Method for preparing oxazolidinone compound and intermediate thereof
One compound and one technology, applied in the field of pharmaceutical chemical synthesis, can solve problems such as unsuitable for industrial production, many side reactions, and low purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1: the preparation of the compound shown in formula IIa
[0080]
[0081] (1), the preparation of 2-cyano-5-bromopyridine:
[0082] Dissolve 100 grams of 2,5-dibromopyridine in 1 liter of dimethylformamide, add 32 grams of copper cyanide and 17.8 grams of sodium cyanide to the solution at room temperature, and stir the solution at 150 °C for 7 hours to react. After cooling to room temperature, water was added to the reaction mixture, followed by extraction with ethyl acetate. The organic layer was washed with brine and dried, filtered and concentrated in vacuo to afford 54 g of the title compound, 70% yield. 1 H-NMR (CDCl 3 ) 8.76 (s, 1H), 7.98 (dd, 1H), 7.58 (dd, 1H).
[0083] (2), the preparation of 2-(tetrazol-5-yl)-5-bromopyridine:
[0084] Dissolve 10 grams of 2-cyano-5-bromopyridine in 100 milliliters of dimethylformamide, add 5.33 grams of sodium azide and 4.4 grams of ammonium chloride to the solution at room temperature, and the solution is a...
Embodiment 2
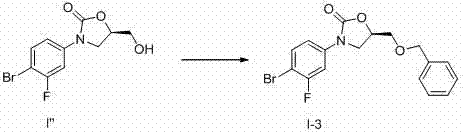
[0089] Example 2: Preparation of (R)-3-(4-iodo-3-fluorophenyl)-2-oxo-5-oxazolidinylmethanol (i.e., compound i'):
[0090]
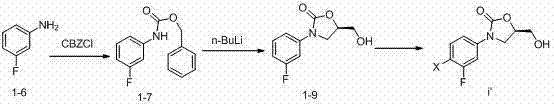
[0091] (1), the preparation of N-benzyloxyformyl-3-fluoroaniline (1-7):
[0092] 100 g of 3-fluoroaniline was dissolved in 1 liter of tetrahydrofuran (THF), and 150 g (1.8 mol) of bicarbonate
[0093] Sodium (NaHCO 3 ) into the solution, and after cooling to 0°C, 154 ml of N-benzooxycarbonyl chloride (CbzCl) was slowly added into the solution for reaction. The reaction mixture was continuously reacted at 0°C for 2 hours with stirring, and then the reaction system was extracted with 0.5 liter of ethyl acetate. After separation, the organic layer was washed with brine, washed with anhydrous magnesium sulfate (MgSO 4 ) was dried and concentrated in vacuo, and the residue was washed twice with n-hexane to obtain 132 g of the title compound as white crystals in a yield of 85%.
[0094] (2) Preparation of (R)-3-(3-fluorophenyl)-2-oxo-5-oxazolidinylmethano...
Embodiment 3
[0099] Example 3: Preparation of tedizolid (compound shown in TD):
[0100]
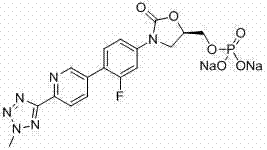
[0101] In a 500ml three-neck flask equipped with a reflux condenser and a thermometer, add 1.57g of Pd(OAc) 2 , 3.7g PPh 3 Dissolve in 150 ml DMF, replace with nitrogen, then add 33.75 ml triethylamine, stir at 70°C until the solution turns red and black, add 47.2 g of (R)-3-(4-iodo-3 prepared in Example 2 -fluorophenyl)-2-oxo-5-oxazolidinylmethanol (compound i') and 34.4g of the compound shown in formula IIa prepared in Example 1, dissolved in 100ml DMF solution, under nitrogen protection, at 90°C Stir the reaction for 2h, monitor the reaction by TLC, and filter it through celite while it is hot. Concentrate to 50ml at 70°C, add 500ml of purified water, stir for 1.0h, filter, wash the filter cake with 50ml of 50% aqueous methanol (volume concentration), and dry at 50°C for 8h. Add 430ml of 50% methanol aqueous solution (volume concentration) to the obtained solid, heat to 70°C for beating for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com