrate, adjust the pH to 7 with 36% acetic acid, extract with dichloromethane, wash the organic layer with water, evaporate the organic layer to dryness, add acetone, add petroleum ether, stir well, freeze, filter, and dry 5.65 g of 5-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridyl)-methylthio]-1H-benzimidazole was obtained with a yield of 86.7%, Content 98.9%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com